Abstract
Background:
Since embryogenesis, plants deal with environmental changes, which might affect their growth and development. Plant autophagy has been shown to function in various stress responses, immunity, development, and senescence. Acquired thermotolerance or thermopriming is enhanced resistance to the elevated temperature following heat stress.
Objectives:
Potential contribution of autophagy mechanism after thermopriming was investigated in shoot apical meristem (SAM) of Arabidopsis thaliana.
Materials and Methods:
Transcriptic expression of Autophagy related Genes (ATGs) were analyzed by qRT-PCR data in 5-day old Arabidopsis thaliana (Col0) seedlings at 4 h and 24 h after thermopriming. Autophagy induction was confirmed by confocal microscopy.
Results:
Expression patterns of 39 ATGs and ATG-receptors were described and relevant thermopriming induced autophagy genes were identified according to their highest expression fold changes during the time after treatment. Significantly, ATG8A, ATG8B, ATG8G, ATG8H, ATI1, ATI2, NBR1, and TSPO genes were identified as the most relevant thermopriming-associated autophagy genes especially in SAM of young seedlings. This mainly implies the role of ATG8 core proteins and their receptor interactors in the regulation of autophagy in form of selective or non-selective during environmental stresses.
Conclusions:
Autophagy, a conserved mechanism for cell survival in plants will be activated in response to the thermopriming which is a promoted acquired resistance stimulus. Determined key genes and components of autophagy associated with thermal priming signaling pathway could be noteworthily employed to study transcriptional regulation of autophagy and integrated defense system against environmental stresses for the improvement of plant thermal tolerance and resistance to the pathogens.
Keywords: Autophagy, Gene Expression, Meristem, Priming, Stress
1. Background
Plants have developed different mechanisms to resist pathogens and environmental stresses. Autophagy is a survival mechanism that protects cells against undesirable environmental conditions such as microbial pathogen infections, nutrient starvation, salt and drought stresses, oxidative stress, aggregated damaged proteins, etc. Autophagy proceeds the degrading of invading agents like bacteria and viruses and old and damaged organelles and therefore leads to reduce cell consumption and uses their raw to produce new components and material ( 1 - 5 ).
Studies regarding the role of autophagy in plant pathology are mainly limited to the function of some genes such as ATG2, ATG5, ATG6, ATG7, ATG8, ATG9, ATG10, ATG18a against model pathogens and abiotic stresses ( 6 - 12 ); However, some pathogens have obtained the ability to use their own or host autophagy mechanism to overcome the autophagic host defense as their pathogenicity factor ( 13 , 14 ). Hence, it is important to study autophagy in immunity and defense responses upon plant stresses.
High temperature as an important climate change factor retards plant growth and notably reduces crop yields ( 15 ). Therefore, uncovering the molecular basis of plant responses and tolerance to heat stress will help genetic breeders to maximize crop yields under adverse environmental conditions. One possible strategy for improving the plant’s ability to withstand heat stress is to stimulate the plant by moderate heat stress treatment (heat priming). This helps the plant physiologically to cope with subsequent exposure to normally lethal levels of heat stress. In another word, it establishes a stress memory and plant acquire thermotolerance ( 16 ). While without priming, plants will meet dead or very weak states after a severe stress ( 17 - 19 ). Priming enhances multiple defence responses and primed plants display longer-lasting activation or attenuated repression of defence upon challenge than unprimed plants ( 20 ).
Autophagy is efficiently related to priming. Research has shown that autophagy mediates the specific degradation of heat shock proteins (HSPs) at later stages of the thermorecovery phase, leading to accumulation of protein aggregates after the second heat shock and a compromised heat tolerance. Also, autophagy mutants retain HSPs longer than wild type and concomitantly display improved thermomemory ( 21 ).
Plant response to stresses is somehow complicated and including transcriptomic changes which lead to physiological differences. Abiotic stresses may affect plant susceptibility to pests and pathogens. Activation of multidirectional defence signals would provide the beneficial effect of integrated disease-pest management. It is controlled by a wide range of molecular mechanisms working together in a complex regulating network. Forty ATG and receptor ATG (rATG) are recognized in Arabidopsis incorporating in autophagy mechanism, but their function, as well as transcription regulators, are mostly unknown. The first step to study the regulating of autophagy transcriptome is to find expression patterns of autophagy-related genes in response to a stimulus (thermopriming) which is preferably able to exhibit a balance of immunity and growth threshold. Transcriptomic changes versus an autophagy-inducing treatment start normally in few minutes to hours. Therefore, identifying the key genes responding in this system is aimed in this study.
