Abstract
Two β-lactamase gene regions were characterized by DNA sequencing in eight clinical isolates of Klebsiella oxytoca. The blaOXY-2a region encoded a β-lactamase nearly identical to OXY-2 (one amino acid residue substituted) and conferred aztreonam and cefuroxime resistance on the K. oxytoca isolates. Overproduction of OXY-2a was caused by a G-to-A substitution of the fifth nucleotide in the −10 consensus sequence of blaOXY-2a. The blaOXY-1a was identified in a susceptible strain, and the OXY-1a enzyme differed from OXY-1 by two amino acid residues.
Since the early 1980s, isolates of Klebsiella oxytoca have been recognized as clinically significant and an indication for therapy (17). Clinical isolates of Klebsiella spp. resistant to cefotaxime, ceftazidime, or aztreonam have been increasingly reported (6, 12, 14). These bacteria can develop resistance to the newer cephalosporins and aztreonam by acquisition of plasmid-located extended-spectrum β-lactamases (ESBLs) (7, 18, 25) or by mutations giving rise to the hyperproduction of chromosomal β-lactamases (15). In wild-type K. oxytoca, chromosomal β-lactamases are constitutively produced at low levels which are sufficient to affect the organism’s susceptibility to ampicillin, amoxicillin, carbenicillin, and ticarcillin (17). Overproduction of these β-lactamases, however, confers resistance to penicillins, cephalosporins, and aztreonam (8); in most cases this overproduction results from a mutation in the promoter region of the β-lactamase gene (9, 11). Molecular cloning and DNA sequencing have shown that the chromosomal β-lactamase genes in K. oxytoca can be divided into two main groups: blaOXY-1 and blaOXY-2 (11). These two categories of β-lactamase genes show 89.7% homology in DNA sequences and belong to functional group 2be of the ESBLs (4) and Ambler class A (1).
In previous studies (27, 28), 11 clinical isolates of K. oxytoca were found to be resistant to aztreonam and cefuroxime (MIC of >16 μg/ml) but susceptible to cefotaxime, ceftazidime, and imipenem (MICs of <4 μg/ml). The bacteria isolated in these previous studies belonged to three subgroups based on their plasmid profiles (27, 28). By isoelectric focusing, a single, common β-lactamase with a pI of 5.25, designated KH, was observed in the strains. The resistance could not be transferred to Escherichia coli XAC and C600 by transformation and conjugation, suggesting a chromosomal location for the β-lactamase gene. Furthermore, the substrate profile of the KH β-lactamase exhibited the hydrolysis of aztreonam characteristic of the enzymes of the K. oxytoca OXY family (10, 28). The aim of the present study was to characterize the genetic determinant for the ESBL produced by the K. oxytoca isolates recovered from hospitalized patients in the Stockholm area.
Seven of the previously reported cefuroxime- and aztreonam-resistant K. oxytoca isolates (27, 28) were further investigated in the present study. One susceptible strain of K. oxytoca isolated during the same period from a patient in Karolinska Hospital was also included. E. coli DH5α was the host for the cloning experiment. Plasmid pACYC177 was used to construct cloning vector pLSK (low copy number, single cloning site, and kanamycin resistance) (Table 1).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | recA endA1 gyrA96 thi-1 hsdR17 supE44 relA1 φ80 dlac ZΔM15 | Bethesda Research Laboratories |
| K. oxytoca | ||
| KH11 | Azr Xmr, clinical isolate | 27, 28 |
| KH26 | Azr Xmr, clinical isolate | 27, 28 |
| KH55 | Azr Xmr, clinical isolate | 27, 28 |
| KH78 | Azr Xmr, clinical isolate | 27, 28 |
| KH93 | Azr Xmr, clinical isolate | 27, 28 |
| KH103 | Azr Xmr, clinical isolate | 27, 28 |
| NBL63 | Azr Xmr, clinical isolate | 27, 28 |
| KH66 | Azs Xms, clinical isolate | This study |
| Plasmids | ||
| pACYC177 | Apr Kanr, cloning vector | 19 |
| pLSK | Kanr, PCR fragment of Kanr and Rep in pACYC177 | This study |
| pLSKSW-7 | pLSK, 1.06-kb PCR fragment from bla of KH11 | This study |
| pLSKSW-8 | pLSK, 1.06-kb PCR fragment from bla of KH66 | This study |
| pKH11 | Plasmid from K. oxytoca KH11 (subgroup 2) | 27, 28 |
| pKH78 | Plasmid from K. oxytoca KH78 (subgroup 1) | 27, 28 |
| pNBL63 | Plasmid from K. oxytoca NBL63 (subgroup 3) | 27, 28 |
Ap, ampicillin, Az, aztreonam; Kan, kanamycin; Xm, cefuroxime; Rep, replication origin; r, resistant; s, susceptible.
