Abstract
Objectives:
To histologically and immunohistochemically assess the pattern of expression of bone morphogenic proteins 2 and 4 (BMP2/4) and proliferating cell nuclear antigen (PCNA) in response to bite jumping appliances in the condylar cartilage and the glenoid fossa.
Materials and Methods:
Fifty-five 4-week-old female Sprague-Dawley rats were randomly divided into four experimental and four control groups. Bite-jumping appliances were fitted to the experimental animals. The rats were sacrificed at 3, 14, 21, and 30 days, and the temporomandibular structures were analyzed histologically and immunohistochemically.
Results:
The expression of BMP2/4 in response to bite-jumping appliances was statistically significant in the condylar cartilage and the glenoid fossa. Cell proliferation was not significant.
Conclusion:
BMP2/4 plays an important role in bone formation in response to mandibular advancement by accelerating and enhancing the differentiation of mesenchymal cells into bone-forming cells.
Keywords: Temporomandibular joint, BMP2/4, PCNA, Mandibular advancement
INTRODUCTION
The major aim of dentofacial orthopedic treatment in Class II individuals with mandibular retrognathia (approximately 70%) is to enhance or optimize the growth of the condyle by functional anterior displacement of the mandible.1,2 The use of functional appliances in adolescents and young adults increases mandibular prognathism, seemingly as a result of changing the biophysical environment of the temporomandibular joint (TMJ) that leads to condylar adaptation3; enhances growth of the condyle4; and initiates remodeling of the glenoid fossa,5–7 thereby reducing the skeletal and soft tissue facial profile convexity.6
Yet to date, despite numerous studies, it has not been established whether treatment success results from therapeutic modification of skeletal growth, genetically determined growth itself, or periodontal and dental reactions, that is, dentoalveolar adaptation.8 The exact mechanism of such a control and whether functional mandibular displacement stimulates mesenchymal cell proliferation are still being debated.1 It is therefore important to understand the cellular response to mandibular advancement in the glenoid fossa and the condyles.
Proliferating cell nuclear antigen (PCNA) functions as a DNA sliding clamp for DNA polymerase delta and is an essential component for eukaryotic chromosomal DNA replication.9 PCNA has therefore been used as a marker for cell proliferation.10 PCNA localizes in the nuclei of chondroblasts of the reserve cell layer and the upper hypertrophic layer. In condylar cartilage, the percentage of PCNA-positive cells is significantly higher when there is an increase in chondrocyte mitosis.11
Bone morphogenic proteins (BMPs) are ubiquitous multifunctional proteins that have widespread effects on cell growth and differentiation in many organ systems.12 BMPs induce not only chondrogenic differentiation but also hypertrophy and mineralization. BMPs also increase Cbfa1/Runx2 expression and stimulate differentiation (transcription factors required for osteogenic commitment and differentiation).13–16 BMPs are molecular cues for osteoprogenitor cells to differentiate into osteoblasts.17,18
Therefore, it is reasonable to speculate that, because of their unique properties, BMPs play a fundamental role in condylar remodeling in response to bite-jumping appliances. This study attempts to clarify the role of mesenchymal cell proliferation in the condylar cartilage and the glenoid fossa and its response to growth modification as well as the role of BMP2/4 in the cellular response to bite-jumping appliance.
MATERIALS AND METHODS
Experimental Model
Fifty-five 3-week-old female Sprague-Dawley rats were selected for this experiment. After an acclimatization period of 1 week, at 4 weeks old the rats were randomly allocated into four experimental groups (n = 35) and four control groups (n = 20) (Table 1). All rats were kept in an environment well controlled for temperature and humidity and given free access to food (ground pellets) and water. The study was approved by the Westmead Animal Ethical Committee (Protocol No. 4113.06-08).
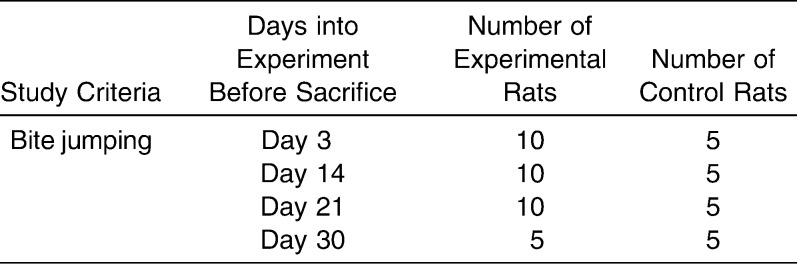
Table 1.
