Abstract
Background/Purpose
Efficacy and safety data of heterologous prime-boost vaccination against SARS-CoV-2 remains limited.
Methods
We recruited adult volunteers for homologous or heterologous prime-boost vaccinations with adenoviral (ChAdOx1, AstraZeneca) and/or mRNA (mRNA-1273, Moderna) vaccines. Four groups of prime-boost vaccination schedules were designed: Group 1, ChAdOx1/ChAdOx1 8 weeks apart; Group 2, ChAdOx1/mRNA-1273 8 weeks apart; Group 3, ChAdOx1/mRNA-1273 4 weeks apart; and Group 4, mRNA-1273/mRNA-1273 4 weeks apart. The primary outcome was serum anti-SARS-CoV-2 IgG titers and neutralizing antibody titers against B.1.1.7 (alpha) and B.1.617.2 (delta) variants on day 28 after the second dose. Adverse events were recorded up until 84 days after the second dose.
Results
We enrolled 399 participants with a median age of 41 years and 75% were female. On day 28 after the second dose, the anti-SARS-CoV-2 IgG titers of both heterologous vaccinations (Group 2 and Group 3) were significantly higher than that of homologous ChAdOx1 vaccination (Group 1), and comparable with homologous mRNA-1273 vaccination (Group 4). The heterologous vaccination group had better neutralizing antibody responses against the alpha and delta variant as compared to the homologous ChAdOx1 group. Most of the adverse events (AEs) were mild and transient. AEs were less frequent when heterologous boosting was done at 8 weeks rather than at 4 weeks.
Conclusion
Heterologous ChAdOx1/mRNA-1273 vaccination provided higher immunogenicity than homologous ChAdOx1 vaccination and comparable immunogenicity with the homologous mRNA-1273 vaccination. Our results support the safety and efficacy of heterologous prime-boost vaccination using the ChAdOx1 and mRNA-1273 COVID-19 vaccines. (ClinicalTrials.gov number, NCT05074368).
Keywords: Adenovirus-vector vaccine, Messenger RNA vaccine, Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), Immune response
Introduction
Coronavirus disease 2019 (COVID-19) has had a tremendous impact on human health, social burden, and economic loss. By January 2022, the World Health Organization (WHO) has estimated the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to have infected 376 million people and caused 5.66 million deaths worldwide.1 Although nonpharmaceutical interventions such as wearing face masks, physical distancing and quarantining at-risk people are important measures to prevent the transmission of SARS-CoV-2, mass vaccination to provide herd immunity is still the most important and fundamental method to reduce the impact of COVID-19. A total of nearly 10 billion vaccine doses have been administered globally as of January 2022.1
In Taiwan, four SARS-CoV-2 vaccines have been made widely available, including the non-replicating adenovirus vector vaccine (ChAdOx1-nCoV-19 [ChAdOx1], AstraZeneca, UK) and the mRNA vaccines (SARS-CoV-2 messenger RNA-1273, Moderna, USA; and BNT-162b2, BioNTech/Pfizer, Germany) and perfusion-stabilized SARS-CoV-2 spike protein (S-2P) adjuvant vaccine (MVC-COV1901, Medigen, Taiwan).2 Current standard immunization protocol based on existing clinical trial data recommends two doses of the same SARS-CoV-2 vaccine at least 3 weeks apart (homologous prime-boost vaccination). The protection afforded by two doses of ChAdOx1 vaccination with an interval of 10–12 weeks is about 81% (60%–91%),3 , 4 and that of two doses of mRNA vaccination with an interval of 28 days is 94%–95%.5 , 6
ChAdOx1 vaccine associated thrombosis with thrombocytopenia syndrome has lead European countries to suggest a heterologous booster with mRNA vaccines for certain age groups who have already received one dose of ChAdOx1 vaccine.7 , 8 Use of heterologous boost vaccination after prime vaccination has been suggested to facilitate mass COVID-19 immunization and avoid possible adverse reactions.9 An observational cohort from Germany showed that SARS-CoV-2–anti-RBD IgG titers were similar between participants receiving homologous BNT-162b2/BNT-162b2 vaccination at a 3-week interval and those receiving heterologous ChAdOx1/BNT-162b2 vaccination at a 10– to 12 week interval, however, the geometric mean of 50% inhibitory dose against B.1.1.7 and B.1.351 variants and SARS-CoV-2 S1 T-cell reactivity were highest among those receiving heterologous vaccines.10 Another small cohort from Sweden compared homologous ChAdOx1/ChAdOx1 with heterologous ChAdOx1/mRNA-1273 vaccination with a 9 to 12 week interval and showed the latter to more efficiently stimulate SARS-CoV-2-specific antibodies and protect against the beta-SARS-CoV-2 variant.11 However, the intervals of prime-boost were variable in the previous analyses, and the correlates of immune protection against emerging SARS-CoV-2 variants by heterologous ChAdOx1/mRNA-1273 vaccination were limited.
To better understand whether heterologous vaccination could induce an enhanced humoral and/or cellular immune response, and to evaluate the immune responses generated specifically against the alpha and the delta SARS-CoV-2 variants, we conducted a prospective study to compare the immunogenicity and safety of heterologous ChAdOx1/mRNA-1273 vaccination versus standard homologous ChAdOx1/ChAdOx1 and mRNA-1273/mRNA-1273 vaccination in Taiwan.
Materials and methods
Study design and participants
Healthy volunteers from two medical centers located in northern Taiwan (National Taiwan University Hospital, Taipei City; Taoyuan General Hospital, Tao-Yuan County) were recruited. The participants were divided into four prime/boost vaccination schedules (Fig. 1 ): homologous ChAdOx1/ChAdOx1 vaccination 8 weeks apart (Group 1); heterologous ChAdOx1/mRNA-1273 vaccination 8 weeks apart (Group 2); heterologous ChAdOx1/mRNA-1273 vaccination 4 weeks apart (Group 3); and homologous mRNA-1273/mRNA-1273 vaccination 4 weeks apart (Group 4). There were 100 participants in each group, and blood was drawn from all participants for SARS-CoV-2 IgG antibody test on the day before the second vaccination, and on the 14th, 28th and 84th day after the second vaccine dose. SARS-CoV-2 neutralizing antibody tests were performed for 32 serum samples randomly selected from each group at each visit. A subset of 25 participants in each group were enrolled to determine the other immunology profiles based on the participants’ willingness to have more blood drawn on the day before and the 28th day after the 2nd vaccination. A standard diary card was designed to evaluate the safety of vaccination according to WHO guidelines.12 All participants were instructed to record any adverse reactions in the standard diary card on the day of the 2nd vaccine dose, daily in first week, then weekly till 84 days after boosting. The records of the diary card were checked at each visit by the physician investigator.
