Abstract
Background
Awake prone positioning has been broadly utilised for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure, but the results from published randomised controlled trials (RCTs) in the past year are contradictory. We aimed to systematically synthesise the outcomes associated with awake prone positioning, and evaluate these outcomes in relevant subpopulations.
Methods
In this systematic review and meta-analysis, two independent groups of researchers searched MEDLINE, Embase, PubMed, Web of Science, Scopus, MedRxiv, BioRxiv, and ClinicalTrials.gov for RCTs and observational studies (with a control group) of awake prone positioning in patients with COVID-19-related acute hypoxaemic respiratory failure published in English from Jan 1, 2020, to Nov 8, 2021. We excluded trials that included patients intubated before or at enrolment, paediatric patients (ie, younger than 18 years), or trials that did not include the supine position in the control group. The same two independent groups screened studies, extracted the summary data from published reports, and assessed the risk of bias. We used a random-effects meta-analysis to pool individual studies. We used the Grading of Recommendations Assessment, Development, and Evaluation approach to assess the certainty and quality of the evidence. The primary outcome was the reported cumulative intubation risk across RCTs, and effect estimates were calculated as risk ratios (RR;95% CI). The analysis was primarily conducted on RCTs, and observational studies were used for sensitivity analyses. No serious adverse events associated with awake prone positioning were reported. The study protocol was prospectively registered with PROSPERO, CRD42021271285.
Findings
A total of 1243 studies were identified, we assessed 138 full-text articles and received the aggregated results of three unpublished RCTs; therefore, after exclusions, 29 studies were included in the study. Ten were RCTs (1985 patients) and 19 were observational studies (2669 patients). In ten RCTs, awake prone positioning compared with the supine position significantly reduced the need for intubation in the overall population (RR 0·84 [95% CI 0·72–0·97]). A reduced need for intubation was shown among patients who received advanced respiratory support (ie, high-flow nasal cannula or non-invasive ventilation) at enrolment (RR 0·83 [0·71–0·97]) and in intensive care unit (ICU) settings (RR 0·83 [0·71–0·97]) but not in patients receiving conventional oxygen therapy (RR 0·87 [0·45–1·69]) or in non-ICU settings (RR 0·88 [0·44–1·76]). No obvious risk of bias and publication bias was found among the included RCTs for the primary outcome.
Interpretation
In patients with COVID-19-related acute hypoxaemic respiratory failure, awake prone positioning reduced the need for intubation, particularly among those requiring advanced respiratory support and those in ICU settings. Awake prone positioning should be used in patients who have acute hypoxaemic respiratory failure due to COVID-19 and require advanced respiratory support or are treated in the ICU.
Funding
OpenAI, Rice Foundation, National Institute for Health Research, and Oxford Biomedical Research Centre.
Introduction
Prone positioning has been shown to improve oxygenation and mortality for intubated patients with moderate to severe acute respiratory distress syndrome (ARDS),1, 2 and it has become the standard of care since the PROSEVA trial was published.3 Prone positioning of non-intubated patients, so-called awake prone positioning, has been attempted for patients with acute hypoxaemic respiratory failure, and it has been shown to improve oxygenation and potentially avoid intubation.4, 5 Similar mechanisms (ie, better ventilation–perfusion matching and a better homogeneity of lung stress and strain) as pertaining to intubated patients are likely to be involved during awake prone positioning.6
Since the early phases of the COVID-19 pandemic, awake prone positioning has been broadly performed worldwide, and it has been recommended by multiple societies,7, 8, 9 because of its potential benefits, low risk, and easy implementation. Furthermore, if it is successfully performed outside of the intensive care unit (ICU), the intervention could spare the very precious resource of ICU bed availability. Multiple observational studies reported improved oxygenation with awake prone positioning, but none of them convincingly demonstrated a benefit in avoiding intubation or reducing mortality.10 In August, 2021, an international randomised controlled meta-trial with more than 1100 patients found that awake prone positioning significantly reduced intubation need and treatment failure for patients with COVID-19-related acute hypoxaemic respiratory failure requiring support with high-flow nasal cannula.11 However, before this publication, six randomised controlled trials (RCTs) with a smaller sample size than the meta-trial,12, 13, 14, 15, 16, 17 and two completed but unpublished multi-centre RCTs that enrolled 248 patients18 and 293 patients,19 respectively, did not find that awake prone positioning reduced the intubation need for patients with COVID-19; and, three RCTs12, 13, 18 found that patients' adherence to awake prone positioning was low. In the two most recent meta-analyses that included RCTs,20, 21 Fazzini and colleagues included two RCTs11, 14 and 12 observational studies, and they reported uncertainty regarding the effects of awake prone positioning on intubation and survival,20 whereas Beran and colleagues included five RCTs11, 12, 13, 14, 16 and nine observational studies, and they reported that awake prone positioning has a benefit on mortality.21
Research in context.
