Abstract
Background
18F-fluorodeoxyglucose (FDG) PET/CT is recommended for evaluation of intermediate-risk indeterminate pulmonary nodules (IPNs). While highly sensitive, the specificity of FDG remains suboptimal for differentiating malignant from benign nodules, particularly in areas where fungal lung diseases are prevalent. Thus, a cancer-specific imaging probe is greatly needed. In this study, we tested the hypothesis that a PET radiotracer (S)-4-(3-[18F]-fluoropropyl)-L-glutamic acid (FSPG) improves the diagnostic accuracy of IPNs compared to 18F-FDG PET/CT.
Methods
This study was conducted at a major academic medical center and an affiliated VA medical center. Twenty-six patients with newly discovered IPNs 7-30mm diameter or newly diagnosed lung cancer completed serial PET/CT scans utilizing 18F-FDG and 18F-FSPG, without intervening treatment of the lesion. The scans were independently reviewed by two dual-trained diagnostic radiology and nuclear medicine physicians. Characteristics evaluated included quantitative SUVmax values of the pulmonary nodules and metastases.
Results
A total of 17 out of 26 patients had cancer and 9 had benign lesions. 18F-FSPG was negative in 6 of 9 benign lesions compared to 7 of 9 with 18F-FDG. 18F-FSPG and 18F-FDG were positive in 14 of 17 and 12 of 17 malignant lesions, respectively. 18F-FSPG detected brain and intracardiac metastases missed by 18F-FDG PET in one case, while 18F-FDG detected a metastasis to the kidney missed by 18F-FSPG.
Conclusion
In this pilot study, there was no significant difference in overall diagnostic accuracy between 18F-FSPG and 18F-FDG for the evaluation of IPNs and staging of lung cancer. Additional studies will be needed to determine the clinical utility of this tracer in the management of IPNs and lung cancer.
Introduction
Lung cancer remains the leading cause of cancer-related death in the United States. The overall 5-year survival from lung cancer remains poor at approximately 20% [1]. Early detection is key to survival as mortality increases with advanced stage [2]. One of the factors that contributes to this high mortality is the delay in definitive diagnosis when an indeterminate pulmonary nodule (IPN) is newly discovered. However, even in high-risk patients, most IPNs are benign [3, 4]. Based on risk assessment, one management option for a newly diagnosed intermediate-risk lung nodule is to repeat imaging over time with the risk of delaying treatment and missing a potential chance of cure. The second option is to proceed with tissue diagnosis at the time of discovery, but this strategy leads to unnecessary healthcare spending, invasive procedures, anxiety, and morbidity [2, 5, 6].
18F-FDG PET/CT is a functional imaging modality recommended for the evaluation of intermediate-risk IPNs [7–9], although this practice is controversial [10]. While highly sensitive, the specificity of 18F-FDG remains variable for differentiating malignant from benign lesions, at approximately 40–60%, particularly in areas with endemic fungal lung disease [10–12]. Furthermore, the sensitivity and specificity of 18F-FDG to diagnose suspicious IPNs vary across clinical context, histologic subtype, and nodule size [13–15]. Several other nuclear imaging probes have been tested to differentiate benign vs malignant pulmonary nodules, but none have been validated to show superiority to 18F-FDG PET/CT [16–21]. Thus, there is an unmet need for a cancer-specific imaging probe to better differentiate benign from malignant pulmonary nodules.
(S)-4-(3-[18F]-fluoropropyl)-L-glutamic acid (18F-FSPG) is an 18F-labeled glutamic acid derivative designed to target tumor-specific adaptations of intermediary metabolism. 18F-FSPG is taken up via the xCT transporter, a glutamate-cystine exchanger (SLC7A11/SLC3A2 (CD44) heterodimer) that transports L-cystine (Cys-S-S-Cys) into the cell and L-glutamate to the extracellular compartment [22]. 18F-FSPG has shown promise as a cancer-imaging probe in a variety of preclinical and clinical settings [22–29]. 18F-FSPG has been evaluated in patients with several tumors, including breast, lung, prostate, colorectal, non-Hodgkin lymphoma, head and neck, hepatocellular carcinoma and brain lesions with promising results [24–29]. 18F-FSPG may also have superior accuracy in correctly identifying benign lesions as compared to 18F-FDG [23, 30].
Given these encouraging clinical and preclinical data, we investigated the hypothesis that in patients with IPNs, PET/CT imaging with 18F-FSPG improves diagnostic accuracy compared to 18 F-FDG PET/CT. In patients with newly diagnosed, untreated lung cancer, we also compared their accuracy for staging and correlated 18F FSPG uptake with xCT and CD44 expression.
Methods
Study design
This was a pilot, open-label, prospective non-randomized clinical study. Our primary endpoint was the accuracy of 18F FSPG and 18F FDG PET/CT in discriminating between benign and malignant lesions in patients with newly discovered IPNs. Our secondary endpoint was the accuracy of 18F FSPG and 18F FDG PET/CT for initial staging in patients with newly diagnosed treatment-naïve lung cancer. We also investigated the correlation of 18F FSPG uptake with CD44 and xCT transporter expression in malignant lesions. This study was approved by the Internal Review Boards at Vanderbilt University Medical Center (IRB# 150392) and of the Nashville VA Medical Center (IRB# 1205000) and registered in Clinicaltrials.gov (NCT02448225). Informed consent was obtained from all patients before enrollment.