Figure 1.
Classification of four study groups in this study.
Adults aged 20 to 65 years old without underlying illness or with well controlled comorbidities who had received a priming vaccination with either ChAdOx1 or mRNA-1273 were eligible for recruitment. The exclusion criteria were previous laboratory-confirmed SARS-CoV-2 infection, history of other vaccination within 30 days, pregnancy or breastfeeding, and uncontrolled medical illness and immunosuppression status not suitable for this study which were evaluated and decided by investigators (physicians). Immune compromised adults were excluded on the basis of the presence of active malignancy, organ transplantation, or ever received immunosuppressive therapy include prednisolone greater than 10 mg per day or its equivalent dose, any B-cell depleting agents, tumor necrosis factor α inhibitors, tyrosine kinase inhibitors, or other cytokine inhibitors within 90 days.
For those participants who had received ChAdOx1 prime vaccination 8 weeks ago, they were randomized into homologous ChAdOx1 boost group or heterologous mRNA-1273 boost group. For those participants who had received mRNA-1273 or ChAdOx1 prime vaccination 4 weeks ago, mRNA-1273 boost vaccinations were arranged. Laboratory staff processing the cellular immunity and humoral immunity tests were blinded to the blood samples received.
The adenoviral vectored vaccine ChAdOx1-nCov-19 (AstraZeneca) and the mRNA vaccine SARS-CoV-2 messenger RNA-1273 (Moderna) were used in this study. Vaccination and blood sampling were performed by well-trained research nurses. Participants were observed for 30 minutes after vaccination.
The primary outcome was serum SARS-CoV-2 anti-spike IgG concentration and neutralizing antibody titers at 28th day after boost vaccination. Secondary outcomes included other cellular or humoral immune profiles and solicited local and systemic reactions after boost vaccination.
Ethics declaration
This study has been approved by the Institutional Review Boards (Ethics Committee) of National Taiwan University Hospital (IRB No. 202106039 MINA) and Tao Yuan General Hospital (IRB No. TYGH 110027).
Laboratory tests
Anti-SARS-CoV-2 spike antibody
Anti-SARS-CoV-2 spike (S) IgG was determined by Abbott SARS-CoV-2 IgG II Quant assay (06S60, Abbott, USA). This assay, designed to measure specific IgG antibodies to the receptor binding domain (RBD) of S protein, is a chemiluminescent microparticle immunoassays (CIMA) on the Architect i2000SR analyzer (Abbott, USA). Results were reported as arbitrary units (AU) per milliliter, and the cut-off value was 50.0 AU/mL. The mathematical relationship of the Abbott AU/mL unit to WHO unit (binding antibody unit per mL [BAU/mL]) would follow the equation: BAU/mL = 0.142∗AU/mL.
Neutralizing antibody test
The neutralizing antibody titers in the serum were determined by a 50% tissue culture infectious dose (TCID50)-based neutralization method. Briefly, Vero E6 cells (1 × 104 cells per well) were seeded in 96-well plates and incubated in DMEM containing 10% FBS for 18 to 24 hours. The medium was replaced with 100 μL of fresh DMEM containing 2% FBS for 1 hour before infection. Serum samples were inactivated at 56 °C for 30 min before use. Serial two-fold dilutions of sera were mixed with an equal volume of 100 TCID50 SARS-CoV-2 virus suspension. The mixture was incubated for 2 hours at 37 °C. After that, the virus-antibody mixture was transferred onto a monolayer of Vero E6 cells, and the cells were incubated with the mixture for 3 days. Cells were fixed with 10% formalin (HT501128, Sigma–Aldrich, USA) and stained with 0.5% crystal violet (0528, VWR International, USA). Serum neutralization titers (NT50) were calculated and expressed as the reciprocals of the highest serum dilution that inhibits 50% of cytopathic effects. The neutralization titers for a panel of serum samples whose titers in IU/mL have been determined after comparison with the WHO IS sera (20/130, 20/136, and 20/268) was used. The results from the reference panel were used to convert NT50 to IU/mL for our test sera. Both B.1.1.7 (alpha) and the B.1.617.2 (delta) SARS-CoV-2 variants were used in the neutralizing antibody test.
Cell isolation, stimulation, and analysis of spike-specific T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by density Ficoll gradient centrifugation, and subsequently cryopreserved in aliquots of up to 1 × 107 cells in heat-inactivated fetal bovine serum (FBS) (Hyclone) containing 10% DMSO. One day before stimulation, PBMCs were thawed and washed once in RPMI 1640 (Gibco) supplemented with 10% FBS, and then rested overnight at a concentration of 1 × 107 cells/ml. The next day, 2.5 × 106 PBMCs were resuspended in RPMI 1640 supplemented with 10% human AB serum (Lonza), 1 mM sodium pyruvate (Gibco), NEAA (100:1; Gibco), HEPES (100:1; Gibco), and 50 μM β-mercaptoethanol, and stimulated with SARS-CoV-2 spike protein overlapping peptides pools 1 and 2 (2 μg/ml; JPT, Berlin, Germany) in the presence of agonistic antibodies against CD28 (1 μg/ml; BD Biosciences) and CD49d (1 μg/ml; BD Biosciences) for 2 hours, and were subsequently incubated with Golgi Stop (BD Biosciences) for 5 hours. The SARS-CoV-2 spike glycoprotein overlapping peptide pool 1 covering the N-terminal amino acid residues 1–643 (abbreviated to ‘S-I’ [N-term]) contains 158 15-mers that overlapped by 11 amino acids. The SARS-CoV-2 spike glycoprotein pool 2 covers the C-terminal amino acid residues 633–1273 with 157 peptides in total. PBMCs stimulated with anti-CD3 (1 μg/ml; BD Biosciences) and CD28 (1 μg/ml; BD Biosciences) serve as a positive control, and those treated with DMSO as a negative control in maximal volume corresponding to the DMSO amount in peptide pools. After stimulation, cells were first stained with fluorescent-conjugated antibodies against surface antigens, including anti-CD3 PE-Cy7, anti-CD4 BV605, anti-CD8 PerCP-Cy5.5, anti-CD69 APC-H7. Cells were then fixed and permeabilized with BD Cytofix/Cytoperm™ (BD Biosciences), and subsequently stained with anti-IFN-γ BV421 and TNF-α BUV395. Samples were analyzed by BD FACS LSRFortessa™ cytometer. For Th1/Th2 cytokine measurement, PBMCs were stimulated with SARS-CoV-2 spike protein overlapping peptides pools and incubated for 24 hours, and the secreted cytokines (IL-2, IL-6, IL-10, IFN-γ, TNF-α, IL-5, IL-13, IL-4) in the resultant supernatants were measured by LEGENDplex bead-based immunoassays (BioLegend).