Evidence before this study
A large meta-trial demonstrated that awake prone positioning in non-intubated hypoxaemic patients with COVID-19 pneumonia significantly reduced the intubation rate but not the mortality rate, although other randomised controlled trials (RCTs) showed contradictory results. Two independent groups searched MEDLINE, Embase, PubMed, Web of Science, Scopus, MedRxiv, BioRxiv, and ClinicalTrials.gov for RCTs, observational studies (with a control group), and meta-analyses of awake prone positioning in patients with COVID-19-related acute hypoxaemic respiratory failure published in English from Jan 1, 2020, to Nov 8, 2021. We included published RCTs comparing awake prone positioning (intervention group) with the supine position (control group) for non-intubated adult (older than 18 years) patients hospitalised for COVID-19 pneumonia. We excluded trials that included patients intubated before or at enrolment, paediatric patients (younger than 18 years), or trials that did not include the supine position in the control group.
Following screening and exclusion of the search results, we identified ten RCTs (three unpublished from ClinicalTrials.gov), 19 observational studies, and 11 meta-analyses. Only two meta-analyses included RCTs; one included two RCTs and 12 observational studies, and it reported uncertainty in regard to the effects of awake prone positioning on intubation rate and survival; the other meta-analysis included five RCTs and nine observational studies, and it reported that awake prone positioning had a benefit on mortality; this result was probably driven by observational studies.
Key search terms were (prone position*) AND (awake OR non-intubated) AND (COVID-19 OR SARS-CoV-2). In general, the quality of the included RCTs was good in terms of randomisation but blinding was not possible due to the nature of prone positioning in awake non-intubated patients. No publication bias was observed among the RCTs for the primary outcome.
Added value of this study
Aggregating the data of all the available RCTs showed that awake prone positioning reduced the need for intubation in patients with COVID-19-related acute hypoxaemic respiratory failure. We separately analysed the observational studies, and the findings were similar, which strengthens the external validity of these results. Moreover, we found that the reduction in intubation was significant when awake prone positioning was performed in patients in an intensive care unit setting at enrolment and in patients receiving advanced respiratory support (high-flow nasal cannula or non-invasive ventilation). It was not significant for patients in general wards or in patients receiving conventional oxygen therapy.
Implications of all the available evidence
Awake prone positioning should be used in patients with COVID-19-related acute hypoxaemic respiratory failure, in particular when they are managed in the intensive care unit and receive advanced respiratory support.
It is crucial to resolve ongoing uncertainties regarding the effects of awake prone positioning on intubation and all-cause mortality for patients with COVID-19-related acute hypoxaemic respiratory failure. Essential to this is to evaluate the effect of awake prone positioning in subpopulations who are likely to benefit differently. These findings can help clinicians in their daily practice and maximise the benefits for patients with COVID-19 during the ongoing pandemic. We performed a systematic review and meta-analysis of all clinical trials with a control group to assess the effect of awake prone positioning on the risk of intubation for patients with COVID-19-related acute hypoxaemic respiratory failure.
Methods
Search strategy and selection criteria
We conducted a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) recommendations.22 Two independent groups of investigators (group 1 was JLi, WT, and JLu; group 2 was YP and IP) searched MEDLINE, Embase, PubMed, Web of Science, Scopus, MedRxiv, BioRxiv, Google Scholar, and ClinicalTrials.gov for eligible studies from Jan 1, 2020, to Nov 8, 2021. The key search terms were (prone position*) AND (awake or non-intubated) AND (COVID-19 OR SARS-CoV-2), with the detailed search strategy available in the appendix (appendix p 5). Only publications in English were included and there was no limit in geographical locations. Summary data were extracted. The status of the trials registered in ClinicalTrials.gov were reviewed, and the authors of RCTs that were completed but not yet published were contacted to obtain their aggregate results.
