Abstract
Background:
The “second wave” of the COVID-19 pandemic hit India from early April 2021 to June 2021. We describe the clinical features, treatment trends, and baseline laboratory parameters of a cohort of patients with SARS-CoV-2 infection and their association with the outcome.
Methods:
This was a retrospective cohort study. Multivariate logistic regression models were fitted to identify clinical and biochemical predictors of developing hypoxia, deterioration during the hospital stay, and death.
Results:
A total of 2080 patients were included. The case fatality rate was 19.5%. Among the survivors, the median duration of hospital stay was 8 (5–11) days. Out of 853 (42.3%%) of patients who had COVID-19 acute respiratory distress syndrome at presentation, 340 (39.9%) died. Patients aged >45 years had higher odds of death as compared to the 18–44 years age group. Vaccination reduced the odds of death by 40% (odds ratio [OR] [95% confidence interval [CI]]: 0.6 [0.4–0.9], P = 0.032). Patients with hyper inflammation at baseline as suggested by leukocytosis (OR [95% CI]: 2.1 [1.5–3.1], P < 0.001), raised d-dimer >500 mg/dL (OR [95% CI]: 3.2 [2.2–4.7], P < 0.001), and raised C-reactive peptide >0.5 mg/L (OR [95% CI]: 3.7 [2.2–13], P = 0.037) had higher odds of death. Patients who were admitted in the 2nd week had lower odds and those admitted in the 3rd week had higher odds of death.
Conclusion:
This study shows that vaccination status and early admission during the inflammatory phase can change the course of illness of these patients. Improving vaccination rates and early admission of patients with moderate and severe COVID-19 can improve the outcomes.
KEY WORDS: COVID-19, SARS-CoV-2, predictors of outcomes, vaccination
INTRODUCTION
The SARS-CoV-2 infection has been declared a pandemic by the World Health Organization. To date, more than 237 million people have been infected by the virus and more than 4.85 million have died.[1]
The disease manifestation ranges from being completely asymptomatic and being detected only on screening due to a history of contact or may present with rapidly progressive hypoxic respiratory failure due to pneumonia and acute respiratory distress syndrome (ARDS).
The clinical features, demographic profile, severity at baseline, and the case-fatality rates differ between various geographic regions. We present these features and the predictors of outcomes in the Indian scenario during the “second wave” of the COVID-19 pandemic in patients who were admitted to All India Institute of Medical Sciences, Jhajjar COVID-19 treatment facility. In the period from April to June 2021, this part of India was significantly affected by COVID-19 with many patients with nearly 30% positivity rates of tests conducted. The objectives of this study were to characterize the demographic, clinical, laboratory and imaging features, treatment trends, and hospital outcomes in patients SARS-CoV-2 infection and to study the factors determining the outcomes in patients with SARS-CoV-2 infection.
METHODS
This was a retrospective cohort study conducted at a tertiary care teaching institute at the National Cancer Institute, All India Institute of Medical Sciences (AIIMS), Jhajjar, India. The study protocol was approved by the Institutional review board. The protocol was designed keeping in mind the STROBE checklist for observational studies. The National Cancer Institute, AIIMS, Jhajjar is a dedicated oncology center that was converted into a designated COVID-19 treatment facility. This referral institute catered to a wide area of the northern part of India and COVID-19 patients were referred here from Delhi as well as from the nearby states of Haryana, Punjab, Rajasthan, Uttar Pradesh, and Bihar.
From the hospital electronic database, we included all consecutive patients admitted from the COVID-19 screening area of the hospital. We retrospectively abstracted the clinical data using a structured data capture form from the case files, screening forms, and treatment sheets. Laboratory parameters and results of other biochemical and microbiological reports were obtained from the hospital’s electronic patient information portal. The demographic parameters such as age, gender, comorbidity status, vaccination status, vital parameters including oxygen saturation at presentation, treatment administered, course during the hospital stay, laboratory parameters at baseline and during the hospital stay, and the outcomes in terms of discharge or death at end of hospitalization were collected. June 21 was considered to be the cutoff date to calculate the case-fatality rates.
Case definitions
SARS-CoV-2 infection
Patients with Patients with Severe acute respiratory syndrome Corona Virus 2 Ribonucleic acid (SARS-CoV-2 RNA) detected by real time reverse transcriptase polymerase chain reaction (RT PCR) or nucleic acid amplification test (NAAT) or SARS-CoV-2 antigen detected on rapid antigen test.
Hypoxia
Any patient with oxygen saturation <94% on room air or needing oxygen >21% to maintain saturation on a ventilator was considered to be hypoxic.
COVID-pneumonia
Patients with SARS-CoV-2 infection as described above with breathlessness and chest infiltrates on chest X-ray or computed tomography scan of the chest.
COVID-19 ARDS
Patients with SARS-CoV-2 pneumonia as described above with symptoms and hypoxia developing in 7 or fewer days from onset along with ARDS as per Berlin Definition 2012.[2]
COVID-19 severity
Asymptomatic SARS-CoV-2 infection
Patients without symptoms of COVID-19 and positive for SARS-CoV-2 as described above.[3]
Mild COVID-19
Patients with baseline oxygen saturation ≥94% without breathlessness but with other symptoms suggestive of COVID-19 such as fever, sore throat, myalgia, and fatigue.[3]
Moderate COVID-19
Patients with breathlessness and other symptoms suggestive of COVID-19 as described above and with oxygen saturation ≥94%.[3]
Severe COVID-19
Patients with COVID-19 symptoms as described above with an oxygen saturation <94% or PaO2/FiO2 <300 or respiratory rate >30/min.[3]
Renal dysfunction
Biochemistry report with a creatinine >1 mg/dL during the hospital stay or reduced urine output <0.5 mL//kg/h or <400 mL/day or requiring hemodialysis for metabolic acidosis, hyperkalemia, or encephalopathy due to renal dysfunction, as described above.
Hospital-acquired infection
Biological samples from tracheal aspirates, urine, or blood cultures showing pathogens known to be associated with nosocomial infections.
Critical illness
Patients who develop COVID-19 necessitating mechanical ventilation for respiratory failure, need for vasopressor support to maintain systemic perfusion or renal replacement therapy for acute kidney injury which developed after the onset of COVID-19.
Deteriorated during hospital stay
Patients who were not hypoxic at presentation, but went on to develop hypoxia; those who were on a face mask or nonrebreather mask receiving oxygen who went on to need high flow oxygen devices, noninvasive or invasive mechanical ventilation or those who needed renal replacement therapy for acute kidney injury which developed during the course of COVID-19.
Death
Patients who died due to any cause during the hospital stay.
Death due to COVID-19 death
Death in which COVID-19 is the proximate or underlying cause of death according to the International guidelines for certification and classification (coding) of COVID-19 as cause of death.[4]
COVID-19-associated death
Cases where the associated COVID-19 infection could have aggravated the consequences of the primary illness or accident leading to death according to the International guidelines for certification and classification (coding) of COVID-19.[4]
Discharge
Persons with SARS-CoV-2 infection who were discharged alive from the hospital. This includes those who were discharged home, those who left against medical advice and those who were transferred to another medical facility as described below.
Discharged home
Patients with SARS-CoV-2 infection who were discharged alive from the hospital after recovery and the destination from the hospital was home.
Left against medical advice
Patients with SARS-CoV-2 infection who were discharged alive from the hospital before reaching the discharged home criteria as described above. Such patients may continue their treatment at another hospital or choose to go home.
Transfer
Patients with SARS-CoV-2 infection who were discharged alive from the hospital to another health-care facility to allow continued medical care for their primary illness or COVID-19-related complications.
The data collected for the purpose of the study were de-identified and analyzed. The patients included in this analysis will also be used in other reports to study subgroups and to answer other research questions.
Statistical analysis
The data were summarized using means and standard deviations for normal data and medians and interquartile ranges (p25-p75) for nonparametric data, and means were compared using the “t-test” and medians using the Wilcoxon rank-sum test. The categorical data were summarized as proportions and compared using the Chi-square test or Fisher’s exact as appropriate. All statistical tests were performed with the use of a two-sided type I error rate of 5%. Missing data were not imputed and the summary parameters were calculated with the available data, and the denominators (n) for each parameter were mentioned.
