Abstract
Objective:
To detail the nasolabial morphologic characteristics of North Sudanese subjects with Down syndrome (DS).
Materials and Methods:
Nasolabial morphology was assessed three-dimensionally in 64 North Sudanese subjects with DS aged 4 to 34 years and in 682 sex- and age-matched controls. Three-dimensional facial coordinates were collected using a laser scan, and selected distances, angles, areas, and volumes were computed. Subject and reference data were compared by computing z-scores and Student's t-tests.
Results:
The nose was significantly smaller (area) in subjects with DS than in reference subjects, and it had a different shape (more flat angle of alar slope, more acute nasal tip angle). The vertical (nasal bridge length, nose height) and anteroposterior (nasal tip protrusion) dimensions were reduced, while the horizontal dimensions (alar base width, inferior widths of the nostrils) were increased. The nasolabial angle was increased. The cutaneous lip volume was significantly smaller, while the vermilion lip area was larger in the subjects with DS. The mouth and philtrum widths were significantly reduced, while the vermilion height was significantly increased.
Conclusion:
Analyzed subjects with DS had a hypoplastic nose and different upper and lower lips than did reference, normal subjects.
Keywords: Anthropometry, Syndrome, Face
INTRODUCTION
Facial phenotype is modified in several genomic and chromosomal alterations, and often the specific phenotypic characteristics are used for clinical diagnosis. Down syndrome (DS, Mendelian Inheritance in Man [MIM] #190685)1 is the most frequent live-born autosomal aneuploidy in humans. DS, first described in 1866, is produced by complete or partial trisomy of chromosome 21.1–4 Various morphologic and functional abnormalities of body structures, from cellular organelles to multiorgan systems, characterize DS and are present in variable proportions in affected individuals.2,4 Among the most constant features is a distinctive and immediately recognizable craniofacial phenotype.1
The main alterations are an overall reduction in head size together with a modification of head shape; a decrement in the interorbital distance, small palpebral fissures, and prominent forehead; a hypoplastic facial middle third (maxilla) with a less prominent nose; a reduced facial lower third with a prominent mandible; and smaller ears.3,5–10
Specific characteristics of the craniofacial phenotype of DS subjects can be identified before birth, and ultrasonographic assessment of intrauterine facial morphology is currently a useful diagnostic tool.11–14
After birth, the three-dimensional (3D) facial dimensions of DS subjects have been obtained by conventional anthropometry,15–19 or by digital, computerized instruments.5–10,20 Among these, optical instruments appear the most suitable for fast data collection in disabled persons.21–24
Among the other intrauterine phenotypic markers of DS is delayed nasal development, with hypoplastic or absent nasal bone.12,14,25 This characteristic persists after birth, and the absence of nasal bones in adult subjects with trisomy 21 has been reported.26 Indeed, a depressed nasal bridge is one of the most common stigmata of DS, and alterations in nasal size and shape have been quantitatively described in adult Northern American White, Croatian, and Italian subjects with DS. Overall, the nose is significantly smaller (volume, area) in subjects with DS than in reference subjects, and it has a different shape. Nasal vertical and anteroposterior dimensions are reduced, while horizontal dimensions are increased.5,15,17,26 Therefore, in White DS subjects, the nose is shorter and less protruding, but with larger nostrils, a flatter angle of alar slope, and a more acute nasal tip angle.5
Differences in the mouth and lip region have been described in White subjects with DS; mouth width is reduced, with a relatively smaller lower lip and a larger upper lip; the upper lip increment is particularly evident for the vermilion area and height. The lips are prominent, with reduced nasolabial, interlabial (soft tissues), and interincisal angles.3,7,16,19,26
In contrast, no data on the craniofacial phenotype of African subjects with DS have been published so far. Prefumo et al.13 reported that the ethnic origin of the mother significantly influenced the rate of visualization of fetal nasal bones in the first trimester of pregnancy. In particular, in women of African origin, failure to visualize fetal nasal bones was significantly greater than in women of White origin. Therefore, the facial postnatal phenotype of African subjects with DS, as compared with normal subjects, may not present the same characteristics described so far for White subjects with DS.