We included RCTs published in English comparing awake prone positioning (intervention group) with the supine position (control group) for non-intubated adult (older than 18 years) patients hospitalised for COVID-19 pneumonia. Observational studies of awake prone positioning that included a control group (the supine position) were included as a sensitivity analysis. To evaluate the effects of awake prone positioning on the intubation risk for adult patients with COVID-19, we excluded trials that included patients intubated before or at enrolment, paediatric patients (younger than 18 years), or trials that did not include the supine position in the control group management; and excluded records that were not observational studies or RCTs—eg, protocols, opinions, editorials, reports. The two investigator groups screened the study titles and abstracts, and reviewed full texts to select the studies; and they independently assessed the risk of bias using the Cochrane collaboration risk of bias tool for RCTs,23 which considers allocation sequence generation, concealment of allocation, masking of participants and investigators, incomplete outcome reporting, selective outcome reporting, and other sources of bias. Each potential source of bias was graded as high, low, or unclear, which determined whether the studies were considered at high, low, or moderate risk of bias. The Newcastle-Ottawa Scale was used to assess the risk of bias of observational studies, and full details are provided in the appendix (appendix p 19). Any disagreement regarding study selection, data extraction, or quality assessments was resolved by a consensus discussion with all five investigators and three other members from the meta-analysis group. The protocol is available online.
Data analysis
Data were extracted by the two independent investigator groups in a blinded way, as recommended by the Cochrane Collaboration.23 Duplicate publications were excluded during screening. A case record form designed for the purpose of the study was used to record information on the following variables: age, sex, body-mass index (BMI), comorbidities, baseline oxygenation, oxygen devices at enrolment, enrolment location, target and actual awake prone positioning duration, and the use of corticosteroids. Data from each study were tabulated and checked by a third independent author (ET) before inclusion in the analysis. Categorical outcomes were extracted as the number of patients who had each outcome and the total number of patients in each group (denominator). Continuous outcomes were extracted as sample size and mean (SD) or median (IQR) provided in the studies, with the conversion of medians to estimated mean (SD).24 Intention-to-treat datasets were selected if more than one set of results was reported. Authors were contacted in cases of missing data or if the reporting format was not suitable for the meta-analysis—eg, if the data was only presented in a figure. The primary analysis was conducted separately on RCTs and observational studies.
We prespecified outcomes for efficacy on the basis of the consensus of the meta-analysis group. The primary outcome was the cumulative intubation risk across RCTs; the measure of effect was risk ratio (RR; 95% CI). Secondary outcomes included the reported all-cause mortality; the need for escalating respiratory support, which was defined as progression to a higher level of oxygen or respiratory support (room air<conventional oxygen therapy<high-flow nasal cannula<non-invasive ventilation [NIV]<invasive ventilation) according to the included studies;11, 12, 13, 14, 15, 16, 17, 18, 19 the duration of ICU and hospital stay; and safety outcomes, including cardiac arrest, vomiting, and central or arterial line dislodgement. We also performed a post-hoc analysis on ICU admission among patients who were not admitted to the ICU at enrolment and added it as a secondary outcome.
We performed data analysis according to the recommendations in chapter 10 of the Cochrane Handbook for Systematic Reviews and Interventions. 23 Funnel plots were depicted to assess publication bias using the random-effects trim and fill method (R0 estimator) to correct for small study effects and Egger's test. Effect estimates were calculated as RRs for dichotomous outcomes and mean difference for continuous outcomes. Studies with no events contributed no information for dichotomous outcomes. Data were combined with random effects to account for heterogeneity and fixed effects to evaluate the influence of small studies. The random-effects model was considered as the main analysis. The Mantel-Haenszel method was used for the fixed-effects model. 25 The restricted maximum-likelihood estimator was used to estimate the between study variance for the inverse variance method in random-effects models.26 Statistical heterogeneity assessment between studies was performed by Cochran's Q test and reported with the I 2 and Chi squared (χ2) statistics, in which I 2 indicated the degree of heterogeneity as follows: insignificant heterogeneity (0–30%), moderate heterogeneity (30–60%), substantial heterogeneity (50–90%), and considerable heterogeneity (75–100%). The threshold for significance for p values was 0·05.