Allocation of Rats into the Four Groups
Bite-jumping appliances were bonded to the lower incisors of the experimental animals in an identical inclination plane causing a fixed magnitude of downward and forward positioning of the mandible. The appliances were worn 24 hours a day; hence, a continuous mandibular protrusion was exhibited (Figure 1). The appliances were fitted under anesthesia. The control animals were monitored under natural growth.
Figure 1.
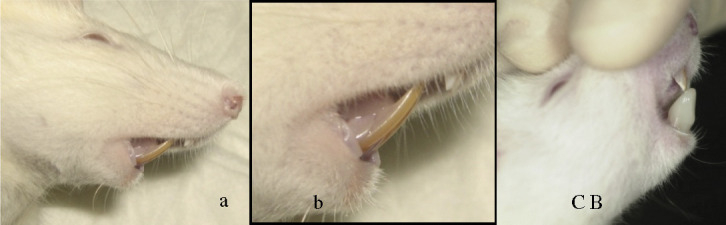
Incisal relations. (a) Normal incisal relation of the rats. (b) Normal incisal relation of the rats. (c) Incisal relation after placement of the bite-jumping appliance.
Histologic Tissue Preparation
The rats in each experimental group and matched control group were sacrificed on days 3, 14, 21, and 30 after placement of the appliance (Table 1). The rats were euthanized by carbon dioxide gas (Aligal 2, Air Liquid, Sydney, Australia). Immediately after death, the heads were fixed in 4% paraformaldehyde for 24 hours and decalcified in 20% ethylenediamine tetra-acetic acid (pH 7.38) at 4°C for 4–5 weeks. Next, the right TMJ was dissected, excess tissues were removed, and the specimens were dehydrated by passing through a series of ethanol and then embedded in paraffin in identical positions. The entire TMJ was then cut at the sagittal plane into serial sections of 5 µm using a rotary microtome (Leitz 1516, Leica Microsystems, Wetzlar, Germany) and floated onto poly-L-lysine coated glass slides and examined by histopathologic (hematoxylin and eosin) and immunohistochemical techniques.
Immunohistochemistry
Immunohistochemical examination was carried out on the condylar cartilage and the glenoid fossa using two primary antibodies: proliferating cell nuclear antigen (PCNA) primary antibody (Mouse PCNA Unconjugated Purified 200 µg, 13-3900, supplied by Invitrogen (Invitrogen Australia pty Ltd, Mulgrave, Victoria, Australia); PCNA: 1/100). Bone morphogenetic protein primary antibody (BMP 2/4 affinity purified goat antibody, GT 15053, supplied by Neuromics; Edina, MN); BMP2/4: 1/100). Immunoglobulin horseradish peroxidase in 10% fetal calf serum was used as a secondary antibody. 3′-diaminobenzidine (DAB) in chromogen solution (Dako Liquid DAB+ Substrate Chromogen System, Code K3467, Dako, Glostrup, Denmark) was applied for the observation of immunohistochemical activity. Finally, a light Meyer's hematoxylin counterstain was applied. Then clearing protocol were followed: sections were dehydrated down to histoclear, and the slides were covered by mounting medium (Fisher Scientific Permount, Fisher Scientific, Pittsburgh, PA) for mounting and long-term storage of slides, SO-P-15, 500 mL-1.1 pt., USA) and a cover slip. We included immunohistochemical staining using IgG2a in place of the primary antibody as a negative control for PCNA and rabbit biotin as a negative control for BMP2/4.
Quantitative Analysis
The expressions of PCNA and BMP2/4 were quantified by manually counting positively stained cells. This was conducted via a computer-assisted image analyzing system, using a Leica Digital Imaging Microscope (Leica application Suit Software). This system can acquire high-definition digital images from the specimens in 10×, 20×, and 60× magnification of the condylar cartilage and glenoid fossa. Measurements were conducted in the posterior region of the condyle and the glenoid fossa, The sections were evaluated at a total magnification of 20×, using the fixed measurement frame of middle quarter of the posterior condylar cartilage and the glenoid fossa.
Statistical Analysis
Statistical analysis was processed with SPSS for Windows (Version 16.0, SPSS Inc. Chicago, Ill) for analysis of variance. One month later, the data were collected again by the same observer to test the method of error. There was no significant difference among the registrations.
RESULTS
Mandibular condylar cartilage is histologically composed of four cell layers: (1) articular layer, (2) proliferative layer, (3) chondrogenic layer, and (4) hypertrophic layer.
