Abstract
Neuronal cell cultures have been used as an essential tool for studying pathomechanisms of toxicity of chemotherapeutic drugs and to develop neuroprotective approaches. They offer the opportunity to dissect disease mechanisms and molecular pathways while allowing precise control of a variety of confounding factors of the physio-chemical environment. As such, a growing number of in vitro studies are published each year to decipher mechanisms of neurotoxicity of taxanes, vinca alcaloids, proteasome inhibitors and platin derivatives and/or to test neuroprotective strategies. Here, we provide a review of cell culture techniques and outcome measures that have been used in the past or are currently employed to model chemotherapy induced neuropathy in vitro. Furthermore, we discuss their advantages as well as their limitations and ways to enhance efficiency and reproducibility of cell culture studies in the field of toxic neuropathy.
Keywords: CIPN, Stem cell, Neurotoxicology, Regeneration, Cell culture technique, Cell culture conditions, Viability, Dorsal root ganglion neuron, Neuronal cell culture
1. Introduction
Peripheral neuropathy due to chemotherapeutic agents (CIPN) represents a particular challenge in the management of cancer patients. CIPN was first described in the late 1960s in patients receiving vincristine (Gottschalk et al., 1968; Moress et al., 1967) and since then has been recognized as the major dose-limiting side effect of many chemotherapeutic agents. Despite advancements in the development of novel treatment approaches such as immune checkpoint inhibitors (Assi et al., 2018; Ok and Young, 2017), antibodies against vascular-endothelial growth receptors (Shitara, 2017) and small molecules against intracellular targets, neurotoxic agents such as taxanes, platin-derivatives, vincristine and bortezomib will still be backbones of chemotherapy for solid cancer and hematological malignancies in the foreseeable future. CIPN is associated with reduced quality of life, due to pain, and increased risk of falls (Ewertz et al., 2015; Kolb et al., 2016). So far, no specific agents have been shown to be able to prevent CIPN and as such, the American Society of Clinical Oncology has recommended no agent for the prevention of CIPN.
Cell cultures are one of the most basic tools to study cellular and molecular effects of conditional changes in the immediate environment including exposure to potentially toxic compounds. Therefore, cell cultures are also frequently employed to model toxic effects of cytostatic compounds to cells of the peripheral nervous system (PNS). They allow controlling the physio-chemical environment such as pH, temperature, exposure to light and supply of nutrients. Moreover, they allow fast replication of an experiment under the same conditions and allow one to evaluate reproducibility. One of the major disadvantages of cell cultures in terms of translation, however, is that they are considered artificial. Usually, as a deconstructed model, only cells of interest are studied thereby neglecting the complex interaction of different cell types. For example, for CIPN research, dorsal root ganglion (DRG) neurons that are cultured in the presence of nerve growth factor (NGF), are often used as primary cell culture, and this cell culture condition selects NGF-responsive small diameter neurons. However, mammalian DRG contain many neuron subpopulations including small and large-diameter neurons in addition to perineuronal satellite cells, Schwann cells, resident macrophages and fibroblasts. Unless a co-culture system is used, cell culture using just neurons do not recapitulate the in vivo interactions among these cell types. In addition, cell culture does not adequately model pharmacokinetics of organ exposure, specifically the entry, distribution and exit of compounds to cells of the PNS which are - like the central nervous system (CNS) - protected by a blood-nerve-barrier (BNB). Finally, pure neuronal cell culture models fall short in modeling the effects of neurotoxic compounds that may be metabolically activated in the liver (Harry and Tiffany-Castiglioni, 2005). This is not relevant for the “traditional” neurotoxic chemotherapy agents but should be taken into account when novel drugs are examined.
Thus, effects that are observed in vitro are usually considered to be preliminary and require validation in a living organism. In such experiments, mostly rodents are used, which on the other hand may not adequately replicate the situation in humans. This dilemma favors the use of in vitro systems with human cells, although they are less frequently used compared to murine and rat neuronal cell cultures. Compared to animal models, cell cultures are more cost-effective and can be used as drug screening platforms. A further aspect that emphasizes the utility of cell culture models is the ethical dilemma of animal experimentation that is encountered by the adherence to the 3R principle (replacement, reduction and refinement). An overview of advantages and disadvantages of cell culture models and animal models are provided in Table 1.
Table 1.
Pros (in green) and Cons (in red) for different experimental approaches.
| Cell culture approach | Animal experimentation |
|---|---|
| fast | time consuming |
| separate effects can be better studied (deconstructed approach) | complex interactions / microenvironment less well controlled |
| simplistic approach, only cells of interest are studied | less artificial due to interaction of different functional systems /organs / cell types |
| control of potentially confounding factors | potentially confounding factors are less well controlled |
| cheap | expensive |
| does not reflect potential metabolism of compounds to active metabolites in non-neuronal tissues | More suitable for PK/PD studies |
| ethical dilemma |
In this review we will discuss cell culture models that are commonly used to study neurotoxic effects of chemotherapeutic agents with a focus on in vitro studies that use chemotherapeutic drugs vincristine, paclitaxel, cisplatin and bortezomib.
2. Cell culture models: Pros and cons
A literature search based on the before mentioned substances in conjunction with keywords “in vitro”, “cell culture”, and “neuropathy” revealed a total of 116 studies that evaluated pathomechanisms or preventive approaches in cell culture systems (Table 2).
Table 2.
Search strategy for reviewed papers. Causes of exclusion were other article types such as review, clinical trials etc., or research unrelated to CIPN.
| Keywords used | n | Included |
|---|---|---|
|
| ||
| Cisplatin AND neuropath* AND (in vitro OR cell culture) | 88 | 38 |
| Cisplatin AND neuropath* AND stem cell | 38 | 4 |
| bortezomib AND neuropath* AND (in vitro OR cell culture) | 33 | 10 |
| Bortezomib AND neuropath* AND stem cell | 153 | 2 |
| Vincristine AND neuropath* AND (in vitro OR cell culture) | 39 | 13 |
| vincristine AND neuropath* AND stem cell | 43 | 3 |
| Paclitaxel AND neuropath* AND (in vitro OR cell culture) | 129 | 43 |
| Paclitaxel AND neuropath* AND Stem cell | 33 | 3 |
Analysis of the studies revealed that in the majority of studies, primary cell cultures derived from rodents were used, mostly rat DRG neurons. Murine neuronal cells were also employed, but to a much lesser extent (Fig. 1). Twenty five percent of the analyzed studies used neuronal cell lines, most often PC12 cells. Human cell lines that were used include SH-SY5Y neuroblastoma cell line (Bavari et al., 2016) and more recently human iPSC derived neurons (Morrison et al., 2016; Wheeler et al., 2015; Wing et al., 2017).
Fig. 1.