Meristem is including a pool of pluripotent stem cells able to maintain themselves and produce cells needed for organ development. Hence, cell division, stem cell maintenance, and their integration into organ meristems are the basis of plant development after embryogenesis. Thereby, plant development under stress is dependent on the meristem ( 22 , 23 ). In these conditions, the shoot apical meristem (SAM) of plant seedling is dominant to the other tissues and has a pivotal role in defence signalling and memorizing to survive the plant. SAM ultrastructural changes have been observed due to heat and oxidative stress in Arabidopsis ( 24 ) and salinity stress and autophagy activity in Canola ( 25 ), but the molecular integration of stress and autophagy in SAM has not been studied so far.
2. Objectives
In the context of plant-stress molecular interaction, investigation of autophagy and priming that has several aspects including temperature, growth and development, reproductivity, defence signalling, and also finding a balance between them is of great importance. Hence, this study aimed to investigate the autophagy genes expression level in given time-points after thermoprimig phases, to determine the effectiveness of autophagy on thermopriming induced defence mechanism in SAM of young Arabidopsis.
3. Materials and Methods
3.1. Plant Source and Seed
Shoot apices including SAM tissue of 5-day old Arabidopsis were aimed to study. For this, Arabidopsis thaliana Columbia 0 ecotype (Col0) seeds were disinfected for 10 min with sterilization suspension containing 5 mL ethanol 70% and two drops of Triton X-100. Seeds were stored for 2 days in the darkroom at 4 °C for stratification. Then, 60 seeds were sown in square plates (12×12 cm) containing 70 mL Murashige & Skoog (MS) culture media (pH ~ 5.7) plus 1% sucrose. Seedlings were grown continuously for 5 days in long days conditions (16-h light/8-h dark cycle, start at 9 am, light intensity 160 μmoL m-2 s-1), at 22 °C with 60% relative humidity.
3.2. Thermopriming
Each biological replicate was including 4 plates, of which for control (without priming) and others for priming treatment according to the protocol published in ( 18 ). Briefly, thermopriming treatment was consisting of 90 min at 37 °C, followed by 90 min at 22 °C, 45 min at 44 °C. Waterproof Leukopor tape was removed and plates returned to phytotron 22 °C until sample harvest time.
3.3. Plant Tissue Harvest
Shoot apices of 5-day-old Col-0 seedlings (5 days after sowing) were harvested under the microscope at 4 h and 24 h after subjecting to the thermopriming treatment. For each time point, a primed (P) and unprimed (C) plate was harvested to their corresponding sample microtubes. Three biological replicates were collected for each sample and there contained approximately fifty SAM tissues. Finally, 4 samples were prepared for each biological replicate: 4 h control (4C), 4 h primed (4P), 24 h control (24C), and 24 h primed (24P).
3.4. RNA Isolation-cDNA Synthesis
RNA isolation of the three biological replicates was performed using Qiagen RNasy mini kit (Qiagen, Hilden, Germany). RNA quantity (ηg. µL-1) and quality (A260/280 ratio ~ 2.0) for each sample were calculated by NanoDrop system ND-1000 UV–Vis spectrophotometer (Nano-Drop Technologies, Böblingen, Germany). Genomic DNA digestion was performed via TURBO DNA- freeTM kit (Ambion/ Applied BiosystemsTM, Lithuania, Vilnius), and cDNA synthesis was carried out by RevertAid First Strand cDNA Synthesis kit (Thermo Fisher ScientificTM/ Invitrogen, Darmstadt, Germany). All of these were performed according to the manufacturer’s instructions.
3.5. Quantitative Real-Time /Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Real-time PCR detection system CFX Connect (Bio-Rad Laboratories, California, USA) was used to carry out qRT-PCR for cDNA qualification and gene expression measurements. The reactions were performed in triplicates with SYBR® Green-PCR Master Mix (Applied Biosystems/ThermoFisher Scientific, Massachusetts, Vereinigten Staaten, USA) and specific qRT-PCR primers. Name and accession number of 40 Gene of interest (GOI) ATG and ATG-receptors and 4 Reference Gene (Ref) in Arabidopsis thaliana was obtained from The Arabidopsis Information Resource (TAIR) database. Then, published resources were used for qRT-PCR primer sequences for GOI ( 21 , 26 , 27 ) and Ref. ( 28 - 30 ) and ordered to Eurofins genomics company (Berlin, Germany). Each measurement was performed on 10 µL volume sample solution including 5 µL 2× buffer, 4 µL mix forward and reverse primer (0.5 µM) and 1 µL cDNA in sterile 96-well qRT-PCR plates. Reactions were performed in a program including 1 cycle of 50 °C for 2 min, 1 cycle of 95 °C for 5 min, 40 cycles of 95 °C for 10 sec followed by 60 °C for 45 sec. and 1 cycle of 95 °C for 10 sec. In order to qualification and measurement analysis, raw data were exported by CFX Manager Software version 3.0 according to cycle threshold (Ct) calculated in Excel 2010.