Sudan is the largest country in Africa and is known for its multiethnic mix. Three major ethnic groups exist: those of Arab descent in the North, Nilotic tribes in the South, and West African tribes in the region of Darfur and Eastern Tribes; these tribes have marked facial morphologic differences.
In the present investigation, the faces of a group of North Sudanese subjects with DS were imaged in three dimensions using laser scanning, and the morphologic features of their nose and lips were quantitatively analyzed. Data were compared with values collected in normal, healthy individuals of the same age, sex, and ethnicity. We wanted to detail the nasolabial morphologic characteristics of North Sudanese subjects with DS, and to assess similarities and differences in White subjects with the same syndrome.
MATERIALS AND METHODS
Subjects
Data from 64 subjects with DS (18 women, age range 5–34 y; 46 men, age range 4–33 y) were collected in the present study. All subjects were Northern Sudanese residing in Khartoum, the capital of Sudan, and had undergone no craniofacial surgical procedures. In Sudan, patients affected by DS usually attend special needs schools, but have no other special service provision in terms of specialist health or educational centers or groups. In the current study, special needs schools were approached, and consent was obtained from the head teacher and parents, who were informed about the study in writing. For all subjects, DS was verified from the clinical features, as well as by school records. Only subjects of Northern African origin were included in the current study.
Reference data were collected on 682 (343 women, 339 men) normal subjects of the same sex, ethnic group, and age from a sample of randomly selected reference groups; no subjects with a previous history of craniofacial trauma or with congenital anomalies were included. The reference group was collected from Northern Sudanese subjects attending preschools, schools, and universities in Khartoum State.
All analyzed individuals and the parents/legal guardians of DS subjects and of all reference subjects younger than 18 years of age gave their written informed consent to the experiment. All procedures were noninvasive, did not provoke damage, risk, or discomfort to subjects, and were preventively approved by the local ethics committee (Ethical Approval Committee at Elrazi Dental School; Reference number: Dent/01).
Collection of 3D Facial Landmarks
Data collection was performed using a portable hand-held laser scanner (FastSCAN Cobra, Polhemus Inc, Colchester, Vt); this was followed by off-line calculations. Scanning technology and accuracy were previously assessed.21,22 The technique is noninvasive and produces a detailed 3D facial model. During the digitization process, the subject is required to remain still for several seconds, while the scanner acquires details of the subject's head. Scans with excessive inaccuracies due to the subject's inability to keep the head still were discarded. The reported precision of the laser scanner is approximately 0.5 mm, and the time of exposure from ear to ear, trichion to menton scan, is 20 to 30 seconds.
The files of the 3D facial scans were stored on magnetic media. The image was further manipulated and visualized by applying 3D spatial modeling with interactive 3D modeler software (Rhinoceros Nurbs Modeling for Windows 4.0, McNeel North America, Seattle, Wash). Based on nonuniform rational B-splines, the geometric shape can be modeled as a 3D parameter that accurately reflects the facial shape; biologically important landmark coordinates (x, y, z) can then be extracted.
Subsequently, a set of 50 standardized landmarks was identified on digital facial reconstruction, and custom computer programs were used for all off-line calculations.5,24
Reproducibility of facial digitization and landmark identification were tested in 10 subjects. Repeated digitizations of each scan were carried out 1 month apart by the same operator. The complete set of 50 landmarks was identified on each paired acquisition, and 18 linear distances were calculated. Systematic errors in repeated acquisition/landmark identification were assessed by Student's t-tests for paired samples; random errors were estimated by calculating the technical error of measurement (TEM):
where D is the difference between each couple of replicate measurements, and n is the number of couples.