Univariate analysis was done to compare the various parameters between those who were discharged and those who died. Multivariate logistic regression analysis was done with models developed by including those that were found to be significant on univariate analysis as well as the parameters of clinical relevance. We also included those parameters which we thought would influence the outcomes based on the available scientific literature. Sensitivity analysis was done by dropping such parameters and by comparing the various models obtained by dropping them. Kaplan–Meier survival probabilities were estimated by baseline severity status and were compared. All analyses were performed using STATA-Version 13.0 software, (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
RESULTS
A total of 2080 patients were admitted to our COVID-19 facility during the period from April–June 2021. Among these, 17 were admitted as caregivers of the patients and 46 were still admitted at the facility as on the cutoff date of the study. Figure 1 shows the sample recruitment for the analysis of this study.
Figure 1.
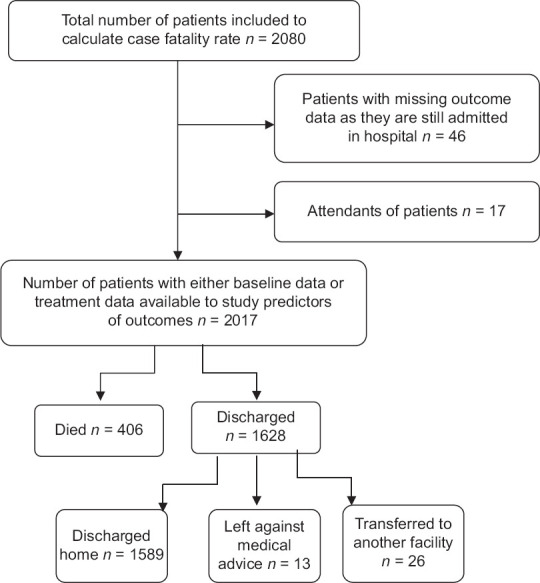
Patient recruitment and outcomes
Case fatality rate and cause of death
Out of the 2080 admitted patients, there were 406 deaths, which amounted to a case fatality rate of 19.5%. In our cohort, 89 (21.9%) patients died <48 h after admission. The majority of patients 342 (84.2%) died due to refractory hypoxia. We had 28 patients (6.9%) who died due to myocarditis or sudden cardiac events such as pulmonary thromboembolism or myocardial infarction. Three patients were dead when they arrived at the emergency department. A minority of patients had acute kidney injury (5/406) and chronic kidney disease and uremia (2/406) as the cause of death. The other proximate causes of death were fall with head injury in one patient, SLE and related complications in two patients, febrile neutropenia and other complications of malignancy or chemotherapy (COVID-19-associated deaths) in eight patients, fungal pneumonia in 2 patients and mucor with fungal sepsis in four patients. The time to death and fraction of patients who were discharged at each time point have been categorized based on the severity of COVID-19 at presentation and the unadjusted odds are depicted in Figure 2.
Figure 2.
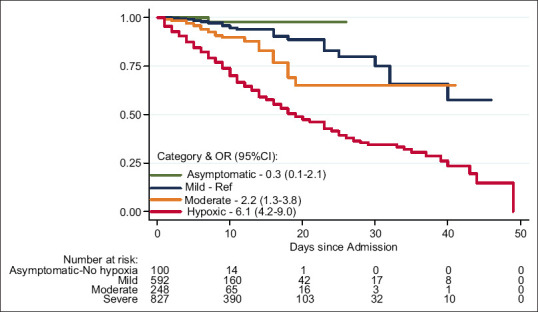
Kaplan–Meier survival estimates by COVID-19 baseline severity. Asymptomatic patients without symptoms of COVID-19. Mild patients with symptoms of COVID-19, but no breathlessness, SpO2 at baseline ≥94%. Moderate patients with COVID-19 pneumonia and breathlessness, SpO2 at baseline ≥94%. Severe patients with COVID-19 and severe hypoxia, SpO2 at baseline <94%
Clinical features and baseline characteristics
Table 1 shows the sample characteristics of the 2017 patients who form the analysis cohort [Figure 1]. Since this was a retrospective cohort study, we had missing data and it has been mentioned as to what proportion of the data points were missing for each of the parameters. Thousand seven hundred and sixty-three (87.3%) patients were admitted due to COVID-19 symptoms and 99 (4.9%) patients were admitted for primarily non-COVID-19 indications such as malignancy and pancreatitis and were found to be positive for SARS-CoV-2 during routine testing during the hospital stay. In our cohort, males 1355 (65%) outnumbered females 725 (35%). We had 1572 patients who were discharged from the hospital cured or improved, 13 patients who left against medical advice, and 26 patients who were transferred to other medical facilities for continuing care.
Table 1.
Demographic and clinical profile of patients at baseline
| Variable | Total, n (%) | Discharge, n (%) | Death, n (%) | P |
|---|---|---|---|---|
| Age (n=2017), mean (SD) | 47.4 (17.6) | 44.8 (17.2) | 57.5 (15.4) | <0.001 |
| Days of hospital stay (n=2017), median (IQR) | 7 (5-11) | 8 (5-11) | 7 (3-12) | 0.022 |
| Sex (n=2017) | ||||
| Female | 697 (34.6) | 565 (35.1) | 132 (32.5) | 0·333 |
| Male | 1320 (65.4) | 1046 (64.9) | 274 (67.5) | |
| Primary indication for admission (n=1862) | ||||
| COVID | 1748 (93.8) | 1369 (94.0) | 379 (93.4) | 0·203 |
| Mucormycosis associated with COVID-19 | 15 (0.8) | 14 (1) | 1 (0.3) | |
| Non-COVID | 99 (5.3) | 73 (5) | 26 (6.4) | |
| COVID symptoms at baseline (n=2004) | ||||
| Asymptomatic | 125 (6.2) | 118 (7.4) | 7 (1.7) | <0.001 |
| Symptomatic | 1879 (93.8) | 1482 (92.6) | 397 (98.3) | |
| Among symptomatic - Time to admission (1867) | ||||
| Week 1 | 1183 (63.4) | 941 (63.9) | 242 (61.3) | 0.002 |
| Week 2 | 603 (32.3) | 480 (32.6) | 123 (31.1) | |
| Week 3 or more | 81 (4.3) | 51 (3.5) | 30 (7.6) | |
| Vaccine protection (n=1818) | ||||
| Not vaccinated | 1314 (72.3) | 1020 (71.2) | 294 (76.4) | 0.037 |
| Symptoms <2 weeks of dose 1 | 215 (11.8) | 173 (12.1) | 42 (10.9) | |
| Partially vaccinated | 258 (14.2) | 210 (14.7) | 48 (12.5) | |
| Fully vaccinated | 31 (1.7) | 30 (2.1) | 1 (0.3) | |
| Symptoms (n=1794) | ||||
| Fever | 1371 (76.4) | 1084 (77.6) | 287 (72.3) | 0.028 |
| Breathlessness | 910 (50.7) | 625 (44.7) | 285 (71.8) | <0.001 |
| Dry cough | 945 (52.7) | 725 (51.9) | 220 (55.4) | 0.215 |
| Cough with expectoration | 214 (11.9) | 168 (12) | 46 (11.6) | 0.812 |
| Rhinitis | 75 (4.2) | 65 (4.7) | 10 (2.5) | 0.061 |
| Sore throat | 335 (18.7) | 282 (20.2) | 53 (13.4) | 0.002 |
| Fatigue | 293 (16.3) | 241 (17.3) | 52 (13.1) | 0.048 |
| Myalgia | 338 (18.8) | 282 (20.2) | 56 (14.1) | 0.006 |
| Chest pain | 134 (7.5) | 114 (8.2) | 20 (5) | 0.037 |
| Gastrointestinal (nausea/vomiting/diarrhea) | 220 (12.3) | 199 (14.2) | 21 (5.3) | <0.001 |
| Drowsiness | 17 (1) | 9 (0.6) | 8 (2) | 0.013 |
| Loss of smell | 148 (8.3) | 131 (9.4) | 17 (4.3) | 0.001 |
| Loss of taste | 149 (8.3) | 139 (10) | 10 (2.5) | <0.001 |
| Comorbidity status (n=1873) | n=1873 | |||
| Any comorbidity | 978 (52.2) | 715 (48.6) | 263 (65.3) | <0.001 |
| 1 comorbidity | 615 (32.8) | 470 (32) | 145 (36) | <0.001 |
| ≥2 comorbidities | 363 (19.4) | 245 (16.7) | 118 (29.3) | |
| Hypertension | 457 (24.4) | 329 (22.4) | 128 (31.8) | <0.001 |
| Diabetes | 437 (23.3) | 308 (21) | 129 (32) | <0.001 |
| CAD | 96 (5.1) | 62 (4.2) | 34 (8.4) | 0.001 |
| Neurological | 29 (1.6) | 25 (1.7) | 4 (1) | 0.308 |
| CLD | 5 (0.3) | 4 (0.3) | 1 (0.3) | 0.934 |
| Malignancy | 102 (5.5) | 75 (5.1) | 27 (6.7) | 0.211 |
| Asthma/COPD | 54 (2.9) | 36 (2.5) | 18 (4.5) | 0.032 |
| Hematological | 13 (0.7) | 9 (0.6) | 4 (1) | 0.495 |
| CKD | 25 (1.3) | 15 (1) | 10 (2.5) | 0.024 |
| Hypothyroid | 86 (4.6) | 63 (4.3) | 23 (5.7) | 0.227 |