Analysis of spike-specific B cells
For detection of antigen-specific B cells, spike protein (R&D Systems) was biotinylated using the biotin conjugation kit (Abcam), and was subsequently labeled for 1 hour at 4 °C with streptavidin (SA)-allophycocyanin (APC)/phycoerythrin (PE) (Miltenyi Biotec) at a 5:1 mass ratio and with SA-FITC (Miltenyi Biotec) at a 4:1 mass ratio, respectively. Ten million resting PBMCs and SA-fluorescence-labeled biotinylated proteins (protein probes) were treated with 5 mM of d-biotin (Cayman Chemical) for 15 min at room temperature, and then stained with protein probes (0.6 μg spike-PE/APC; 0.4 μg receptor binding domain derived from spike protein (RBD-FITC) for 30 min. The RBD-FITC was from Dr. Mi-Hua Tao at Academia Sinica, Taipei, Taiwan. Cells were then washed twice with staining buffer, and stained by a panel of surface marker antibodies, including anti-CD3 PE-Cy7, anti-CD19 BV421, anti-CD20 PE-CF594, anti-IgG APC-H7, and anti-IgM BUV395. Finally, cells were analyzed using BD FACS LSRFortessa™ cytometer.
Statistical analysis
Categorical variables were presented as numbers and percentages and were compared using the chi-square test or Fisher exact test. Continuous variables were presented as median (range) and mean (standard deviations), and were compared using the Student's t-test. The average values of binding antibody titers were expressed as geometric means with 95% confidence interval. Mann–Whitney U test was performed to compare the antibody titers between groups. All analyses were set at a 2-tailed significance level of 0.05. All statistics were conducted by Stata software (version 14; StataCorp, College Station, Texas, USA).
Results
Between July 1 and August 31, 2021, 400 participants were recruited and ultimately, 399 were enrolled in this study. One participant in Group 4 was excluded because of immunosuppressant (rituximab) use before the second vaccine dose. Demographic characteristics and concurrent medications are shown in Table 1 . The median age of 399 participants was 41 years (interquartile range, 33–48 years) with 75% women. Baseline characteristics were balanced across the four groups except the Group 4 having a higher proportion of men participants (Table 1). During the study period, neither hospitalization nor acquisition of SARS-CoV-2 infection occurred in any participants. Only one participant in Group 3 had an out-patient clinic visit because of persistent generalized skin rashes and later was diagnosed as psoriasis vulgaris on skin biopsy.
Table 1.
Baseline characteristics by vaccine schedule in prime-boost interval study groups.
| Group 1 (n = 100) |
Group 2 (n = 100) |
Group 3 (n = 100) |
Group 4 (n = 99) |
P-value | |
|---|---|---|---|---|---|
| Age (Mean ± SD) | 41.1 ± 10.7 | 42.3 ± 10.7 | 40.9 ± 10.6 | 42.2 ± 8.9 | 0.702 |
| Male (n, %) | 20 (20.0%) | 19 (19.0%) | 22 (22.0%) | 40 (40.4%) | 0.001 |
| Past history, n (%) | |||||
| DM under OHA | 4 (4.0%) | 2 (2.0%) | 4 (4.0%) | 1 (1.0%) | 0.477 |
| DM under insulin | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0.394 |
| Hypertension | 8 (8.0%) | 9 (9.0%) | 3 (3.0%) | 5 (5.0%) | 0.278 |
| Coronary arterial disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Congestive heart failure | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Stroke | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Chronic lung disease | 0 (0.0%) | 2 (2.0%) | 4 (4.0%) | 2 (2.0%) | 0.254 |
| Chronic viral hepatitis | 5 (5.0%) | 4 (4.0%) | 0 (0.0%) | 4 (4.0%) | 0.195 |
| Decompensated hepatic insufficiency | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Chronic kidney disease | 2 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.111 |
| ESRD under dialysis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.0%) | 0.386 |
| Hyperthyroidism | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Hypothyroidism | 1 (1.0%) | 3 (3.0%) | 4 (4.0%) | 0 (0.0%) | 0.167 |
| Rheumatoid arthritis | 2 (2.0%) | 2 (2.0%) | 0 (0.0%) | 1 (1.0%) | 0.528 |
| Ankylosing spondylitis | 1 (1.0%) | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0.572 |
| Antiphospholipid syndrome | 2 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.111 |
| Systemic lupus erythematosus | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0.392 |
| Sjogren Syndrome | 2 (2.0%) | 3 (3.0%) | 0 (0.0%) | 1 (1.0%) | 0.338 |
| Solid organ malignancy | 2 (2.0%) | 3 (3.0%) | 2 (2.0%) | 2 (2.0%) | 0.953 |
| Seronegative spondyloarthritis | 1 (1.0%) | 3 (3.0%) | 0 (0.0%) | 1 (1.0%) | 0.280 |
| Autoimmune thyroiditis | 0 (0.0%) | 1 (1.0%) | 2 (2.0%) | 0 (0.0%) | 0.298 |
| Current medication, n (%) | |||||
| Methotrexate | 1 (1.0%) | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0.572 |
| Plaquenil | 5 (5.0%) | 4 (4.0%) | 0 (0.0%) | 6 (6.1%) | 0.122 |
| Rituximab | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| NSAID except COX-2 | 0 (0.0%) | 1 (1.0%) | 1 (1.0%) | 4 (4.0%) | 0.104 |
| COX-2 inhibitor | 3 (3.0%) | 3 (3.0%) | 2 (2.0%) | 1 (1.0%) | 0.746 |
| Sulfasalazine | 2 (2.0%) | 1 (1.0%) | 2 (2.0%) | 1 (1.0%) | 0.881 |
| Steroid | 1 (1.0%) | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0.572 |
| OPD visit for adverse effect | 0 (0.0%) | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0.392 |
SD, standard deviation; DM, diabetes mellitus; OHA, oral hypoglycemic agent; ESRD, end stage renal disease; NSAID, non-steroidal anti-inflammatory drug; COX-2, cyclooxygenase-2; OPD, out-patient department.
