Abstract
Background and Objective:
High-flow nasal cannula (HFNC), a relatively new technique in acute hypoxemic respiratory failure (AHRF), is gaining popularity in intensive care units (ICUs). Our study aims to identify the predictive factors for failure of HFNC.
Materials and Methods:
This is a 5-year retrospective cohort study in patients with AHRF using HFNC in an ICU of a regional hospital in Hong Kong. The primary outcome is to identify the predictive factors for failure of HFNC which is defined as escalation of treatment to noninvasive ventilation, mechanical ventilation, extracorporeal membrane oxygenation, or death.
Results:
Of the 124 ICU patients with AHRF, 69 (55.65%) failed in the use of HFNC. The patients failing HFNC had higher Acute physiology and Chronic Health Evaluation IV scores, lower Glasgow Coma Scale scores, lower platelet counts and serum sodium levels upon ICU admission, and higher pH on day of HFNC commencement. They had higher respiratory rates before HFNC and higher heart rates before and 1 h after HFNC. The respiratory rate-oxygenation (ROX) index which is defined as a ratio of SpO2/FiO2 to respiratory rate was significantly lower in the failure group 1 h and 12 h after HFNC. By multivariate binary logistic regression, failure of HFNC is associated with lower ROX index at 12 h after HFNC.
Conclusion:
ROX index at 12 h serves as a valuable tool to monitor the responsiveness to HFNC treatment. Close monitoring is required to identify patient failing using HFNC.
KEY WORDS: Clinical respiratory medicine, critical care medicine, ventilation
INTRODUCTION
High-flow nasal cannula (HFNC), a relatively new technique to provide support in patients with respiratory distress, is gaining popularity in intensive care units (ICUs). HFNC has several advantages: (I) the high flow of gas reduces the entrainment of room air and dilution of oxygen;[1,2] (II) it creates a positive pressure effect;[3] (III) it washes out carbon dioxide in the upper airway and reduces the anatomic dead space;[4,5] (IV) the heat and humidification improve mucociliary motion and sputum clearance;[6,7] (V) it reduces upper airway resistance and work of breathing and improves thoracoabdominal synchrony;[8,9,10] and (VI) it is better tolerated compared with other devices like noninvasive ventilation (NIV).
Researchers began to evaluate the role of HFNC in adult patients with acute hypoxemic respiratory failure (AHRF).[10] The FLORALI trial, a multicenter randomized control trial comparing HFNC and other oxygenation strategies, found a lower ICU and 90-day mortality and longer ventilator-free days in patients receiving HFNC.[11] The post hoc analysis found a lower intubation rate in the patients receiving HFNC in the subgroup of patients with a P/F ratio <200.
Kang, in his retrospective cohort of 175 HFNC failure patients, found that late intubation (beyond 48 h after HFNC) had a higher ICU mortality, a lower success rate in ventilator weaning, and fewer ventilator-free days compared to early intubation (within 48 h after HFNC).[12] Therefore, it is ideal to know the accurate predictive factors for failure of HFNC, so that physicians can early identify patients failing HFNC and timely escalate the ventilatory support.
The predictive factors for HFNC failure are, however, not well investigated, with inconsistent results in different studies. We performed a retrospective cohort study to identify factors for HFNC failure in ICU patients.
MATERIALS AND METHODS
Study population
This retrospective cohort study was conducted in the ICU of Pamela Youde Nethersole Eastern Hospital in Hong Kong. The hospital records of patients admitted to ICU between May 2012 and April 2017 were retrospectively evaluated, and patients were included if they had matched keywords of “Optiflow,” “Airvo,” or “HFNC” as the oxygen device in the Clinical Information System (CIS, Philips IntelliSpace Critical Care and Anesthesia). Patients were excluded if they were (1) given HFNC as a tool to wean from mechanical ventilation (MV), (2) given HFNC as a palliative management in malignancies, and (3) considered not suitable for enrollment by the investigators.