| Temperature at baseline (n=1611) | ||||
| Febrile at baseline | 15 (0.9) | 14 (1.1) | 1 (0.3) | 0.186 |
| Heart rate at baseline (n=1704) | ||||
| Tachycardia at baseline | 801 (47) | 604 (44.1) | 197 (59) | <0.001 |
| Respiratory rate at baseline (n=1679) | ||||
| Tachypnea at baseline | 335 (20) | 222 (16.4) | 113 (35.2) | <0.001 |
| Oxygen status at presentation (n=1819) | ||||
| On oxygen | 415 (22.8) | 244 (17.1) | 171 (43.3) | <0.001 |
| On room air | 1404 (77.2) | 1180 (82.9) | 224 (56.7) | |
| Baseline COVID severity (n=1801) | ||||
| Asymptomatic_no hypoxia | 100 (5.6) | 99 (7.0) | 1 (0.3) | <0.001 |
| Mild (symptomatic_no hypoxia) | 599 (33.3) | 571 (40.6) | 28 (7.1) | |
| Moderate (SpO2 ≥94%/COVID-19 pneumonia/breathlessness) | 249 (13.8) | 224 (15.9) | 25 (6.4) | |
| Severe (hypoxic-SpO2 <94%/COVID-19 ARDS) | 853 (47.4) | 513 (36.5) | 340 (86.3) |
SD: Standard deviation, IQR: Interquartile range, CLD: Chronic liver disease, COPD: Chronic obstructive pulmonary disease, CKD: Chronic kidney disease, SpO2: Oxygen saturation, ARDS: Acute respiratory distress syndrome, CAD: Coronary artery disease
We found that around half of the patients (47%) had severe disease at presentation (oxygen saturation of <94% at baseline). Tachycardia, tachypnea, and hypoxia at presentation were associated with increased odds of mortality on univariate analysis. Patients who had breathlessness and those who were drowsy or in altered sensorium had a higher odds of death. Sore throat, fatigue, myalgia, chest pain, gastrointestinal symptoms, and loss of smell and taste were reported in higher proportions by patients who survived. This is likely due to the higher proportion of patients with severe disease, who probably could not narrate the history due to their respiratory distress. Breathlessness and altered sensorium on the other hand can be assessed by the examiner and could have been more accurately captured thus attaining significant differences between those who survived and those who died. Of note, 13.8% of patients presented with COVID-19 pneumonia and 47.4% of patients presented with COVID-19-related ARDS.
Laboratory parameters
The biochemical parameters of the cohort at baseline are detailed in Table 2. We found that patients with hyper inflammation as evidenced by leukocytosis, elevated C-reactive peptide, and d-dimer were associated with higher odds of death.
Table 2.
Laboratory parameters at baseline
| Parameter | Discharges | Deaths | P* | ||
|---|---|---|---|---|---|
|
|
|
||||
| Available observations | Mean (SD) or median (p25-p75) | Available observations | Mean (SD) or median (p25-p75) | ||
| Hemoglobin (mg/dL) | 1322 | 12.9 (2.2) | 334 | 12.7 (2.2) | 0.051 |
| Leucocyte count (×103 cells/mm3) | 1320 | 6.8 (4.8-9.9) | 332 | 11.6 (8.1-15.9) | <0.001 |
| Neutrophil (×103 cells/mm3) | 1287 | 4.8 (2.8-7.8) | 320 | 10.0 (6.6-13.9) | <0·001 |
| Lymphocytes (×103 cells/mm3) | 1288 | 1.0 (0.7-1.5) | 320 | 0.6 (0·4-0.8) | <0·001 |
| LDH (U/L) | 1159 | 328 (241-460) | 271 | 654 (501-890) | <0.001 |
| Total·bilirubin (mg/dL) | 1341 | 0.5 (0.4-0.7) | 339 | 0.6 (0.4-0.9) | <0.001 |
| ALT (U/L) | 1329 | 45 (27-79) | 338 | 53 (31-91) | 0.003 |
| AST (U/L) | 1329 | 42 (30-68) | 338 | 61 (39-98) | <0.001 |
| Globulin (g/dL) | 1315 | 2.6 (0.4) | 336 | 2.7 (0.5) | 0.002 |
| A/G ratio | 1314 | 1.5 (1.4-1.7) | 334 | 1.3 (1.1-1.5) | <0.001 |
| Albumin (g/dL) | 1329 | 3.9 (0.5) | 337 | 3.5 (0.5) | <0.001 |
| Urea (mg/dL) | 1351 | 30 (21.4-43.0) | 338 | 59.9 (42.8-87.7) | <0.001 |
| Creatinine (mg/dL) | 1351 | 0.7 (0.6-0.9) | 338 | 0.9 (0.7-1.3) | <0.001 |
| Calcium (mg/dL) | 1323 | 8.6 (3.6) | 338 | 8.1 (0.8) | <0.001 |
| Phosphate (mg/dL) | 1323 | 3.0 (2.5-3.5) | 338 | 3.1 (2.5-4.0) | 0.005 |
| Sodium (mEq/L) | 1341 | 137.2 (8.3) | 339 | 139.2 (9.7) | <0.001 |
| Potassium (mEq/L) | 1341 | 4.5 (4.1-4.9) | 339 | 4.7 (4.2-5.2) | <0.001 |
| Inflammatory markers | |||||
| Ferritin (ng/mL) | 473 | 365.4 (135.2-878) | 116 | 1096.5 (712.5-1650) | <0.001 |
| D (ng/mL) | 1154 | 201.5 (121-347) | 271 | 622 (337-2550) | <0.001 |
| CRP (mg/dL) | 1286 | 2.6 (0.8-7.4) | 301 | 9.0 (5.1-16.0) | <0.001 |
| IL-6 (pg/mL) | 581 | 9.1 (3.4-22.6) | 195 | 40.5 (14.4-104.2) | <0.001 |
|
| |||||
| Categorical | n | n (%) | n | n (%) | χ 2 |
|
| |||||
| Hemoglobin <12 g/dl | 1322 | 378 (28.6) | 334 | 111 (33.2) | 0.097 |
| Thrombocytopenia (<1.5 Lac) | 1329 | 269 (20.4) | 334 | 74 (22.2) | 0.439 |
| Leucopenia (<4000 cells/mm3) | 1320 | 199 (15.1) | 332 | 15 (4.5) | <0.001 |
| LDH >246 U/L | 1159 | 853 (73.6) | 271 | 269 (99.3) | <0.001 |
| Total bilirubin >1.2 mg/dl | 1341 | 60 (4.5) | 339 | 29 (8.6) | 0.003 |
| ALT >49 U/L | 1329 | 605 (45.5) | 338 | 179 (53.0) | 0.014 |
| AST ≥34 U/L | 1329 | 889 (66.9) | 338 | 275 (81.4) | <0.001 |
| Urea ≥50 mg/dl | 1351 | 242 (17.9) | 338 | 213 (63.0) | <0.001 |
| Creatinine ≥1.0 mg/dl | 1351 | 53 (3.9) | 338 | 88 (26.0) | <0.001 |
| Hyperferritinemia (>322 ng/mL) | 473 | 250 (52.9) | 116 | 105 (90.5) | <0.001 |
| d-dimer >500 ng/ml | 1154 | 188 (16.3) | 271 | 155 (57.2) | <0.001 |
| IL-6 >4.4 pg/ml | 581 | 410 (70.6) | 195 | 188 (95.4) | <0.001 |
| CRP >0.5 mg/dl | 1286 | 1031 (80.2) | 301 | 298 (99.0) | <0.001 |
P for means are calculated by t-test and for medians by Wilcoxon rank-sum test. SD: Standard deviation, CRP: C-reactive protein, ALT: Alanine amino transferase, AST: Aspartate amino transferase, IL-6: Interleukin 6, A/G: Albumin/Globulin ratio, LDH: Lactate dehydrogenase
Treatment trends
The patients were treated by the team of intensive care physicians according to the clinical condition based on the national guidelines, institute protocols, and latest scientific literature subject to availability of the interventions. In summary, almost all patients who were hypoxic and were on oxygen were treated with corticosteroids. All hypoxic patients without significant thrombocytopenia or other clear contraindications were treated with prophylactic doses of anticoagulation. The proportion of patients who were discharged and those who expired receiving various medications is detailed in Table 3. As expected, patients with severe disease were more likely to be treated with drugs such as pulse methylprednisolone, tocilizumab, and remdesivir. Out of 256 patients who were treated with noninvasive ventilation, 221 (86.3%) succumbed while only 13.7% of patients survived. Similarly, 250 patients received invasive mechanical ventilation, thereby representing 13.7% of the total cohort. Only 2.4% of those who received mechanical ventilation could be discharged and the rest succumbed to the illness.