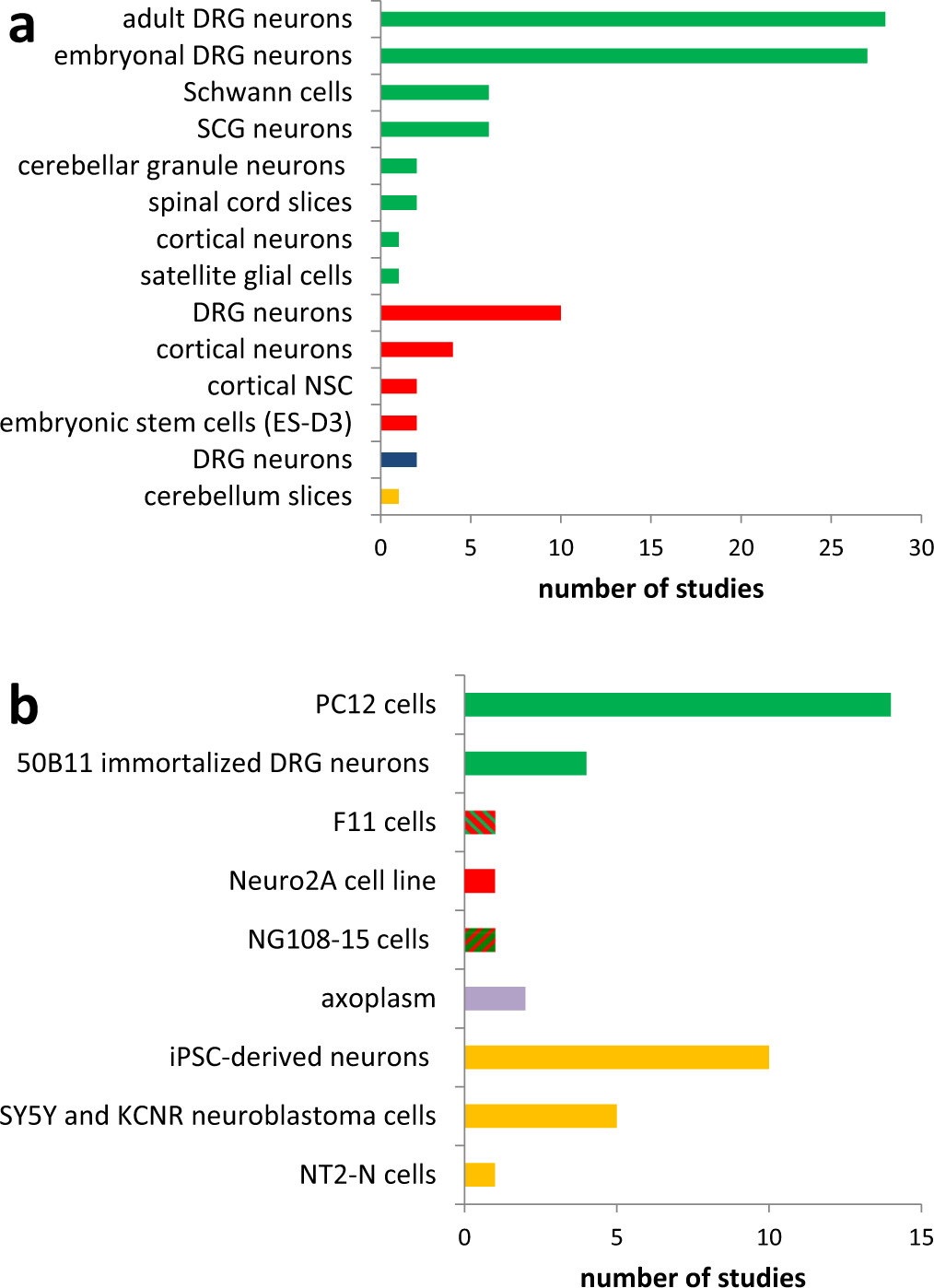
Overview about employed cell culture systems to model chemotherapy induced peripheral neuropathy. a: primary cell cultures, b: cell lines. Species are labeled with colors (green = rat, red = mouse, blue = chicken, purple = squid, yellow = primate/human).
The frequent use of primary cell cultures, derived from DRG neurons requires a closer look at the methodology of this cell culture system, its advantages and its limitations. Generally, DRG as a source of neurons for in vitro experiments has a long history (Bunge et al., 1980). Usually DRGs from embryonic or early postnatal rat pups are prepared by surgical preparation and are used as whole DRG explants or are subsequently dissociated by use of trypsin and/or collagenase. Even slight deviation from commonly used cell preparation protocols may result in different functional properties of cell cultures. For instance, trypsination followed by gentle mechanical dissociation has been shown to alter size and changes of endogenous potassium currents in HEK293 cells (Ponce et al., 2018).
In most studies DRG neurons are cultured in the presence of NGF, with concentration ranging from 3 ng/ml up to 100 ng/ml (Bobylev et al., 2016; Hol et al., 1994; Podratz et al., 2011). One has to bear in mind, that the surgical removal and dissociation of DRG already induces an experimental bias by “artificial” axotomy that activates similar signaling pathways seen after chronic constriction injury leading to hyperexcitability of dissociated neurons (Zheng et al., 2007) and the process of DRG dissociation itself upregulates TrkA, and TrKC receptors (demonstrated for trigeminal ganglia) (Genç et al., 2005).
Age of neurons represents an important confounding parameter for cell culture experiments (Ng and Lozano, 1999). For example, postnatal neurons rapidly downregulate TrkA receptor, which is required for NGF signaling (Bennett et al., 1996; Molliver and Snider, 1997), and may respond dierently to molecules of the environment, as demonstrated for myelin associated glycoprotein (MAG), which inhibits neurite outgrowth in postnatal, but promotes axon elongation in embryonic neurons (Filbin, 1995; Mukhopadhyay et al., 1994). Also postnatally, sensory neurons still mature with changing expression of signaling molecules and transcription factors, for instance in nociceptive neurons for CaMKIIα or TRPV1 (Isensee et al., 2017) and in mechanoreceptive neurons Runt related transcription factors (Runx) 1 and 3 (Yoshikawa et al., 2013). Thus, DRG neurons derived from adult animals are probably more appropriate for modeling CIPN, which primarily affects adults whose sensory neurons are fully matured.
Cell culture studies that are based on animal-derived tissue always raise the question to what extent these results can be extrapolated to the situation in humans. Recently, Schwaid and colleagues compared the proteome of rat and human dorsal root ganglia and found that the DRG proteome is largely (> 75%) congruent which supports the concept of principal translatability, within the constraints that potential differences in protein quantity, function and dynamic changes are not taken into account (Schwaid et al., 2018)(Fig. 2). Species differences have been reported regarding the response to cisplatin and bortezomib in DRG neuronal cell cultures derived from either rat or mice, to the extent that mouse DRG neurons are more resistant to toxic effects (Podratz et al., 2016).
Fig. 2.