The following clinical and laboratory data were collected: demographic data; diagnoses and the causes of respiratory failure; clinical parameters 1 h before, 1 h after, and 12 h after the use of HFNC; usage of vasopressor before commencement of HFNC; laboratory data including white blood cells, hemoglobin, platelets, prothrombin time, renal function tests, arterial blood gases upon ICU admission, and on the day of HFNC use; details of oxygen therapy or MV before and after HFNC; the settings of HFNC including oxygen fraction and flow at commencement and 1 h and 12 h after; time of commencement and termination of HFNC; time of MV, NIV, extracorporeal membrane oxygenation, or death in that index admission; and Acute Physiology and Chronic Health Evaluation (APACHE IV) scores upon admission.
Outcomes
The primary outcome of the study is to identify the factors associated with failure of HFNC which is defined as treatment escalation to NIV, MV, extracorporeal membrane oxygenation, or death within 28 days from the commencement of HFNC.
Statistical analysis
Statistical analysis was performed with the IBM SPSS Statistics for windows version 19, Armonk, NY: IBM Corp. Baseline characteristics were expressed as mean (standard deviation) or median (interquartile range). Comparisons of continuous data for analysis were performed with Student’s t-test or Mann–Whitney U-test as appropriate. Fisher’s exact test was used in small expected count. Comparisons of categorical data were made with Chi-square test. Discriminative power of predicting failure of HFNC was evaluated by the receiver operating characteristic curve. P <0.05 was considered statistically significant in univariate analysis and multivariate analysis.
This retrospective study was performed in compliance with ethical standard of the Helsinki Declaration and approved by the Research Ethics Committee of the Hospital Authority in Hong Kong, reference number HKECREC-2018-002. Written informed consent was waived.
RESULTS
Patient characteristics
During the study period, 6782 patients admitted to the ICU were screened. One hundred and thirty-nine patients had the keywords of “Optiflow,” “Airvo,” or “HFNC” matched in the Clinical Information System. Twelve patients received HFNC for weaning of MV; one patient for palliative care in terminal malignancy (n = 1) and two patients for awake extracorporeal membrane oxygenation (ECMO) care were all excluded [Figure 1]. The baseline characteristics of the 124 eligible patients are summarized in Table 1. The majority (77.4%) suffered from pneumonia as the primary cause of respiratory failure, followed by fluid overload/congestive heart failure (CHF) (9.7%) and interstitial lung disease (4.8%). Before commencement of HFNC, 72 patients (58.1%), 35 patients (28.2%), 14 patients (11.3%), and 1 patient (0.8%) received nonrebreathing mask, nasal cannula, NIV, and Hudson mask, respectively. There were two patients receiving HFNC since admission in ICU. The median flow rate was 8 (IQR: 6–11) L/min 1 h before commencement of HFNC. The median respiratory rate and the mean heart rate were 28 (IQR: 23.75–32) and 102.7 (SD 20.43) per minute, respectively 1 h before HFNC. One hundred and nine patients (87.9%), 14 patients (11.3%), and 1 patient (0.8%) were receiving no vasopressor, noradrenaline, and dopamine, respectively. Among those receiving noradrenaline, the median dosage was 0.098 (IQR: 0.057–0.244) mcg/kg/min. The median APACHE IV score upon ICU admission was 68.5 (IQR: 56.25–89.75). At commencement of HFNC, the median flow of HFNC was 40 (IQR: 40–40) L/min and the median FiO2 was 0.5 (IQR: 0.45–0.6).
Figure 1.
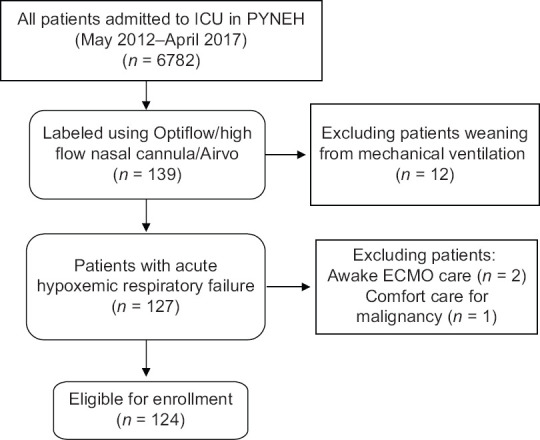
The enrollment of the study participants
Table 1.