SARS-CoV-2 anti-spike IgG titers
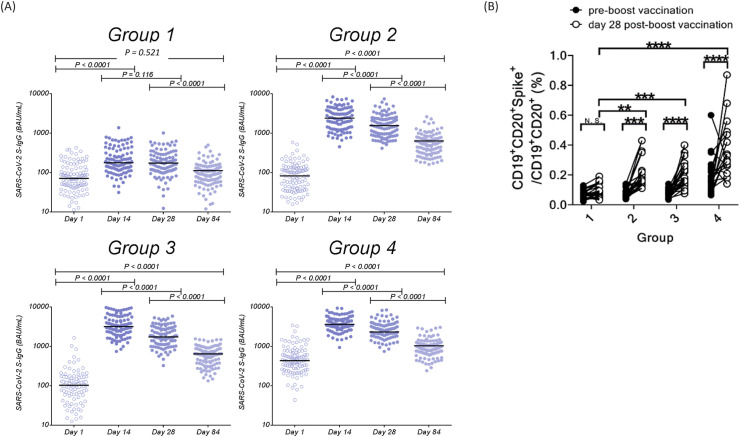
The SARS-CoV-2 anti-spike IgG titers of the four groups of participants before and after booster vaccination are shown in Table 2 and Fig. 2 A. In each group, the SARS-CoV-2 anti-spike IgG titers increased significantly at 14th day, 28th day, and 84th day after booster vaccination compared to the baseline titer before boost vaccination (all P < 0.0001). The results showed that immunological response could be augmented either by homologous or heterologous vaccinations. Except for homologous ChAdOx1/ChAdOx1 vaccination (P = 0.116), a significant decrease in SARS-CoV-2 anti-spike IgG titers were found on day 28 after boosting compared with titers on day 14 (P < 0.0001), suggesting the peak antibody response is likely to occur between these two timepoints. The anti-spike IgG titers were further decreased on day 84 in all 4 groups. On day post boost, the SARS-CoV-2 anti-spike IgG titers (geometric mean, 95% confidence interval, binding antibody units [BAU/mL]) of both heterologous vaccinations (Group 2, 1534.82 [1350.72–1744.02] BAU/mL], and Group 3, 1789.50 [1588.75–2015.62] BAU/mL]) were significantly higher than that of homologous ChAdOx1/ChAdOx1 vaccination (Group 1, 170.09 [146.79–197.08] BAU/mL). Homologous mRNA-1273/mRNA-1273 vaccination had significantly higher geometric mean antibody titer (Group 4, 2516.60 [2285.50–2771.06] BAU/mL) compared to other 3 groups (Table 2 and Supplementary Table 1). The homologous ChAdOx1/ChAdOx1 group (Group 1) had lower anti-spike IgG antibody titers than the other 3 groups throughout.
Table 2.
Anti-SARS-CoV-2 antibody responses of 4 vaccine groups at Day 1, Day 14, Day 28, and Day 84 post booster dose.
| SARS-CoV-2 S-IgG (BAU/mL) Geometric mean (95% CI) |
Geometric mean neutralization titer (NT50) (IU/mL) |
|||
|---|---|---|---|---|
| Alpha Variant | Delta Variant | |||
| Group 1 | Day 1 | 72.10 (60.06–86.56) | 8.73 | 1.07 |
| Day 14 | 194.07 (165.50–227.57) | 125.94 | 3.73 | |
| Day 28 | 170.09 (146.79–197.08) | 97.02 | 4.72 | |
| Day 84 | 88.96 (77.50–102.12) | 32.22 | 3.66 | |
| Group 2 | Day 1 | 76.38 (64.82–89.99) | 4.35 | 1.00 |
| Day 14 | 2330.81 (2038.83–2664.60) | 1237.61 | 274.55 | |
| Day 28 | 1534.82 (1350.72–1744.02) | 928.72 | 204.42 | |
| Day 84 | 517.36 (456.46–586.39) | 282.36 | 73.51 | |
| Group 3 | Day 1 | 93.47 (76.77–113.80) | 11.03 | 1.42 |
| Day 14 | 3283.76 (2905.02–3711.87) | 993.21 | 263.07 | |
| Day 28 | 1789.50 (1588.75–2015.62) | 510.66 | 89.81 | |
| Day 84 | 553.68 (494.20–620.31) | 180.98 | 35.01 | |
| Group 4 | Day 1 | 449.28 (383.46–526.40) | 51.13 | 2.41 |
| Day 14 | 3791.72 (3457.41–4158.35) | 1524.16 | 342.12 | |
| Day 28 | 2516.60 (2285.50–2771.06) | 961.98 | 195.36 | |
| Day 84 | 903.10 (813.62–1002.43) | 403.75 | 105.72 | |
BAU, binding antibody units; CI, confidence interval; NT50, 50% neutralization titer; IU, international unit.
Antibody values were transformed to log values, and the average values were expressed as geometric means with 95% confidence interval.
Figure 2.
SARS-CoV-2 anti-spike IgG responses and spike-specific memory B cell responses after boost dose among four groups. (A) Distributions of SARS-CoV-2 anti-spike IgG responses at the day before and 14th, 28th, and 84th days after boost vaccination; (B) Responses of spike-specific memory B cells at the day before and 28th days after boost dose among four groups. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001).
Spike protein-specific memory B cells
The frequency and phenotypes of the memory B cells carrying membrane-bound immunoglobulin-specific for spike protein are shown in Fig. 2B. The frequencies of spike protein-specific memory B cells before boost vaccination in the three ChAdOx1 prime vaccination groups (Groups 1, 2, and 3) were all around 0.07%, which is not significantly different between each other, but is significantly lower than that in mRNA-1273/mRNA-1273 prime vaccination group (Group 4) (0.23%). In contrast, on day 28 after booster vaccination, the frequencies of the spike protein-specific memory B cells increased significantly in the two heterologous ChAdOx1/mRNA-1273 vaccination groups (Group 2 and Group 3) and homologous mRNA-1273/mRNA-1273 group (Group 4), but not in the ChAdOx1/ChAdOx1 group (Group 1). Moreover, on day 28 post boosting, the frequencies of spike protein-specific memory B cells in the heterologous ChAdOx1/mRNA-1273 vaccination groups (Group 2 and Group 3) and homologous mRNA-1273/mRNA-1273 group (Group 4) were significantly higher than that of ChAdOx1/ChAdOx1 group (Group 1). Fold-increase of spike protein-specific memory B cells on day 28 post boost vaccination in the four groups were 1.4, 2.8, 3.3, and 2.6, respectively.