All standard nucleic acid techniques were carried out essentially as described by Ausubel et al. (2) and Sambrook et al. (20). Plasmid DNA was prepared by use of the Wizard Plus Minipreps or Midipreps DNA purification system (Promega, Madison, Wis.). Chromosomal DNA from the K. oxytoca isolates was prepared according to the procedure of Wilson (26). The quantity of recovered DNA was measured with a GeneQuant RNA-DNA calculator (Pharmacia Biotech, Ltd., Cambridge, England). Plasmid DNA was introduced into E. coli by transformation with CaCl2-treated E. coli DH5α, as recommended by Ausubel et al. (2). The DNA sequence was determined by the dideoxy-chain termination method (21) with an automated DNA-sequencing system (model 377; PE/ABI, Foster City, Calif.). Nucleotide and deduced amino acid sequences were analyzed with the GCG program (Genetics Computer Group, Madison, Wis.) and Lasergene software (DNASTAR, Madison, Wis.).
Primer oligonucleotides are shown in Table 2. To detect the blaOXY-1 gene, primers C and D were used to generate a 668-bp amplicon. Primers L and M were employed to detect blaOXY-2, producing a 723-bp PCR fragment. Primers KHUBI and KHDBI were designed based on blaOXY-2 (11) and blaRBI (13) sequences, and these primers were used to amplify the region covering the complete KH β-lactamase gene for cloning. Primers CYC7BI1 and CYC7BI2 were designed and employed to construct plasmid pLSK from plasmid pACYC177 (19). The 5′ nucleotides of the primers KHUBI, KHDBI, CYC7BI1, and CYC7BI2 were modified to create a BamHI restriction site (Table 2) in order to ligate the PCR products. The PCR was made with a PCR reagent kit according to the standard reaction recommended by the manufacturer (Perkin-Elmer Cetus, Branchburg, N.J.). Therefore, 100 ng of the chromosomal DNA from each of the K. oxytoca isolates and 40 pmol of each primer were included in a 100-μl reaction mixture. PCR amplification was performed in a DNA Thermal Cycler 480 (Perkin-Elmer Cetus) with the following temperature profiles: 94°C for 5 min; 30 cycles of 94°C for 1 min, 60 (for blaOXY-1) or 55°C (for blaOXY-2) for 1 min, 72°C for 1 min, and 72°C for 5 min; and holding at 4°C (16).
TABLE 2.
Oligonucleotides for detection and amplification of blaOXY regions
| Primer | Sequence | Positiona | Reference (accession no.) |
|---|---|---|---|
| C | 5′-GCGTAGCGCTGATTAACACG-3′ | 494–513 | 9 (Z30177) |
| D | 5′-CCTGCTGCGGCTGGGTAAAA-3′ | 1143–1162 (comp) | 9 (Z30177) |
| L | 5′-CAGATCTCGAGAAGCGTTCA-3′ | 421–440 | 11 (Z49084) |
| M | 5′-ACCTCTTTGCGGTTTTTCGC-3′ | 1125–1144 (comp) | 11 (Z49084) |
| KHUBI | 5′-CCAGGATCCTGGCGAGACACTATAAGC-3′b | 174–193 | 11 (Z49084) |
| KHDBI | 5′-CCTGGATCCAGAGTGCAGAGTGTTGCA-3′ | 994–1012 (comp) | 13 (D84548) |
| CYC7BI1 | 5′-CAAGGATCCAGGTGAAGATCCT-3′ | 680–701 | 19 (X06402) |
| CYC7BI2 | 5′-TCGGGATCCACCAGAATGACATC-3′ | 3133–3158 (comp) | 19 (X06402) |
Comp, complementary strand of DNA sequence.