Patients selection (inclusion/exclusion criteria)
Vanderbilt University Medical Center’s (VUMC) and Nashville VA Medical Center’s (VAMC) Institutional Review Boards (IRBs) approved this study under the purview of the US Food and Drug Administration investigational new drug (IND) application 124202 ([18F]FSPG). Prospective participants from VUMC and VAMC were recruited by the study team members. Inclusion criteria included adult patients, age 40 to 80, with a newly detected IPN (7-30mm in diameter) that had not previously been evaluated, or newly diagnosed, untreated primary lung cancer 7 mm or more in diameter, and who had an indication for 18FDG PET/CT as part of standard of care evaluation. Exclusion criteria included known active lung infection, pregnant or lactating patients, body weight greater than 400 pounds, a body habitus or disability that would not permit the imaging protocol to be performed, previous radiation or systemic treatment for cancer of any type within 1 year, and, if no tissue diagnosis, expected lifespan less than 2 years, which would preclude appropriate follow up.
PET/CT protocol
All patients underwent a standard of care whole body 18F-FDG PET/CT scan for the evaluation of an IPN or lung mass. A similar protocol was followed for obtaining the 18F-FSPG PET/CT images. 18F-FSPG scans were performed within 30 days of the 18F-FDG scans with no intervening treatment of the lesion, except for 2 patients who had 34 and 63 days in between scans but no intervening treatment. The patients were asked to have nothing to eat or drink (NPO) after midnight on the day of the scans. Fasting blood glucose levels were measured prior to initiation of the exam to ensure that the levels were below 200 mg/dL. Standard of care scan from the vertex to mid-thigh was performed approximately 60 minutes after the IV administration of 18F-FDG (dose range 10.1 to 24.5 mCi) or 18F-FSPG (dose range 7.5 to 9 mCi) with the patient in the supine position. Images were acquired either on a GE Discovery STE (VUMC) or GE Discovery VCT (VAMC) PET/CT scanner with emission imaging in 3D mode with scatter correction. Low dose CT scans were obtained for attenuation correction and anatomic localization.
Imaging analysis
18F-FDG and 18F-FSPG PET/CT studies were reviewed independently by two dual-trained diagnostic radiology and nuclear medicine physicians blinded to each other’s interpretation. A third radiologist was available for ties, but this was not necessary. Maximum standardized uptake values (SUVmax) were normalized to lean body mass and measured with a 1 cm diameter round region of interest over the area of greatest uptake in the lesion being measured. Regions of interest were drawn around the IPNs as well as any suspicious areas of metastasis. SUVmax values from the IPNs, metastases and blood pool in the ascending aorta were recorded. These measurements were performed by using Xeleris 4.0 workstation hardware and software from GE Healthcare, Waukesha, MI, USA.
Radiotracer production
18F-FSPG was produced by the Vanderbilt Center for Molecular Probes Radiochemistry Core Laboratory in accordance with the Chemistry, Manufacturing, and Control (CMC) sections of the IND referenced above. Tracers met all USP <823> requirements for sterile, injectable PET radiopharmaceuticals. The tracers used for this PET study were previously evaluated in humans at VUMC and other sites with no adverse side effects, such as anaphylactic reactions, allergic reactions, any morbidity, or mortality observed. 18F-FSPG is obtained by radiolabeling of the protected precursor di-tertbutyl (2S,4S)-2-(3-((naphthalen-2-ylsulfonyl)oxy)propyl)-4-(tritylamino)pentanedioate) (PI-021) with [18F]fluoride. After acidic deprotection, the tracer is purified over cartridges and finally formulated for intravenous injection by passing the solution through a 0.22-μm sterile filter. The synthesis is performed within an automated synthesis module in a lead-shielded hot cell.
Immunohistochemistry of xCT transporter and CD44 expression
Tumor tissue from biopsies or surgical excisions performed as standard of care for pathologic examinations were obtained before or after PET/CT imaging for immunohistochemistry (IHC) studies for xCT and CD44 expression levels. Tissue processing and IHC analysis of formalin-fixed, paraffin-embedded tissue sections were performed by VUMC Translational Pathology Shared Resource and as detailed in previous publications [31, 32]. Briefly, 5-um thick whole tissue sections were transferred onto poly-L-lysine-coated adhesive slides and dried at 60°C for 60 minutes. Slides were then placed on the Leica Bond-Rx IHC stainer. All steps besides dehydration, clearing and coverslipping were performed on the Leica Bond-RX. Slides were deparaffinized. Heat induced antigen retrieval was performed on the Bond-Rx using their Epitope Retrieval 1 solution for 20 minutes (anti-CD44) and Epitope Retrieval 2 solution for 20 minutes (anti-xCT). Slides were incubated with anti-CD44 (cat# 3570S, Cell Signaling Technology, Danvers, MA) for one hour at a dilution of 1:500 and with anti-xCT (cat# 12691S, Cell Signaling Technology, Danvers, MA) for one hour at a dilution of 1:100. The Leica Bond Refine (Polymer) detection system was used for visualization. Slides were then dehydrated, cleared and coverslipped. The level of expression of xCT and CD44 proteins in the cytoplasmic membrane of tumor cells were examined by an experienced pathologist who was blinded to any patient and imaging information. The percentage of tumor cells positive for the marker and the intensity of staining were evaluated, the latter using a scale of 0 (none), 1+ (weak), 2+ (intermediate), and 3+ (strong) with a sample being reported as positive if greater than 10% of the tumor cells in the sample were positively stained with any intensity. The correlation between the intensity of IHC staining and SUVmax of the corresponding lesion on the PET/CT was assessed using Spearman correlation.