3.6. Data Analysis
Raw data were qualified and analyzed by Eq. 1 and 2 ( 30 ) and Eq. 3 ( 31 ). ACTIN2 (AT3G18780) and UBQ10 (AT4G05320) were used as marker genes for cDNA quantification (20< Ct <18) and GAPDH (AT1G13440) for qualification (Eq. 1). Data were normalized to the housekeeping (reference) gene SAND (AT2G28390) as the internal control. Gene expression values were calculated at 40-∆Ct, 2 ∆∆Ct, and log2FCh using the comparative Ct method for approximately 40 ATG genes, relative to their biological control ( 31 ).
For each biological replicate, an average of three or two converge technical replicates were calculated for all of GOI and Ref. then, the relative expression of each gene was determined with an average of normalized Ct data. Statistical analysis was performed by a two-tailed Student’s t-test. Data presented as the mean ± standard error (SE) and p<0.05 considered as significant differences between experimental and control treatments.
3.7. In Vivo Observation of Autophagy Induction
Seeds of Arabidopsis thaliana (Col-0) transgenic by PromUBQ10:GFP-ATG8a construct ( 21 ) (carrying coding region of the reporter gene of green fluorescent protein (GFP) fused to ATG8A as a molecular marker of autophagy) was sown and grown as described above for non-transgenic seeds. Plates containing 7-day old seedlings were subjected to thermopriming and then to the growth chamber for about 48 hours. Seedlings were treated in an MS culture medium containing 1 µM conconamycin A to raise the pH in vacuolar lumens to inhibit vacuolar hydrolases. This resulted in the accumulation of autophagic bodies in the vacuoles during imaging. Pictures were taken 48 h after thermopriming by Leica DM6000B/SP5 confocal laser scanning microscope (CLSM, Leica Microsystems, Wetzlar, Germany). SAM of three seedlings was screened for each primed and unprimed (control) state.
4. Results
4.1. Autophagy-Related Genes Induction Upon Thermopriming in SAM of Arabidopsis Thaliana
Transcript analysis of high-quality cDNA of ATGs (data not shown) in shoot apices of 5-day old Arabidopsis seedlings indicated the presence of autophagy induction upon thermopriming in two-time points of 4h (first) and 24h (second). The results presented in Figure 1 shows that thermopriming-induced autophagy doesn’t have the same effect on the expression of all genes. Most of the ATGs expressions were increased compared to the control. That implies the effect of thermopriming on autophagy induction in SAM. Fast upregulation of ATGs after 4h indicated the impact of defensive autophagy mechanism subsequent to thermopriming as an inducer.
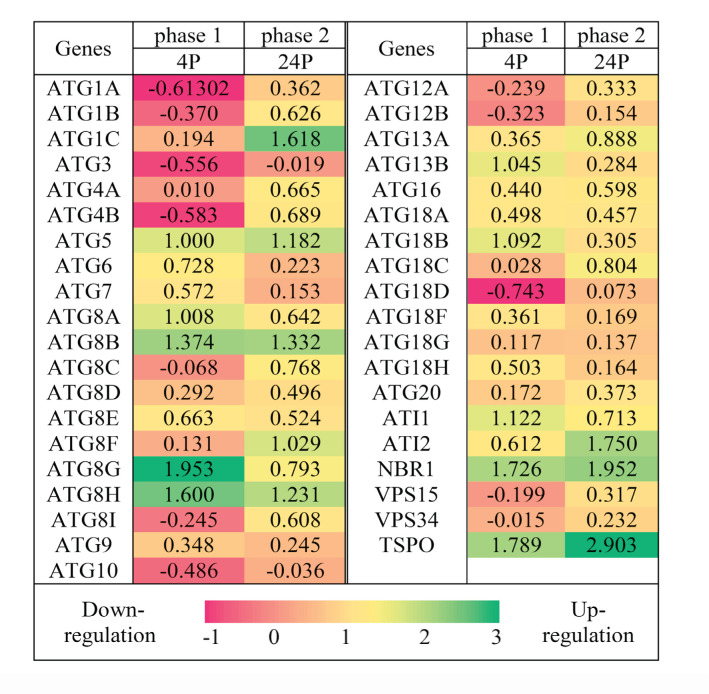
Figure 1.