Underlined sequences indicate BamHI restriction sites.
The bacterial strains were grown in Luria-Bertani medium (Difco Laboratories, Detroit, Mich.) with aeration at 37°C. Kanamycin (Sigma, St. Louis, Mo.) was added at 50 μg/ml for selection and maintenance of pLSK plasmids. MICs of cefuroxime, aztreonam, cefotaxime, ceftazidime, and imipenem for the K. oxytoca isolates and E. coli transformants were determined by a microdilution method (27).
To determine whether the gene for KH β-lactamase is located on a chromosome or a plasmid, one strain from each plasmid subgroup of the K. oxytoca isolates was studied. Chromosomal and plasmid DNA preparations from strains KH78 (group 1), KH11 (group 2), and NBL63 (group 3) were used as templates for PCR amplification. Under the recommended conditions, PCR products of 0.68 kb for blaOXY-1 (primer pair C-D) and 0.72 kb for blaOXY-2 (primer pair L-M) were obtained from the chromosomal DNA templates of strains KH11, KH78, and NBL63. However, no PCR product was obtained for blaOXY-1 (primer pair C-D) or for blaOXY-2 (primer pair L-M) when the plasmid DNA preparations of these strains were used as templates.
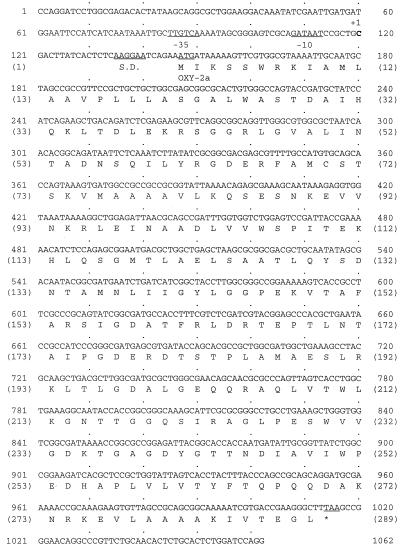
The region including the entire KH β-lactamase gene was amplified from the chromosomal DNA of the K. oxytoca isolates by using primers KHUBI and KHDBI. All PCR products from strains KH11, KH66, KH78, and NBL63 were sequenced. The 1,062-bp nucleotide sequence containing the β-lactamase gene from strain KH11 and the deduced amino acid sequence are shown in Fig. 1. The 867-bp nucleotide sequence (positions 147 to 1016) encoded a β-lactamase of 289 amino acid residues. The nucleotide sequence of this β-lactamase was nearly identical (99.8%) to that of the wild-type OXY-2 β-lactamase from K. oxytoca SL911 (11). The amino acid sequence of the β-lactamase was different from that of OXY-2 by only one residue; Ala-13 (ABL Ala-10) (1) was absent in the enzyme from strain KH11. On the basis of this high degree of similarity, KH β-lactamase was redesignated OXY-2a, and the KH determinant was redesignated blaOXY-2a. The blaOXY-2a gene was preceded by a promoter, in which TTGTCA and GATAAT were the −35 and −10 consensus sequences, respectively, and by a putative Shine-Dalgarno sequence (AAGGAA). The nucleotide and amino acid sequences of the β-lactamase genes and proteins of strains KH78 and NBL63 were identical to those of strain KH11. The β-lactamase from the susceptible K. oxytoca strain KH66 was highly similar to wild-type OXY-1 in amino acid sequence (99.3% identity), and the DNA equences encoding the two proteins were also very similar (98.9% identity) (9). The major difference between the proteins was that Leu-262 (ABL Leu-261) and Glu-278 (ABL Glu-277) (1) in OXY-1 were respectively substituted with Pro-262 (ABL 261) and Lys-278 (ABL 277) in KH66. Accordingly, the KH66 protein was designated OXY-1a.