Statistical analysis
Descriptive statistics, reported as means and standard deviations or median and interquartile range for continuous variables and percentages and frequencies for categorical parameters, were calculated. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and receiver operating characteristic (ROC) curves were generated for 18F-FDG and 18F-FSPG. McNemar test was used to compare sensitivity, specificity, and accuracy. Bootstrap method was used to compare the AUCs of the two ROC curves. Spearman correlation was used to assess the association between immunohistochemical staining (CD44 and xCT expression levels) and the SUVmax of 18F-FDG and 18F-FSPG tests. Statistical analysis was conducted using R version 3.6.1.
Results
This study was terminated before the target 30-patient goal due to the SARS CoV 2 pandemic and difficulty with subject enrollment and funding. Forty-six patients were enrolled in the study, of which 27 completed both 18F-FSPG and 18F-FDG PET scans. One patient was excluded from the analysis because of treatment of the nodule of interest in between scans. Baseline characteristics of the participants are presented in Table 1. Twenty-six subjects underwent 18F-FSPG and 18F-FDG PET scans with a median time between the scans of 17 days (IQR, 12–21). The median age at the time of the scans was 65 years old, and 69% of the subjects were males. Most patients (24/26) were either current or former smokers. The median pack-year-history was 50 (IQR, 45–60) in those diagnosed with malignancy compared to 40 (IQR, 0–50) in those with benign lesions. A total of 17 patients were diagnosed with cancer while 9 patients had benign lesions. Of these, 5 patients had no evidence of malignancy on biopsy, and 4 patients had nodule stability or regression after 2 years of follow up. All IPNs in this cohort were solid, except for one ground glass nodule that was diagnosed as adenocarcinoma. The median lesion size was similar in benign lesions (1.6 cm) and malignant lesions (1.5 cm), and 81% of them were located in the upper lobes.
Table 1. Baseline characteristics.
| Characteristics | Benign (N = 9) | Malignant (N = 17) |
|---|---|---|
| Age—years, Median (IQR) | 62 (54–68) | 66 (58–70) |
| Male sex–no. (%) | 6 (67) | 12 (71) |
| Smoking History | ||
| Current–no. (%) | 5 (56) | 6 (35) |
| Former–no. (%) | 2 (22) | 11 (65) |
| Never–no. (%) | 2 (22) | 0 (0) |
| Pack Year, Median (IQR) | 40 (0–50) | 50 (45–60) |
| BMI, Median (IQR) | 33 (22–41) | 28 (23–34) |
| COPD–no. (%) | 3 (33) | 10 (59) |
| Days Between Scans, Median (IQR) | 19 (14–20) | 15 (11–23) |
| Lesion Size (cm), Median (IQR) | 1.6 (0.9–1.8) | 1.5 (1.2–3.8) |
| Spiculation–no. (%) | 2 (22) | 7 (41) |
| Nodule Location | ||
| Lower lobe–no. (%) | 2 (22) | 4 (24) |
| Upper lobe–no. (%) | 7 (78) | 13 (76) |
| Histologic Diagnosis | ||
| NSCLC–no. | 12 | |
| Malignant (Unknown type)–no. | 2 | |
| Renal cell carcinoma–no. | 1 | |
| Sarcomatoid carcinoma–no. | 1 | |
| SCLC–no. | 1 | |
| Stage | ||
| IA–no. | 5 | |
| IIB–no. | 1 | |
| III–no. | 5 | |
| IV–no. | 3 | |
| Stage IV (SCLC)–no. | 1 | |
| Metastatic from RCC–no. | 1 | |
| Unknown–no. | 1 |
BMI-Body Mass Index
COPD-Chronic Obstructive Pulmonary Disease
NSCLC-Non-Small Cell Lung Cancer
SCLC-Small Cell Lung Cancer
RCC-Renal Cell Carcinoma
Of the malignant lesions, 12 were NSCLC. The other histologic diagnoses included 1 recurrent metastatic renal cell carcinoma, 1 sarcomatoid carcinoma, 1 small cell carcinoma, and 2 unknowns. One of the unknowns was empirically radiated for presumed malignancy given the nodule characteristics and very high-risk patient. The other unknown was also empirically radiated after nodule growth over time and biopsy with highly atypical cells. Of the patients with confirmed NSCLC, 8 had advanced stage (III and IV) while 4 patients had Stage I or II disease.