Table 3.
Treatment offered and in-hospital complications
| Parameter (n) | Total, n (% of observations who received the intervention) | Discharged, n (%)* | Died, n (%)* | χ2/exact, P |
|---|---|---|---|---|
| Oxygen (n=1831) | 1025 (56.0) | 626 (61.1) | 399 (38.9) | <0.001 |
| HFNC (n=1808) | 138 (7.6) | 42 (30.4) | 96 (69.6) | <0.001 |
| NIV (n=1823) | 256 (14.0) | 35 (13.7) | 221 (86.3) | <0.001 |
| Invasive MV (n=1830) | 250 (13.7) | 6 (2.4) | 244 (97.6) | <0.001 |
| ICU admission (n=2017) | 239 (11.9) | 99 (41.4) | 140 (58.6) | <0.001 |
| Corticosteroid use (n=1813) | 1094 (60.3) | 722 (66.0) | 372 (34.0) | <0.001 |
| Pulse methylprednisolone (n=1752) | 165 (9.4) | 52 (31.5) | 113 (68.5) | <0.001 |
| Inhaled steroids (n=1761) | 357 (20.3) | 247 (69.2) | 110 (30.8) | <0.001 |
| Anticoagulation therapy (n=1796) | 988 (55.0) | 658 (66.6) | 330 (33.4) | <0.001 |
| Ivermectin (n=1758) | 283 (16.1) | 235 (83.0) | 48 (17.0) | 0.115 |
| Doxycycline (n=1757) | 324 (18.4) | 246 (75.9) | 78 (24.1) | 0.072 |
| Minocycline (n=1757) | 38 (2.2) | 29 (76.3) | 9 (23.7) | 0.615 |
| Azithromycin (n=1754) | 247 (14.1) | 195 (79.0) | 52 (21.1) | 0.750 |
| Ceftriaxone (n=1757) | 286 (16.3) | 172 (60.1) | 114 (39.9) | <0.001 |
| Levofloxacin (n=1760) | 234 (13.3) | 98 (41.9) | 136 (58.1) | <0.001 |
| Tocilizumab (n=1755) | 37 (2.1) | 8 (21.6) | 29 (78.4) | <0.001 |
| Remdesivir (n=1755) | 403 (23.0) | 257 (63.8) | 146 (36.2) | <0.001 |
| Zinc (n=1687) | 486 (28.8) | 440 (90.5) | 46 (9.5) | <0.001 |
| Hyperglycemia (n=1660) | 402 (24.2) | 258 (64.2) | 144 (35.8) | <0.001 |
| Renal dysfunction (n=1831) | 340 (18.6) | 152 (44.7) | 188 (55.3) | <0.001 |
| Hypotension (n=1800) | 95 (5.3) | 3 (3.2) | 92 (96.8) | <0.001 |
| Barotrauma and pneumothorax (n=1816) | 12 (0.7) | 0 | 12 | <0.001 |
| Myocarditis, MI and PTE (n=1816) | 28 (1.5) | 0 | 28 | <0.001 |
| Invasive fungal infections (n=1816) | 6 (0.3) | 4 (0.3) | 2 (0.5) | 0.62 |
| Hemodialysis (n=2017) | 20 (1.0) | 6 (30.0) | 14 (70·0) | <0.001 |
| Dialysis for AKI | 5 (0.25) | 1 (20.0) | 4 (80·0) | <0.001 |
*n denotes the number of patients who received the intervention and percentage denotes the proportion of patients who were discharged or died after receiving the intervention. HFNC: High-flow nasal cannula, NIV: Noninvasive ventilation, MV: Mechanical ventilation, ICU: Intensive care unit, MI: Myocardial infarction, AKI: Acute kidney injury, PTE: Pulmonary thromboembolism
Predictors of outcome
We analyzed the predictors of (i) developing severe illness needing oxygen therapy, (ii) deterioration during the hospital stay as defined above using the clinical and laboratory parameters at baseline are depicted in Tables 4 and 5, respectively. Predictors for the development of critical illness (as defined above) are depicted in Supplementary Table 1. The independent predictors of death were derived using logistic regression analysis and 4 models were created using the clinically relevant parameters as covariates. Adjusting for baseline clinical characteristics and inflammatory parameters [Table 6, Model-2], Age ≥45 years, having comorbid conditions, severe illness at presentation and hospitalization in the 3rd week of illness were independently associated with increased odds of death. Another model which adjusted for the baseline inflammatory markers in addition to model 1 found that vaccination (odds ratio [OR] 0.6 95% confidence interval [CI] 0.4–0.9 P = 0.032) and hospitalization in the 2nd week of illness (OR 0.6 95% CI 0.4–0.9 P = 0.003) reduced the odds of death by 40% each. The factors predicting deterioration in patients who were not hypoxic at baseline are described in Supplementary Table 2. The clinical and laboratory parameters associated with hospitalization at various time points following symptom onset has been detailed in Supplementary Table 3.
Table 4.
Factors predicting the development of hypoxia requiring oxygen support
| Oxygen support during the hospital stay | ||
|---|---|---|
|
| ||
| OR (95% CI), P | ||
|
| ||
| Model-1 | Model-2 | |
| Age (Reference: 18-45 years) | ||
| <18 years | 0.4 (0.2-0.9), 0.023 | 0.2 (0.1-0.8), 0.023 |
| 45-60 years | 2.9 (2.1-3.8), <0.001 | 2.0 (1.3-2.9), 0.001 |
| >60 years | 2.9 (2.1-4), <0.001 | 1.6 (1.0-2.6), 0.037 |
| Male (Reference: Female) | 1.2 (1.0-1.6), 0.077 | 1.1 (0.8-1.5), 0.730 |
| Vaccinated | 0.6 (0.5-0.8), <0.001 | 0.7 (0.5-1.0), 0.050 |
| Symptom onset to hospitalization (Reference: 1 week) | ||
| 2 weeks | 1.2 (0.9-1.5), 0.254 | 1.0 (0.7-1.4), 0.961 |
| 3 or more weeks | 1.2 (0.6-2.3), 0.614 | 0.7 (0.2-1.9), 0.451 |
| Asymptomatic | 0.3 (0.2-0.6), <0.001 | 0.1 (0.04-0.3), <0.001 |
| Symptoms reported (Reference: No) | ||
| Breathlessness | 6.3 (5-8.1), <0.001 | 3.7 (2.7-5.2), <0.001 |
| Dry cough | 1.3 (1-1.7), 0.024 | |
| Rhinitis | 0.5 (0.3-0.9), 0.012 | |
| Sore throat | 0.7 (0.5-0.9), 0.026 | |
| Fatigue | 0.7 (0.5-0.9), 0.036 | |
| Comorbidities (Reference: No) | ||
| 1 | 1.3 (1-1.8), 0.032 | 1.3 (0.9-1.9), 0.171 |
| 2 or more | 1.6 (1·2-2.3), 0.006 | 1.5 (0.9-2.4), 0.092 |
| Lab parameters (Reference: No) | ||
| Leucocyte count (Reference: Normal) | ||
| Leukopenia | 0.4 (0.2-0.6), <0.001 | |
| Leukocytosis | 2.6 (1.7-4.0), <0.001 | |
| D-Dimer >500 (Reference:=<500) | 2.5 (1.7-3.9), <0.001 | |
| CRP high (Reference: Normal) | 5.9 (3.4-9.9), <0.001 | |
| Creatinine >1.0 mg/dl | 1.8 (1.1-2.9), 0.018 | |
Model-1: Adjusting for baseline clinical parameters, Model-2: Model-1 plus baseline lab parameters Age, gender and comorbidities are included in all models Only symptoms and lab parameters with significant P value are included in these final models. OR: Odds ratio, CI: Confidence interval, CRP: C-reactive protein
Table 5.