Neutralizing antibody tests
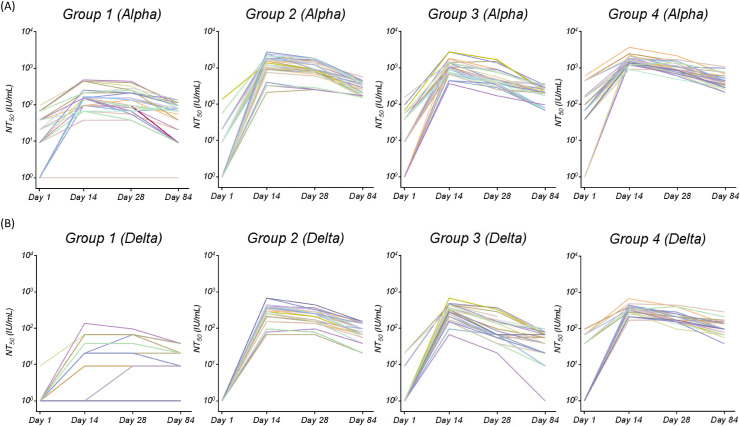
The neutralizing antibody titers against SARS-CoV-2 alpha and delta variants, determined by a TCID50-based neutralization method, were converted into IU/mL using a WHO reference panel. The neutralizing antibody titers against the alpha and delta variants are shown in Fig. 3 A and 3B, respectively. Lower neutralizing antibody titers against the delta variant were observed in both the heterologous and homologous prime-boost groups when compared to those against the alpha variant. For those receiving at least one dose of mRNA-1273 vaccine, their neutralizing antibody titers against alpha or delta variants were all significantly higher than those of Group 1 (Fig. 2 and Supplementary Table 1). For those receiving heterologous mRNA vaccines, Group 2 with a longer interval between the two doses had better neutralizing antibody titers than Group 3 on days 24 and 84 after the second dose, and comparable neutralizing antibody titers as compared to those receiving homologous mRNA-1273 vaccine (Supplementary Table 1). Group 3 with a shorter interval between heterologous mRNA boosting had a significant lower mean titer of neutralizing antibodies than those of Group 4 at all three timepoints post 2nd vaccine dose. All 4 groups had lower neutralizing antibody titers by day 84 compared to days 14 and 28 post 2nd vaccine dose.
Figure 3.
The serum neutralizing antibody titers (NT50) of 4 Groups of study subjects against SARS-CoV-2 (A) Alpha variant, and (B) Delta variant.
Spike protein-specific T cells
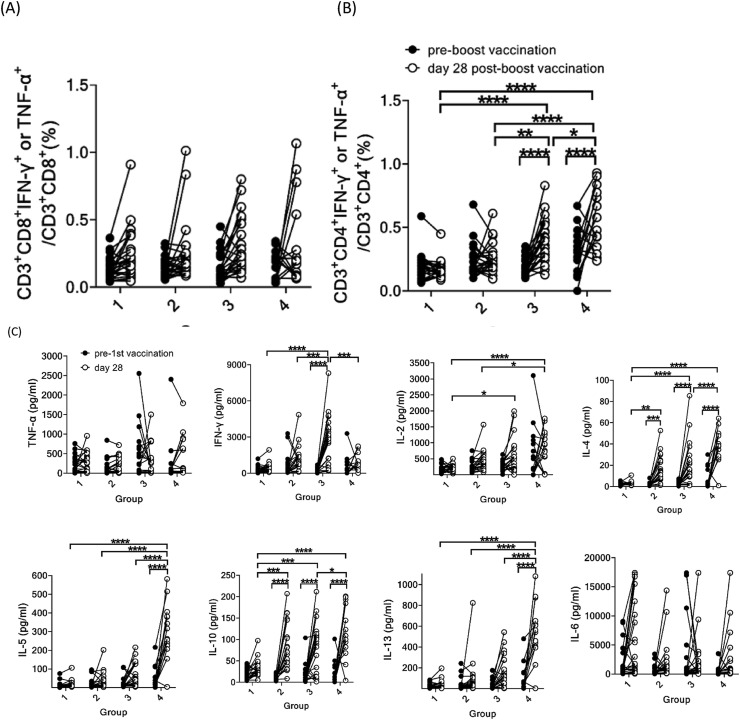
The spike protein-specific TNF-α- or IFN-γ-secreting CD8+ T cells on day 28 post boost vaccination did not increase significantly in all 4 groups (Fig. 4 A). However, the spike protein-specific TNF-α- or IFN-γ-secreting CD4+ T cells increased significantly in Groups 3 and 4, but not in Groups 1 and 2. In addition, the frequencies of spike protein-specific TNF-α- or IFN-γ-secreting CD4+ T cells on day 28 post boosting in Groups 3 and 4 are significantly higher than that in the ChAdOx1/ChAdOx1 group (Group 1). The homologous mRNA-1273/mRNA-1273 group (Group 4) had the highest frequency of spike protein-specific TNF-α- or IFN-γ-secreting CD4+ T cells after 2nd dose, which was significantly higher than those of the other 3 groups (Fig. 4B). We also analyzed the Th1/Th2 cytokine production by T cells, and found that the two heterologous ChAdOx1/mRNA-1273 vaccination groups (Groups 2 and 3) and homologous mRNA-1273/mRNA-1273 group (Group 4) had higher IL-4 and IL-10 production than the homologous ChAdOx1/ChAdOx1 group (Group 1) (Fig. 4C). Groups 3 and 4 also had significantly higher IL-2 production than Group 1. Interestingly, Group 3 exhibited the highest IFN-γ production, whereas Group 4 exhibited the highest production of IL-4, IL-5, IL-13 and IL-10, primarily Th2 cytokines. However, TNF-α and IL-6 production did not differ significantly between groups, and between pre- and post-booster vaccination. These results indicate that the heterologous ChAdOx1/mRNA-1273 vaccination schedule was superior to the homologous ChAdOx1/ChAdOx1 vaccination schedule in terms of humoral and cellular responses.
Figure 4.
Immunological response of SARS-CoV-2 spike-specific memory T cells before and at 28th day post boost vaccination among four groups. Intracellular staining of cytokines in spike-specific CD8+ T cells (A) and spike-specific CD4+ T cells (B). (C) Th1/Th2 cytokine production by T cells using bead-based cytokine assay. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001).