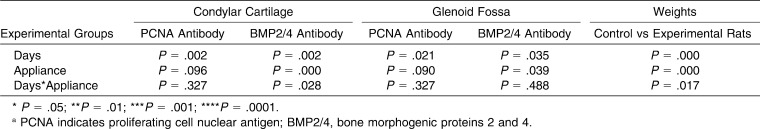
The results of the statistical analysis are summarized in (Table 2). Statistical analysis indicated that BMP2/4 expression was significant (P < .05) in the condylar cartilage (P = .000) and the glenoid fossa (P = .090), but PCNA expression was not significant for condylar cartilage (P = .096) and glenoid fossa (P = .090) in experimental groups compared with the control groups.
Table 2.
Results of Statistical Analysisa
Immunostaining in the Condyle
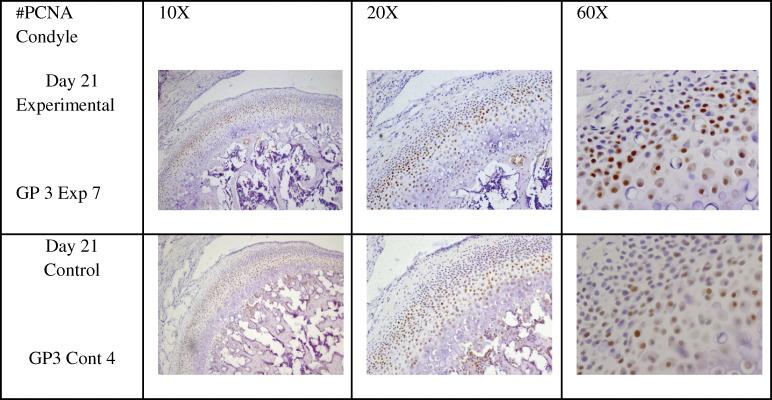
Immunostaining for PCNA was mainly expressed in the proliferative and chondrogenic layers (Figure 2). PCNA expression in the condyle of experimental animals was not statistically significant at P = .096.
Figure 2.
Proliferating cell nuclear antigen staining of the proliferative and chondrogenic layers of the mandibular condylar cartilage (arrow) in experimental and control Sprague-Dawley rats, showing the levels of staining at 10×, 20×, and 60×.
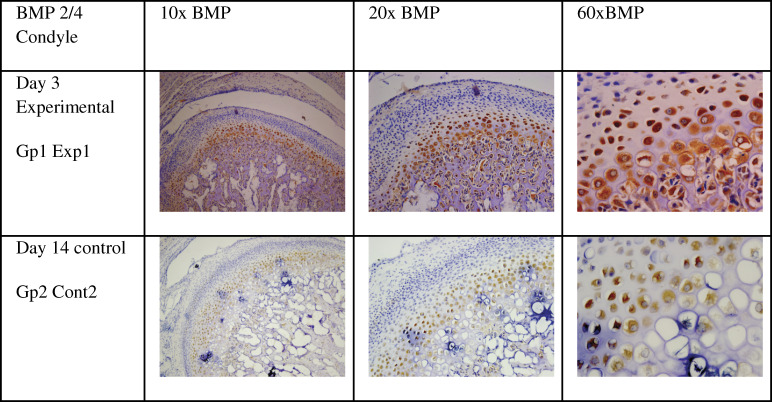
Although immunohistostaining for BMP2/4 was mainly expressed in the chondrogenic and hypertrophic layers (Figure 3) Overall the level of BMP2/4 expression was higher in experimental than in controls, this level was statistically significant with the P-value of (P = .000).
Figure 3.
Immunohistochemical stain of bone morphogenic proteins 2 and 4 in the condyle at 10×, 20×, and 60× (arrow) showing staining in the chondrogenic and hypertrophic layers.
The level of PCNA expression gradually increased from day 3 to a maximum on day 21, after which it declined on day 30 (Figure 4). BMP 2/4 reached maximum levels on day 3 and then gradually declined to a minimum on day 30 (Figure 5).
Figure 4.
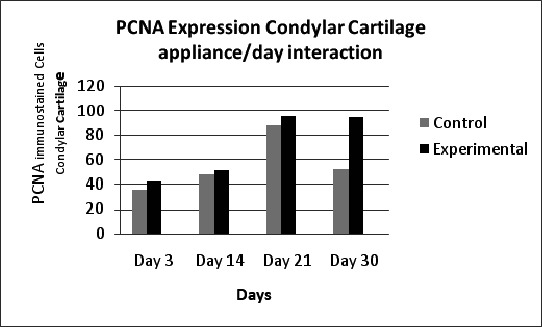
Proliferating cell nuclear antigen expression in condylar cartilage showing that expression gradually increased from day 3 to 21 and then declined sharply on day 30 in the control group; (0 = control rats), (1 = experimental rats).