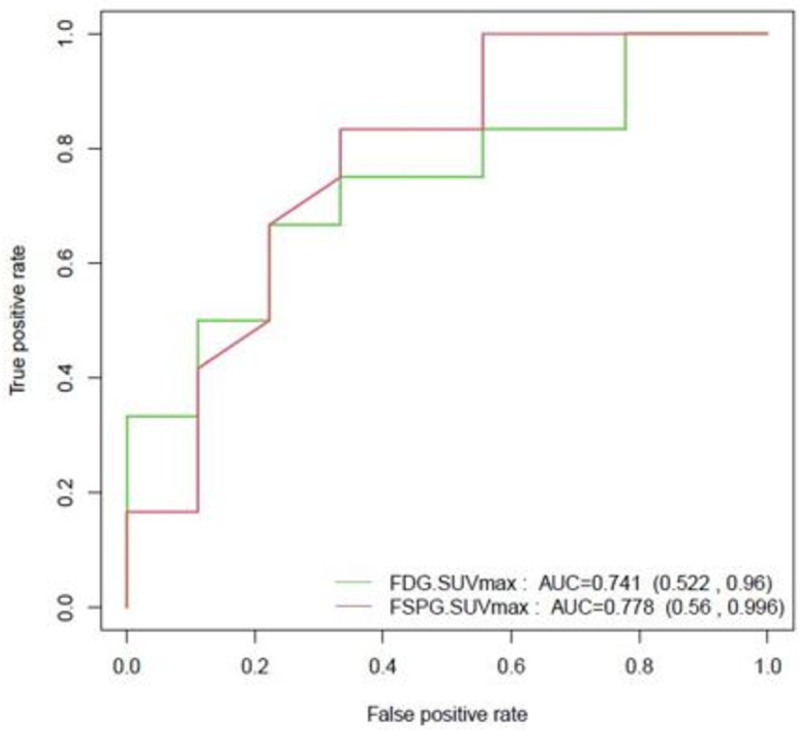
18F-FSPG and 18F-FDG PET/CT were positive in 14 of 17 and 12 of 17 malignant lesions respectively. When subdivided into IPNs (7-30mm) and lung masses (>30mm), 18F-FSPG and 18F-FDG PET/CT were positive in 9 of 12 and 7 of 12 malignant IPNs respectively, and 5 of 5 lung masses. The two nodules that were negative with 18F-FDG but positive on 18F-FSPG were solid. One nodule measured 1.7cm and was diagnosed as Stage IIB NSCLC. The other nodule measured 1.2 cm and was presumed to be malignant (interval growth and highly atypical cells on biopsy) and empirically radiated. 18F-FSPG was negative in 6 of 9 benign lesions compared to 7 of 9 with 18F-FDG. The sensitivity of 18F-FSPG and 18F-FDG for IPNs was 75% and 58% respectively, the specificity was 67% and 78% respectively, and the accuracy was 71% and 67% respectively. Sensitivity, positive predictive value (PPV) and accuracy slightly improved for both tracers after adding lung masses to the analysis. The median SUVmax was lower in benign lesions for both 18F-FSPG and 18F-FDG. 18F-FSPG SUVmax was lower in both benign and malignant lesions compared to 18F-FDG. These results are presented in Tables 2 and 3. The ROC curves for 18F-FSPG and 18F-FDG are shown in Fig 1. Overall, there was no significant difference between the two AUCs (p = 0.64).
Table 2. 18F-FSPG and 18F-FDG evaluation of primary lesion.
| IPNs (7-30mm) | Mass (>30mm) | ||
|---|---|---|---|
| Malignant (N = 12) | Benign (N = 9) | Malignant (N = 5) | |
| 18 F-FDG | |||
| Positive–no. (%) | 7 (58) | 2 (22) | 5 (100) |
| Negative–no. (%) | 5 (42) | 7 (78) | 0 |
| SUVmax, Median (IQR) | 2.2 (1.6–4.3) | 1.4 (0.7–1.8) | 11.3 (10.1–17.1) |
| 18 F-FSPG | |||
| Positive–no. (%) | 9 (75) | 3 (33) | 5 (100) |
| Negative–no. (%) | 3 (25) | 6 (67) | 0 |
| SUVmax, Median (IQR) | 1.2 (1–1.7) | 0.7 (0.4–1.0) | 8.5 (6.7–8.8) |
SUVmax–Maximum standardized uptake value
18F-FSPG SUVmax >1 and 18F-FDG SUVmax >2 defines a positive result
Table 3. Performance Comparison of 18F-FSPG and 18F-FDG for IPNs and combined IPNs and lung masses.
| IPNs (7-30mm) N = 21 | All Pulmonary Lesions N = 26 | |||
|---|---|---|---|---|
| 18F-FDG (95% CI) | 18F-FSPG (95% CI) | 18F-FDG (95% CI) | 18F-FSPG (95% CI) | |
| Sens (%) | 58 (0.30–0.86) | 75 (0.51–1.00) | 71 (0.49–0.92) | 82 (0.64–1.00) |
| Spec (%) | 78 (0.51–1.00) | 67 (0.36–0.98) | 78 (0.51–1.00) | 67 (0.36–0.98) |
| PPV (%) | 78 (0.51–1.00) | 75 (0.51–1.00) | 86 (0.67–1.00) | 82 (0.64–1.00) |
| NPP (%) | 58 (0.30–0.86) | 67 (0.36–0.98) | 58 (0.30–0.86) | 67 (0.36–0.98) |
| Accuracy (%) | 67 (0.47–0.87) | 71 (0.52–0.91) | 73 (0.56–0.90) | 77 (0.61–0.93) |
Fig 1. 18F-FSPG and 18F-FDG ROC curves for IPNs (N = 21).