Adverse events
The adverse events of four study groups are shown in Table 3 . Except skin rashes and gastrointestinal discomfort, heterologous ChAdOx1/mRNA-1273 vaccination groups (Group 2 and Group 3) and homologous mRNA-1273/mRNA-1273 vaccination group (Group 4) had higher rates of local (pain, erythema, swelling) and systemic (fever, chills, headache, myalgia, fatigue, arthralgia/arthritis) adverse reactions compared to homologous ChAdOx1/ChAdOx1 vaccination group (Group 1) (Supplementary Table 2). In general, there were no significant differences of adverse events between Groups 3 and 4, except for a higher proportion of swelling and moderate chills in Group 3. As to Groups 2 and 3, shorter interval of heterologous vaccination group (Group 3) seemed to increase the prevalence of adverse events, such as pain, swelling, fever, myalgia, and fatigue than Group 2. There were no serious adverse events occurred across all four groups during the observation period. Only one participant had a clinic visit due to persistent generalized skin rashes as described above.
Table 3.
Adverse events of anti-SARS-CoV-2 vaccination schedule within 84 days after boost dose.
| Group 1 (n = 100) |
Group 2 (n = 100) |
Group 3 (n = 100) |
Group 4 (n = 99) |
P-value | ||
|---|---|---|---|---|---|---|
| Pain, n (%) | Yes | 62 (62.0%) | 74 (74.0%) | 96 (96.0%) | 94 (94.9%) | <0.001 |
| Grade 2 or 3 | 14 (14.0%) | 50 (50.0%) | 44 (44.0%) | 40 (40.4%) | <0.001 | |
| Grade 3 | 2 (2.0%) | 15 (15.0%) | 9 (9.0%) | 3 (3.0%) | 0.001 | |
| Erythema, n (%) | Yes | 7 (7.0%) | 14 (14.0%) | 23 (23.0%) | 17 (17.2%) | 0.016 |
| Swelling, n (%) | Yes | 6 (6.0%) | 25 (25.0%) | 42 (42.0%) | 23 (23.2%) | <0.001 |
| Fever, n (%) | Yes | 8 (8.0%) | 32 (32.0%) | 55 (55.0%) | 42 (42.4%) | <0.001 |
| Chills, n (%) | Yes | 16 (16.0%) | 50 (50.0%) | 51 (51.0%) | 41 (41.1%) | <0.001 |
| Grade 2 or 3 | 4 (4.0%) | 20 (20.0%) | 28 (28.0%) | 12 (12.1%) | <0.001 | |
| Grade 3 | 1 (1.0%) | 4 (4.0%) | 0 (0.0%) | 0 (0.0%) | 0.034 | |
| Headache, n (%) | Yes | 30 (30.0%) | 54 (54.0%) | 53 (53.0%) | 42 (42.4%) | 0.002 |
| Grade 2 or 3 | 8 (8.0%) | 22 (22.0%) | 26 (26.0%) | 17 (17.2%) | 0.007 | |
| Grade 3 | 1 (1.0%) | 1 (1.0%) | 1 (1.0%) | 1 (1.0%) | 1.000 | |
| Myalgia, n (%) | Yes | 46 (46.0%) | 68 (68.0%) | 91 (91.0%) | 87 (87.9%) | <0.001 |
| Grade 2 or 3 | 9 (9.0%) | 39 (39.0%) | 33 (33.0%) | 26 (26.3%) | <0.001 | |
| Grade 3 | 1 (1.0%) | 6 (6.0%) | 1 (1.0%) | 2 (2.0%) | 0.074 | |
| Fatigue, n (%) | Yes | 55 (55.0%) | 69 (69.0%) | 91 (91.0%) | 84 (84.8%) | <0.001 |
| Grade 2 or 3 | 13 (13.0%) | 42 (42.0%) | 30 (30.0%) | 28 (28.3%) | <0.001 | |
| Grade 3 | 2 (2.0%) | 4 (4.0%) | 1 (1.0%) | 3 (3.0%) | 0.560 | |
| Rashes, n (%) | Yes | 5 (5.0%_ | 11 (11.0%) | 8 (8.0%) | 6 (6.1%) | 0.393 |
| Grade 2 or 3 | 1 (1.0%) | 5 (5.0%) | 4 (4.0%) | 4 (4.0%) | 0.445 | |
| Generalized rashes, n (%) | Yes | 0 (0.0%) | 2 (2.0%) | 1 (1.0%) | 1 (1.0%) | 0.569 |
| Arthralgia/arthritis, n (%) | Yes | 1 (1.0%) | 11 (11.0%) | 12 (12.0%) | 7 (7.1%) | 0.015 |
| GI upset (nausea/vomit), n (%) | Yes | 2 (2.0%) | 7 (7.0%) | 8 (8.0%) | 5 (5.1%) | 0.259 |
| Others, n (%) | Yes | 10 (10.0%) | 21 (21.0%) | 19 (19.0%) | 19 (19.2%) | 0.162 |
Others include chest tightness (n = 11), anorexia (9), dizziness/vertigo (7), lymphadenitis (6), abdominal pain/epigastralgia (5), diarrhea (5), palpitation (5), insomnia (4), limbs numbness (4), drowsiness (4), ecchymosis (3), epistaxis (2), conjunctivitis (1), asthma/bronchitis (1), dysuria (1) and night sweats (1).
SD, standard deviation; GI, gastrointestinal.
Discussion
Understanding the immunogenicity and safety of heterologous vaccination enables governments to make rational policy of vaccination for COVID-19 and selection of alternative vaccine in individuals who had severe adverse events after their first dose. Our results revealed that heterologous ChAdOx1/mRNA-1273 vaccination provided strong humoral and cellular immune responses comparable to standard homologous mRNA-1273/mRNA-1273 vaccination and approximately 10-fold higher SARS-CoV-2 anti-spike IgG levels than that of homologous ChAdOx1/ChAdOx1 vaccination. Similar differences were also observed for neutralizing antibody activity, vaccine induced spike-specific memory B cell, and spike-specific T cell responses, and associated cytokine production in our present study. Although significant higher proportion of adverse reactions was observed in heterologous vaccination groups, mostly were mild and well tolerated. Our results support the safety and immunogenicity of heterologous ChAdOx1/mRNA-1273 vaccination with cross protection against both alpha and delta variants of SARS-CoV-2.