Baseline characteristics of patients (n=124)
| n (%) | |
|---|---|
| Age | 65 (55-78) |
| Body weight (kg) | 54.2 (44.28-59.95) |
| Height (cm)¥ | 157.86 (10.03) |
| Physical parameters upon ICU admission | |
| O2 flow (LPM) | 8 (6-15) |
| Respiratory rate | 26 (22-32) |
| SpO2 | 94 (91-97) |
| Temp (°C) | 37.6 (36.93-38.3) |
| Heart rate¥ | 107.54 (23.13) |
| MAP (mmHg) | 85 (71.25-97.75) |
| Physical parameters before HFNC | |
| FiO2 1 h before HFNC (LPM) | 8 (6-11) |
| Respiratory rate before HFNC | 28 (23.75-32) |
| SpO2 before HFNC | 92 (90-94) |
| GCS before HFNC | 15 (15-15) |
| Flow of HFNC at commencement (LPM) | 40 (40-40) |
| Physical parameters 1 h after HFNC | |
| Flow rate (LPM) | 40 (40-40) |
| FiO2 | 0.5 (0.45-0.6) |
| GCS | 15 (14-15) |
| Heart rate | 104.28 (21.61) |
| Respiratory rate | 26 (23-32) |
| SpO2 | 92 (90-94) |
| Physical parameters 12 h after HFNC | |
| Heart rate¥ | 96.2 (21.4) |
| Respiratory rate | 27.25 (23-31) |
| ROX index | |
| ROX 1 h | 6.45 (4.95-8.24) |
| ROX 12 h | 7.14 (5.61-9.20) |
| Blood parameters on day of HFNC | |
| pH | 7.46 (7.42-7.49) |
| PaCO2 (kPa) | 4.37 (3.86-5.18) |
| PaO2 (kPa) | 9.93 (8.66-11.6) |
| HCO3 (mmol/L) | 22.4 (19.8-26.0) |
| Hemoglobin (g/dL) | 10.2 (9.1-11.9) |
| White cell count ×109/L | 12.2 (8.31-17.64) |
| Platelet×109/L | 209 (108-288) |
| Sodium (mmol/L) | 137 (133-140) |
| Potassium (mmol/L) | 3.8 (3.5-4.1) |
| Urea (mmol/L) | 6.9 (4.8-11) |
| Creatinine (µmol/L) | 69 (55-127) |
| Bilirubin (mmol/L) | 14 (9.23-22) |
| ICU stay days | 6.6 (3.99-14.88) |
| APACHE IV score | 68.5 (56.25-89.75) |
| Time from admission to HFNC (h) | 21.36 (5.04-54.72) |
| HFNC duration (h) | 27.41 (11.61-64.48) |
| Categorical baseline characteristics | |
| Sex¶ | |
| Male | 82 (66.7) |
| Cause of respiratory failure¶ | |
| Pneumonia | 96 (77.4) |
| Cancer or carcinomatosis | 4 (3.2) |
| Interstitial lung disease | 6 (4.8) |
| Fluid overload/CHF | 12 (9.7) |
| ARDS | 2 (1.6) |
| Hemoptysis | 1 (0.8) |
| Pulmonary embolism | 1 (0.8) |
| Pleural effusion | 1 (0.8) |
| Atelectasis | 1 (0.8) |
| Cause of pneumonia¶ | |
| Nonspecific, bacterial | 85 (88.5) |
| Influenza | 3 (3.1) |
| CMV | 1 (1) |
| Aspiration | 3 (3.1) |
| PCP | 1 (1) |
| Adenovirus | 1 (1) |
| Other viruses | 2 (2.1) |
| Modality of O2 delivery before HFNC¶ | |
| Nasal cannula | 35 (28.2) |
| Nonrebreathing mask | 72 (58.1) |
| Hudson mask | 1 (0.8) |
| Optiflow | 2 (1.6) |
| NIV | 14 (11.3) |
| Choice of vasopressor 1 h before HFNC¶ | |
| None | 109 (87.9) |
| Noradrenaline | 14 (11.3) |
| Dopamine | 1 (0.8) |
| Choice of vasopressor 12 h after HFNC¶ | |
| None | 112 (90.3) |
| Noradrenaline | 12 (9.7) |