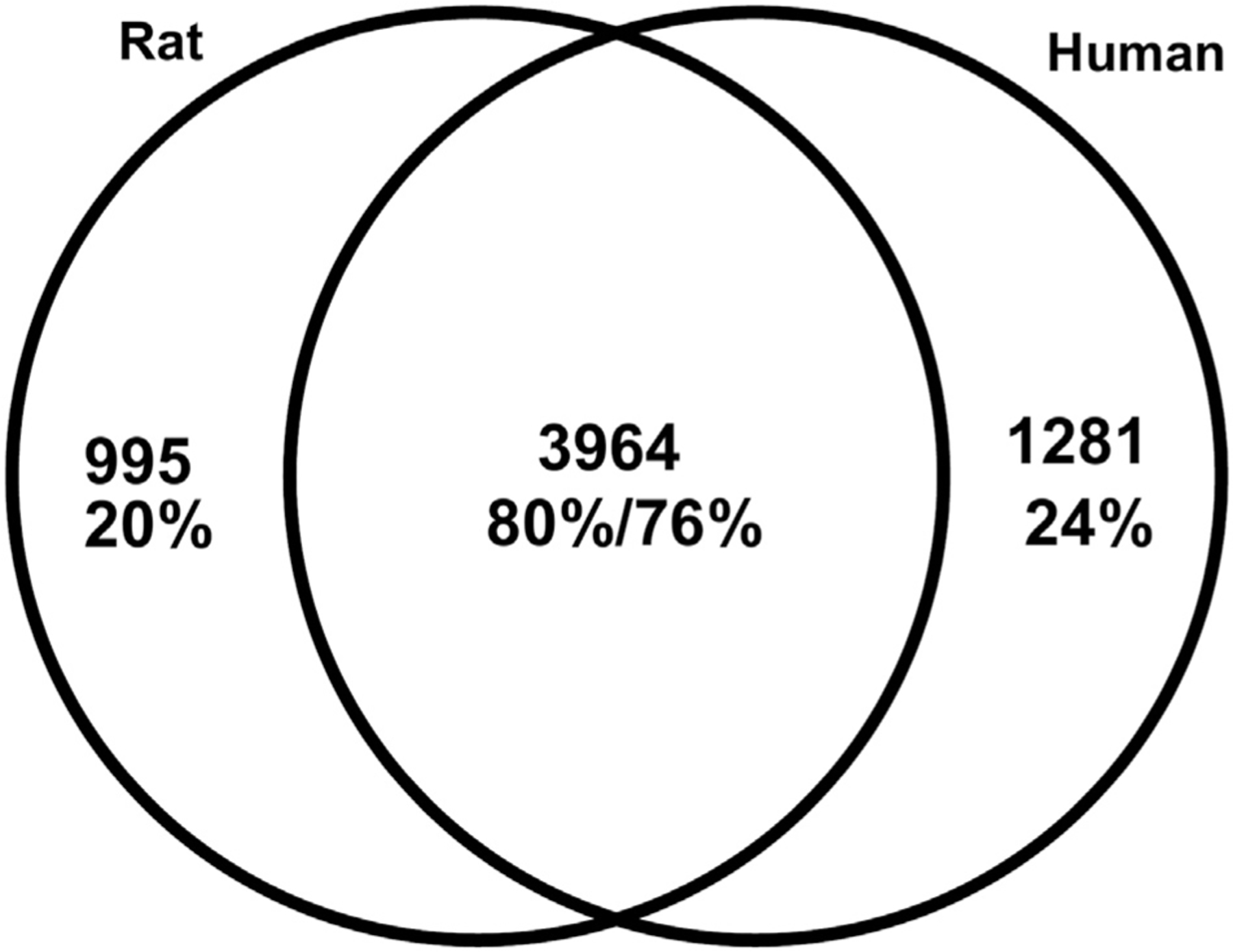
Venn-Diagram of common and unique expressed proteins in rat and human DRG proteome. There is a large overlap in the proteome of both species (reprinted with permission from (Schwaid et al., 2018)).
Moreover, also among mouse strains, the sensitivity to toxic effects varies; for example, cisplatin is more toxic to neurons derived from C57BL/6J mice compared to those stemming from C3H/HeJ mice (Podratz et al., 2016). Despite the above shortcomings, the use of sensory neurons in vitro allows dissecting pathomechanisms at a molecular level, which explains its attractiveness as cell culture system to model CIPN. Compared to a cell line, such as PC12 cells, it may replicate more accurately the situation in vivo, since DRG neurons represent the cell type that is targeted by chemotherapeutics. Furthermore, conducting mechanistic experiments with DRG neurons in vitro is more efficient in terms of time and costs compared to in vivo animal experiments. However, the use of NGF as growth factor selects only NGF responsive (p75 and TrkA positive) neurons, which correspond to small, un- or only thinly myelinated nerve fibers in vivo. It has to be considered that DRGs of higher vertebrates comprise > 20 different subtypes of sensory neurons (Friedel et al., 1997). Moreover, there is abundant clinical and autopsy evidence that the neuropathy caused by paclitaxel, cisplatin and bortezomib affects also, if not predominantly, large sensory nerve fibers (Chaudhry et al., 2008; Krarup-Hansen et al., 1993; Krarup-Hansen et al., 2007; Sahenk et al., 1994). A way to bypass this selectivity of explored neuronal subpopulation and to more closely model the disease condition, one could use neurotrophins such neurotrophin 3 (NT3), glial derived neurotrophic factor (GDNF) (Gavazzi et al., 1999) and brain-derived neurotrophic factor (BDNF) that support other sensory neuronal lineages.
DRG explants and even dissociated neuronal cell cultures contain almost invariably to some degree non-neuronal cells such as Schwann cells and fibroblasts. Depending on the desired cell culture condition, depletion of these cells can be achieved by mechanical approaches (Jirsova et al., 1997) or with mitotic inhibitors such as Floxuridine (FUdR) (Malin et al., 2007) or cytosine arabinoside (AraC) (Wood, 1976), but these agents may be toxic to neurons as well (Wallace and Johnson Jr., 1989; Zhuo et al., 2018) and thus confound experimental outcomes. Neuronal cell cultures that are basically free from non-neuronal cells may be better monitorable, (i.e. measuring direct response to neurotoxic drugs), and may be analyzed by standard methods like Western-Blot and ELISA to monitor intracellular signaling cascades. However in the nervous system of vertebrates, virtually all peripheral axons are engulfed by Schwann cells, even without assembling myelin, and also DRG contain, apart from neurons, a myriad of non-neuronal cells including macrophages, fibroblasts, satellite glial cells and Schwann cells. Therefore, mixed co-cultures must be considered less artificial compared to pure neuronal cell cultures. Mixed cultures, on the other hand, may require advanced single cell analysis techniques in addition to immunostaining, such as single cell RT-PCR (Ho and O'Leary, 2011) or a quantitative automated microscopy (Andres et al., 2010), when signaling cascades and response to stimuli are investigated. Markers that are often used to label specific cell populations are β III tubulin for neurons and S100 for Schwann cells. Same limitations must be considered when pure Schwann cell cultures, in the absence of any neurons, are used (Campana et al., 1998; Imai et al., 2017).
3. Cell lines to model CIPN
The most frequently used cell lines to model CIPN in vitro are PC12 cells, (SH)-SY5Y cells, and immortalized DRG neurons. PC12 cells originate from rat phaeochromocytoma, divide and secrete catecholamines (mostly dopamine and noradrenaline). When exposed to NGF, PC12 cells stop dividing and develop to a neuronal phenotype that extend neurites, become electrically excitable, and establish synapses when co-cultured with muscle cells (Fujita et al., 1989). Although being a cell line, variability exists among PC12 clones in terms of protein expression and extension of neurites (Clementi et al., 1992; Koike et al., 2017). High number of passages alters the sensitivity to toxic compounds and may lead to misinterpretation of toxic or neuroprotective interventions (Kinarivala et al., 2017). Despite these concerns, studies that used PC12 reported overall consistent effects; for example for cisplatin (in a dose of 32 μM), a reduction of undifferentiated PC12 cell viability ranging from 40 to 50% over 24 h can be expected (Li et al., 2015; Li et al., 2019; Mendonca et al., 2009).