Figure 5.
Bone morphogenic proteins 2 and 4 in condylar cartilage showing gradual decline in experimental groups.
Immunostaining in the Glenoid Fossa
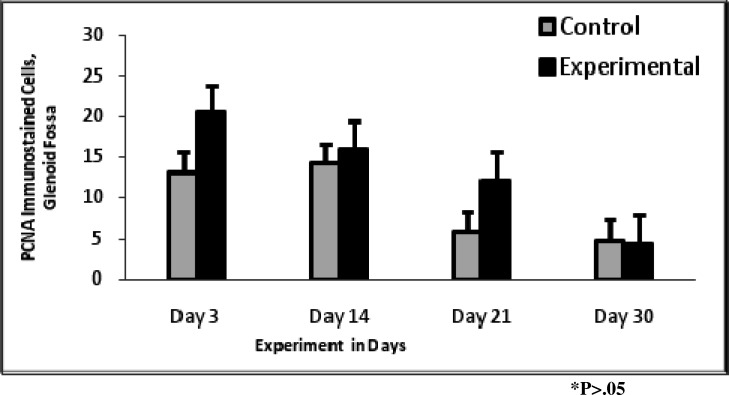
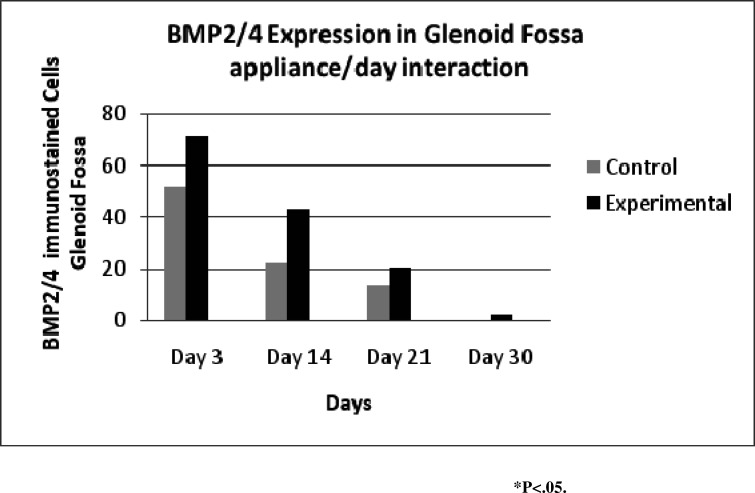
PCNA expression (Figure 6) gradually declined from day 3 to day 30, and experimental rats maintained a higher level of expression than control rats except on day 30 (Figure 7). The expression of PCNA was not significant overall for glenoid fossa (P = .090). The expression of BMP2/4 (Figure 8) was statistically significant for glenoid fossa (P = .039).
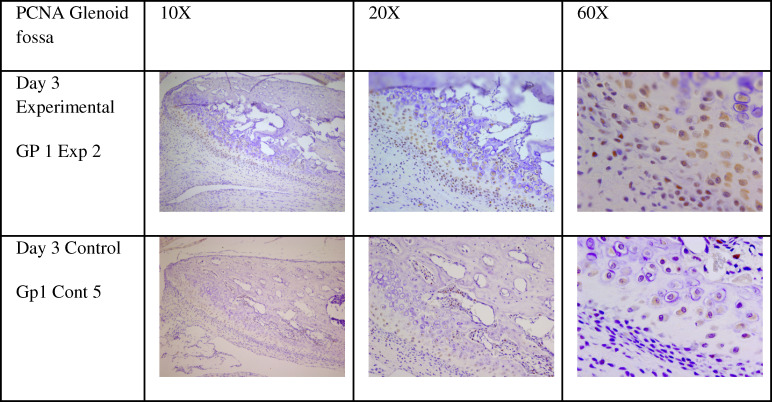
Figure 6.
Proliferating cell nuclear antigen expression in the glenoid fossa in experimental and control rats at 10× (left), 20× (center), and 60× (right).
Figure 7.
Proliferating cell nuclear antigen expression in the glenoid fossa; it generally declined from day 3 to day 30.