| Dopamine | 0 (0) |
| Modality of O2 delivery after HFNC¶ | |
| Nasal cannula | 48 (38.7) |
| NRM | 5 (4) |
| Noninvasive ventilation | 21 (16.9) |
| Mechanical ventilation | 43 (34.7) |
| Death | 2 (1.6) |
| ECMO | 1 (0.8) |
| Direct high-flow nasal cannula to general ward | 4 (3.2) |
| Success¶ | 55 (44.35) |
| Failure¶ | 69 (55.6) |
| Direct to mechanical ventilation | 43 (34.7) |
| Direct to NIV (in which 6 patients later escalate to mechanical ventilation) | 21 (16.9) |
| Direct to ECMO | 1 (0.8) |
| Death during HFNC | 2 (1.6) |
| HFNC to Nasal cannula or NRM to mechanical ventilation | 2 (0.8) |
| Mortality¶ | 31 (25) |
Results shown as median (IQR) unless otherwise specified, ¶n (%) ¥Mean±SD. FiO2: Fraction of inspired oxygen, SpO2: Peripheral capillary oxygen saturation, MAP: Mean arterial pressure, HFNC: High-flow nasal cannula, GCS Glasgow Coma Scale, CHF: Congestive heart failure, ROX: Ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate, APACHE: Acute Physiology and Chronic Health Evaluation, AKI: Acute kidney injury, ARDS: Acute respiratory distress syndrome, CMV: Cytomegalovirus, PCP: Pneumocystis pneumonia, NRM: Nonrebreathing mask, NIV: Noninvasive ventilation, ECMO: Extracorporeal membrane oxygenation, IQR: Interquartile range, ICU: Intensive care unit, SD: Standard deviation
Forty-eight patients (38.7%), 5 patients (4%), 21 patients (16.9%), 43 patients (34.7%), and 1 patient (0.8%) received nasal cannula, nonrebreathing mask, NIV, MV, and extracorporeal membrane oxygenation, respectively, after HFNC. Two patients (1.6%) died and four patients (3.2%) were transferred to general wards while receiving HFNC. The median HFNC duration was 27 (IQR: 11.61–64.48) h and the median time from admission to HFNC commencement was 21.36 (IQR: 5.04–54.72) h. Sixty-nine patients (55.6%) were defined as failure which was defined as any escalation to NIV, MV, ECMO, or death within 28 days after commencement of HFNC.
Primary endpoints
Compared to the 55 patients who succeeded with the use of HFNC, the 69 patients with HFNC failure had higher APACHE IV scores and lower Glasgow Coma Scale (GCS) scores upon ICU admission (P = 0.002, 0.024). They had higher respiratory rates 1 h before HFNC (P = 0.032) and heart rates 1 h before and 1 h after HFNC (P = 0.011, P < 0.001). They had lower platelet counts (P = 0.012) and serum sodium levels (P = 0.011) upon ICU admission and a higher pH on the day of HFNC (P = 0.029).
The respiratory rate-oxygenation (ROX) index which is defined as a ratio of SpO2/FiO2 to respiratory rate was significantly lower in the failure group at 1 h and 12 h after HFNC (P = 0.014, 0.014) [Table 2].
Table 2.