Factors predicting deterioration during the hospital stay
| Deterioration during the hospital stay, OR (95% CI), P | ||
|---|---|---|
|
| ||
| Model-1 | Model-2 | |
| Age (Reference: 18-45 years) | ||
| <18 years | 0.6 (0.3-1.3), 0.179 | 0.5 (0.1-2.0), 0.343 |
| 45-60 years | 1.7 (1.3-2.3), <0.001 | 1.5 (1.0-2.2), 0.039 |
| >60 years | 2.6 (1.9-3.6), <0.001 | 1.8 (1.2-2.8), 0.006 |
| Male (Reference: Female) | 1.2 (0.9-1.5), 0.149 | 0.9 (0.7-1.3), 0.694 |
| Primary condition (Reference: COVID) | ||
| Non-COVID | 2.1 (1.2-3.8), 0.010 | 0.9 (0.4-2.2), 0.850 |
| Mucor | 2.1 (0.2-24.0), 0.546 | |
| Vaccinated | 0.8 (0·6-1.1), 0.131 | 0.8 (0.6-1.1), 0.237 |
| Time to admission from symptom onset (Reference: 1 week) | ||
| 2 weeks | 0.7 (0.5-0.8), 0.001 | 0.6 (0.4-0.8), 0.001 |
| 3 or more weeks | 1.4 (0.8-2.5), 0.206 | 0.8 (0.4-2.0), 0.675 |
| Asymptomatic | 1.0 (0.3-3.7), 0.952 | 2.3 (0.1-40.0), 0.569 |
| Comorbidities (Reference: No) | ||
| 1 | 1.3 (1.1-1.7), 0.030 | 1.3 (0.9-1.9), 0.110 |
| 2 or more | 1.5 (1.1-2.1), 0.005 | 1.4 (0.9-2.1), 0.147 |
| Baseline COVID-19 severity (Reference: Mild) | ||
| Asymptomatic - No hypoxia | 0.2 (0.1-0.8), 0.026 | 0.1 (0.0-2.2), 0.144 |
| Moderate | 2.4 (1.7-3.4), <0.001 | 1.6 (1.0-2.5), 0.055 |
| Severe | 3.1 (2.4-4.1), <0.001 | 1.1 (0.7-1.6), 0.678 |
| Symptoms reported (Reference: No) | ||
| Fever | 0.7 (0.5-0.9), 0.021 | |
| Dry cough | 1.3 (1.0-1.7), 0.021 | 1.6 (1.2-2.2), 0.002 |
| Lab parameters (Reference: No) | ||
| Thrombocytopenia | 1.8 (1.2-2.6), 0.003 | |
| Leucocyte count (Reference: Normal) | ||
| Leukopenia | 0.5 (0.3-0.8), 0.006 | |
| Leukocytosis | 2.1 (1.5-3.0), <0.001 | |
| D-Dimer >500 (Reference: ≤500) | 2.5 (1.8-3.4), <0.001 | |
| CRP high (Reference: Normal) | 6.0 (2.8-13.0), <0.001 | |
| Creatinine >1.0 mg/dl | 2.3 (1.5-3.3), <0.001 | |
Model-1: Adjusting for baseline clinical parameters, Model-2: Model-1 plus baseline lab parameters·Age, gender and comorbidities are included in all models·Only symptoms and lab parameters with significant P value are included in these final models. OR: Odds ratio, CI: Confidence interval, CRP: C-reactive protein
Supplementary Table 1.
Logistic regression model for developing critical illness
| OR (95%CI), P | ||
|---|---|---|
|
| ||
| Model-1 | Model-2 | |
| Age (Reference: 18-45 years) | ||
| <18 years | 0.8 (0.2-3.0), 0.765 | - |
| 45-60 years | 1.9 (1.3-2.7), 0.001 | 2·3 (1·4-3.8), 0·002 |
| >60 years | 2.7 (1.8-4.1), <0.001 | 2·5 (1·5-4·4), 0·001 |
| Male (Reference: Female) | 1.3 (1.0-1.8), 0.055 | 1·3 (0·9-1·9), 0·240 |
| Vaccinated | 0.7 (0.5-1.0), 0.054 | 0·7 (0·4-1.0), 0·057 |
| Symptom onset to hospitalization (Reference: 1 week) | ||
| 2 weeks | 0.7 (0.6-0.9), 0.040 | 0.8 (0.5-1.1), 0.158 |
| 3 or more weeks | 2.3 (1.3-4.2), 0.006 | 1.1 (0.4-2.8), 0.830 |
| Asymptomatic | 1.3 (0.4-4.8), 0.695 | 7.4 (0.3-165.0), 0.206 |
| Comorbidities (Reference: No) | ||
| 1 | 1.4 (1.0-2.0), 0.027 | 1.5 (1.0-2.3), 0.080 |
| 2 or more | 1.8 (1.3-2.6), 0.001 | 1.7 (1.1-2.7), 0.036 |
| Baseline COVID-19 severity (Reference: Mild) | ||
| Asymptomatic - No hypoxia | 0.2 (0.02-2.4), 0.213 | 0.03 (0.01-1.4), 0.074 |
| Moderate | 2.3 (1.2-4.3), 0.011 | 1.7 (0.8-3.6), 0.137 |
| Severe | 11 (6.7-17.0), <0.001 | 4.9 (2.8-8.8), <0.001 |
| Symptoms reported (Reference: No) | ||
| Gastrointestinal | 0.6 (0.4-1.0), 0.059 | 0.5 (0.2-1.0), 0.053 |
| Lab parameters (Reference: No) | ||
| Leucocyte count (Reference: Normal) | ||
| Leukopenia | 0.6 (0.3-1.5), 0.303 | |
| Leukocytosis | 2.1 (1.5-3.1), <0.001 | |
| Thrombocytopenia <1.5 lac (Reference: ≥1.5 lac) | 2.5 (1.5-4.0), <0.001 | |
| D-Dimer >500 ng/ml (Reference: ≤500 ng/ml) | 2.8 (1.9-4.0), <0.001 | |
| CRP >0.5 mg/dl (Reference: ≤0.5 mg/dl) | 3.9 (1.1-13.0), 0.032 | |
| Creatinine >1.0 mg/dl (Reference: ≤1.0 mg/dl) | 2.1 (1.4-3.2), 0.001 | |
Model-1: Adjusting for baseline clinical parameters, Model-2: Model-1 plus baseline lab parameters·Age, gender and comorbidities are included in all models·Only symptoms and lab parameters with significant P value are included in these final models. OR: Odds ratio, CI: Confidence interval, CRP: C-reactive protein
Table 6.