18F-FSPG and 18F-FDG PET/CT were positive in all patients with metastatic disease except for two patients. One patient had stage II NSCLC cancer who had positive LN involvement on biopsy but negative PET scans. Notably, this was one of the subjects who had a false negative 18F-FDG PET. The other patient had multiple ground-glass nodules that were falsely negative with both tracers and ultimately diagnosed with stage III adenocarcinoma on biopsy. 18F-FSPG scans were positive in all sites where 18F-FDG PET/CT showed signs of metastasis except in 2 patients. 18F-FSPG detected brain and intracardiac metastases not visualized by 18F-FDG PET/CT in one case [33] while a biopsy proven metastatic lesion to the kidney was not seen on the 18F-FSPG PET/CT but seen on 18F-FDG PET/CT.
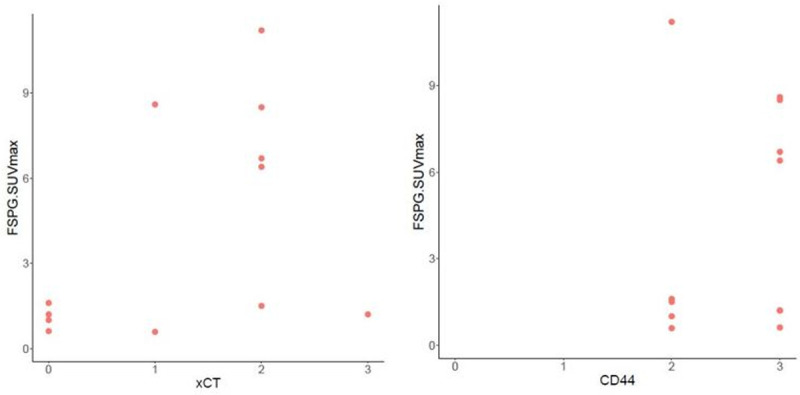
Pathology slides for IHC analysis were available on 12 of 17 patients with cancer, including the renal cell cancer case. We found no correlation between 18F FSPG SUVmax and the intensity of staining of xCT (Spearman correlation 0.440, p = 0.153) and between 18F FSPG SUVmax and the intensity of staining of CD44 (Spearman correlation 0.172, p = 0.594) (Fig 2). Of note, CD44 intensity was moderate or high (+2 or +3) in all our samples.
Fig 2. 18F-FSPG SUVmax correlation with xCT (spearman correlation 0.440, p = 0.153) and CD44 (spearman correlation 0.172, p = 0.594) in cancer cases.
Representative images from 4 subjects showing positive and negative 18F-FSPG and 18F-FDG PET/CT scans are shown in Figs 3–6.
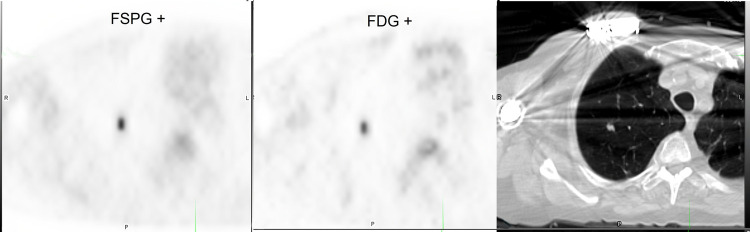
Fig 3. Subject with positive 18F-FSPG (SUVmax 3.5) and 18F-FDG (SUVmax 7.4) PET/CT.
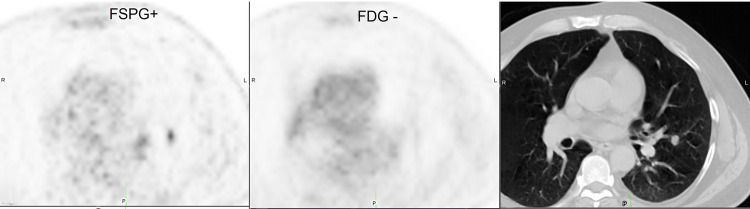
Fig 6. Subject with positive 18F-FSPG (SUVmax 2.02) and negative 18F-FDG (SUVmax 0.7) PET/CT.
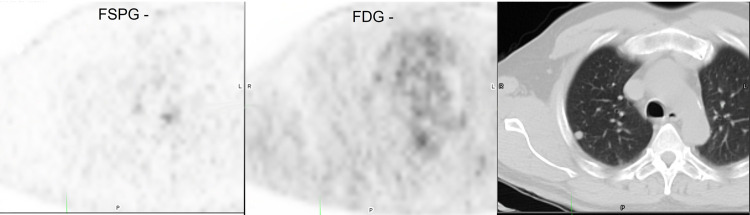
Fig 4. Subject with negative 18F-FSPG (SUVmax 0.4) and 18F-FDG (SUVmax 0.54) PET/CT.
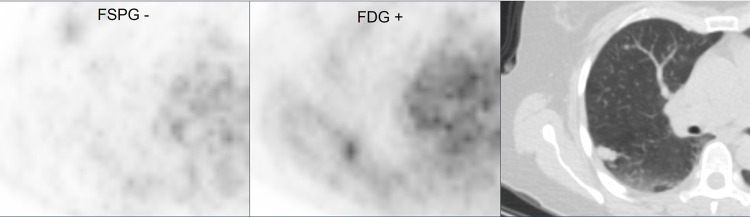
Fig 5. Subject with negative 18F-FSPG (SUVmax 0.9) and positive 18F-FDG (SUVmax 2.4) PET/CT.
Discussion
In this prospective pilot study, we compared the accuracy of 18F-FSPG PET/CT to 18F-FDG PET/CT in discriminating between benign and malignant lesions in patients with newly discovered IPNs. We found that both tracers had similar accuracy in our small cohort; however, we were underpowered to detect a significant difference between these two tracers. In patients diagnosed with lung cancer, we also found no significant difference between the two tracers for initial lung cancer staging.