Data Analysis
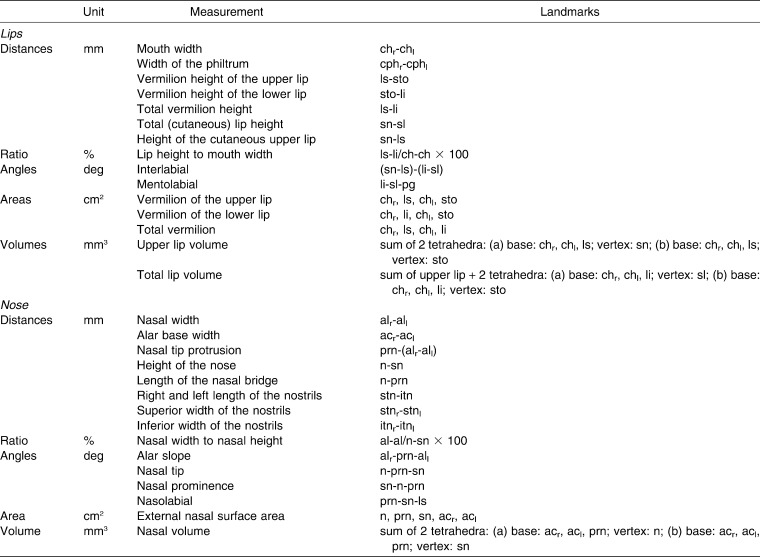
According to the geometric model of the lips and nose defined by Ferrario et al.,5 the x, y, z coordinates of a subset of landmarks obtained on each subject (Table 1) were used to calculate several nasal and labial parameters (Table 2, Figure 1).
Table 1.
Three-Dimensional Soft Tissue Facial Landmarks Used in the Analysis
Table 2.
Nasolabial Measurements Calculated From the Digitized Landmarks
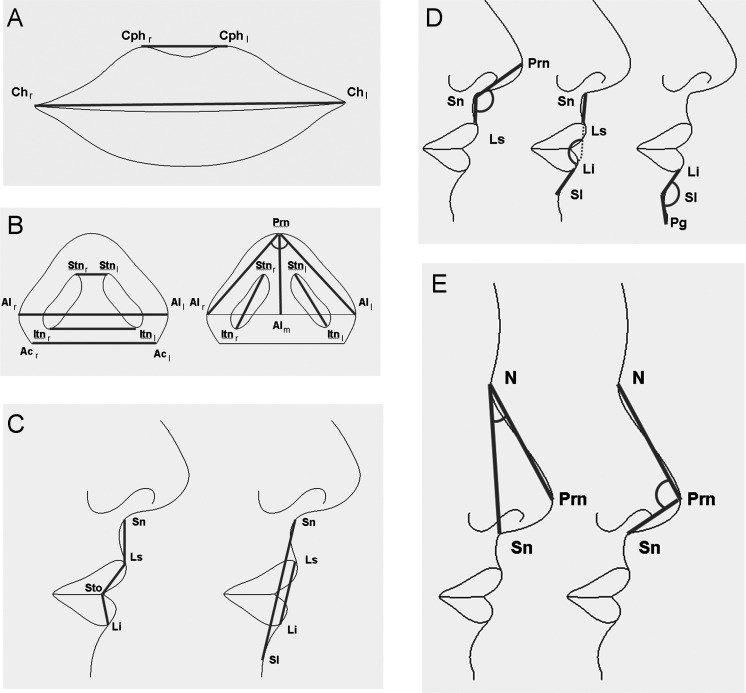
Figure 1.
Landmarks identified in the nasolabial region and three-dimensional (3D) linear distances and angles computed in the analyzed subjects: (A) mouth width (chr-chl) and width of the philtrum (cphr-cphl); (B) superior width of the nostrils (stnr-stnl), nasal width (alr-all), inferior width of the nostrils (itnr-itnl), alar base width (acr-acl), nasal tip protrusion (prn-alm), right and left length of the nostrils (stn-itn), and alar slope angle (alr-prn-all); (C) height of the cutaneous upper lip (sn-ls), vermilion height of the upper lip (ls-sto), vermilion height of the lower lip (sto-li), total (cutaneous) lip height (sn-sl), and total vermilion height (ls-li); (D) nasal angles: nasolabial (prn-sn-ls), interlabial ([sn-ls]-[li-sl]), and mentolabial (li-sl-pg); and (E) nasal angles: nasal prominence (sn-n-prn) and nasal tip (n-prn-sn).