FIG. 1.
Nucleotide sequence of the blaOXY-2a region of K. oxytoca KH11. Numbering starts at the 5′ end of primer KHUBI and ends at the complementary nucleotide of the 5′ end of primer KHDBI. The start codon and consensus sequences are underlined. The stop codon is designated with an asterisk. The deduced polypeptide sequence is indicated. S.D., Shine-Dalgarno site.
The promoter regions from each of the eight K. oxytoca isolates were sequenced. The promoters for the β-lactamase genes in all the isolates but KH66 had the same consensus sequence as that for blaOXY-2a in the strain KH11. Contrasting with the promoter sequences of wild-type blaOXY-2 (TTGTCA for −35 and GATAGT for −10), the blaOXY-2a promoters had a substitution (G→A) of the fifth base in the −10 consensus sequence. The promoter of blaOXY-1a in KH66 was identical to that of wild-type blaOXY-1 (9).
The DNA fragment containing the replication origin and kanamycin resistance gene in plasmid pACYC177 was PCR amplified by using the primers CYC7BI1 and CYC7BI2. After digestion with BamHI, the fragment was ligated with BamHI digests of PCR-generated blaOXY-2a and blaOXY-1a regions from Klebsiella strains KH11 and KH66 to form recombined plasmids pLSKSW-7 and pLSKSW-8, respectively. The two plasmids were then transformed into E. coli DH5α for the expression of resistance to β-lactam antibiotics. The E. coli (pLSKSW-7) transformant and K. oxytoca KH11 showed similar antibiograms which indicated resistances to aztreonam and cefuroxime and intermediate susceptibilities or susceptibilities to cefotaxime, ceftazidime, and imipenem (Table 3). The E. coli (pLSKSW-8) transformant and K. oxytoca KH66 similarly displayed no significant resistances to the β-lactam antibiotics tested (Table 3).
TABLE 3.
Antibiotic susceptibility of K. oxytoca strains and E. coli transformants
| Strain | MIC (μg/ml) ofa:
|
||||
|---|---|---|---|---|---|
| IPM | CAZ | ATM | CTX | CXM | |
| K. oxytoca | |||||
| KH11 | 0.5 | 1.0 | >64 | 2.0 | >64 |
| KH26 | 1.0 | 0.5 | >64 | 2.0 | >64 |
| KH55 | 1.0 | 1.0 | >64 | 2.0 | >64 |
| KH78 | 1.0 | 1.0 | >64 | 2.0 | >64 |
| KH93 | 1.0 | 1.0 | >64 | 4.0 | >64 |
| KH103 | 1.0 | 1.0 | >64 | 4.0 | >64 |
| NBL63 | 1.0 | 1.0 | >64 | 4.0 | >64 |
| KH66 | 0.5 | 0.25 | 0.25 | <0.064 | 4.0 |
| E. coli | |||||
| DH5α/pLSKSW-7 | 0.5 | 2.0 | >64 | 8.0 | >64 |
| DH5α/pLSKSW-8 | 0.25 | 0.25 | 1.0 | 0.125 | 8.0 |
| DH5α/pLSK | 0.25 | 0.125 | 0.25 | 0.125 | 8.0 |
| ATCC 25922 | 0.25 | 0.25 | 0.25 | 0.125 | 4.0 |
IPM, imipenem; CAZ, ceftazidime; ATM, aztreonam; CTX, cefotaxime; CXM, cefuroxime.