Relative expression of the ATG genes in shoot apices of 5-day old Arabidopsis thaliana (Col-0) seedlings, 4 h and 24 h after thermopriming. Data were normalized by SAND as a reference gene. Heat map showing the fold change (log2 basis) of the relative expression compared to their controls (4C for 4P in the first phase and 24C for 24P in the second phase). Values are means ± SE (n=3). Green: upregulated; red: downregulated; the scale bar shows the fold change value. Abbreviations: Control 4 h (4C); Primed 4 h (4P); Control 24 h (24C); Primed 24 h (24P).
Figure 1 is the heat map of up-and down-regulation of ATGs expression relative to their phasic control, 4P to 4C and 24P to 24C. This represents the differentiated expression of ATG1, ATG5, ATG8A, ATG8B, ATG8F, ATG8G, ATG8H, ATG13B, ATG18B, also rATG including ATI1 (ATG8-Interacting Protein 1), ATI2, NBR1 (Neighbour of BRCA Gene1), and TSPO (Tryptophan-Rich Sensory Protein-related Outer Membrane).
The expression pattern of the genes would be described as following. ATG8A, ATG8G, ATG8H, ATG13B, ATG18B, and ATI1 were induced upon thermopriming and their expression has been increased in the first phase but decreased in the second phase. So, those are introduced as early-short response ATGs (ESRGs). Induction of ATG5 and ATG8B caused ascending expression in 4h after thermopriming and stayed constantly high till 24 h, and would be grouped as early-long response ATGs (ELRGs). While ATG8F and ATG1C induction didn’t have a big fold change in 4h and increased expression was visible in 24 h, and would be grouped in late response ATGs (LRGs). ATI2, NBR1, and TSPO which have been strongly induced during two phases would be grouped as strong response genes (SRGs) which are more related to selective autophagy. Some genes like ATG1A, ATG1B, ATG3, ATG4B, and ATG8I had a (little) descending expression in the first phase but were ascending in the second phase, hence grouped as negative short response ATGs (NSRGs). VPS15 (Vacuolar Protein Sorting 15) and VPS30 expression have not been much notably changed during the first two phases, but their expression tends to follow a mild trend of NSRGs. Also, the expression of ATG8D, ATG8E, ATG9, ATG16, ATG18G, ATG18A, and ATG20 has not been significantly changed after thermopriming phases.
4.2. Thermopriming-Associated Autophagy Genes
Autophagy induction upon thermopriming revealed several expression groups of genes. Hereby, core ATG8s which coexpressed rATG would be introduced as thermopriming-associated autophagy-related genes and dominant ATGs in SAM. Figure 2 (40-∆Ct expression level), shows the high expression level of these ATGs in SAM, that first of all presents the pivotal role of autophagy in SAM and emphasizes the assumed importance of choosing this target tissue. Core ATG8s had significant expression compared to their control in the first phase while this was observed for rATG in the second phase and especially for TSPO in both phases.