Predictive factors for success of high-flow nasal cannula
| Success | Failure | P | |
|---|---|---|---|
| Heart rate | |||
| Before HFNC¥ (mean±SD) | 97.53±19.20 | 106.96±20.56 | 0.011* |
| 1 h after HFNC¥ (mean±SD) | 96.76±19.07 | 110.34±21.76 | <0.001* |
| 12 h after HFNC¥ | 94.06 (20.76) | 99.10 (22.12) | 0.268 |
| Respiratory rate | |||
| Before HFNC | 27 (23-30) | 30 (25-33) | 0.032* |
| 1 h after HFNC | 25 (22-30) | 28 (23-35) | 0.079 |
| 12 h after HFNC | 26.5 (22.25-30.75) | 28 (23-34) | 0.164 |
| GCS upon ICU | 15 (14-15) | 15 (10-15) | 0.024* |
| GCS 12 h after HFNC | 15 (15-15) | 15 (13.75-15) | 0.027* |
| pH on day of HFNC | 7.44 (7.41-7.48) | 7.47 (7.43-7.5) | 0.029* |
| PCO2 on day of HFNC | 4.43 (4.04-5.19) | 4.28 (3.72-4.98) | 0.284 |
| HCO3 on day of HFNC | 22.8 (19.8-25.7) | 22.25 (19.88-26.38) | 0.925 |
| Platelet upon ICU | 238 (152-299) | 163 (100.5-251) | 0.012* |
| Na upon ICU | 137 (133-139) | 134 (130.5-137) | 0.011* |
| Bilirubin on day of HFNC | 11.5 (9-19) | 16.5 (10-24.85) | 0.051 |
| APACHE IV score | 62 (49-82) | 75 (60.05-106.5) | 0.002* |
| APACHE IV risk | 0.18 (0.09-0.31) | 0.26 (0.14-0.62) | 0.003* |
| ROX 1 h | 7.13 (5.98-9.47) | 6.93 (4.59-7.66) | 0.014* |
| ROX 12 h | 7.39 (6.42-9.90) | 6.10 (4.73-8.06) | 0.014* |
| Time from admission to HFNC | 1.01 (0.35-2.02) | 0.68 (0.17-0.68) | 0.422 |
| HFNC duration (h) | 47 (25.47-72) | 18 (5.18-42.69) | <0.001* |
| ICU stay (days) | 4.99 (3.40-7.06) | 10.97 (5.33-23.04) | <0.001* |
| 28-day mortality (%) | 0 | 31 (44.9) | <0.001* |
| Cause of respiratory failure§ | 0.629 | ||
| Oxygen modality before HFNC | 0.646 | ||
| Time from admission to HFNC | 24.28 (8.32-48.43) | 16.3 (4.09-59.61) | 0.422 |
*Clinical significance P<0.05, §Categorical. Results shown as median (IQR) unless otherwise specified, n (%) ¥Mean±SD. HFNC: High-flow nasal cannula, GCS: Glasgow Coma Scale, ROX: Ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate, APACHE: Acute Physiology and Chronic Health Evaluation, IQR: Interquartile range, ICU: Intensive care unit, SD: Standard deviation
There was no statistically significant association between HFNC failure and different causes of respiratory failure (P = 0.629) or modalities of oxygen therapy before HFNC (P = 0.646) or the time from admission to HFNC initiation (P = 0.422).
Multivariate analysis
By multivariate binary logistic regression, HFNC failure is only associated with lower ROX index at 12 h after HFNC commencement (P = 0.012, odds ratio [OR]: 0.802) [Table 3].
Table 3.
Multivariate analysis of the predictive factors for success of high-flow nasal cannula
| OR | P | |
|---|---|---|
| ROX at 12 h | 0.802 | 0.012* |
*Clinical significance P<0.05. ROX: Respiratory rate-oxygenation index, OR: Odds ratio
The receiver operating characteristic (ROC) curve has its largest area under curve if ROX index at 12 h is used to predict the success of HFNC in our patients (AUC = 0.659) [Figure 2]. The sensitivity and specificity would be 0.88 and 0.41, respectively, if cutoff value for ROX 12 h is set to be 5.626
Figure 2.