To our knowledge, this is the first prospective and largest study of 18F-FSPG PET/CT for the evaluation of IPNs and staging of lung cancer. Unlike prior studies, we enrolled patients with newly discovered IPNs, which allowed us to compare the performance of 18F-FDG and 18F-FSPG prospectively. 18F-FSPG detected more malignant IPNs (14 of 17 cancers) compared to 18F-FDG (12 of 17 cancers). If we exclude the RCC case that was missed by both tracers, 18F-FSPG detected 14 of 16 lung cancers and 18F-FDG 12 of 16 lung cancers. The sensitivity observed with 18F-FDG (71% overall) is partly explained by the histology of the cases missed and the nodule size, both known to decrease the sensitivity of 18FDG PET [14]. Beak and colleagues evaluated 18F-FSPG in 10 patients with known NSCLC and a positive 18F-FDG PET/CT and found that all patients had positive 18F-FSPG scans [25]. Our results differ from this study as we had a few false negatives; however, the patients in Beak, et al, had positive 18F-FDG PET/CT, were known to have lung cancer and had a larger average lesion size compared to our study, which included patients with undiagnosed IPNs >7mm.
18F-FSPG appears to have the potential to correctly identify benign lesions [23, 30] and thus potentially better specificity compared to 18F-FDG. In this study, both tracers appear to have similar specificity, although we only had 9 benign nodules and were underpowered to detect a difference. While the overall SUVmax was lower with 18F-FSPG, this did not result in better discrimination between benign and malignant lesions. Of the 3 patients in our cohort with benign lesions that had positive 18F-FSPG scans, 2 underwent bronchoscopies to obtain biopsies and one was followed with interval imaging. Although biopsies did not show cancer, no firm diagnosis was secured in either case. Histoplasma is an endemic fungus in the Mississippi and Ohio River Valleys where our institutions are located, and a common cause of false positive results on 18F-FDG PET [12]. It is possible that some of these false positives could represent granulomatous inflammation secondary to Histoplasma akin to what is seen in sarcoidosis, which has shown to have high 18F-FSPG uptake [30].
One potential strength of 18F-FSPG appears to be its ability to detect metastatic foci from lung cancer in tissues with high physiologic 18F-FDG uptake such as the brain, which is a major limitation of 18F-FDG. In a small pilot study that included patients with NSCLC metastatic to the brain, all 3 patients with metastatic brain lesions from NSCLC had a positive 18F-FSPG scan [27]. In our study, we had a patient with cardiac and cerebellar metastases that were not evident with 18F-FDG but easily identified with 18F-FSPG [33]. While these results are encouraging, additional studies involving patients with metastatic disease to the brain will be needed to confirm these early findings. Although the kidneys are not a common metastatic site for NSCLC [34], we had one patient in our cohort with an intensely 18F-FDG avid right renal metastasis that was not seen in the 18F-FSPG scan.
18F-FSPG is taken up via the xCT transporter, a glutamate-cystine exchanger (SLC7A11/SLC3A2 (CD44) heterodimer) that transports L-cystine (Cys-S-S-Cys) into the cell and L-glutamate to the extracellular compartment [22]. Overexpression of xCT provides tumor cells a survival advantage as this allows them to maintain high levels of glutathione synthase to detoxify reactive oxygen species efficiently [22]. In a pilot study by Beak and colleagues that included 10 patients with 18F-FDG PET positive NSCLC and 5 patients with breast cancer, immunohistochemical (IHC) analyses on these subjects’ pathology samples showed significant correlation between 18F-FSPG uptake and protein expression of both the SLC7A11 subunit of system xC- and the stem cell marker CD44. In breast tumor samples specifically, IHC showed that absence of CD44 correlated with low signal from 18F-FSPG-PET, even if the SLC7A11 subunit was present, indicating possible importance of CD44 co-expression for system xC- function [25]. In our study, we found no correlation between the intensity of staining of xCT and CD44 with 18F-FSPG SUVmax. Even though no statistically significant correlation was found between xCT intensity and FSPG SUVmax, most cases with low xCT IHC score had a relatively low FSPG SUVmax while most cases with moderate or high xCT IHC score had a relatively high 18F-FSPG SUVmax (Fig 2). The lack of significant correlation might be explained by the small sample size. CD44 IHC score was high (+2 or +3) in all of our cases. One possible explanation, as previously noted, is that CD44 co-expression is necessary for the system to properly function [25], but it seems that high CD44 does not lead to high FSPG uptake if the xCT subunit is not present or is in low concentrations. A lab error could also account for the high CD44 IHC score, although we followed the same procedures described in our previously published studies [31, 32]. Given we do not have low CD44 IHC score, we could not assess whether low or absent CD44 could be associated with lower FSPG uptake regardless of xCT expression as seen in other studies [25].
Besides lung cancer, 18F-FSPG has been evaluated in patients with several other tumors including breast, prostate, colorectal, non-Hodgkin lymphoma, head and neck, hepatocellular carcinoma, and brain lesions [24–29]. Like these studies, our study highlights the promising clinical potential of 18F-FSPG, but the similar accuracies found in our study should be interpreted as hypothesis generating given our small sample and limited statistical power to detect a difference.