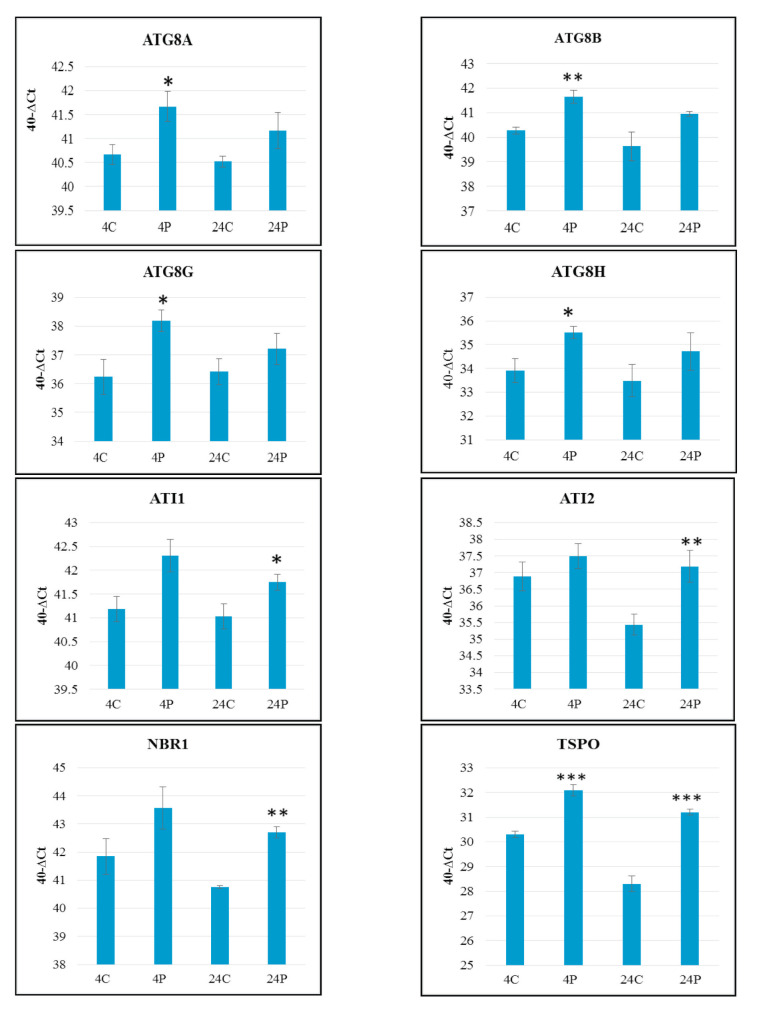
Figure 2.
Relative expression level of the ATG selected genes in shoot apices of 5-day old Arabidopsis (Col-0), 4 h and 24 h after thermopriming presented as 40-∆Ct. Data were normalized by SAND as a reference gene. Values are means ± SE (n=3). Statistical significance was calculated using Student’s T-test (*: p<0.05; **: p<0.01; ***: p<0.001). Abbreviations: Control 4 h (4C); Primed 4 h (4P); Control 24 h (24C); Primed 24 h (24P).
ATG8G is the most elevated ATG8 despite decreased expression during the recovery phase, so that remained still high in 24h. ATG8 A, B, and H that had similar expression trends are headings of three classes in evolutionary Neighbour–Joining tree of ATG8s as published by ( 32 ). Out of nine ATG8s, Core ATG8s are differentially expressed in SAM. This implies that each ATG8 homolog may have a distinct function instead of redundancy. Also, this is declared by ( 32 ) because of different expressions of ATG8s in different regions of root apical meristem.
4.3. Autophagy Detection after Thermopriming in SAM of Arabidopsis Thaliana
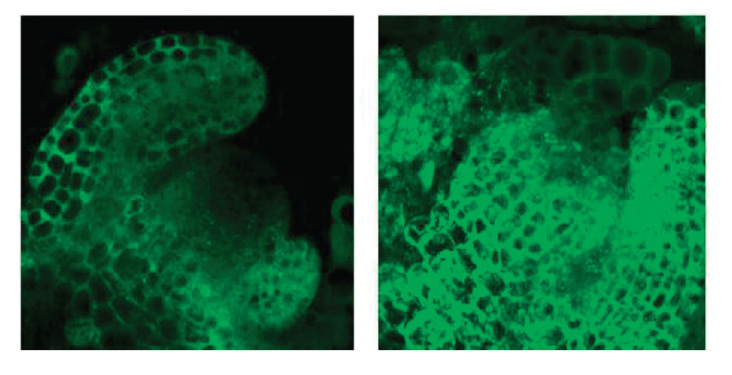
In addition to transcript level, autophagy induction would be assayed in vivo at protein state in SAM. For this purpose, Col0 plants expressing AtATG8A-GFP protein were used to investigate the autophagosome formation in the shoot apices of Col-0 plants in both control and primed conditions under the confocal microscope. GFP fluorescence was observed in many ring-shaped and punctate structures, which corresponded to autophagosomes and their intermediates. Because of autophagy disruption and no autophagosome formation after thermopriming in transgenic atg5/GFP-ATG8a Arabidopsis plant, autophagic bodies structure in Col-0/GFP-ATG8a could determine autophagy induction ( 21 ). Accumulation of autophagic bodies in the SAM seems more abundant in primed condition compared to the control after 48 h of thermopriming (Fig. 3). It shows the induction of autophagy subsequent to thermopriming (in primed) and the presence of the basic level of autophagy as a housekeeping work (in control). These are in the proteomic level that confirms the measured transcription level in SAM explained in section 4.1.
Figure 3.