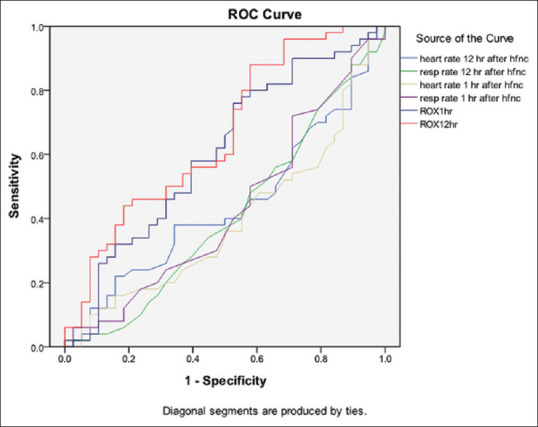
Receiver operating characteristic curves predicting high-flow nasal cannula success
DISCUSSION
AHRF is a fatal complication of many diseases and it contributes to 30% of ICU admissions.[13,14] It has been increasingly recognized that MV is associated with various adverse events and the hospital mortality remained as high as 30%.[15,16] NIV is an established treatment to improve gas exchange and to decrease intubation rate and mortality in chronic obstructive pulmonary disease and CHF.[17,18] However, the use of NIV in AHRF is debatable and is even shown to be detrimental in some studies.
| Area under curve | |
|---|---|
| ROX at 12 h after HFNC | 0.659 |
| ROX at 1 h after HFNC | 0.605 |
| Heart rate 1 h after HFNC | 0.393 |
| Respiratory rate 1 h after HFNC | 0.429 |
| Heart rate 12 h after HFNC | 0.440 |
| Respiratory rate 12 h after HFNC | 0.411 |
The sensitivity and specificity would be 0.88 and 0.41 if cutoff value for ROX 12 h would be 5.626. HFNC: High-flow nasal cannula
HFNC appears to be a good alternative to avoid MV in AHRF. Patients when receiving HFNC were found to have a higher PR ratio, and a lower respiratory rate, work of breathing and thoracoabdominal asynchrony (TAA), compared with other oxygen devices.[10,19,20] Sztrymf et al. reported a significant decrease in TAA at 1 h in patients receiving HFNC (P = 0.0007),[10] and found that patients exhibiting higher percentage of TAA as early as 30 min after HFNC initiation were more likely to require endotracheal intubation.
Frat, in the FLORALI trial,[11] found lower ICU mortality and 90-day mortality rates, and longer ventilator-free days in the patients receiving HFNC, compared to patients receiving standard oxygen therapy or NIV. In the post hoc analysis of the subgroup of patients with P/F ratio <200, intubation rate was significantly lower in the patients receiving HFNC. Compared to standard oxygen therapy, HFNC was associated with significant reduction in the intubation rate (OR: 0.52, 95% confidence interval [CI]: 0.34–0.79, P = 0.002) in a meta-analysis by Zhao,[21] despite no difference in mortality (OR: 1.01, 95% CI: 0.67–1.53, P = 0.96).