In the 682 reference, normal individuals, descriptive statistics were calculated for each variable separately for each age and sex. Individual measurements obtained in the 64 DS subjects were transformed to z-scores by subtracting each from its sex and age reference mean value, and dividing by the relevant reference standard deviation.5,8,16–19
Statistical Calculations
Descriptive statistics were computed for the values of the z-scores separately for men and for women, as well as for the pooled sample.
Statistical comparisons were performed with paired Student's t-tests (null hypothesis: the z-scores should be zero if the values in DS subjects do not differ from the reference population matched for sex and age; alternative hypothesis: z-scores are significantly different from zero) and unpaired Student's t-tests (null hypothesis: male values do not differ from female values; alternative hypothesis: male values are different from female values). For all analyses, significance was set at 5%.
RESULTS
The mean technical error of measurement on 18 facial distances made on the repeated scans was 0.755 mm. No statistically significant systematic errors were found (paired Student's t-test >.05 on all occasions).
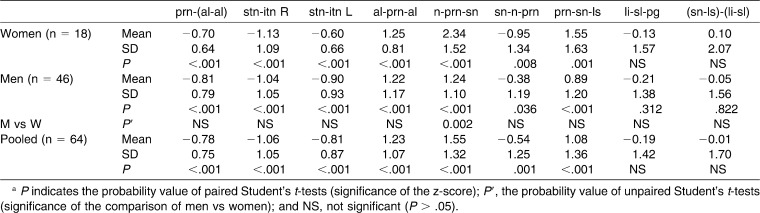
On average, the ages of analyzed male and female subjects with DS did not differ (Student's t-test for independent data P > .05, Table 3).
Table 3.
Descriptive Statistics of the z-Score Values of the Analyzed Areas and Volumesa
Sixteen linear distances (six from homologous landmarks in the left-right direction, seven in the vertical direction, and three in the anteroposterior direction), two linear distance ratios, six angles, four areas, and three volumes were computed in the 64 analyzed subjects with DS. Analyzed persons had a wide range in age, encompassing childhood, adolescence, and young and middle adulthood. To allow a global assessment, z-scores were computed using the mean values of normal, reference individuals of the same age, sex, and ethnicity (Tables 3 through 6).
Table 4.
Descriptive Statistics of the z-Score Values of the Analyzed Horizontal Distancesa
Table 5.
Descriptive Statistics of the z-Score Values of the Analyzed Vertical Distances and Ratiosa
Table 6.
Descriptive Statistics of the z-Score Values of the Analyzed Sagittal Distances and Anglesa
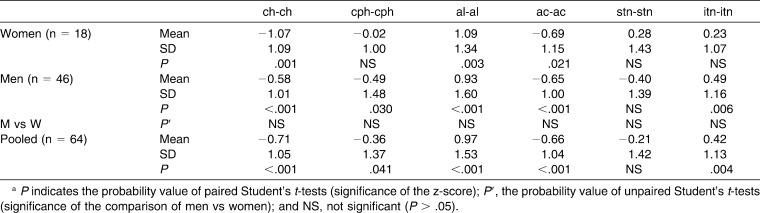
In general, more z-scores were significantly different from 0 in men than in women, but only one sex-related difference was observed: the discrepancy in the n-prn-sn angle was significantly larger in women than in men (Student's t-test for independent samples, P = .002). Pooled data (male and female) were therefore computed.