Factors predicting death
| OR (95% CI), P | ||||
|---|---|---|---|---|
|
| ||||
| Model-1 | Model-2 | Model-3 | Model-4 | |
| Age (Reference: 18-45 years) | ||||
| <18 years | 0.5 (0.1-1.7), 0.236 | 0.6 (0.1-5·9), 0.696 | 2.3 (0.4-13), 0.355 | 2 (0.2-18), 0.521 |
| 45-60 years | 1.7 (1.2-2.5), 0.004 | 2.0 (1.2-3.3), 0.012 | 1.6 (0.9-3.0), 0.141 | 1.5 (0.7-2.8), 0.294 |
| >60 years | 3.5 (2.4-5.2), <0.001 | 3.0 (1.7-5.1), <0.001 | 2.7 (1·4-5.2), 0.003 | 2.6 (1.3-5.1), 0.006 |
| Male (Reference: Female) | 1.2 (0.9-1.6), 0.199 | 1 (0.7-1.5), 0.980 | 1 (0.6-1.7), 0.943 | 1.1 (0.7-1.9), 0.708 |
| Primary condition (Reference: COVID) | ||||
| Non-COVID | 3.5 (1.7-7.2), 0.001 | 1.2 (0.4-3.5), 0.705 | 0.8 (0.3-2·6), 0.729 | 0.5 (0.1-1.8), 0.266 |
| Mucor | 0.5 (0.1-4.0), 0.472 | 0.9 (0.1-11), 0.940 | ||
| Vaccinated | 0.7 (0.5-1.0), 0.060 | 0.6 (0.4-0.9), 0.032 | 0.5 (0.3-0.9), 0.014 | 0.6 (0.3-0.9), 0.042 |
| Symptom onset to hospitalization (Reference: 1 week) | ||||
| 2 weeks | 0.6 (0.5-0.9), 0.003 | 0.6 (0.4-0.9), 0.027 | 0.5 (0.3-0.8), 0.002 | 0.5 (0.3-0.8), 0.003 |
| 3 or more weeks | 1.9 (1.1-3.4), 0.032 | 0.9 (0.4-2.2), 0.828 | 0.6 (0.2-1.9), 0.372 | 0.5 (0.1-1.9), 0.336 |
| Asymptomatic | 1.1 (0.3-4.2), 0.886 | 6.5 (0.3-152), 0.242 | 13 (0.5-344), 0.126 | 0.01 (NR) 0.984 |
| Comorbidities (Reference: No) | ||||
| 1 | 1.5 (1.1-2), 0.017 | 1.6 (1.0-2.5), 0.037 | 2.1 (1.3-3.7), 0.005 | 1.9 (1.1-3.3), 0.031 |
| 2 or more | 1.8 (1.2-2.5), 0.002 | 1.5 (0.9-2.5), 0.087 | 2.0 (1.1-3.6), 0.029 | 1.6 (0·9-3.1), 0.130 |
| Baseline COVID-19 severity (Reference: Mild) | ||||
| Asymptomatic - No hypoxia | 0.1 (0.01-0.9), 0.048 | 0.03 (0.01-1.1), 0.054 | 0.02 (0.01-0.82), 0.039 | 5837 (NR) 0.897 |
| Moderate | 2.1 (1.2-3.9), 0.013 | 1.5 (0.8-3.2), 0.241 | 1.5 (0.6-3.3), 0.382 | 1.3 (0.6-3.3), 0.522 |
| Severe | 12 (7.6-18), <0·001 | 4.7 (2.7-8.2), <0.001 | 2.4 (1.2-4.9), 0.011 | 2.4 (1.2-5.1), 0.017 |
| Symptoms reported (Reference: No) | ||||
| Fever | 0.6 (0.4-0.8), 0.002 | |||
| Gastrointestinal | 0.5 (0.3-0.9), 0.017 | 0.4 (0.2-0.9), 0.030 | 0.3 (0.1-0.8), 0.006 | 0.3 (0.1-0.7), 0.009 |
| Loss of taste | 0.4 (0.2-0.9), 0.018 | |||
| Lab parameters (Reference: No) | ||||
| Leucocyte count (Reference: Normal) | ||||
| Leukopenia | 0.6 (0.2-1.4), 0.218 | 0.7 (0.3-2.0), 0.533 | 0.9 (0.3-2.4), 0.778 | |
| Leukocytosis | 2.1 (1.5-3.1), <0.001 | 1.9 (1.2·1-3), 0.010 | 2.0 (1·2-3·3), 0.009 | |
| Thrombocytopenia <1.5 lac (Reference: ≥1.5 lac) | 2.5 (1.5-4), <0.001 | 2.3 (1.3-4.1), 0.006 | 2·1 (1·1-3·9), 0.018 | |
| d-dimer >500 (Reference: ≤500) | 3.2 (2.2-4.7), <0.001 | 4.5 (2.9-7.2), <0.001 | 4.6 (2.8-7.5), <0.001 | |
| CRP high (Reference: Normal) | 3.7 (2.2-13), 0.037 | |||
| Creatinine >1.0 mg/dl | 2.7 (1.7-4.1), <0.001 | 3.2 (1.9-5·5), <0.001 | ||
| Treatment (Reference: No) | ||||
| Methylprednisolone pulse (>250 mg/day) | 6.4 (3.3-12), <0.001 | 5.9 (3.0-12), <0.001 | ||
| Levofloxacin | 4.9 (2.8-8.6), <0.001 | 3.7 (2-6.9), <0.001 | ||
| Tocilizumab | 6.6 (1.4-32), 0.019 | 5.5 (0.9-29), 0.047 | ||
| Zinc | 0.2 (0.1-0.4), <0.001 | 0.3 (0.2-0.5), <0.001 | ||
| Anticoagulant therapy | 2.5 (1.2-5.0), 0.012 | 2.1 (1.0-4.3), 0.052 | ||
| In hospital complications | ||||
| Renal dysfunction | 3.8 (2.2-6·6), <0.001 | |||
| Hypotension | 30 (6.4-13), <0.001 | |||
Model-1: Adjusting for baseline clinical parameters, Model-2: Model-1 plus baseline lab parameters, Model-3: Model-2 plus treatment characteristics, Model-4: Model-3 plus in-hospital complications·Age, gender and comorbidities are included in all models·Only symptoms, lab and treatment parameters with significant P value are included in these final models. OR: Odds ratio, CI: Confidence interval, CRP: C-reactive protein, NR=Not reported
Supplementary Table 2.
Factors predicting deterioration during hospital stay amongst those with no hypoxia at Baseline
| OR (95% CI), P | ||
|---|---|---|
|
| ||
| Model-1 | Model-2 | |
| Age (Reference: 18-45 years) | ||
| <18 years | 0.78 (0.32-1.88), 0.578 | 0.5 (0.1-2.1), 0.365 |
| 45-60 years | 2.1 (1.38-3.19), 0.001 | 1.4 (0.8-2.4), 0.188 |
| >60 years | 2.44 (1.52-3.91), <0.001 | 1.5 (0.8-2.7), 0.213 |
| Male (Reference: Female) | 1.53 (1.07-2.18), 0.02 | 1.1 (0.72-1.8), 0.588 |
| Vaccinated | 0.71 (0.48-1.05), 0.085 | 0.8 (0.49-1.3), 0.317 |
| Comorbidities (Reference: No) | ||
| 1 | 1.5 (1.02-2.22), 0.041 | 1.2 (0.7-1.9), 0.553 |
| 2 or more | 2.13 (1.31-3.45), 0.002 | 1.4 (0.8-2.6), 0.258 |
| Baseline COVID-19 severity (Reference: Mild) | ||
| Asymptomatic - No hypoxia | 0.36 (0.14-0.94), 0.036 | |
| Moderate | 2.22 (1.56-3.15), <0.001 | |
| Symptoms reported (Reference: No) | ||
| Dry cough | 1.49 (1.05-2.1), 0.025 | |
| Gastrointestinal symptoms | 1.95 (1.28-2.97), 0.002 | |
| Fever | 2.3 (1.3-4), 0.003 | |
| Lab parameters (Reference: No) | ||
| Leucocyte count (Reference: Normal) | ||
| Leukopenia | 0.5 (0.3-0.9), 0.026 | |
| Leukocytosis | 2.3 (1.3-4.0), 0.003 | |
| D-Dimer >500 ng/ml (Reference: ≤500 ng/ml) | 1.7 (1.0-3.0), 0.047 | |
| CRP >0.5 mg/dl (Reference: ≤0.5 mg/dl) | 9.4 (3.7-24.0), <0.001 | |
| Creatinine >1.0 mg/dl (Reference: ≤1.0 mg/dl) | 2.2 (1.2-3.9), 0.010 | |
Notes: Model-1: Adjusting for baseline clinical parameters, Model-2: Model-1 plus baseline lab parameters·Age, gender and comorbidities are included in all the models·Only symptoms and lab parameters with significant P value are included in these final models. OR: Odds ratio, CI: Confidence interval, CRP: C-reactive protein
Supplementary Table 3.