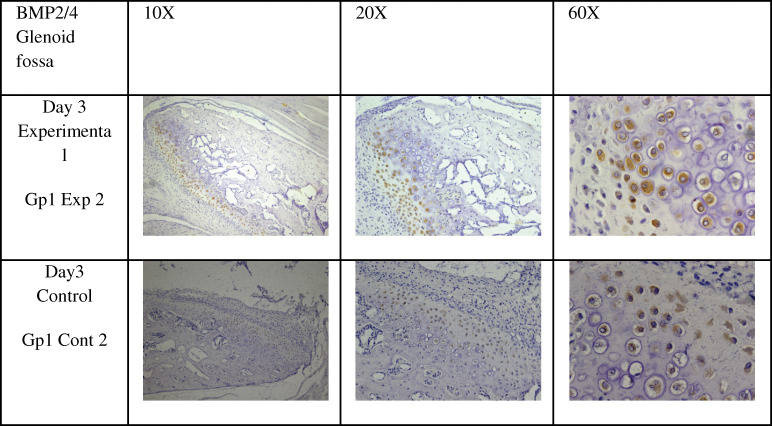
Figure 8.
Immunohistochemical stain of bone morphogenic proteins 2 and 4 in the glenoid fossa, at 10×, 20×, and 60×.
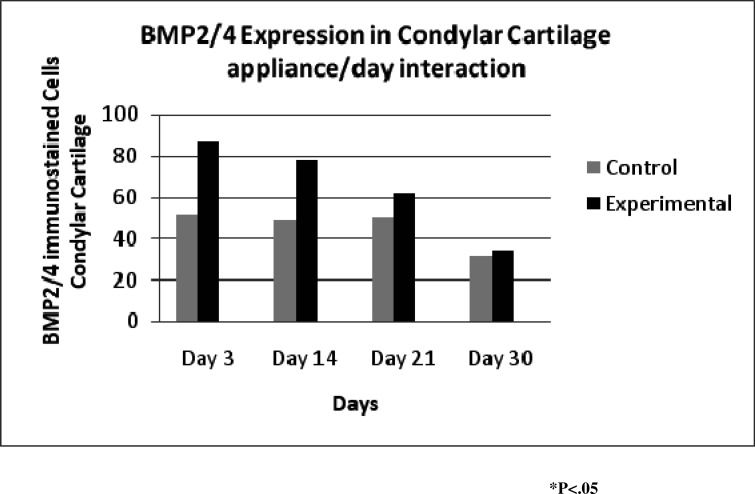
Day-appliance interaction (Figure 9) showed the maximum expression on day 3 and declined to a minimum on day 30. The experimental rats maintained a significantly higher level of expression than control rats on all days.
Figure 9.
Expression of bone morphogenic proteins 2 and 4 in the glenoid fossa showing that expression sharply declined from day 3 to day 30.
Weight Gain
Duration and appliance significantly decreased the body weight of experimental rats (P < .001; Table 2; Figure 10).
Figure 10.
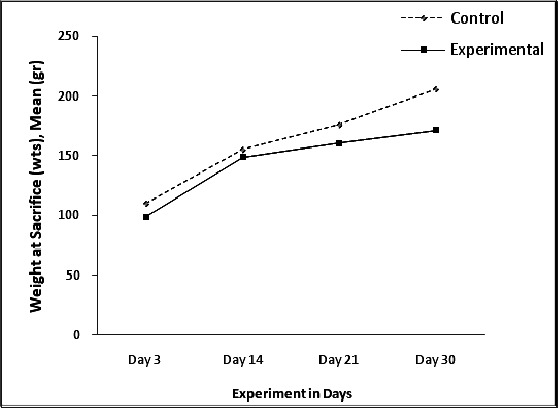
Weights increased over time; however, the experimental rats had lower weights generally.
DISCUSSION
It is difficult to elucidate the real effects of orthopedic therapy on condylar growth by deducting the attribution of condylar natural growth from the overall treatment changes, because these elements always overlap. This might be one of the reasons why the skeletal effects of bite-jumping therapy remain in dispute.19
Experiments conducted by Charlier and coworkers20 and Petrovic and coworkers21 at the University of Strasbourg showed an increase in the mitotic activity of mesenchymal cells in the condyle in response to mandibular advancement. Attempts to reproduce these results using biochemical, histomorphometric, and autoradiographic methods have been unsuccessful.22–24 A study of the mandibular condyle of Macaca mulatta25 reported significant broadening of cartilage zones in all experimental animals; however, there was no increase in cell proliferation compared with controls.