Overall, when compared with normal, reference subjects, subjects with DS had a significantly smaller external nasal surface area, coupled with significantly smaller length of the nasal bridge (n-prn), height of the nose (n-sn), and nasal tip protrusion (prn-[alr-all]). Alar base width (ac-ac) was smaller, with smaller nostrils (stn-itn), but nasal width (al-al) and the inferior width of the nostrils (itnr-itnl) were increased (different inclination of nostril axes). The width-to-length ratio was increased, with larger angles of alar slope and nasal tip (more flat) than in the reference subjects. Nasal prominence was reduced, with a flatter nasolabial fold (angle prn-sn-ls).
In subjects with DS, lip volume was reduced, but total upper and lower lip vermilion areas were increased. Cutaneous lip heights were reduced, but with increments in the vermilion heights of the upper (ls-sto), lower (sto-li), and total (ls-li) lip. Mouth (ch-ch) and philtrum (cph-cph) widths were reduced. No differences in interlabial angle were noted (sn-ls)-(li-sl), and the labiomental fold was unchanged (angle li-sl-pg).
DISCUSSION
In the present investigation, quantitative data on the nasolabial area (volume, area, linear dimensions, angles) of a group of Sudanese subjects with DS were compared with data collected in normal individuals matched for age, sex, and ethnic group. Data were obtained using a hand-held portable laser scanner, which allowed fast detection of coordinates of the landmarks of interest.21,22 Dedicated computer programs computed the conventional 3D linear distances and angles and were used for the assessment of more complex values (nose and lip areas and volume).5,7
Overall, the nose was significantly smaller (volume, area) in the analyzed subjects with DS than in the reference subjects, and it had a different shape, thus substantiating previous investigations performed in White subjects with DS.5,7,15–19 The vertical (length of the nasal bridge, height of the nose) and anteroposterior (nasal tip protrusion, width of the nostrils) dimensions were reduced, while the horizontal dimensions (alar base width, superior and inferior widths of the nostrils) were increased, as reported by Ferrario et al.,5 for White subjects with DS. The increment in alar base width was not associated with modifications in nasal width (al-al), as previously reported.5,15 The reduction in nose area and height and the decrement in nasal tip protrusion appear in good accord with literature findings.5,15,17
The different modifications in the spatial dimensions of the nose were paralleled by alterations in the measured nasal angles: the angle of the alar slope was significantly more flat, in accord with Ferrario et al.,5 and the angle of the nasal tip was more acute—a finding that contrasts with data reported for White subjects with DS.
In the analyzed subjects with DS, the mouth was reduced in width.5,15,19 In the vertical direction, the upper and lower lips had increased vermilion areas and heights. In contrast, the cutaneous part of the upper lip (sn-ls) was significantly reduced in height, with a subsequent reduction in lip volume.
The upper lip length was reported by Quintanilla et al.3 to be within normal ranges, but Ferrario et al.5 found a reduced dimension in DS subjects. Quintanilla et al.3 found an additional protrusion of the lower lip, determined by a proinclination of the lower incisors. Overall, the current modifications in upper and lower lip dimensions were only partially in accord with previous data reported in White subjects with DS. The principal difference among the groups is race/ethnicity, but age range (4–34 y in the current study; 12–45 y in the study by Ferrario et al.5; and 7–18 y in the study by Quintanilla et al.3), method of measurement (three-dimensional assessment vs two-dimensional projection on the midsagittal plane), and type of dental support all may play a role in the different findings.
Indeed, the wide age range in the current investigation should be acknowledged as a potential limit. Unfortunately, the reduced sample size did not allow us to group patients into preadolescent, adolescent, and postadolescent groups, and additional assessments should be made regarding the potential effect of age on facial discrepancies in subjects with DS.
In the present study, only one significant difference was found between z-scores computed in male and female subjects, revealing similar behavior in the two sexes. The effects of sex are difficult to compare: Farkas et al.16–19 reported only pooled values, and Bagic and Verzak15 and Ferrario et al.5 found no significant sex-related differences in the morphology of the nasiolabial region of White subjects with DS.
Most of the differences found in the nose were similar to those previously found in White subjects with DS, but variations in the lips and mouth were less marked in North Sudanese than in White subjects with DS. Therefore, prenatal observations of variation in the incidence of nasal hypoplasia found in the different ethnicities13 seem to be partially confirmed by ethnic differences even after birth.
