Antibody waning against SARS-CoV-2 over time after vaccination, together with the emergence of new viral variants, pose great challenges for ending the pandemic. To our knowledge, no previous work has assessed the long-term prevalence of anti-SARS-CoV-2 antibodies in individuals vaccinated with Sputnik V (Gam-COVID-Vac).1 We assessed the persistence of anti-spike IgG antibodies and their neutralising capacity against the original SARS-CoV-2 lineage (B.1) and a local isolate of the BA.1 lineage of the omicron (B.1.1.529) variant in a longitudinal cohort during 1 year after Sputnik V vaccination in Argentina.
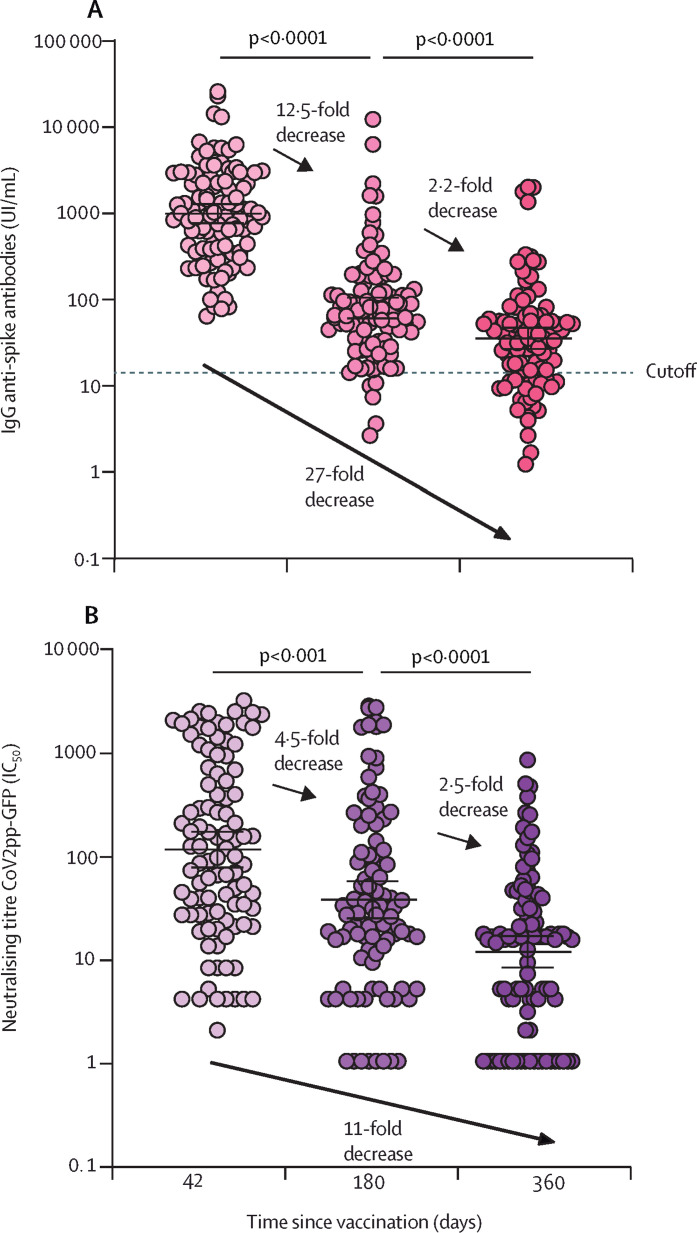
We used 400 paired serum samples (100 samples at each timepoint, including at baseline before vaccination) from 100 volunteers who received two doses of Sputnik V that were obtained between Jan 1, 2021, and Jan 15, 2022. Participants with current or previous SARS-CoV-2 infection, determined by assessing seropositivity to nucleocapsid protein, were excluded from the analysis. The geometric mean (GM) of international units of IgG anti-spike antibodies2 per mL (IU/mL) were 994 (95% CI 769–1285) at 42 days, 80 (60–106) at 180 days, and 36 (27–47) at 360 days after completion of the two-dose vaccination scheme (figure A ; appendix p 2). Overall, a 27-fold reduction in IgG was observed 1 year after Sputnik V vaccination.
Figure.
Longitudinal analysis of humoral response up to 1 year after two doses of Sputnik V vaccine
(A) IgG anti-spike antibody concentrations quantified according to the WHO International Standard. Antibodies were measured at 42 (n=100), 180 (n=100), and 360 (n=100) days after completion of the two-dose vacination schedule. (B) Neutralising titres measured at 50% inhibition against the pseudotyped virus (CoV2pp GFP) for the same cohort as in panel A. Each datapoint indicates one volunteer, the horizontal lines at each timepoint show the mean titre, with error bars showing 95% CIs. Wilcoxon matched-pair test was used.
We assessed the GM half-maximal neutralising titre (GMT, IC50) using a pseudotyped vescicular stomatitis virus carrying the spike of a viral isolate from Wuhan at the early stage of the pandemic (appendix p 4). The GMT at 42 days after vaccination was 133 (95% CI 92–193), at 180 days was 28 (19–39), and at 360 days was 11 (8–16; figure B).
Considering previous studies indicating that antibody responses undergo a maturation process,3, 4 we analysed the serum neutralising activity over time against the omicron variant. To this aim, we assessed the neutralising activity elicited by the Sputnik V vaccine5 using the original B.1 isolate and a local isolate of BA.1 omicron. For this analysis, we used 60 samples (20 samples per timepoint) with the highest neutralising GMT for the original B.1 virus. For all timepoints analysed, we found a substantial decrease in the serum neutralising capacity against the omicron variant compared with the B.1 lineage (64-fold reduction at 42 days, 32-fold reduction at 180 days, and 28-fold reduction at 360 days after vaccination; appendix p 2). Six (30%) of the 20 immunised individuals remained positive for neutralising antibodies against omicron at 42 days after vaccination. This proportion increased to 45% (nine of 20) at 360 days. Similar results have been obtained using other vaccines against SARS-CoV-2. Studies in individuals vaccinated with mRNA vaccines reported a similar decrease at 6 months after vaccination in both the concentration of IgG antibodies directed to the spike protein and the serum neutralising capacity against the original B.1 variant.6, 7 A substantial reduction in neutralising capacity against the omicron variant was also reported with mRNA vaccines.8
Overall, our data suggest that maturation of the antibody response observed over time after standard Sputnik V vaccination is unable to overcome the ability of omicron to escape the humoral response induced by the vaccine, emphasising the need to administer a booster dose urgently. Booster vaccination combining other vaccine platforms would be an option to further increase neutralising antibody levels against the omicron variant.
LS, SOR, MP, and DSO contributed equally. We declare no competing interests.
Supplementary Material
References
- 1.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojeda DS, Gonzalez Lopez Ledesma MM, Pallarés HM, et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruell H, Vanshylla K, Tober-Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 omicron variant. Nat Med. 2022 doi: 10.1038/s41591-021-01676-0. published online Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez Lopez Ledesma MM, Sanchez L, Ojeda DS, et al. Longitudinal study after Sputnik V vaccination shows durable SARS-CoV-2 neutralizing antibodies and reduced viral variant escape to neutralization over time. mBio. 2022 doi: 10.1128/mbio.03442-21. published online Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muik A, Lui BG, Wallisch AK, et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375:678–680. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.