Standard homologous ChAdOx1/ChAdOx1 had been effective in preventing symptomatic COVID-19 when administered with a 4 to 12 weeks interval between doses.4 The WHO recommended interval for ChAdOx1/ChAdOx1 is 8–12 weeks.13 Vaccine efficacy was higher in participants with a longer prime-boost interval (≥12 weeks, 81.3%) compared to those with a short interval (<6 weeks, 55.1%).4 Our results showed an 8-week interval homologous ChAdOx1/ChAdOx1 vaccination group had lower immunogenic response than heterologous ChAdOx1/mRNA-1273 vaccination groups. Whether a longer interval (more than 8 weeks) of ChAdOx1/ChAdOx1 vaccination could provide comparable immune response against SARS-CoV-2 compared to heterologous ChAdOx1/mRNA-1273 (4–8 weeks apart) needs further investigation.
Immunogenicity following heterologous boosting (3rd dose) with mRNA vaccines compared to homologous mRNA vaccines have been reported recently.14 In a large, open-label clinical trial, 458 adult participants who had completed two doses of COVID-19 vaccine regimen at least 12 weeks earlier, received a booster with one of three vaccines included mRNA-1273, Ad26.COV2.S (Johnson & Johnson-Janssen), or BNT-162b2 at least 12 weeks apart. They found a similar increased trends of geometric mean binding antibody titers among participants who had received homologous or heterologous prime-booster mRNA vaccines.14 In contrast, our results revealed homologous mRNA-1273 vaccination had significantly higher antibody titers compared with heterologous mRNA-1273 vaccination at 4-week and 8-weeks apart. Whether a longer 12-week interval yields a similar result between participants receiving homologous and heterologous mRNA-1273 vaccine remains unclear.
The titers of both RBD-binding and neutralizing antibodies were found well correlated with protection against symptomatic disease.15 , 16 A small cohort from Sweden assessed 37 health care workers who had received ChAdOx1/ChAdOx1 with an interval of 9–12 weeks, compared with 51 heterologous ChAdOx1/mRNA-1273 prime-boost vaccination.11 The results showed elevations of SARS-CoV-2 anti-spike IgG titers at 7 to 10 days after boost compared with the titers on the day of the boost were much higher in heterologous compared to homologous group (115-folds versus 5-folds, P < 0.001). Similarly, the serum neutralization titers of the 50% inhibitory dilution (ID50) at 7 to 10 days after the heterologous ChAdOx1/mRNA-1273 boost was 20-fold elevations than that on the day of the boost, compared with a two-fold elevation of ID50 at 7 to 10 days after homologous ChAdOx1/ChAdOx1 boost vaccination. Another report from Germany showed that heterologous ChAdOx1/BNT-162b2 (BioNTech/Pfizer) vaccination with an interval of 11.2 ± 1.3 weeks led to a significantly higher elevation of SARS-CoV-2 anti-spike IgG titers compared with homologous ChAdOx1/ChAdOx1 vaccination with an interval of 10.8 ± 1.4 weeks (3630 BAU/mL versus 404 BAU/mL, P < 0.0001)17 and similar to homologous BNT-162b2/BNT-162b2 vaccination with an interval of 4.3 ± 1.1 weeks (4932 BAU/mL) at a median of 14 days after boost.
A Spanish study recruited participants of aged 18 to 60 years to receive a boost dose of BNT-162b2 vaccine 2 to 3 months after priming with ChAdOx1. They found a 37-fold increase in SARS-CoV-2 anti-spike IgG at 14 days post boost, higher than the 22-fold increase at 7 days and 19-fold increase at 28 days post boost.18 A prospective cohort study in Germany, which compared healthcare workers received BNT-162b2/BNT-162b2 at a 3-week interval or ChAdOx1/BNT-162b2 at an 8 to 12-week interval, showed similar concentrations of binding antibody at 3 weeks post boost and higher cellular responses in the ChAdOx1/BNT-162b2 recipients.10 Most of these studies, the interval of prime and boost vaccination were a range of several weeks. Consistent with their results, our study results, with fixed duration of interval of 4 or 8 weeks, also demonstrated that heterologous ChAdOx1/mRNA-1273 prime-boost vaccination schedule induced robust antibody production against SARS-CoV-2. In addition, we also found that the heterologous prime-boost ChAdOx1/mRNA-1273 vaccination with an 8-week interval and the homologous mRNA-1273 vaccination with a 4-week interval had similar mean neutralizing antibody titers, which are significantly higher than the 4-week interval of heterologous ChAdOx1/mRNA-1273 vaccination on day 28 after boosting.
Among a cohort of healthcare workers whose immune responses following ChAdOx1-prime and 3 weeks later boost with ChAdOx1 or BNT-162b2, the heterologous ChAdOx1/BNT162b2 vaccination induced significantly higher frequencies of spike-specific CD4+ and CD8+ T cells responses and higher titers of neutralizing antibodies against the alpha, beta and gamma variants (all P < 0.0001).19 Similarly, a small German study that compared an 8-week interval of prime-boost ChAdOx1/BNT-162b2 with homologous BNT-162b2/BNT-162b2 vaccination found significantly higher neutralization titers against alpha variant and equivalent neutralizing activities against beta and delta variants.20 Our study adds to the growing evidence that heterologous ChAdOx1/mRNA-1273 produces better neutralizing activities against both the alpha and delta variants than homologous ChAdOx1/ChAdOx1 vaccination when administered at 4-weeks or 8-weeks apart. Consistently, the analysis of spike-specific T cells and associated cytokines showed that the heterologous ChAdOx1/mRNA-1273 and the homologous mRNA-1273/mRNA-1273 vaccination stimulated more spike-specific cytokine-producing CD4+ T cells and more cytokine secretion than the homologous ChAdOx1/ChAdOx1 vaccination. The increased frequency of spike-specific CD4+ T helper cells has been linked to the enhanced antibody response against SARS-CoV-2 in heterologous ChAdOx1/BNT162b2 vaccination.21
Regarding adverse events, a multicenter, randomized prime-boost COVID-19 vaccination study that enrolled 830 healthy participants aged 50 years and older in United Kingdom reported the frequency of systemic reactions to be higher after the priming dose of ChAdOx1/ChAdOx1 group and after the booster dose of BNT-162b2/BNT-162b2 group.22 Both heterologous vaccination schedules induced significant higher systemic reactions following the booster dose than their homologous counterparts. For example, fever was reported in 34% of ChAdOx1/BNT-162b2 group compared with 10% of ChAdOx1/ChAdOx1 group, and 41% of BNT-162b2/ChAdOx1 group compared with 21% of BNT-162b2/BNT-162b2 group (all P values were <0.05), respectively. However, they found the systemic or local reactions were mild and transient, and occurring mostly within 48 hours after vaccination. In a study enrolling 330 healthcare workers who received prime vaccination with BNT162b2 or ChAdOx1, the adverse events among homologous BNT162b2/BNT162b2 or ChAdOx1/ChAdOx1 vaccinations and heterologous ChAdOx1/BNT162b2 vaccinations were not different for local reactions but variable for systemic reactions, such as fatigue, myalgia, headache, feverishness or chills, which were more frequent after priming with ChAdOx1 (86%) and after homologous BNT162b2 booster immunization (65%).10 Heterologous ChAdOx1/BNT162b2 had similar rates of systemic reactions and antipyretic use as homologous ChAdOx1/ChAdOx1 and BNT162b2/BNT162b2 groups. Compatible with these reports, our results support the safety of heterologous prime ChAdOx1 and boost mRNA vaccination. Most adverse reactions of our participants were also reported within 7 days after vaccination. Shorter interval of heterologous vaccination (4 weeks) seemed to give rise to more adverse events, such as pain, swelling, fever, myalgia, and fatigue than longer interval (8 weeks) in our study.