In the present study, by PCR-based cloning and sequencing techniques, the chromosomally encoded OXY-2a was confirmed to be responsible for β-lactam resistance in the K. oxytoca isolates recovered from patients in the Stockholm area during 1987 (27, 28). The high identities of the OXY-2a coding regions and promoter sequences in the K. oxytoca strains suggest a common origin for the β-lactam-resistant K. oxytoca isolates. The mutation observed in the blaOXY-2a promoters may have resulted in the overexpression of this β-lactamase, thereby conferring resistance to β-lactam antibiotics on K. oxytoca. Similar findings have been reported by others (3, 8, 9, 17, 22–24).
Of the two substitutions observed in OXY-1a, one of them (Pro-261) is located in a hydrophobic pocket and the other (Lys-277) is quite close to the homologous active site (Asp-276) of TEM β-lactamases (5).
In this study, the primers specific to OXY-1 and OXY-2 cross-amplified. This may have been caused by our use of a large amount of template DNA and the high homology of the two genes. This cross-amplification suggests that PCR conditions should be carefully established. The parallel PCRs for blaOXY-1 and blaOXY-2 should be set with the intention of differentiating the two genes. On the other hand, PCR might be performed with one of the primer pairs, and the PCR product might be directly sequenced to identify the genes.
The effort to construct a recombinant plasmid by ligating two PCR-generated DNA fragments was successful and demonstrated a simple, fast, and straightforward approach for cloning.
Nucleotide sequence accession numbers.
The nucleotide sequences described in this paper have been submitted to EMBL-GenBank under accession no. Y17714 for blaOXY-2a and no. Y17715 for blaOXY-1a.
Acknowledgments
We thank Robyn T. Bilinski for the valuable effort she made in the preparation of the manuscript.
REFERENCES
- 1.Ambler R P, Coulson A F, Frere J M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. New York, N.Y: John Wiley; 1992. [Google Scholar]
- 3.Buirma R J, Horrevorts A M, Wagenvoort J H. Incidence of multi-resistant gram-negative isolates in eight Dutch hospitals. The 1990 Dutch surveillance study. Scand J Infect Dis Suppl. 1991;78:35–44. [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caniça M M, Caroff N, Barthélémy M, Labia R, Krishnamoorthy R, Paul G, Dupret J-M. Phenotypic study of resistance of β-lactamase-inhibitor-resistant TEM enzymes which differ by naturally occurring variations and by site-directed substitution at Asp276. Antimicrob Agents Chemother. 1998;42:1323–1328. doi: 10.1128/aac.42.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanal C M, Sirot D L, Petit A, Labia R, Morand A, Sirot J L, Cluzel R A. Multiplicity of TEM-derived β-lactamases from Klebsiella pneumoniae strains isolated at the same hospital and relationships between the responsible plasmids. Antimicrob Agents Chemother. 1989;33:1915–1920. doi: 10.1128/aac.33.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chardon H, Fosse T, Labia R, Nicolas M H, Poyart-Salmeron C, Sirot J, Sirot D, Vernon M. Multifactorial analysis of the phenotypes for β-lactams of 1044 Escherichia coli strains. Pathol Biol (Paris) 1993;41:337–342. [PubMed] [Google Scholar]
- 8.Fournier B, Arlet G, Lagrange P H, Philippon A. Klebsiella oxytoca: resistance to aztreonam by overproduction of the chromosomally encoded β-lactamase. FEMS Microbiol Lett. 1994;116:31–36. doi: 10.1111/j.1574-6968.1994.tb06671.x. [DOI] [PubMed] [Google Scholar]