SH-SY5Y is a human neuroblastoma cell line and can be differentiated by retinoic acid, dibutyryl cyclic AMP (dbcAMP), or neurotrophins into mature human neurons that express neurites (Kovalevich and Langford, 2013). Due to expression of tyrosine hydroxylase and acetylcholine receptors they are frequently used as a model for dopaminergic or cholinergic, but a less common model for sensory neurons. Nevertheless, a comparison of the transcriptome between SH-SY5Y and murine DRG revealed, that SH-SY5Y express many markers that are present in peripheral sensory neurons such as RET, GDNF receptor tyrosine kinase, and TrkA, although the expression profile is not characteristic for a specific subclass of peripheral sensory neurons (Yin et al., 2016). Importantly, they lack the nociceptive neuron marker, transient receptor potential vanilloid family-1 (TRPV1), and the peptidergic neuron marker CGRP, which makes this cell line not suitable for drug screening approaches related to CIPN associated neuropathic pain. A clear advancement in this regard is the establishment of an immortalized rat DRG neuronal line, 50B11 (Chen et al., 2007). These cells can also be differentiated into a neurite-extending phenotype, and express markers such as p75, TrkA, c-ret and GFRa1. After exposure to cisplatin or paclitaxel, these cells show a reduction in neurite length (Vencappa et al., 2015; Zhu et al., 2013), and mitochondrial dysfunction (Galley et al., 2017).
4. Human cell-based models
4.1. hESC-derived neuronal cells
Despite substantial similarity in the protein expression of human and rat sensory neurons, the use of human neurons is preferred and considered less artificial. The stem cell technology offers the opportunity to culture human neurons. One approach is the use of human embryonic stem cells (ESCs) that are totipotent and induced into neurons by use of specific cell culture conditions (Jones et al., 2018). It has been demonstrated that sensory neurons derived from hESC are heterogeneous with regard to expression of specific markers for neuronal subpopulation and as such comparable to the situation in vivo (Alshawaf et al., 2018).
4.2. hiPSC-derived neuronal cells
Human induced pluripotent stem cells (hiPSCs) offer an alternative to generate sensory neurons and have already been employed by several research groups to study neurotoxicity (Hoelting et al., 2016; Wheeler et al., 2015; Wing et al., 2017). Interestingly, neurons derived from genetically different hiPSCs also displayed differences in terms of neurite outgrowth in the presence of paclitaxel which emphasizes its suitability as a genetically diverse human cellular model for CIPN (Wheeler et al., 2015). Rana and colleagues recently demonstrated the use of hiPSCs as tool for high throughput screening (Rana et al., 2017). Limitations of hiPSC include tedious and time intensive cull culture conditions with low efficiency in terms of reprogramming and genetic instability. Direct conversion of somatic cells (i.e. fibroblasts) to functional neurons may offer an alternative to circumvent these hurdles (Hoelting et al., 2016).
5. In vitro outcome measures and their relevance to human disease
Just as there are major differences in the use of cell culture systems and techniques, there are also major differences regarding the use of in vitro outcome measures. These include mainly measures of cell viability, axon morphology and biochemical assays. Although all these measures have their advantages in terms of feasibility, robustness, and reproducibility, these returns do not necessarily correspond to relevance and usefulness for further translation to in vivo models or to the human condition CIPN. In humans, CIPN is a predominantly sensory axonal neuropathy that is neuropathologically characterized by a “dying back” axon degeneration that proceeds in a distal-to-proximal fashion. An exception to this is cisplatin induced neuropathy, which causes a sensory neuronopathy, with neuronal cell death at the level of the DRG (Staff, N.P, et al., 2017). Therefore, an assay that measures axonal degeneration might be more suitable to address paclitaxel induced neuropathy, whereas a “live-dead” assay that measures cell viability might be more translatable to cisplatin induced neuropathy. In the following sections we will discuss the most frequently employed assays.
5.1. Cell viability
Viability of neuronal cell lines or non-neuronal cells is a frequently used endpoint in in vitro studies. A conceptual limitation of cell viability assays is that other mechanisms than just neuronal cell death may contribute to the neurotoxic effects in vivo and functional impairment may occur even at sublethal concentrations (Harry and Tiffany Castiglioni, 2005). Moreover, cell populations studied in vitro are usually not in the same stage of their cell cycle. Thus they may respond differently to cytostatics with some cells undergoing cell death while other (non-dividing) cells may survive. This is particular important for primary neuronal cell cultures that use (immature) embryonal DRG neurons but heterogeneous cell cycle may also bias cytotoxicity experiments in neuronal cell lines. To bypass this phenomenon, experimental paradigms with varying (i.e. longer) incubation times and multiple time points of measurements are recommended (Ramirez et al., 2010).
Cell viability can be assessed with fluorescent probes such as propidium iodide (to label dead cells) (Ustun et al., 2018), fluorescein diacetate (FDA, producing green fluorescence in living cells) (Aubert et al., 2008), colorimetric WST-8 assay (Kawashiri et al., 2018), or TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining (Melli et al., 2008). A drawback of assays that are based on dyes that enters the compromised membranes of dying cells is that a proportion of these cells may still retain their membrane integrity for a substantial period of time after injury (Ramirez et al., 2010). In CIPN models with primary cell lines, a common problem is to identify apoptotic neurons in mixed cultures from non-neuronal cells, which requires an additional staining and washing procedure which may detach dead cells and hence introduce bias. These markers could be NeuN, which is expressed exclusively in neuronal cell nuclei or Tuj-1 which stains neuronal cell bodies and neurites. Vimentin is a specific biomarker, compared to S100 and GFAP, to stain cell bodies and proprocesses of non-neuronal cells according to a study by Guo and colleagues, (Guo et al., 2017).
Viability assays that are based on assessment of metabolic function are the MTT assay and quantification of ATP. The MTT assay is based on enzymatic conversion of a tetrazolium compound to insoluble formazan crystals, indicating living cells (van Tonder et al., 2015). Other modified tetrazolium-based assays are the MTS and WST assays. The MTT assay is considered gold standard for cytotoxicity assessment, but might be biased by mitochondrial number and function that influence the conversion to formazan crystals (van Tonder et al., 2015), medium conditions including serum and albumin, and growth state of cells (confluent or exponential) (Liu and Dalgleish, 2009; Stepanenko and Dmitrenko, 2015). Notably, Ulukaya and colleagues reported that cisplatin and paclitaxel can increase absorbance values of the MTT assay, which may lead to false positive overestimation of cell viability (Ulukaya et al., 2004). A way to improve accurateness of cell viability, combination of assays might be considered either by trypan blue staining or by adding conventional microscopic assay (Garg et al., 2018). Cell death goes along with rapid depletion of ATP, which is exploited by luciferase-based ATP detection assays. However, different cell types may have different amounts of ATP and cell culture conditions may further influence ATP content by contact inhibition at high densities. A major advantage of cell viability assays is that they are easily adaptable for high throughput screening and may be used in an initial screen for potential neuroprotective compounds using robotic systems (Schmidt et al., 2017).