Heart rate, respiratory rate, SOFA score, APACHE II score, oxygenation, delirium, and thoracoabdominal asynchrony have been inconsistently identified as predictive factors for HFNC failure in different studies.[9,10,22,23,24,25]
In our study, the only factor associated with HFNC failure by multivariate analysis is the ROX index at 12 h after HFNC commencement (OR: 0.802, P = 0.012). The ROX index, a ratio of pulse oximetry (SpO2)/fraction of inspired oxygen (FiO2) to respiratory rate, was proposed by Roca to predict the HFNC failure.[26] In his 4-year observational cohort study, ROX index demonstrated the best prediction accuracy (AUROC, 0.74) at 12 h after HFNC initiation, with the best cutoff value for the ROX index estimated to be 4.88. It was also better than other physiological parameters to predict failure when measured at 18 h and 24 h after HFNC initiation. Compared to other clinical parameters, our study showed the greatest area under receiver operating characteristic curve (AUROC = 0.659) if ROX at 12 h with the sensitivity and specificity of 0.88 and 0.41 if cutoff value was set at 5.626. Our study shared similar findings with Roca’s study that the ROX index at 12 h was better than other physiological parameters to predict HFNC failure. ROX index appears to be a useful tool and can be easily incorporated in routine clinical monitoring in patients using HFNC. We would also like to point out that the ROX index at 1 h after HFNC was significantly lower in patients with HFNC failure (P = 0.014). Although the ROX index at 1 h only has an AUROC of 0.605 only, it may be still worthy of calculating the ROX index as early as 1 h after HFNC.
According to our study, heart rates before and 1 h after HFNC were predictive of HFNC failure. Apart from being an hemodynamic parameter, patient heart rate also reflects the degree of stress and the dosage of vasopressors. Interestingly, Frat also found the heart rate 1 h after HFNC commencement was the only factor associated with intubation in the post hoc analysis of the FLORALI study.[25]
We found that HFNC failure patients had significantly higher APACHE IV scores and lower GCS scores upon ICU admission (P = 0.002, 0.024). Obviously, a higher APACHE score signifies higher illness severity, and it has been identified as a factor for failure in previous studies. Imai, in his retrospective cohort, found delirium as a predictor of failure in HFNC in 106 patients with acute respiratory failure.[22] Impaired consciousness in ICU patients may lead to a lower threshold for endotracheal intubation, and on the other hand, it may reflect the severity of underlying illnesses.
The failure rate of 55.65% in our study seems to be high when compared to the intubation rate of 38% in the FLORALI study.[11] However, the definition of failure of HFNC differed in the two studies. Apart from intubation, we also regard escalation to NIV and 28-day mortality as HFNC failure. After excluding these patients, 43 patients (34.7%) required MV and the intubation rate was comparable to that in the FLORALI study. Interestingly, among 21 patients with escalation to NIV, a majority of 15 patients (71.4%) did not need escalation to MV. The role of NIV as escalation of support after failure of HFNC has never been investigated and may warrant further studies.
In our study, patients with higher APACHE IV scores, more deranged physical parameters including high heart rates and respiratory rates and blood parameters of low platelet counts, sodium levels, and higher pH were at higher risk of HFNC failure. Close monitoring of clinical response is deemed important in patients receiving HFNC. As early as 1 h after HFNC initiation, the heart rate can provide additional information to predict treatment failure. ROX index at 12 h has a valuable role in clinical monitoring. As supported by findings from Roca’s cohort[26] and our study, escalation of treatment has to be considered if ROX index is lower than the cutoff value or patient condition deteriorates. Because HFNC has an advantage of improving patient comfort and patients probably may tolerate for long period of time, physicians should beware of delaying endotracheal intubation.
Our study had several limitations. First, it is a retrospective study without predetermined protocol for the indication, initiation, and cessation of HFNC. Second, patients’ comfort, dyspnea, and TAA were not assessed as they were not routinely documented in the medical record. Third, the ROX index at 12 h, as a tool to predict failure, is unable to identify patients failing HFNC within 12 h. Fourth, our study has a small sample size and is prone to be underpowered. Finally, there is heterogeneity in the causes of AHRF, though no relationship was found in our study between HFNC failure and the etiology of the respiratory failure.