Considering the increasing number of subjects with DS living in the community,2,4 and the multiple ethnicities living side by side in contemporary society, assessment of the characteristics of persons with DS of various ethnic origins may be of help to clinicians and basic researchers all over the world.
CONCLUSIONS
North Sudanese subjects with DS had a hypoplastic nose, with horizontal dimensions prevalent over vertical and anteroposterior ones.
North Sudanese subjects with DS had reduced total cutaneous lip volume and dimensions.
North Sudanese subjects with DS had increased lip vermilion area and relevant dimensions.
REFERENCES
- 1.OMIM Online Mendelian Inheritance in Man. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM Accessed February 20, 2010. [Google Scholar]
- 2.Desai S. S. Down syndrome: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:279–285. doi: 10.1016/s1079-2104(97)90343-7. [DOI] [PubMed] [Google Scholar]
- 3.Quintanilla J. S, Biedma B. M, Rodriguez M. Q, Mora M. T, Cunqueiro M. M, Pazos M. A. Cephalometrics in children with Down's syndrome. Pediatr Radiol. 2002;32:635–643. doi: 10.1007/s00247-002-0703-x. [DOI] [PubMed] [Google Scholar]
- 4.Roizen N. J, Patterson D. Down's syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- 5.Ferrario V. F, Dellavia C, Colombo A, Sforza C. Three-dimensional assessment of nose and lip morphology in subjects with Down syndrome. Ann Plast Surg. 2004;53:577–583. doi: 10.1097/01.sap.0000130702.51499.6b. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario V. F, Dellavia C, Zanotti G, Sforza C. Soft tissue facial anthropometry in Down syndrome subjects. J Craniofac Surg. 2004;15:528–532. doi: 10.1097/00001665-200405000-00037. [DOI] [PubMed] [Google Scholar]
- 7.Ferrario V. F, Dellavia C, Serrao G, Sforza C. Soft tissue facial angles in Down's syndrome subjects: a three-dimensional non-invasive study. Eur J Orthod. 2005;27:355–362. doi: 10.1093/ejo/cji017. [DOI] [PubMed] [Google Scholar]
- 8.Sforza C, Dellavia C, Zanotti G, Tartaglia G. M, Ferrario V. F. Soft tissue facial areas and volumes in subjects with Down syndrome. Am J Med Genet. 2004;130A:234–239. doi: 10.1002/ajmg.a.30253. [DOI] [PubMed] [Google Scholar]
- 9.Sforza C, Dellavia C, Dolci C, Donetti E, Ferrario V. F. A quantitative three-dimensional assessment of abnormal variations in the facial soft tissues of individuals with Down syndrome. Cleft Palate Craniofac J. 2005;42:410–416. doi: 10.1597/04-005.1. [DOI] [PubMed] [Google Scholar]
- 10.Sforza C, Dellavia C, Tartaglia G. M, Ferrario V. F. Morphometry of the ear in Down's syndrome subjects: a three-dimensional computerized assessment. Int J Oral Maxillofac Surg. 2005;34:480–486. doi: 10.1016/j.ijom.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Borenstein M, Persico N, Kagan K. O, Gazzoni A, Nicolaides K. H. Frontomaxillary facial angle in screening for trisomy 21 at 11 + 0 to 13 + 6 weeks. Ultrasound Obstet Gynecol. 2008;32:5–11. doi: 10.1002/uog.5334. [DOI] [PubMed] [Google Scholar]
- 12.Cicero S, Longo D, Rembouskos G, Sacchini C, Nicolaides K. H. Absent nasal bone at 11–14 weeks of gestation and chromosomal defects. Ultrasound Obstet Gynecol. 2003;22:31–35. doi: 10.1002/uog.170. [DOI] [PubMed] [Google Scholar]
- 13.Prefumo F, Sairam S, Bhide A, Penna L, Hollis B, Thilaganathan B. Maternal ethnic origin and fetal nasal bones at 11–14 weeks of gestation. BJOG. 2004;111:109–112. doi: 10.1046/j.1471-0528.2003.00025.x-i1. [DOI] [PubMed] [Google Scholar]