- 9.Fournier B, Lu C Y, Lagrange P H, Krishnamoorthy R, Philippon A. Point mutation in the Pribnow box, the molecular basis of β-lactamase overproduction in Klebsiella oxytoca. Antimicrob Agents Chemother. 1995;39:1365–1368. doi: 10.1128/aac.39.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier B, Roy P H. Variability of chromosomally encoded β-lactamases from Klebsiella oxytoca. Antimicrob Agents Chemother. 1997;41:1641–1648. doi: 10.1128/aac.41.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier B, Roy P H, Lagrange P H, Philippon A. Chromosomal β-lactamase genes of Klebsiella oxytoca are divided into two main groups, blaOXY-1 and blaOXY-2. Antimicrob Agents Chemother. 1996;40:454–459. doi: 10.1128/aac.40.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutmann L, Kitzis M D, Billot-Klein D, Goldstein F, Tran Van Nhieu G, Lu T, Carlet J, Collatz E, Williamson R. Plasmid-mediated β-lactamase (TEM-7) involved in resistance to ceftazidime and aztreonam. Rev Infect Dis. 1988;10:860–866. doi: 10.1093/clinids/10.4.860. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K, Arakawa Y, Ohsuka S, Ito H, Suzuki K, Kurokawa H, Kato N, Ohta M. Molecular aspects of high-level resistance to sulbactam-cefoperazone in Klebsiella oxytoca clinical isolates. Antimicrob Agents Chemother. 1996;40:1988–1994. doi: 10.1128/aac.40.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11:315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- 15.Labia R, Morand A, Guionie M, Heintz M, Pitton J S. Klebsiella oxytoca β-lactamases: study of their action on 3d-generation cephalosporins. Pathol Biol (Paris) 1986;34:611–615. [PubMed] [Google Scholar]
- 16.Liu Y, Mee B J, Mulgrave L. Identification of clinical isolates of indole-positive Klebsiella spp., including Klebsiella planticola, and a genetic and molecular analysis of their β-lactamases. J Clin Microbiol. 1997;35:2365–2369. doi: 10.1128/jcm.35.9.2365-2369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reig R, Roy C, Hermida M, Teruel D, Coira A. A survey of beta-lactamases from 618 isolates of Klebsiella spp. J Antimicrob Chemother. 1993;31:29–35. doi: 10.1093/jac/31.1.29. [DOI] [PubMed] [Google Scholar]
- 19.Rose R E. The nucleotide sequence of pACYC177. Nucleic Acids Res. 1988;16:356. doi: 10.1093/nar/16.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah P M, Asanger R, Kahan M. Incidence of multi-resistance in gram negative aerobes from intensive care units of 10 German hospitals. Scand J Infect Dis. 1991;78(Suppl.):22–34. [PubMed] [Google Scholar]
- 23.Sirot D L, Goldstein F W, Soussy C J, Courtieu A L, Husson M O, Lemozy J, Meyran M, Morel C, Perez R, Quentin-Noury C, Reverdy M E, Scheftel J M, Rosenbaum M, Rezvani Y. Resistance to cefotaxime and seven other β-lactams in members of the family Enterobacteriaceae: a 3-year survey in France. Antimicrob Agents Chemother. 1992;36:1677–1681. doi: 10.1128/aac.36.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snydman D R. Clinical implications of multi-drug resistance in the intensive care unit. Scand J Infect Dis. 1991;78(Suppl.):54–63. [PubMed] [Google Scholar]
- 25.Venezia R A, Scarano F J, Preston K E, Steele L M, Root T P, Limberger R, Archinal W, Kacica M A. Molecular epidemiology of an SHV-5 extended-spectrum beta-lactamase in enterobacteriaceae isolated from infants in a neonatal intensive care unit. Clin Infect Dis. 1995;21:915–923. doi: 10.1093/clinids/21.4.915. [DOI] [PubMed] [Google Scholar]
- 26.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. Brooklyn, N.Y: Wiley Interscience; 1987. pp. 2.4.1–2.4.5. [Google Scholar]
- 27.Wu S W, Dornbusch K, Goransson E, Ransjo U, Kronvall G. Characterization of Klebsiella oxytoca septicaemia isolates resistant to aztreonam and cefuroxime. J Antimicrob Chemother. 1991;28:389–397. doi: 10.1093/jac/28.3.389. [DOI] [PubMed] [Google Scholar]
- 28.Wu S W, Dornbusch K, Norgren M, Kornvall G. Extended spectrum beta-lactamase from Klebsiella oxytoca, not belonging to the TEM or SHV family. J Antimicrob Chemother. 1992;30:3–16. doi: 10.1093/jac/30.1.3. [DOI] [PubMed] [Google Scholar]