Our study has several limitations. First, the study was terminated before achieving our 30-patient goal due to the SARS CoV2 pandemic and difficulty with enrollment. Only 9 subjects were diagnosed with benign lesions in this cohort, and to show superior specificity, a larger number of benign nodules was needed. Additionally, we have no formal diagnosis for patients with benign lesions, and pathology slides for IHC were available in 12 of 17 patients diagnosed with malignancy and none in those diagnosed with benign disease. Time between scans could potentially bias the results toward correct diagnosis with 18F-FSPG as malignant nodules might grow during this time interval and benign nodules might decrease in size. There was a total of 6 subjects that underwent 18F-FSPG scans more than 3 weeks after the 18F-FDG scans. One subject had a benign nodule with negative 18F-FSPG and 18F-FDG scans. This nodule did not significantly change during this time interval. The other 5 subjects had cancer and were all positive with both tracers. Only two subjects had their scans more than 1 month apart: one with stage IV sarcomatoid carcinoma (34 days apart) and one with stage IA adenocarcinoma (63 days apart). Although the nodule diagnosed as sarcomatoid carcinoma grew from 1.7cm to 2cm, we believe this likely did not affect the results given the high avidity of the nodule. The other nodule did not significantly change in the size over this time interval. Our investigation used identical PET/CT imaging protocols for both 18F-FSPG and 18F-FDG. Further work, especially in animal models, might demonstrate superior performance of 18F-FSPG if the imaging protocol were maximized for this radiopharmaceutical, such as using earlier or later imaging after injection, and/or imaging of dynamic uptake and/or washout. Thus, with proper maximization of the imaging protocol and larger sample size, 18F-FSPG might show superior accuracy to 18F-FDG for PET/CT evaluations of IPNs.
Conclusion
In this pilot study, there was no significant difference in overall diagnostic accuracy between 18F-FSPG and 18F-FDG for the evaluation of IPNs and staging of lung cancer, but we were limited by the small sample size. Additional studies will be needed to determine the clinical utility of this tracer in the management of IPNs and lung cancer.
Supporting information
(PDF)
(DOC)
(DOCX)
Acknowledgments
We acknowledge the Translational Pathology Shared Resource
In loving memory of our beloved colleague, mentor, and friend, Pierre P. Massion.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by Piramal Imaging and NCI UO1 CA 152662 to Pierre P Massion, MD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Howlander N NA, Krapcho M, et al. SEER. Surveillance, Epidemiology, and End Results (SEER) Program: Mortality—All COD, Aggregated With State, Total U.S. (1969–2017). Underlying mortality data provided by NCHS (www.cdc.gov/nchs). 2019. 2020 [Available from: https://seer.cancer.gov/statfacts/html/lungb.html.
- 3.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503–13. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 5.Lokhandwala T, Bittoni MA, Dann RA, D’Souza AO, Johnson M, Nagy RJ, et al. Costs of Diagnostic Assessment for Lung Cancer: A Medicare Claims Analysis. Clin Lung Cancer. 2017;18(1):e27–e34. doi: 10.1016/j.cllc.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 6.Freiman MR, Clark JA, Slatore CG, Gould MK, Woloshin S, Schwartz LM, et al. Patients’ Knowledge, Beliefs, and Distress Associated with Detection and Evaluation of Incidental Pulmonary Nodules for Cancer: Results from a Multicenter Survey. J Thorac Oncol. 2016;11(5):700–8. doi: 10.1016/j.jtho.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228–43. doi: 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. doi: 10.1378/chest.12-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70 Suppl 2:ii1–ii54. [DOI] [PubMed] [Google Scholar]
- 10.Deppen SA, Blume JD, Kensinger CD, Morgan AM, Aldrich MC, Massion PP, et al. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: a meta-analysis. Jama. 2014;312(12):1227–36. doi: 10.1001/jama.2014.11488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe VJ, Fletcher JW, Gobar L, Lawson M, Kirchner P, Valk P, et al. Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol. 1998;16(3):1075–84. doi: 10.1200/JCO.1998.16.3.1075 [DOI] [PubMed] [Google Scholar]
- 12.Deppen S, Putnam JB Jr., Andrade G, Speroff T, Nesbitt JC, Lambright ES, et al. Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg. 2011;92(2):428–32; discussion 33. doi: 10.1016/j.athoracsur.2011.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiga AW, Deppen SA, Mercaldo SF, Blume JD, Montgomery C, Vaszar LT, et al. Assessment of Fluorodeoxyglucose F18-Labeled Positron Emission Tomography for Diagnosis of High-Risk Lung Nodules. JAMA Surg. 2018;153(4):329–34. doi: 10.1001/jamasurg.2017.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalaf M, Abdel-Nabi H, Baker J, Shao Y, Lamonica D, Gona J. Relation between nodule size and 18F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol. 2008;1:13. doi: 10.1186/1756-8722-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004;45(1):19–27. doi: 10.1016/j.lungcan.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 16.Walker R, Deppen S, Smith G, Shi C, Lehman J, Clanton J, et al. 68Ga-DOTATATE PET/CT imaging of indeterminate pulmonary nodules and lung cancer. PLoS One. 2017;12(2):e0171301. doi: 10.1371/journal.pone.0171301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44(9):1426–31. [PubMed] [Google Scholar]