CONCLUSION
HFNC is an excellent modality of respiratory support with advantages of simplicity and excellent tolerance, with proven benefit in terms of patient physiological parameters and clinical outcome. Close monitoring of the physical parameters is crucial. The ROX index has a predictive role in treatment failure and can be easily employed as a routine monitoring parameter for patients on HFNC. Physicians should beware of delayed intubation which was shown to have worse clinical outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103:886–90. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: Mechanisms of action. Respir Med. 2009;103:1400–5. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Wagstaff TA, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia. 2007;62:492–503. doi: 10.1111/j.1365-2044.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura M. High-flow nasal cannula oxygen therapy in adults: Physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61:529–41. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 5.Sotello D, Rivas M, Mulkey Z, Nugent K. High-flow nasal cannula oxygen in adult patients: A narrative review. Am J Med Sci. 2015;349:179–85. doi: 10.1097/MAJ.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 6.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: Mechanisms of action and clinical implications. Chest. 2015;148:253–61. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: A critical review of the literature. Intensive Care Med. 2013;39:247–57. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 8.Cuquemelle E, Pham T, Papon JF, Louis B, Danin PE, Brochard L. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxemic respiratory failure. Respir Care. 2012;57:1571–7. doi: 10.4187/respcare.01681. [DOI] [PubMed] [Google Scholar]
- 9.Itagaki T, Okuda N, Tsunano Y, Kohata H, Nakataki E, Onodera M, et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients. Respir Care. 2014;59:70–4. doi: 10.4187/respcare.02480. [DOI] [PubMed] [Google Scholar]
- 10.Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: A prospective pilot study. Intensive Care Med. 2011;37:1780–6. doi: 10.1007/s00134-011-2354-6. [DOI] [PubMed] [Google Scholar]
- 11.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–96. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 12.Kang BJ, Koh Y, Lim CM, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–32. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 13.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 14.Cook D, Brower R, Cooper J, Brochard L, Vincent JL. Multicenter clinical research in adult critical care. Crit Care Med. 2002;30:1636–43. doi: 10.1097/00003246-200207000-00039. [DOI] [PubMed] [Google Scholar]
- 15.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA. 2002;287:345–55. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 16.Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care. 2005;50:714–21. [PubMed] [Google Scholar]
- 17.Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982–93. doi: 10.1001/jamainternmed.2014.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: Systematic review and meta-analysis. JAMA. 2005;294:3124–30. doi: 10.1001/jama.294.24.3124. [DOI] [PubMed] [Google Scholar]
- 19.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–13. [PubMed] [Google Scholar]
- 20.Schwabbauer N, Berg B, Blumenstock G, Haap M, Hetzel J, Riessen R. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: Effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV) BMC Anesthesiol. 2014;14:66. doi: 10.1186/1471-2253-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Wang H, Sun F, Lyu S, An Y. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy but not to noninvasive mechanical ventilation on intubation rate: A systematic review and meta-analysis. Crit Care. 2017;21:184. doi: 10.1186/s13054-017-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai R, Tsugitomi R, Jinta T, Yamada U, Aoki K, Tamura T. Delirium as a predictor of high flow nasal cannula failure in patients with acute respiratory failure. Eur Respir J. 2018;52:2295. [Google Scholar]
- 23.Kim WY, Sung H, Hong SB, Lim CM, Koh Y, Huh JW. Predictors of high flow nasal cannula failure in immunocompromised patients with acute respiratory failure due to non-HIV pneumocystis pneumonia. J Thorac Dis. 2017;9:3013–22. doi: 10.21037/jtd.2017.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azoulay E, Pickkers P, Soares M, Perner A, Rello J, Bauer PR, et al. Acute hypoxemic respiratory failure in immunocompromised patients: The Efraim multinational prospective cohort study. Intensive Care Med. 2017;43:1808–19. doi: 10.1007/s00134-017-4947-1. [DOI] [PubMed] [Google Scholar]
- 25.Frat JP, Ragot S, Coudroy R, Constantin JM, Girault C, Prat G, et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med. 2018;46:208–15. doi: 10.1097/CCM.0000000000002818. [DOI] [PubMed] [Google Scholar]
- 26.Roca O, Messika J, Caralt B, García-de-Acilu M, Sztrymf B, Ricard JD, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J Crit Care. 2016;35:200–5. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]