- 14.Sonek J. D. Nasal bone evaluation with ultrasonography: a marker for fetal aneuploidy. Ultrasound Obstet Gynecol. 2003;22:11–15. doi: 10.1002/uog.182. [DOI] [PubMed] [Google Scholar]
- 15.Bagic I, Verzak Z. Craniofacial anthropometric analysis in Down's syndrome patients. Coll Antropol. 2003;27:23–30. [PubMed] [Google Scholar]
- 16.Farkas L. G, Katic M. J, Forrest C. R. Surface anatomy of the face in Down's syndrome: anthropometric proportion indices in the craniofacial regions. J Craniofac Surg. 2001;12:519–526. doi: 10.1097/00001665-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Farkas L. G, Katic M. J, Forrest C. R, Litsas L. Surface anatomy of the face in Down's syndrome: linear and angular measurements in the craniofacial regions. J Craniofac Surg. 2001;12:373–379. doi: 10.1097/00001665-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Farkas L. G, Katic M. J, Forrest C. R. Age-related changes in anthropometric measurements in the craniofacial regions and in height in Down's syndrome. J Craniofac Surg. 2002;13:614–622. doi: 10.1097/00001665-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Farkas L. G, Katic M. J, Forrest C. R. Surface anatomy of the face in Down's syndrome: age-related changes of anthropometric proportion indices in the craniofacial regions. J Craniofac Surg. 2002;13:368–374. doi: 10.1097/00001665-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Shaner D. J, Peterson A. E, Beattie O. B, Bamforth J. S. Assessment of soft tissue facial asymmetry in medically normal and syndrome-affected individuals by analysis of landmarks and measurements. Am J Med Genet. 2000;93:143–154. doi: 10.1002/1096-8628(20000717)93:2<143::aid-ajmg12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Aung S. C, Ngim R. C, Lee S. T. Evaluation of the laser scanner as a surface measuring tool and its accuracy compared with direct facial anthropometric measurements. Br J Plast Surg. 1995;48:551–558. doi: 10.1016/0007-1226(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy R. J, Kinsella A, Waddington J. L. 3D laser surface scanning and geometric morphometric analysis of craniofacial shape as an index of cerebro-craniofacial morphogenesis: initial application to sexual dimorphism. Biol Psychiatry. 2002;51:507–514. doi: 10.1016/s0006-3223(01)01327-0. [DOI] [PubMed] [Google Scholar]
- 23.Seager D. C, Kau C. H, English J. D, Tawfik W, Bussa H. I, Ahmed A. Y. M. Facial morphologies of an adult Egyptian population and an adult Houstonian White Population compared using 3D imaging. Angle Orthod. 2009;79:991–999. doi: 10.2319/111408-579.1. [DOI] [PubMed] [Google Scholar]
- 24.Sforza C, Ferrario V. F. Soft-tissue facial anthropometry in three dimensions: from anatomical landmarks to digital morphology in research, clinics and forensic anthropology. J Anthropol Sci. 2006;84:97–124. [Google Scholar]
- 25.Bergann A, Bamberg C, Eder K, Proquitté H, Hartung J. P, Bollmann R, Kalache K. D. Mid-facial anthropometry in second-trimester fetuses with trisomy 21: a three-dimensional ultrasound study. Prenat Diagn. 2006;26:158–162. doi: 10.1002/pd.1362. [DOI] [PubMed] [Google Scholar]
- 26.Tuxen A, Keeling J. W, Reintoft I, Fischer Hansen B, Nolting D, Kjaer I. A histological and radiological investigation of the nasal bone in fetuses with Down syndrome. Ultrasound Obstet Gynecol. 2003;22:22–26. doi: 10.1002/uog.152. [DOI] [PubMed] [Google Scholar]