Other studies on rats found a broadened condylar cartilage zone, increased metabolic activity and proliferation in the condylar cartilage in response to lateral functional shift,26,27 and a significant increase in mesenchymal cell proliferation in both condylar cartilage and glenoid fossa in response to mandibular advancement.28
Several researchers have reported that functional appliance therapy accelerates growth, which is then followed by subnormal growth in the posterior regions of both the condyle and the glenoid fossa.29,30
Somatic growth puberty for Sprague–Dawley rats occurs between 35 and 56 days of age.31 The rats in the present study received bite-jumping appliances from 28 days of age to the final group at 58 days old, which is when these rats are expected to be experiencing their maximum growth spurt. The use of growing rats in which the mandibular is displaced forward permits the investigation of the adaptive remodeling process of the condylar cartilage.
Our study shows that throughout the experimental period, from the age of 28 days (day 0) to 58 days (day 30), mandibular advancement maintained a nonsignificant level of replicating mesenchymal cells in both the condyle (P = .096) and the glenoid fossa (P = .090) (Table 2).
Our results agree with those of others who also showed an insignificant increase in cellular proliferation in the condylar cartilage and the glenoid fossa.8,22–25,32
A most interesting finding in the present study is that although the number of replicating mesenchymal cells did not significantly increase in experimental rats, it did not decrease in response to bite-jumping appliances. Cellular proliferation is expected to increase in both groups as the animal grows, and that correspond with the somatic puberty growth period.
We also found that forward mandibular positioning caused a significant increase in cartilage and bone forming cells, in both the condyle and glenoid fossa. This was represented by a significant expression of BMP2/4 in the condylar cartilage (P = .002) and the glenoid fossa (P = .039).
Although many cytokines and growth factors are capable of regulating specific aspects of bone development and metabolism, to date BMPs are the only factors known to induce bone formation in vivo, among them BMP2 and BMP4.33–35
It has been shown that the BMPs are highly osteoinductive and are effective in promoting bone healing in animals. It has been suggested that BMP2 mediates the differentiation of mesenchymal cells into osteoblasts and chondroblasts.36,37
Studies on fracture healing found that the more primitive uncommitted cells have the highest concentration of staining and thus may be initially responsible for turning on BMP production.38 The more mature differentiated cells have significantly less BMP associated with them, and therefore BMP may play a less significant role in their functioning.38 This may explain why BMP 2/4 expression gradually declines in both the condyle and glenoid fossa.
It has been suggested that cellular growth curves show an initial period of rapid progenitor cell proliferation with numbers increasing at an exponential rate.39 Cowan and Morris40 have proposed that exponential growth lasts as long as the curve of log cell number plot against time becomes linear. However, as soon as the cells differentiate, according to Urist,41 differentiation curtails the population size of mesenchymal cells; as a consequence, proliferative activity slows as development continues.42 Our study found that mandibular advancement elicited an increase in bone formation in the condyle and the glenoid fossa. As more cells differentiate, this can affect the population size of mesenchymal cells. This may explain the insignificant increase in mesenchymal cell proliferation in experimental vs control rats.
CONCLUSIONS
In response to bite-jumping appliances, BMP2/4 may initiate the cascade of cellular events that eventually lead to bone formation by accelerating and enhancing the differentiation of mesenchymal cells into bone-forming cells.
The statement that the number of replicating mesenchymal cells influences the growth potential of the condyle and the glenoid fossa is still debatable.