5.2. Morphological measures
5.2.1. Neurite length
Measurement of neurite length is one of the most commonly used assays to investigate axon degeneration in vitro. Usually dissociated cell cultures are stained with a neuronal marker (β III tubulin, neurofilament) and length of neurites, either total or longest, is measured by use of an image analyzing program, optionally facilitated by semi-automatic imaging processing programs (Long et al., 2017). Furthermore, several automated image processing algorithms allow one to develop high content screening assays to examine potential neuroprotective compounds (Chen et al., 2015; Rudhard et al., 2015). In case DRG explants are used, radial neurite length is assessed. Depending on how this assay is structured it can measure actual axon degeneration (e.g. by allowing the axons to extend for a period of time before adding the toxic and/or protective compounds) or inhibition of further neurite outgrowth (i.e. secondary regeneration after the initial injury induced by culturing of DRG neurons). Despite this conceptual shortcoming, the neurite length is often considered to be an assessment tool for axon injury in general. In studies that used microtubule stabilizers, also the branching of axons is assessed by counting the number of branching segments per a given field (Pittman et al., 2016).
5.2.2. Neurofilament
Axons contain intermediate filaments composed of neurofilament (NF) subunits, of different size, namely, NF-L (68 kDa), NF-M (150 kDa) and NF-H (190–210 kDa)(Hares et al., 2011). Phosphorylation of NF is a marker of axon integrity and dot blot assay has been proposed as an in vitro assessment (Hares et al., 2011). In vivo assessment of NF-L in body fluids has emerged as novel biomarker for axonal damage in many neuropathic conditions, including CIPN (Mariotto et al., 2018; Meregalli et al., 2018). In vitro studies have used staining for NF-L as marker for axonal loss (Jackson et al., 2018), but to date it is unknown if an immunoassay for NF-L in cell supernatants may also serve as a marker to assess axon integrity in neuronal cell cultures.
5.3. Electrophysiological recordings
Whole and dissociated DRGs can be used to perform electrophysiological recordings by use of patch clamp technique. It allows studying channel and receptor function of neurons in vitro. Conceptually, it is anticipated that ion channels expressed in the membrane of the cell soma are congruent to those of nerve terminals which are not accessible to microelectrodes (Passmore, 2005). Basically, a glass electrode is sealed onto a membrane patch to measure rapid ion channel-mediated conductance changes across a neuronal membrane (Hoerbelt and Heifets, 2018). Likewise, intracellular activity can be recorded by use of appropriate sharp glass electrodes.
By use of these techniques, it could be demonstrated that paclitaxel evokes ectopic spontaneous activity in neurons in vitro which is caused an increased expression of the ion channel Nav1.7 (Li et al., 2018; Zhang and Dougherty, 2014). Another example includes oxaliplatin, which alters currents of voltage-gated calcium channels (Leo et al., 2017; Schmitt et al., 2018). An extension to these single cell recordings is the use of multi-well multielectrode arrays to record spontaneous activity in whole DRG cell cultures (Newberry et al., 2016).
6. Summary and future directions
Over several decades cell cultures have served as a valuable tool to study neurotoxicity with a growing diversity in terms of used cell lineages, outcome measures and molecular/biochemical methods. As much as this diversity is to be welcomed, it makes it more difficult to compare observations and to assess its overall significance. Some form of standardization in the field is necessary. For example, approaches to align in vitro testing for CNS toxicity has already been proposed and validated around 30 years ago (Atterwill et al., 1993; Atterwill and Walum, 1989; Williams et al., 1994). This approach employed a three tiered in vitro testing procedure for neurotoxicity with screening experiments based on human and neuroblastoma cell lines and rat primary mixed neural cell cultures with endpoints including MTT reduction and LDH release (first tier). As second tier organotypic cultures were proposed for investigation of kinetics, specificity and patho-mechanisms. A third phase included experiments using specific neural and astrocytic cell cultures. A potential modification for the purpose to study CIPN in vitro is shown in Fig. 3. However, such general recommendations and guidelines for conducting experiments are only useful if they are implemented over the long term, and widely accepted in the research community (the above mentioned papers were last referenced more than ten years ago). Furthermore such recommendations would need to be quickly adapted and regularly updated in line with the latest methodological and scientific developments. Some of these future developments might be the more extensive application of stem cell technologies or novel cell culture techniques such as 3D cell cultures. Particularly the latter may help to improve translation of findings to the next pre-clinical level in small animals. Examples include the use of gelatin blocks with capillary structure to model the 3D structure of nerves (Anderson et al., 2018) or spheroids consisting of Schwann cells and neurons (Kraus et al., 2015).
Fig. 3.
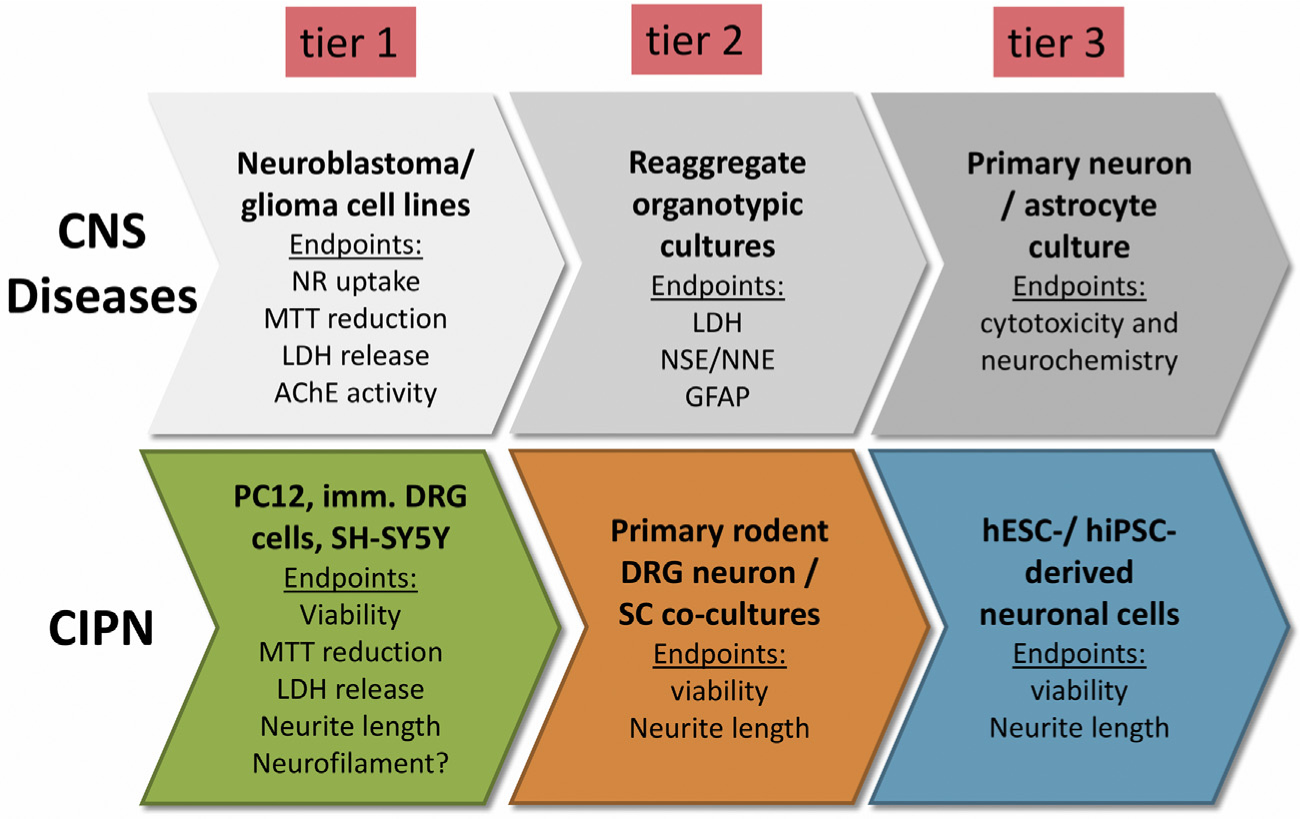
Three-tiered-test model to study cytotoxicity. The upper sequence shows a three-tiered test model to study CNS cytotoxicity (modified after (Atterwill et al., 1993; Williams et al., 1994), the lower line a potential modification to study CIPN in vitro. NNE = non-neuronal enolase. NSE = neuron specific enolase.