Autophagy induction 48h after thermopriming in shoot apical meristem of Arabidopsis thaliana (Col-0/GFP -ATG8a). Left: control; Right: primed.
5. Discussion
Defence signalling in SAM usually processes through reactive oxygen species, mitogen-activated protein kinases, and phytohormones to respond to environmental stresses while doing cell division ( 33 ). According to the described results, autophagy is recommended as a potential protection system for the homeostasis of SAM. Hence, more investigations are needed to identify the role of autophagy in the induction of immunity and defence response networks in SAM.
Induced autophagy following the priming in SAM (Fig. 1) showed that the expression level of each gene varies in different fold changes that confirm the presence of a regulating network in autophagy transcriptome level. Although, some key regulators in the human and yeast autophagy system are identified, regulating transcription factors of plant autophagy are still almost unknown. Thereby, more investigation of plant autophagy regulators and their regulating network of ATGs are proposed. Since transcription factors (TFs) of some ATG8s by gene ontology (GO) enrichment analysis have been indicated responsive to various stresses and hormones and involved in salicylic acid-mediated systemic acquired resistance, cellular response to glucose stimulus, and abscisic acid-activated signalling pathway ( 34 ), it would be suggested to unravel linking points between autophagy and phytohormone defensive pathway and even reactive oxygen species.
The expression pattern of ATGs described in several groups of genes considers the intensity and time of the induction of genes. The increasing expression level of several ATGs in the second phase shows that the induction still exists after 24 h. However, it is known that autophagy is not active for more than 2 days in transcriptomic or 3 days in proteomic levels upon thermopriming ( 21 ). Remarkably, the increasing trend of expression of NSRGs is so milder than SRGs. This would be related to the function of SRGs that are involved in selective autophagy.
ATI1, ATI2, NBR1, and TSPO are involved in selective autophagy as receptors incorporating with ATG8s response in biotic and abiotic stresses, through recognition of targeted cell cargos by specific interaction with special receptors in a delicate programmed pathway ( 35 - 43 ). Furthermore, it has been assessed that ATG8s are responsive to various abiotic stresses and have a distinct expression pattern in the parts of plants ( 32 , 34 , 44 ). However, their functional role was not studied in SAM.
Herein, core ATG8s are introduced as capital ATGs in SAM between 40 autophagy-related genes. Also, ATG8 A, B, F, G, and H are highlighted as the relevant thermopriming-expressed ATG8s corresponding with elevated rATG expression implying the probable presence of selective autophagy in SAM. Therefore, more investigation on autophagy flux at the ultrastructural level of SAM is proposed to identify any evidence for the presence of selective autophagy in SAM at the protein level.
6. Conclusion
This work showed that by a mild stress (thermopriming), autophagy as one of its subsequent signalling networks has been activated in plant apical meristem and relevant thermopriming-induced autophagy genes were identified. Autophagy, bulk or selective, as a plant protective mechanism that may induce broad-spectrum or specific defence response could be considered a great solution for enhancing plant resistance against pathogens and abiotic stressors. Therefore, uncovering potential molecular links between the defence signalling pathways mediated by autophagy would be an interesting challenge in the field of plant stress co-interactions. Identification of key regulators of both biotic and abiotic stress response linking defence pathways provides opportunities to achieve stress-resistant crops with a wide range of stresses and leading to enhanced yield.
Acknowledgement
This work was supported by Lorestan University, Khorramabad, Iran. Also, the scholarship provided by the Ministry of Science, Research and Technology of Iran for research studies abroad is appreciated by authors.