- 18.Kaira K, Oriuchi N, Shimizu K, Ishikita T, Higuchi T, Imai H, et al. Correlation of angiogenesis with 18F-FMT and 18F-FDG uptake in non-small cell lung cancer. Cancer Sci. 2009;100(4):753–8. doi: 10.1111/j.1349-7006.2008.01077.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, et al. 18F-FMT uptake seen within primary cancer on PET helps predict outcome of non-small cell lung cancer. J Nucl Med. 2009;50(11):1770–6. doi: 10.2967/jnumed.109.066837 [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Das SK, Fels DR, Hansen KS, Wong TZ, Dewhirst MW, et al. PET with 62Cu-ATSM and 62Cu-PTSM is a useful imaging tool for hypoxia and perfusion in pulmonary lesions. AJR Am J Roentgenol. 2013;201(5):W698–706. doi: 10.2214/AJR.12.9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata H, Nomori H, Uno K, Iyama K, Tomiyoshi K, Nakashima R, et al. 11C-acetate for positron emission tomography imaging of clinical stage IA lung adenocarcinoma: comparison with 18F-fluorodeoxyglucose for imaging and evaluation of tumor aggressiveness. Ann Nucl Med. 2009;23(7):609–16. doi: 10.1007/s12149-009-0278-9 [DOI] [PubMed] [Google Scholar]
- 22.Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215(3):593–602. doi: 10.1002/jcp.21366 [DOI] [PubMed] [Google Scholar]
- 23.Koglin N, Mueller A, Berndt M, Schmitt-Willich H, Toschi L, Stephens AW, et al. Specific PET imaging of xC- transporter activity using a 1⁸F-labeled glutamate derivative reveals a dominant pathway in tumor metabolism. Clin Cancer Res. 2011;17(18):6000–11. doi: 10.1158/1078-0432.CCR-11-0687 [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Mosci C, Kumar M, Wardak M, Koglin N, Bullich S, et al. Initial evaluation of (4S)-4-(3-[(18)F]fluoropropyl)-L-glutamate (FSPG) PET/CT imaging in patients with head and neck cancer, colorectal cancer, or non-Hodgkin lymphoma. EJNMMI Res. 2020;10(1):100. doi: 10.1186/s13550-020-00678-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek S, Choi CM, Ahn SH, Lee JW, Gong G, Ryu JS, et al. Exploratory clinical trial of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate for imaging xC- transporter using positron emission tomography in patients with non-small cell lung or breast cancer. Clin Cancer Res. 2012;18(19):5427–37. doi: 10.1158/1078-0432.CCR-12-0214 [DOI] [PubMed] [Google Scholar]
- 26.Park SY, Na SJ, Kumar M, Mosci C, Wardak M, Koglin N, et al. Clinical Evaluation of (4S)-4-(3-[(18)F]Fluoropropyl)-L-glutamate ((18)F-FSPG) for PET/CT Imaging in Patients with Newly Diagnosed and Recurrent Prostate Cancer. Clin Cancer Res. 2020;26(20):5380–7. doi: 10.1158/1078-0432.CCR-20-0644 [DOI] [PubMed] [Google Scholar]
- 27.Mittra ES, Koglin N, Mosci C, Kumar M, Hoehne A, Keu KV, et al. Pilot Preclinical and Clinical Evaluation of (4S)-4-(3-[18F]Fluoropropyl)-L-Glutamate (18F-FSPG) for PET/CT Imaging of Intracranial Malignancies. PLoS One. 2016;11(2):e0148628. doi: 10.1371/journal.pone.0148628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baek S, Mueller A, Lim YS, Lee HC, Lee YJ, Gong G, et al. (4S)-4-(3-18F-fluoropropyl)-L-glutamate for imaging of xC transporter activity in hepatocellular carcinoma using PET: preclinical and exploratory clinical studies. J Nucl Med. 2013;54(1):117–23. doi: 10.2967/jnumed.112.108704 [DOI] [PubMed] [Google Scholar]
- 29.Kavanaugh G, Williams J, Morris AS, Nickels ML, Walker R, Koglin N, et al. Utility of [(18)F]FSPG PET to Image Hepatocellular Carcinoma: First Clinical Evaluation in a US Population. Mol Imaging Biol. 2016;18(6):924–34. doi: 10.1007/s11307-016-1007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chae SY, Choi CM, Shim TS, Park Y, Park CS, Lee HS, et al. Exploratory Clinical Investigation of (4S)-4-(3-18F-Fluoropropyl)-L-Glutamate PET of Inflammatory and Infectious Lesions. J Nucl Med. 2016;57(1):67–9. doi: 10.2967/jnumed.115.164020 [DOI] [PubMed] [Google Scholar]
- 31.Hassanein M, Hoeksema MD, Shiota M, Qian J, Harris BK, Chen H, et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19(3):560–70. doi: 10.1158/1078-0432.CCR-12-2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi T, Hassanein M, Amann JM, Liu Q, Slebos RJ, Rahman SM, et al. In-depth proteomic analysis of nonsmall cell lung cancer to discover molecular targets and candidate biomarkers. Mol Cell Proteomics. 2012;11(10):916–32. doi: 10.1074/mcp.M111.015370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magarik MA, Walker RC, Gilbert J, Manning HC, Massion PP. Intracardiac Metastases Detected by 18F-FSPG PET/CT. Clin Nucl Med. 2018;43(1):28–30. doi: 10.1097/RLU.0000000000001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.