On a conceptual level, more rapid dissemination of research findings and closer collaboration with exchange of knowledge in methodology of research groups in the field of neuroscience, but also oncology, may help to design future research in a more efficient way. As such, the recently founded Toxic Neuropathy Consortium (TNC, https://sites.google.com/campus.unimib.it/tncwebsite/home-page) may represent a step forward to optimize pre-clinical research in the field of CIPN.
References
- Alshawaf AJ, Viventi S, Qiu W, D’Abaco G, Nayagam B, Erlichster M, Chana G, Everall I, Ivanusic J, Skafidas E, Dottori M, 2018. Phenotypic and functional characterization of peripheral sensory neurons derived from human embryonic stem cells. Sci. Rep. 8, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WA, Willenberg AR, Bosak AJ, Willenberg BJ, Lambert S, 2018. Use of a capillary alginate gel (Capgel™) to study the three-dimensional development of sensory nerves reveals the formation of a rudimentary perineurium. J. Neurosci. Methods 305, 46–53. [DOI] [PubMed] [Google Scholar]
- Andres C, Meyer S, Dina OA, Levine JD, Hucho T, 2010. Quantitative automated microscopy (QuAM) elucidates growth factor specific signalling in pain sensitization. Mol. Pain 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assi HI, Kamphorst AO, Moukalled NM, Ramalingam SS, 2018. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 124, 248–261. [DOI] [PubMed] [Google Scholar]
- Atterwill CK, Walum E, 1989. Neurotoxicology in vitro: model systems and practical applications. Toxicol. in Vitro 3, 159–161. [DOI] [PubMed] [Google Scholar]
- Atterwill CK, Davenport-Jones J, Goonetilleke S, Johnston H, Purcell W, Thomas SM, West M, Williams S, 1993. New models for the in vitro assessment of neurotoxicity in the nervous system and the preliminary validation stages of a ‘tiered-test’ model. Toxicol. in Vitro 7, 569–580. [DOI] [PubMed] [Google Scholar]
- Aubert N, Vaudry D, Falluel-Morel A, Desfeux A, Fisch C, Ancian P, de Jouffrey S, Le Bigot JF, Couvineau A, Laburthe M, Fournier A, Laudenbach V, Vaudry H, Gonzalez BJ, 2008. PACAP prevents toxicity induced by cisplatin in rat and primate neurons but not in proliferating ovary cells: involvement of the mitochondrial apoptotic pathway. Neurobiol. Dis. 32, 66–80. [DOI] [PubMed] [Google Scholar]
- Bavari M, Tabandeh MR, Najafzadeh Varzi H, Bahramzadeh S, 2016. Neuroprotective, antiapoptotic and antioxidant effects of l-carnitine against caffeine-induced neurotoxicity in SH-SY5Y neuroblastoma cell line. Drug Chem. Toxicol. 39, 157–166. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB, 1996. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur. J. Neurosci. 8, 2204–2208. [DOI] [PubMed] [Google Scholar]
- Bobylev I, Maru H, Joshi AR, Lehmann HC, 2016. Toxicity to sensory neurons and Schwann cells in experimental linezolid-induced peripheral neuropathy. J. Antimicrob. Chemother. 71, 685–691. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Williams AK, Wood PM, Uitto J, Jeffrey JJ, 1980. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J. Cell Biol. 84, 184–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM, Eskeland N, Calcutt NA, Misasi R, Myers RR, O'Brien JS, 1998. Prosaptide prevents paclitaxel neurotoxicity. Neurotoxicology 19, 237–244. [PubMed] [Google Scholar]
- Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I, 2008. Characteristics of bortezomib- and thalidomide-induced peripheral neuropathy. J. Peripher. Nerv. Syst. 13, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mi R, Haughey N, Oz M, Hoke A, 2007. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J. Peripher. Nerv. Syst. 12, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Sun YT, Chen YF, Lee MY, Chang LY, Chang JY, Shen MR, 2015. Integrating image-based high-content screening with mouse models identifies 5-Hydroxydecanoate as a neuroprotective drug for paclitaxel-induced neuropathy. Mol. Cancer Ther. 14, 2206–2214. [DOI] [PubMed] [Google Scholar]
- Clementi E, Racchetti G, Zacchetti D, Panzeri MC, Meldolesi J, 1992. Differential expression of markers and activities in a group of PC12 nerve cell clones. Eur. J. Neurosci. 4, 944–953. [DOI] [PubMed] [Google Scholar]
- Ewertz M, Qvortrup C, Eckhoff L, 2015. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol 54, 587–591. [DOI] [PubMed] [Google Scholar]
- Filbin MT, 1995. Myelin-associated glycoprotein: a role in myelination and in the inhibition of axonal regeneration? Curr. Opin. Neurobiol. 5, 588–595. [DOI] [PubMed] [Google Scholar]
- Friedel RH, Schnurch H, Stubbusch J, Barde YA, 1997. Identification of genes differentially expressed by nerve growth factor- and neurotrophin-3-dependent sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 94, 12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G, 1989. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 80, 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley HF, McCormick B, Wilson KL, Lowes DA, Colvin L, Torsney C, 2017. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J. Pineal Res. 63 (4), e12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Huifu H, Kaul SC, Wadhwa R, 2018. Integration of conventional cell viability assays for reliable and reproducible read-outs: experimental evidence. BMC Res Notes 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I, Kumar RD, McMahon SB, Cohen J, 1999. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur. J. Neurosci. 11, 3405–3414. [DOI] [PubMed] [Google Scholar]
- Genç B, Ulupinar E, Erzurumlu RS, 2005. Differential Trk expression in explant and dissociated trigeminal ganglion cell cultures. J. Neurobiol. 64, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk PG, Dyck PJ, Kiely JM, 1968. Vinca alkaloid neuropathy: nerve biopsy studies in rats and in man. Neurology 18, 875–882. [DOI] [PubMed] [Google Scholar]
- Guo L, Hamre J, Eldridge S, Behrsing HP, Cutuli FM, Mussio J, Davis M, 2017. S highlight: multiparametric image analysis of rat dorsal root ganglion cultures to evaluate peripheral neuropathy-inducing chemotherapeutics. Toxicol. Sci. 156, 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hares K, Kemp K, Gray E, Scolding N, Wilkins A, 2011. Neurofilament dot blot assays: novel means of assessing axon viability in culture. J. Neurosci. Methods 198, 195–203. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Tiffany-Castiglioni E, 2005. Evaluation of neurotoxic potential by use of in vitro systems. Expert Opin. Drug Metab. Toxicol. 1, 701–713. [DOI] [PubMed] [Google Scholar]