References
- 1.Bassham DC. Plant autophagy-more than a starvation response. Curr Opin Plant Biol. 2007;10(6):587–593. doi: 10.1016/j.pbi.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20(7):748–762. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Autophagy in infection and immunity. 335: Springer; 2009. pp. 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, et al. Autophagy Negatively Regulates Cell Death by Controlling NPR1-Dependent Salicylic Acid Signaling during Senescence and the Innate Immune Response in Arabidopsis. Plant Cell. 2009;21(9):2914–2927. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong Y, Contento AL, Bassham DC. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J. 2005;42(4):535–546. doi: 10.1111/j.1365-313X.2005.02397.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Dinesh-Kumar SP. Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy. 2008;4(1):20–27. doi: 10.4161/auto.5056. [DOI] [PubMed] [Google Scholar]
- 9.Lenz HD, Haller E, Melzer E, Kober K, Wurster K, Stahl M, et al. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J. 2011;66(5):818–830. doi: 10.1111/j.1365-313X.2011.04546.x. [DOI] [PubMed] [Google Scholar]
- 10.Lai Z, Wang F, Zheng Z, Fan B, Chen Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 2011;66(6):953–968. doi: 10.1111/j.1365-313X.2011.04553.x. [DOI] [PubMed] [Google Scholar]
- 11.Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NH, Mattsson O, et al. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell. 2009;137(4):773–783. doi: 10.1016/j.cell.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Rabiee S, Afsharifar A, Izadpanah K. Effect of RNA silencing suppressors of Barley yellow striate mosaic virus on expression levels of autophagy-related genes in Nicotiana benthamiana16c. Iran J Plant Pathol. 2019;55(4):287–303. [Google Scholar]
- 13.Dagdas YF, Belhaj K, Maqbool A, Chaparro-Garcia A, Pandey P, Petre B, et al. An effector of the Irish potato famine pathogen antagonizes a host autophagy cargo receptor. Elife. 2016; 5: e10856. doi: 10.7554/eLife.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leary AY, Sanguankiattichai N, Duggan C, Tumtas Y, Pandey P, Segretin ME, et al. Modulation of plant autophagy during pathogen attack. J Exp Bot. 2018;69(6):1325–1333. doi: 10.1093/jxb/erx425. [DOI] [PubMed] [Google Scholar]
- 15.Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot. 2010;61(7):1959–1968. doi: 10.1093/jxb/erq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilker M, Schwachtje J, Baier M, Balazadeh S, Bäurle I, Geiselhardt S, et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol Rev. 2016;91(4):1118–1133. doi: 10.1111/brv.12215. [DOI] [PubMed] [Google Scholar]
- 17.Serrano N, Ling Y, Bahieldin A, Mahfouz MM. Thermopriming reprograms metabolic homeostasis to confer heat tolerance. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-018-36484-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedaghatmehr M, Mueller-Roeber B, Balazadeh S. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat Commun. 2016;7(1):12439. doi: 10.1038/ncomms12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biochem Sci. 2012;37(3):118–25. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Medina A, Flors V, Heil M, Mauch-Mani B, Pieterse CM, Pozo MJ, et al. Recognizing plant defense priming. Trends Plant Sci. 2016;21(10):818–822. doi: 10.1016/j.tplants.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Sedaghatmehr M, Thirumalaikumar VP, Kamranfar I, Marmagne A, Masclaux-Daubresse C, Balazadeh S. A regulatory role of autophagy for resetting the memory of heat stress in plants. Plant Cell Environ. 2018;42(3):1054–1064. doi: 10.1111/pce.13426. [DOI] [PubMed] [Google Scholar]
- 22.Steeves TA, Sussex IM. Patterns in plant development. Cambridge University Press: 1989. [DOI] [Google Scholar]
- 23.Ogawa D, Abe K, Miyao A, Kojima M, Sakakibara H, Mizutani M, et al. RSS1 regulates the cell cycle and maintains meristematic activity under stress conditions in rice. Nat Commun. 2011;2(1):1–11. doi: 10.1038/ncomms1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolzblasz A, Smakowska E, Gola EM, Sokołowska K, Kicia M, Janska H. The mitochondrial protease AtFTSH4 safeguards Arabidopsis shoot apical meristem function. Sci Rep. 2016;6(1):1–14. doi: 10.1038/srep28315(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoodzadeh H. Ultrastructural changes in shoot apical meristem of canola (Brassica napus cv. Symbol) treated with sodium chloride. Pak J Biol Sci. 2008;11(8):1161–1164. doi: 10.3923/pjbs.2008.1161.1164. [DOI] [PubMed] [Google Scholar]