Demographic profile and baseline characteristics of patients who presented at varying time windows
| Variable | Week-1, n (%) | Week-2, n (%) | Week ≥3, n (%) | Asymptomatic, n (%) | P |
|---|---|---|---|---|---|
| Age (n=2017), mean (SD) | 49.5 (18.5) | 51.1 (14.7) | 48.9 (16.4) | 37.3 (17.8) | <0.001 |
| Days of hospital stay (n=2017), median (IQR) | 8 (5-11) | 8 (4-12) | 9 (4-16) | 6 (4-8) | 0.002 |
| Sex (n=1992) | |||||
| Female | 414 (35.0) | 208 (34.5) | 26 (32.1) | 40 (32.0) | 0.878 |
| Male | 769 (65.0) | 395 (65.5) | 55 (67.9) | 85 (68.0) | |
| Primary condition (n=1841) | |||||
| COVID | 1009 (94.0) | 563 (97.7) | 69 (89.6) | 90 (79.0) | <0.001 |
| Mucor | 6 (0.6) | 3 (0.5) | 5 (6.5) | 0 (0) | |
| Non-COVID | 59 (5.5) | 10 (1.7) | 3 (3.9) | 24 (21.1) | |
| Vaccine protection (n=1806) | |||||
| Not vaccinated | 769 (72.3) | 406 (72.0) | 59 (81.9) | 74 (69.2) | 0.273 |
| Vaccinated | 294 (27.7) | 158 (28.0) | 13 (18.1) | 33 (30.8) | |
| Symptoms (n=1677) | |||||
| Fever | 813 (77.8) | 486 (87.1) | 67 (90.5) | <0.001 | |
| Breathlessness | 486 (46.5) | 368 (66.0) | 52 (70.3) | <0.001 | |
| Drycough | 594 (56.8) | 312 (55.9) | 35 (47.3) | 0.277 | |
| Cought with expectoration | 128 (12.3) | 78 (14.0) | 8 (10.8) | 0.537 | |
| Rhinitis | 57 (5.5) | 17 (3.1) | 1 (1.4) | 0.036 | |
| Sorethroat | 232 (22.2) | 95 (17.0) | 8 (10.8) | 0.006 | |
| Fatigue | 188 (18.0) | 94 (16.9) | 9 (12.2) | 0.048 | |
| Myalgia | 225 (21.5) | 102 (18.3) | 8 (10.8) | 0.039 | |
| Chest pain | 93 (8.9) | 37 (6.6) | 3 (4.1) | 0.125 | |
| Gastrointestinal symptoms | 153 (14.6) | 61 (10.9) | 4 (5.4) | 0.015 | |
| Drowsiness | 12 (1.2) | 3 (0.5) | 1 (6.3) | 0.457 | |
| Loss of smell | 99 (9.5) | 46 (8.2) | 2 (2.7) | 0.12 | |
| Loss of taste | 95 (9.1) | 51 (9.1) | 2 (2.7) | 0.165 | |
| Comorbidity status (n=1857) | |||||
| Any comorbidity | 564 (51.5) | 304 (53.0) | 46 (59.0) | 51 (46.8) | 0.385 |
| 1 comorbidity | 355 (32.4) | 189 (32.9) | 26 (33.3) | 35 (32.1) | 0.591 |
| ≥2 comorbidities | 209 (19.1) | 115 (20.0) | 20 (25.6) | 16 (14.7) | |
| Temperature at baseline (n=1598) | |||||
| Febrile at baseline | 11 (1.2) | 3 (0.6) | 0 (0) | 1 (1.0) | 0.624 |
| Heart rate at baseline (n=1690) | |||||
| Tachycardia at baseline | 463 (46.5) | 258 (48.9) | 40 (60.6) | 37 (36.6) | 0.018 |
| Respiratory rate at baseline (n=1663) | |||||
| Tachypnea at baseline | 188 (19.2) | 121 (23.4) | 16 (25.8) | 7 (6.7) | 0.001 |
| Oxygen status at presentation (n=1801) | |||||
| On oxygen | 210 (20.0) | 165 (29.4) | 34 (46.0) | 4 (3.5) | <0.001 |
| On room air | 842 (80.0) | 397 (70.6) | 40 (54.1) | 109 (96.5) | |
| Baseline COVID severity (n=1790) | |||||
| Asymptomatic_No hypoxia | 0 | 0 | 0 | 96 (85.0) | <0.001 |
| Mild (symptomatic_No hypoxia) | 425 (40.8) | 123 (21.9) | 16 (21.9) | 0 | |
| Moderate (No hypoxia but report Breathlessness) | 124 (11.9) | 74 (13.2) | 10 (13.7) | 1 (0.9) | |
| Severe (hypoxic-SpO2 <94%) | 494 (47.4) | 364 (64.9) | 47 (64.4) | 16 (14.2) | |
|
| |||||
| Inflammatory markers | n, median (IQR) | n, median (IQR) | n, median (IQR) | n, median (IQR) | P |
|
| |||||
| IL-6 (pg/mL) | 393, 12.4 (5-34.6) | 304, 13.5 (5.0-39.7) | 50, 11.8 (4.8-41.6) | 11.8 (4.8-41.6) | 0.544 |
| CRP (mg/dL) | 932, 3.7 (0.9-8.7) | 487, 4.5 (1.8-11.1) | 55, 3.9 (1.7-8.5) | 97, 0.4 (0.1-3.0) | <0.001 |
| LDH (U/L) | 846, 351 (245-528) | 437, 453 (337-618) | 42, 366 (259-609) | 94, 238 (202-308) | <0.001 |
| Ferritin (ng/mL) | 341, 386.4 (135-947.5) | 184, 739.5 (312.2-1273.9) | 28, 877.5 (274.3-1538.55) | 31, 270.2 (80.1-763.7) | <0.001 |
| D-Dimer (ng/mL) | 852, 215.5 (130.5-433) | 425, 281 (180-548) | 51, 477 (271-2215) | 82, 121.5 (79-318) | <0.001 |
| Fibrinogen (mg/dL) | 856, 405 (334.5-471) | 430, 446 (378-512)] | 54, 433 (347-514) | 85, 321 (278-387) | <0.001 |
SD: Standard deviation, IQR: Interquartile ranges, CRP: C-reactive protein, SpO2: Oxygen saturation, LDH: Lactate dehydrogenase, IL-6: Interleukin 6
Leukocytosis, thrombocytopenia, elevated d-dimer, C-reactive peptide, and creatinine at baseline independently predicted mortality after adjusting for other baseline and treatment characteristics [Table 6, Model-3].
DISCUSSION
This study describes the demography and clinical profile of the patients admitted to our facility during the “second wave” of the COVID-19 pandemic. Few points are worthy of elaboration. Although the vaccination program had been started around 3 months before the rapid ascent in the curve, <2% of the patients who were admitted had received both the doses of vaccine at least 2 weeks before the infection and 14.2% were partially vaccinated. In a subgroup analysis of patients from this cohort who were eligible to get vaccinated, it was found that completing the full course of vaccination had significantly reduced odds of death.[5] The designated COVID-19 facility catered to predominantly patients who were getting admitted due to COVID-19 pneumonia. We had around 5% of patients who had other indications for admission such as malignancy. We found that getting admitted for other indications had a higher odds of death as compared to those who were admitted for COVID-19. Similarly, another interesting finding was that patients who got admitted in the 3rd week (OR 1.9 95% CI 1.1–3.4 P = 0.032) after symptom onset had a higher odds of death as compared to those getting admitted in the first week of onset of symptoms, while the odds of death was significantly lower (OR 0.6 95% CI 0.5–0.9 95% CI 0.003) when admitted during the 2nd week of onset of symptoms compared to week 1. This probably could be because the initial 1st week was associated with fulminant viral pneumonia and those who present with severe disease at this early viremic stage had a higher odds of death. Similarly, those who presented in the 3rd week are likely to be those with inflammatory damage due to the “cytokine storm” which occurs in the 2nd week. These patients who presented in the 3rd week are likely those who had not received corticosteroid therapy in the 2nd week of the illness when the inflammatory phase had set in, probably leading to an increase in mortality. Another possibility is that, since ours was a tertiary care center that had patients referred from other centers, who were sicker patients with poor prognostic factors were selectively admitted to our center, but we do not have the data on the proportion of cases who were referred. However, these hypotheses need further study.
Our findings were different from the study by Chauhan et al.[6] that showed more proportion of fever, fatigue, myalgia, abdominal pain in those who died as compared to those who survived. However, the study by Bairwa et al.[7] concurred with the clinical symptomatology of our study. The case-fatality rate of our cohort was 19.5% which was similar to that in multiple other studies.[6,7,8,9,10] Another cohort from our center during the “first wave” of the pandemic reported a case-fatality rate of 1.4%.[11] However, a study from a tertiary care hospital in New Delhi reported a CFR of 28.3%.[12] The cohort of patients reported by Gupta et al.[13] had a case-fatality rate of 9.5% while the study by Wang et al.[14] reported a CFR of 4.3%. These differences could be due to various factors such as different strains of the virus, vaccination rates, usage of antiviral/anti-inflammatory agents, and monoclonal antibodies, due to study design factors such as the proportion of patients still admitted and undergoing treatment at the end of the study or due to other baseline differences. Similar to these studies, a higher proportion of those with comorbidities and those with severe illness succumbed to the illness.