- Ho C, O’Leary ME, 2011. Single-cell analysis of sodium channel expression in dorsal root ganglion neurons. Mol. Cell. Neurosci. 46, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelting L, Klima S, Karreman C, Grinberg M, Meisig J, Henry M, Rotshteyn T, Rahnenführer J, Blüthgen N, Sachinidis A, Waldmann T, Leist M, 2016. Stem cell-derived immature human dorsal root ganglia neurons to identify peripheral Neurotoxicants. Stem Cells Transl. Med. 5, 476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerbelt P, Heifets BD, 2018. Native system and cultured cell electrophysiology for investigating anesthetic mechanisms. Methods Enzymol. 602, 301–338. [DOI] [PubMed] [Google Scholar]
- Hol EM, Mandys V, Sodaar P, Gispen WH, Bar PR, 1994. Protection by an ACTH4–9 analogue against the toxic effects of cisplatin and taxol on sensory neurons and glial cells in vitro. J. Neurosci. Res. 39, 178–185. [DOI] [PubMed] [Google Scholar]
- Imai S, Koyanagi M, Azimi Z, Nakazato Y, Matsumoto M, Ogihara T, Yonezawa A, Omura T, Nakagawa S, Wakatsuki S, Araki T, Kaneko S, Nakagawa T, Matsubara K, 2017. Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci. Rep. 7, 5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Schild C, Schwede F, Hucho T, 2017. Crosstalk from cAMP to ERK1/2 emerges during postnatal maturation of nociceptive neurons and is maintained during aging. J. Cell Sci. 130, 2134–2146. [DOI] [PubMed] [Google Scholar]
- Jackson TC, Kotermanski SE, Jackson EK, Kochanek PM, 2018. BrainPhys® increases Neurofilament levels in CNS cultures, and facilitates investigation of axonal damage after a mechanical stretch-injury in vitro. Exp. Neurol. 300, 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsova K, Sodaar P, Mandys V, Bar PR, 1997. Cold jet: a method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J. Neurosci. Methods 78, 133–137. [DOI] [PubMed] [Google Scholar]
- Jones I, Yelhekar TD, Wiberg R, Kingham PJ, Johansson S, Wiberg M, Carlsson L, 2018. Development and validation of an in vitro model system to study peripheral sensory neuron development and injury. Sci. Rep. 8, 15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashiri T, Miyagi A, Shimizu S, Shigematsu N, Kobayashi D, Shimazoe T, 2018. Dimethyl fumarate ameliorates chemotherapy agent-induced neurotoxicity in vitro. J. Pharmacol. Sci. 137, 202–211. [DOI] [PubMed] [Google Scholar]
- Kinarivala N, Shah K, Abbruscato TJ, Trippier PC, 2017. Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis. ACS Chem. Neurosci. 8, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Kadoya M, Kaida KI, Ikeda S, Kawagashira Y, Iijima M, Kato D, Ogata H, Yamasaki R, Matsukawa N, Kira JI, Katsuno M, Sobue G, 2017. Paranodal dissection in chronic inflammatory demyelinating polyneuropathy with anti-neuro-fascin-155 and anti-contactin-1 antibodies. J. Neurol. Neurosurg. Psychiatry 88, 465–473. [DOI] [PubMed] [Google Scholar]
- Kolb NA, Smith AG, Singleton JR, Beck SL, Stoddard GJ, Brown S, Mooney K, 2016. The Association of Chemotherapy-Induced Peripheral Neuropathy Symptoms and the risk of falling. JAMA Neurol 73, 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Langford D, 2013. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 1078, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup-Hansen A, Fugleholm K, Helweg-Larsen S, Hauge EN, Schmalbruch H, Trojaborg W, Krarup C, 1993. Examination of distal involvement in cisplatin-induced neuropathy in man. An electrophysiological and histological study with particular reference to touch receptor function. Brain 116 (Pt 5), 1017–1041. [DOI] [PubMed] [Google Scholar]
- Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rorth M, Krarup C, 2007. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain 130, 1076–1088. [DOI] [PubMed] [Google Scholar]
- Kraus D, Boyle V, Leibig N, Stark GB, Penna V, 2015. The neuro-spheroid–a novel 3D in vitro model for peripheral nerve regeneration. J. Neurosci. Methods 246, 97–105. [DOI] [PubMed] [Google Scholar]
- Leo M, Schmitt LI, Jastrow H, Thomale J, Kleinschnitz C, Hagenacker T, 2017. Cisplatin alters the function and expression of N-type voltage-gated calcium channels in the absence of morphological damage of sensory neurons. Mol. Pain 13 (1744806917746565). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Sun JY, Wang K, Zhang S, Hou YJ, Yang MF, Fu XY, Zhang ZY, Mao LL, Yuan H, Fang J, Fan CD, Zhu MJ, Sun BL, 2015. Attenuation of cisplatin-induced neurotoxicity by Cyanidin, a natural inhibitor of ROS-mediated apoptosis in PC12 cells. Cell. Mol. Neurobiol. 35, 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM, 2018. DRG voltage-gated Sodium Channel 1.7 is upregulated in paclitaxel-induced neuropathy in rats and in humans with neuropathic pain. J. Neurosci. 38, 1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zheng M, Sah SK, Mishra A, Singh Y, 2019. Neuroprotective influence of sitagliptin against cisplatin-induced neurotoxicity, biochemical and behavioral alterations in Wistar rats. Mol. Cell. Biochem. 455, 91–97. [DOI] [PubMed] [Google Scholar]
- Liu WM, Dalgleish AG, 2009. MTT assays can underestimate cell numbers. Cancer Chemother. Pharmacol. 64, 861–862. [DOI] [PubMed] [Google Scholar]
- Long BL, Li H, Mahadevan A, Tang T, Balotin K, Grandel N, Soto J, Wong SY, Abrego A, Li S, Qutub AA, 2017. GAIN: a graphical method to automatically analyze individual neurite outgrowth. J. Neurosci. Methods 283, 62–71. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC, 2007. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2, 152–160. [DOI] [PubMed] [Google Scholar]
- Mariotto S, Farinazzo A, Magliozzi R, Alberti D, Monaco S, Ferrari S, 2018. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J. Peripher. Nerv. Syst. 23, 174–177. [DOI] [PubMed] [Google Scholar]
- Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, Taroni F, Lauria G, 2008. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp. Neurol. 214, 276–284. [DOI] [PubMed] [Google Scholar]
- Mendonca LM, Dos Santos GC, Antonucci GA, Dos Santos AC, Bianchi Mde L, Antunes LM, 2009. Evaluation of the cytotoxicity and genotoxicity of curcumin in PC12 cells. Mutat. Res. 675, 29–34. [DOI] [PubMed] [Google Scholar]
- Meregalli C, Fumagalli G, Alberti P, Canta A, Carozzi VA, Chiorazzi A, Monza L, Pozzi E, Sandelius A, Blennow K, Zetterberg H, Marmiroli P, Cavaletti G, 2018. Neurofilament light chain as disease biomarker in a rodent model of chemotherapy induced peripheral neuropathy. Exp. Neurol. 307, 129–132. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Snider WD, 1997. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J. Comp. Neurol. 381, 428–438. [DOI] [PubMed] [Google Scholar]
- Moress GR, D’Agostino AN, Jarcho LW, 1967. Neuropathy in lymphoblastic leukemia treated with vincristine. Arch. Neurol. 16, 377–384. [DOI] [PubMed] [Google Scholar]