- 26.Di Berardino J, Marmagne A, Berger A, Yoshimoto K, Cueff G, Chardon F, et al. Autophagy controls resource allocation and protein storage accumulation in Arabidopsis seeds. J Exp Bot. 2018;69(6):1403–1414. doi: 10.1093/jxb/ery012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinow T, Himbert S, Krenz B, Jeske H, Koncz C. NAC domain transcription factor ATAF1 interacts with SNF1-related kinases and silencing of its subfamily causes severe developmental defects in Arabidopsis. Plant Sci. 2009;177(4):360–370. doi: 10.1016/j.plantsci.2009.06.011. [DOI] [Google Scholar]
- 28.Dong MA, Farré EM, Thomashow MF. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc Natl Acad Sci. 2011;108(17):7241–7246. doi: 10.1073/pnas.1103741108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy DS, Bhatnagar-Mathur P, Reddy PS, Cindhuri KS, Ganesh AS, Sharma KK. Identification and validation of reference genes and their impact on normalized gene expression studies across cultivated and wild cicer species. PLoS One. 2016;11(2):e0148451. doi: 10.1371/journal.pone.0148451.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R. Genome-Wide Identification and Testing of Superior Reference Genes for Transcript Normalization in Arabidopsis. Plant Physiol. 2005;139(1):5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3(6):1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 32.Sláviková S, Shy G, Yao Y, Glozman R, Levanony H, Pietrokovski S, et al. The autophagy-associated Atg8 gene family operates both under favourable growth conditions and under starvation stresses in Arabidopsis plants. J Exp Bot. 2005;56(421):2839–2849. doi: 10.1093/jxb/eri276. [DOI] [PubMed] [Google Scholar]
- 33.Lee H. Stem cell maintenance and abiotic stress response in shoot apical meristem for developmental plasticity. J Plant Biol. 2018;61(6):358–365. doi: 10.1007/s12374-018-0301-6. [DOI] [Google Scholar]
- 34.Wang P, Nolan TM, Yin Y, Bassham DC. Identification of transcription factors that regulate ATG8 expression and autophagy in Arabidopsis. Autophagy. 2019:1–17. doi: 10.1080/15548627.2019.1598753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honig A, Avin-Wittenberg T, Ufaz S, Galili G. A New Type of Compartment, Defined by Plant-Specific Atg8-Interacting Proteins, Is Induced upon Exposure of Arabidopsis Plants to Carbon Starvation. Plant Cell. 2012;24(1):288–303. doi: 10.1105/tpc.111.093112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Michaeli S, Galili G, Peled-Zehavi H. ATG8-interacting (ATI) 1 and 2 define a plant starvation-induced ER-phagy pathway and serve as MSBP1 (MAPR5) cargo-receptors. bioRxiv. 2020 doi: 10.1101/2020.01.29.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haxim Y, Ismayil A, Jia Q, Wang Y, Zheng X, Chen T, et al. Autophagy functions as an antiviral mechanism against geminiviruses in plants. Elife. 2017;6:e23897. doi: 10.7554/eLife.23897.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanhee C, Zapotoczny G, Masquelier D, Ghislain M, Batoko H. The Arabidopsis Multistress Regulator TSPO Is a Heme Binding Membrane Protein and a Potential Scavenger of Porphyrins via an Autophagy-Dependent Degradation Mechanism. Plant Cell. 2011;23(2):785–805. doi: 10.1105/tpc.110.081570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanhee C, Guillon S, Masquelier D, Degand H, Deleu M, Morsomme P, et al. A TSPO-related protein localizes to the early secretory pathway in Arabidopsis, but is targeted to mitochondria when expressed in yeast. J Exp Bot. 2010;62(2):497–508. doi: 10.1093/jxb/erq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehtonen MT, Akita M, Frank W, Reski R, Valkonen JP. Involvement of a class III peroxidase and the mitochondrial protein TSPO in oxidative burst upon treatment of moss plants with a fungal elicitor. Mol Plant Microbe Interact. 2012;25(3):363–371. doi: 10.1094/MPMI-10-11-0265. [DOI] [PubMed] [Google Scholar]
- 41.Guillaumot D, Guillon S, Déplanque T, Vanhee C, Gumy C, Masquelier D, et al. The Arabidopsis TSPO-related protein is a stress and abscisic acid-regulated, endoplasmic reticulum–Golgi-localized membrane protein. Plant J. 2009;60(2):242–256. doi: 10.1111/j.1365-313X.2009.03950.x. [DOI] [PubMed] [Google Scholar]
- 42.Hafrén A, Macia J-L, Love AJ, Milner JJ, Drucker M, Hofius D. Selective autophagy limits cauliflower mosaic virus infection by NBR1-mediated targeting of viral capsid protein and particles. Proc Natl Acad Sci. 2017;114(10):E2026–E2035. doi: 10.1073/pnas.1610687114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hafrén A, Üstün S, Hochmuth A, Svenning S, Johansen T, Hofius D. Turnip mosaic virus counteracts selective autophagy of the viral silencing suppressor HCpro. Plant Physiol. 2018;176(1):649–662. doi: 10.1104/pp.17.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD. Autophagic Nutrient Recycling in Arabidopsis Directed by the ATG8 and ATG12 Conjugation Pathways. Plant Physiol. 2005;138(4):2097–2110. doi: 10.1104/pp.105.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]