REFERENCES
- 1.Meikle M. C. Remodeling the dentofacial skeleton: the biological basis of orthodontics and dentofacial orthopedics. J Dent Res. 2007;86(1):12–24. doi: 10.1177/154405910708600103. [DOI] [PubMed] [Google Scholar]
- 2.Proffit W. R. Malocclusion and dentofacial deformity in contemporary society. In: Proffit W. R, Fields H. W, editors. Contemporary Orthodontics 3rd ed. St Louis, MO: CV Mosby; 2000. pp. 1–22. [Google Scholar]
- 3.Rabie A, Xiong H, Hägg U. Forward mandibular positioning enhances condylar adaptation in adult rats. Eur J Orthod. 2004;26:353–358. doi: 10.1093/ejo/26.4.353. [DOI] [PubMed] [Google Scholar]
- 4.Hagg U, Du X, Rabie A. Initial and late treatment effects of headgear-Herbst appliance with mandibular step-by-step advancement. Am J Orthod Dentofacial Orthop. 2002;122:477–485. doi: 10.1067/mod.2002.128218. [DOI] [PubMed] [Google Scholar]
- 5.Enlow D. H, Hans M. G. Essentials of Facial Growth. Philadelphia, PA: WB Saunders; 1996. [Google Scholar]
- 6.Ruf S, Pancherz H. Dentoskeletal effects and facial profile changes in young adults treated with the Herbst appliance. Angle Orthod. 1999;69:239–246. doi: 10.1043/0003-3219(1999)069<0239:DEAFPC>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Shum L, Rabie A, Hägg U. Vascular endothelial growth factor expression and bone formation in posterior glenoid fossa during stepwise mandibular advancement. Am J Orthod Dentofacial Orthop. 2004;125:185–190. doi: 10.1016/j.ajodo.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Proff P, Gedrange T, Franke R, et al. Histological and histomorphometric investigation of the condylar cartilage of juvenile pigs after anterior mandibular displacement. Ann Anat. 2007;189:269–275. doi: 10.1016/j.aanat.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Paunesku T, Mittal S, Protic M, et al. Proliferating cell nuclear antigen (PCNA): ringmaster of the genome. Int J Radiat Biol. 2001;77:1007–1021. doi: 10.1080/09553000110069335. [DOI] [PubMed] [Google Scholar]
- 10.Tsurimoto T. PCNA binding proteins. Front Biosci. 1999;4:d849–d858. doi: 10.2741/tsurimoto. [DOI] [PubMed] [Google Scholar]
- 11.Sharawy M. M, Ali A. M, Choi W. S. Immunohistochemical localization and distribution of proliferating cell nuclear antigen in the rabbit mandibular condyle following experimental induction of anterior disk displacement. Cranio. 2002;20:111–115. doi: 10.1080/08869634.2002.11746199. [DOI] [PubMed] [Google Scholar]
- 12.Croteau S, Rauch F, Silvestri A, Hamdy R. C. Bone morphogenic proteins in orthopaedics; from basic science to clinical practice. Orthopaedics. 1999;22:686–695. [PubMed] [Google Scholar]
- 13.Kramer J, Hegert C, Guan K, et al. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 14.Worster A. A, Brower-Toland B. D, Fortier L. A, Bent S. J, Williams J, Nixon A. J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 15.Fukada K, Shibata S, Suzuki S, Ohya K. In situ hybridization study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J Anat. 1999;195:321–329. doi: 10.1046/j.1469-7580.1999.19530321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mundlos S, Zabel B. Developmental expression of human cartilage matrix protein. Dev Dynamics. 1994;199:241–252. doi: 10.1002/aja.1001990308. [DOI] [PubMed] [Google Scholar]
- 17.Reddi A. H. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994;4:737–744. doi: 10.1016/0959-437x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 18.Wozney J. M. Bone morphogenetic proteins and their expressions. In: Noda N, editor. Cellular and Molecular Biology of Bone. San Francisco, CA: Academic Press; 1993. pp. 131–165. [Google Scholar]
- 19.Bendeus M, Hägg U, Rabie B. Growth and treatment changes in patients treated with a headgear-activator appliance. Am J Orthod Dentofacial Orthop. 2002;121:376–384. doi: 10.1067/mod.2002.122177. [DOI] [PubMed] [Google Scholar]
- 20.Charlier J. P, Petrovic A, Herman-Stutzmann J. J. The effect of mandibular hyperpropulsion on the prechondroblastic zone of the young rat condyle. Am J Orthod. 1969;55:71–74. doi: 10.1016/s0002-9416(69)90174-2. [DOI] [PubMed] [Google Scholar]
- 21.Petrovic A. G, Stutzmann J, Oudet C. L. Control processes in the postnatal growth of the condylar cartilage of the mandible. In: McNamara J. A Jr, editor. Determinants of Mandibular Form and Growth. Ann Arbor: University of Michigan; 1975. pp. 101–154. [Google Scholar]