- Morrison G, Liu C, Wing C, Delaney SM, Zhang W, Dolan ME, 2016. Evaluation of inter-batch differences in stem-cell derived neurons. Stem Cell Res. 16, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT, 1994. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13, 757–767. [DOI] [PubMed] [Google Scholar]
- Newberry K, Wang S, Hoque N, Kiss L, Ahlijanian MK, Herrington J, Graef JD, 2016. Development of a spontaneously active dorsal root ganglia assay using mul-tiwell multielectrode arrays. J. Neurophysiol. 115, 3217–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WP, Lozano AM, 1999. Neuronal age influences the response to neurite outgrowth inhibitory activity in the central and peripheral nervous systems. Brain Res. 836, 49–61. [DOI] [PubMed] [Google Scholar]
- Ok CY, Young KH, 2017. Checkpoint inhibitors in hematological malignancies. J. Hematol. Oncol. 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore GM, 2005. Dorsal root ganglion neurones in culture: a model system for identifying novel analgesic targets? J. Pharmacol. Toxicol. Methods 51, 201–208. [DOI] [PubMed] [Google Scholar]
- Pittman SK, Gracias NG, Fehrenbacher JC, 2016. Nerve growth factor alters microtubule targeting agent-induced neurotransmitter release but not MTA-induced neurite retraction in sensory neurons. Exp. Neurol. 279, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ, 2011. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 41, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Kulkarni A, Pleticha J, Kanwar R, Beutler AS, Staff NP, Windebank AJ, 2016. Neurotoxicity to DRG neurons varies between rodent strains treated with cisplatin and Bortezomib. J. Neurol. Sci. 362, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce A, Castillo A, Hinojosa L, Martinez-Rendon J, Cereijido M, 2018. The expression of endogenous voltage-gated potassium channels in HEK293 cells is affected by culture conditions. Phys. Rep. 6, e13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez CN, Antczak C, Djaballah H, 2010. Cell viability assessment: toward content-rich platforms. Expert Opin. Drug Discovery 5, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana P, Luerman G, Hess D, Rubitski E, Adkins K, Somps C, 2017. Utilization of iPSC-derived human neurons for high-throughput drug-induced peripheral neuropathy screening. Toxicol. in Vitro 45, 111–118. [DOI] [PubMed] [Google Scholar]
- Rudhard Y, Sengupta Ghosh A, Lippert B, Böcker A, Pedaran M, Krämer J, Ngu H, Foreman O, Liu Y, Lewcock JW, 2015. Identification of 12/15-lipoxygenase as a regulator of axon degeneration through high-content screening. J. Neurosci. 35, 2927–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahenk Z, Barohn R, New P, Mendell JR, 1994. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch. Neurol. 51, 726–729. [DOI] [PubMed] [Google Scholar]
- Schmidt BZ, Lehmann M, Gutbier S, Nembo E, Noel S, Smirnova L, Forsby A, Hescheler J, Avci HX, Hartung T, Leist M, Kobolák J, Dinnyés A, 2017. In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities. Arch. Toxicol. 91, 1–33. [DOI] [PubMed] [Google Scholar]
- Schmitt LI, Leo M, Kleinschnitz C, Hagenacker T, 2018. Oxaliplatin modulates the characteristics of voltage-gated calcium channels and action potentials in small dorsal root ganglion neurons of rats. Mol. Neurobiol. 55, 8842–8855. [DOI] [PubMed] [Google Scholar]
- Schwaid AG, Krasowka-Zoladek A, Chi A, Cornella-Taracido I, 2018. Comparison of the rat and human dorsal root ganglion proteome. Sci. Rep. 8, 13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara K, 2017. Chemotherapy for advanced gastric cancer: future perspective in Japan. Gastric Cancer 20, 102–110. [DOI] [PubMed] [Google Scholar]
- Staff NP, Grisold A, Grisold W, Windebank AJ, 2017. Chemotherapy-induced peripheral neuropathy: a current review. Ann. Neurol. 81, 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanenko AA, Dmitrenko VV, 2015. Pitfalls of the MTT assay: direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 574, 193–203. [DOI] [PubMed] [Google Scholar]
- Ulukaya E, Colakogullari M, Wood EJ, 2004. Interference by anti-cancer chemotherapeutic agents in the MTT-tumor chemosensitivity assay. Chemotherapy 50, 43–50. [DOI] [PubMed] [Google Scholar]
- Ustun R, Oguz EK, Seker A, Korkaya H, 2018. Thymoquinone prevents cisplatin neurotoxicity in primary DRG neurons. Neurotoxicology 69, 68–76. [DOI] [PubMed] [Google Scholar]
- van Tonder A, Joubert AM, Cromarty AD, 2015. Limitations of the 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vencappa S, Donaldson LF, Hulse RP, 2015. Cisplatin induced sensory neuropathy is prevented by vascular endothelial growth factor-a. Am. J. Transl. Res. 7, 1032–1044. [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Johnson EM Jr., 1989. Cytosine arabinoside kills postmitotic neurons: evidence that deoxycytidine may have a role in neuronal survival that is independent of DNA synthesis. J. Neurosci. 9, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler HE, Wing C, Delaney SM, Komatsu M, Dolan ME, 2015. Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS One 10, e0118020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SP, Davenport-Jones J, Egan C, O’Hare S, Cookson M, McClean R, Garle MJ, Pentreath V, Atterwill CK, 1994. Phase 1 of an in vitro neurotoxicological pre-validation trial. Toxicol. in Vitro 8, 799–802. [DOI] [PubMed] [Google Scholar]
- Wing C, Komatsu M, Delaney SM, Krause M, Wheeler HE, Dolan ME, 2017. Application of stem cell derived neuronal cells to evaluate neurotoxic chemotherapy. Stem Cell Res. 22, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PM, 1976. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 115, 361–375. [DOI] [PubMed] [Google Scholar]
- Yin K, Baillie GJ, Vetter I, 2016. Neuronal cell lines as model dorsal root ganglion neurons: A transcriptomic comparison. Mol. Pain 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Murakami Y, Senzaki K, Masuda T, Ozaki S, Ito Y, Shiga T, 2013. Coexpression of Runx1 and Runx3 in mechanoreceptive dorsal root ganglion neurons. Dev Neurobiol 73, 469–479. [DOI] [PubMed] [Google Scholar]
- Zhang H, Dougherty PM, 2014. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology 120, 1463–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JH, Walters ET, Song XJ, 2007. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J. Neurophysiol. 97, 15–25. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen W, Mi R, Zhou C, Reed N, Höke A, 2013. Ethoxyquin prevents chemotherapy-induced neurotoxicity via Hsp90 modulation. Ann. Neurol. 74, 893–904. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gorgun MF, Englander EW, 2018. Neurotoxicity of cytarabine (Ara-C) in dorsal root ganglion neurons originates from impediment of mtDNA synthesis and compromise of mitochondrial function. Free Radic. Biol. Med. 121, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