Patients treated with methylprednisolone pulse therapy and tocilizumab had a higher odds of death, as shown in Table 4. This apparent paradox may be due to confounding by indication. Understandably, patients with severe disease and those with critical illness are more likely to be treated with these agents.
In our cohort, we had 1067 patients who needed oxygen and only 63% of them survived. This implies a mortality rate of 37% in patients who needed some form of oxygen therapy or mechanical ventilation. This reflects the severe nature of COVID-19 pneumonia and ARDS in this cohort of patients. More than 47% of patients of our cohort had ARDS due to COVID-19 pneumonia. Respiratory failure necessitating mechanical ventilation had a very poor prognosis with only 13.7% of those receiving noninvasive ventilation and 2.4% of those receiving invasive mechanical ventilation being discharged. It was disappointing that, almost 86% of patients who had to be ventilated died. This is probably due to the fulminant viral pneumonia in the initial week and hyperinflammatory lung disease during the second and 3rd weeks of the illness. This is different from the historical mortality rates of ARDS.[15] Similarly, all 12 patients who developed barotrauma-related pneumothorax or pneumomediastinum succumbed. The presence of renal dysfunction irrespective of the need for renal replacement therapy and hypotension were also independently associated with increased odds of mortality. These observations could have several reasons. First, it is possible that patients who had predictors of severe illness could have been admitted to our center as seen from the fact that more than half of the patients had at least one comorbidity, leading to poorer outcomes. Second, it is possible that due to the stretched health-care resources, skewed doctor − patient ratios and nurse to patient ratios and other factors that are important in improving outcomes in the critical care setting could have been compromised. Because of the sheer patient load, on several instances, patients who needed ICU care had been managed in the HDU setting and in several cases, in order to avoid delay, intubation and initiation of mechanical ventilation were done by the physicians on duty and the quick response team in the respective wards and then shifted to the HDU/ICU subject to bed availability. However, all the patient care areas had round-the-clock monitoring by physicians and HDU and ICU areas had trained critical care teams. Similarly, although we had procured monitoring devices in bulk during the pandemic, there were few poor quality equipment such as faulty pulse oximeters. We also had a few instances of limited communication due to internet outages. The hospital was completely dependent on internet connectivity through which the laboratories and imaging databases were integrated into patient care areas. We had near misses when oxygen supply was running out, though fortunately, supply was never interrupted because our hospital administration could ensure sufficient quantities to keep the ventilators and flow meters running by working round the clock and liasioning with suppliers.
In another analysis of patients from this cohort, who were hypoxic at presentation, it was found that patients who had silent hypoxia and dyspneic hypoxia had comparable mortality rates.[16]
Our study includes a large cohort of patients which allows us to explore the various factors associated with severe illness, deterioration during hospitalization, and death. However, it has a few limitations. Due to the retrospective nature of the data collection, few files had been misplaced or lost and nearly 10% of data points were missing. However, due to the large sample size of 2080 patients, we were able to find several independent risk factors for these outcomes. Similarly, due to the observational nature of the study, it is not possible to draw the conclusions regarding causation. It is difficult to comment on the efficacy of for example high dose methylprednisolone pulses in preventing in-hospital deterioration because the drug had been administered at varying time points in the patients rather than a protocolized administration. In some patients, it had been administered once they needed oxygen administered through a high-flow nasal cannula while in others it was administered while on the verge of respiratory failure needing noninvasive ventilation. Similarly, the treatment was decided by the individual clinicians based on their discretion and thus subject to confounding by indication, thus precluding definite causation from being inferred. Other inflammatory markers such as raised ferritin and IL-6 were also associated with increased odds of death; however, due to the low number of patients in whom these parameters were available at baseline, it was not included in the logistic regression models.
COCLUSION
This is the largest cohort of COVID-19 patients that has been reported from the South-east Asian region. Our study has also reported the relationship between the time of presentation and its association with mortality. We believe that early admission to the hospital, especially during the inflammatory phase, could make a difference by reducing mortality. Although during the first week or the viremic phase, we have no definite intervention to prevent mortality. Even remdesivir, which is an antiviral drug is effective in patients who are hypoxic but not critically ill and not in the early phase of the illness. Thus, the data showing better outcomes in the 2nd week and poorer outcomes in the third might be an indicator that those who are administered corticosteroids in the inflammatory phase might benefit as compared to those presenting in the 3rd week. However, this could be subject to referral bias as sicker patients could have been admitted later. Based on the available data, we could also conclude that vaccination has an impact on reducing the odds of death. However, once the patient develops ARDS related to COVID-19 necessitating respiratory support, the prognosis was dismal in our study. Administration of corticosteroids early in the inflammatory phase seems to be the only intervention that could have possibly changed the course of illness in our patients. These results described apply to patients who need hospitalization and most patients with mild COVID-19 were still managed at home settings during the second wave of the pandemic in India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.COVID Live Update:237,809,466 Cases and 4,853,001 Deaths from the Coronavirus –Worldometer. [Last accessed on 2021 Oct 09]. Available from: https://www.worldometers.info/coronavirus/
- 2.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Clinical Spectrum. COVID-19 Treatment Guidelines. [Last accessed on 2021 Jul 19]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 4. [Last accessed on 2021 Jul 19]. Available from: https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19.pdf .
- 5.Sagiraju HK, Elavarasi A, Gupta N, Garg RK, Paul SS, Vig S. The effectiveness of SARS-CoV-2 vaccination in preventing severe illness and death-real-world data from a cohort of patients hospitalized with COVID-19. medRxiv. doi: 10.4103/ijcm.ijcm_1388_21. 2021.08.26.21262705. Doi:https://doi.org/10.1101/2021.08.26.21262705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan NK, Shadrach BJ, Garg MK, Bhatia P, Bhardwaj P, Gupta MK, et al. Predictors of clinical outcomes in adult COVID-19 patients admitted to a tertiary care hospital in India: An analytical cross-sectional study. Acta Biomed. 2021;92:e2021024. doi: 10.23750/abm.v92i3.10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bairwa M, Kumar R, Ajmal M, Bahurupi Y, Kant R. Predictors of critical illness and mortality based on symptoms and initial physical examination for patients with SARS-CoV-2: A retrospective cohort study. J Infect Public Health. 2021;14:1028–34. doi: 10.1016/j.jiph.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregoriano C, Koch D, Haubitz S, Conen A, Fux CA, Mueller B, et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: An observational analysis. Swiss Med Wkly. 2020;150:w20316. doi: 10.4414/smw.2020.20316. [DOI] [PubMed] [Google Scholar]
- 9.Albalawi O, Alharbi Y, Bakouri M, Alqahtani A, Alanazi T, Almutairi AZ, et al. Clinical characteristics and predictors of mortality among COVID-19 patients in Saudi Arabia. J Infect Public Health. 2021;14:994–1000. doi: 10.1016/j.jiph.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JJ, Cao YY, Tan G, Dong X, Wang BC, Lin J, et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2021;76:533–50. doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan A, Tiwari P, Bhatnagar S, Patel A, Maurya A, Dar L, et al. Clinico-demographic profile &hospital outcomes of COVID-19 patients admitted at a tertiary care centre in North India. Indian J Med Res. 2020;152:61–9. doi: 10.4103/ijmr.IJMR_1788_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal A, Shrivastava A, Kumar A, Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J Assoc Physicians India. 2020;68:19–26. [PubMed] [Google Scholar]
- 13.Gupta N., Ish P., Kumar R., Dev N., Yadav S. R., Malhotra N., Agrawal S., Gaind R., Sachdeva H. and COVID 2019 working group, *Other members of the S. H.. (2020) “Evaluation of the clinical profile, laboratory parameters and outcome of two hundred COVID-19 patients from a tertiary centre in India”. Monaldi Archives for Chest Disease. 90(4) doi: 10.4081/monaldi.2020.1507. doi:10.4081/monaldi.2020.1507. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in Intensive Care Units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 16.Sirohiya P, Elavarasi A, Raju Sagiraju HK, Baruah M, Gupta N, Garg RK. Silent hypoxia in coronavirus disease-2019: Is it more dangerous?–A retrospective cohort study. medRxiv. doi: 10.4103/lungindia.lungindia_601_21. 2021.08.26.21262668. Doi:https://doi.org/10.1101/2021.08.26.21262668. [DOI] [PMC free article] [PubMed] [Google Scholar]