A longer prime-boost interval of vaccination may offer the potential to enhance and extend humoral immunity in the elderly population. In a report with 172 participants aged over 80 years without previous infections who received homologous BNT162b2/BNT162b2 vaccination either following a standard 3-week interval or delayed to 12 weeks, the peak antibody response was 3.5-fold higher in donors who had undergone delayed interval vaccination, although cellular immune responses were 3.6-fold lower.23 Our results presented here address a 4-week and 8-week schedules with heterologous prime-boost interval of a relatively younger population. Whether a 12 week-interval between prime and boost vaccination could offer potential benefits to enhance and extend humoral immunity in the younger population need further study.
Since there was a very low prevalence rate of COVID-19 in Taiwan and there was only one wave of community outbreak during May to June 2021, none of our vaccinees were infected by SARS-CoV-2 before or after completing their vaccine schedules. Their immune responses were very likely due to a pure vaccination effect, without an occasion for natural boosting. The limitations of our present study include: first, we enrolled only 20–65 years old relatively healthy subjects, and the results cannot be applied to other age groups and immunosuppressed populations from our study. Second, due to the low number of study subjects and the low incidence and endemicity of COVID-19 in Taiwan, we could not compare the efficacy or effectiveness of different vaccination schedules.
In conclusion, our study confirms that heterologous vaccination schedules of ChAdOx1 and mRNA-1273 can induce robust and broad immune responses when administered 4- or 8-weeks apart. The information support flexibility in deploying mRNA and viral vectored vaccines schedules with different prime-boost intervals. This information would be useful, especially in countries with limited supplies of mRNA vaccines or with considerable side effects from the adenoviral based vaccine.
Funding
The funding support of this study included MOST-110-2740-B-002-006, MOST109-2327-B-002-009 from Ministry of Science and Technology Taiwan and a private donation fund to support COVID-19 studies at National Taiwan University, College of Medicine (109F004T). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement
We would like to acknowledge the services provided by the Biosafety Level-3 Laboratory of the First Core Laboratory from National Taiwan University College of Medicine and the Biosafety Level-3 Laboratory from National Taiwan University Hospital. The authors would like to thank Prof. Shin-Ru Shih (Chang-Gung University, Taoyuan, Taiwan) for kindly support of a WHO reference panel; Dr. Aristine Cheng M.D. (National Taiwan University Hospital, Taipei, Taiwan) for her kind and indispensable assistance in the English editing of this manuscript; and Ms.Yu-Yun Wu for her help with statistical analysis. We would also like to express our appreciation to the Central Epidemic Command Center (CECC) of Taiwan for approval of the heterologous COVID-19 vaccination program in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfma.2022.02.020.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.World Health Organization (WHO) WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.Taiwan Centers for Diseases Control (T-CDC) COVID-19 (SARS-CoV-2 infection) https://www.cdc.gov.tw/En
- 3.World Health Organization AZD1222 vaccine against COVID-19 developed by Oxford University and AstraZeneca: background paper. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-background-2021.1
- 4.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization mRNA-1273 vaccine (Moderna) against COVID-19 background document: draft prepared by the strategic advisory group of experts (SAGE) on immunization working group on COVID-19 vaccines. 19 January 2021. https://apps.who.int/iris/handle/10665/338738
- 6.World Health Organization Interim recommendations for use of the Pfizer-BioNTech COVID-19 vaccine, BNT162b2, under emergency use listing. 15 June 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021
- 7.Pottegard A., Lund L.C., Karlstad O., Dahl J., Andersen M., Hallas J., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. Br Med J. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. April 7, 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rarecases-unusual-blood-clots-low-blood (accessed August 20, 2021)
- 10.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., et al. Safety, reactogenicity and immunogenicity of homologous and heterologous prime-boost immunization with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Normark J., Vikstrom L., Gwon T.D., Persson I.L., Edin A., Bjorsell T., et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385:1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Guidelines on clinical evaluation of vaccines: regulatory expectations. https://www.who.int/biologicals/BS2287_Clinical_guidelines_final_LINE_NOs_20_July_2016.pdf
- 13.WHO AstraZeneca ChAdOx1-S/nCoV-19 [recombinant], COVID-19 vaccine. https://www.who.int/publications/m/item/chadox1-s-recombinant-covid-19-vaccine
- 14.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med. 2022 doi: 10.1056/NEJMoa2116414. (Online ahead of print on Jan 26, 2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 16.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borobia A.M., Carcas A.J., Perez-Olmeda M., Castano L., Bertran M.J., Garcia-Perez J., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicenter, open-label, randomized, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Ramos G.M., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grob R., Zanoni M., Seidel A., Conzelmann C., Gilg A., Krnavek D., et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2022;75:103761. doi: 10.1016/j.ebiom.2021.103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozzetto B., Legros V., Djebali S., Barateau V., Guiber N., Villard M., et al. Immunogenicity and efficacy of heterologous ChadOx1/BNT162b2 vaccination. Nature. 2021;600:701–706. doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 22.Shaw R.H., Stuart A., Greenland M., Liu X., Nguyen Van-Tam J.S., Snape M.D., Com-COV Study Group Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry H., Bruton R., Stephens C., Brown K., Amirthalingam G., Hallis B., et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv. 2021 doi: 10.1101/2021.05.15.21257017. (Published online May 15) (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.