- 22.Tonge E, Heath J. K, Meikle M. C. Anterior mandibular displacement and condylar growth; an experimental study in the rat. Am J Orthod. 1982;82:277–287. doi: 10.1016/0002-9416(82)90462-6. [DOI] [PubMed] [Google Scholar]
- 23.Ghafari J, Degroote C. Condylar cartilage response to continuous mandibular displacement in the rat. Angle Orthod. 1986;56:49–57. doi: 10.1043/0003-3219(1986)056<0049:CCRTCM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Tewson D. H, Heath J. K, Meikle M. C. Biochemical and autoradiographical evidence that anterior mandibular displacement in the young growing rats does not stimulate cell proliferation or matrix formation at the mandibular condyle. Arch Oral Biol. 1988;33:99–107. doi: 10.1016/0003-9969(88)90052-0. [DOI] [PubMed] [Google Scholar]
- 25.McNamara J. A, Jr, Hinton R. J, Hoffman D. L. Histologic analysis of temporo-mandibular joint adaptation to protrusive function in young adult rhesus monkeys. Am J Orthod. 1982;82:288–298. doi: 10.1016/0002-9416(82)90463-8. [DOI] [PubMed] [Google Scholar]
- 26.Fuentes M. A, Opperman L. A, Buschang P, et al. Lateral functional shift of the mandible: Part II. Effects on gene expression in condylar cartilage. Am J Orthod Dentofacial Orthop. 2003b;123:160–166. doi: 10.1067/mod.2003.6. [DOI] [PubMed] [Google Scholar]
- 27.Fuentes M. A, Opperman L. A, Buschang P. H, et al. Lateral functional shift of the mandible: Part I. Effects on condylar cartilage thickness and proliferation. Am J Orthod Dentofacial Orthop. 2003a;123:153–159. doi: 10.1067/mod.2003.5. [DOI] [PubMed] [Google Scholar]
- 28.Rabie A. B. M, Wong L, Tsai M. Replicating mesenchymal cells in the condyle and the glenoid fossa during mandibular forward positioning. Am J Orthod Dentofacial Orthop. 2003b;123:49–57. doi: 10.1067/mod.2003.46. [DOI] [PubMed] [Google Scholar]
- 29.Johnston L. E., Jr Functional appliances: a mortgage on mandibular position. Aust Orthod J. 1996;14:154–157. [PubMed] [Google Scholar]
- 30.McNamara J. A, Jr, Bryan F. A. Long-term mandibular adaptations to protrusive function: an experiment study in Macaca mulatta. Am J Orthod Dentofacial Orthop. 1987;92:98–108. doi: 10.1016/0889-5406(87)90364-7. [DOI] [PubMed] [Google Scholar]
- 31.Lane-Petter W. The UFAW Hand Book on the Care and Management of Laboratory Animals. Edinburgh: Churchill Livingstone; 1976. The laboratory rat; pp. 210–217. [Google Scholar]
- 32.Degroote C. W. Alterability of Mandibular Condylar Growth in the Young Rat and its Implications [dissertation] Leuven, Belgium: Katholieke Universiteit; 1984. [Google Scholar]
- 33.Wang E. A, Rosen V, D'Alessandro J. S, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wozney J. M, Rosen V, Celeste A. J, Mitsock L. M, Whitters M. J, Kriz R. W, Hewick R. M. Wang EA Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 35.Wozney J. M. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1:267–280. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 36.Si X, Jin Y, Yang L, Tipoe G. L, White F. H. Expression of BMP-2 and TGF-B1 mRNA during healing of the rabbit mandible. Eur J Oral Sci. 1997;105:325–330. doi: 10.1111/j.1600-0722.1997.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 37.Marukawa K, Ueki K, Alam S, Shimada M, Nakagawa K, Yamamoto E. Expression of bone morphogenetic protein-2 and proliferating cell nuclear antigen during distraction osteogenesis in the mandible in rabbits. Br J Oral Maxillofac Surg. 2006;44:141–145. doi: 10.1016/j.bjoms.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Bostrom M. P, Lane J. M, Berberian W. S, et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13:357–367. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- 39.Rabie A, Bakr M, Zhao Z, et al. Osteogenesis in the glenoid fossa in response to mandibular advancement. Am J Orthod Dentofacial Orthop. 2001;119:390–400. doi: 10.1067/mod.2001.112875. [DOI] [PubMed] [Google Scholar]
- 40.Cowan R, Morris V. B. Cell population dynamics during the differentiation phase of tissue development. J Theor Biol. 1986;122:205–244. doi: 10.1016/s0022-5193(86)80082-0. [DOI] [PubMed] [Google Scholar]
- 41.Urist M. R. Bone morphogenetic proteins. Part 2. In: Rabie A. M. B, Urist M. R, editors. Bone Formation and Repair. Amsterdam, Netherlands: Elsevier; 1997. pp. 23–33. [Google Scholar]
- 42.Bruder S. P, Fink D. J, Caplan A. I. Mesenchymal stem cells in bone development, bone repair and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]