Trial sequential analysis (TSA) was performed by TSA, version 0.9.5.10 to further test the effect of awake prone positioning, which was achieved by defining the required information size, adjusting the thresholds for statistical significance each time a trial was included using the O'Brien-Fleming boundaries, and introducing the threshold for futility. The subgroup analyses on intubation need were based on respiratory support level before randomisation and patient location at enrolment. With regards to respiratory support level, conventional oxygen therapies without positive pressure such as nasal cannula or a mask were classified as conventional oxygen therapy, and positive airway pressure such as high-flow nasal cannula and NIV were classified as an advanced level of respiratory support. The location at enrolment was ICU versus non-ICU. Intermediate ICU or the emergency department was categorised as ICU, considering the intensity of the care and the ratio of nurses to patients in the emergency department, whereas non-ICU represented general wards.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) method was used to grade the quality or certainty of the outcomes and the strength of recommendations.27
Meta-analyses were conducted using meta package (version 5.0.1) in R (version 4.0.3). The review protocol was prospectively registered with PROSPERO, CRD42021271285.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
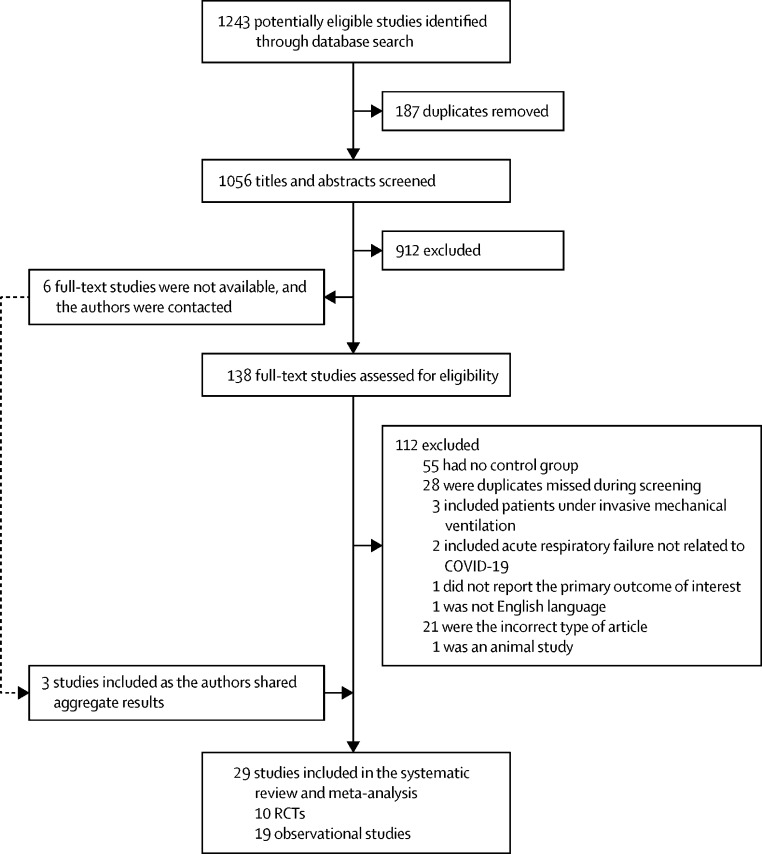
We identified a total of 1243 studies with our search strategy. We screened 1056 titles and abstracts after 187 duplicates were removed. Of 1056 titles and abstracts, 138 full-text articles were assessed for eligibility. In addition, we contacted the authors of six RCTs who had not published their studies results at the time of conducting this systematic review and meta-analysis and received the aggregated results of three RCTs. We excluded 112 full-text articles; therefore, 29 studies were included in the study (figure 1 ). We included ten RCTs11, 12, 13, 14, 15, 16, 17, 18, 19 (NCT04853979) that included a total of 1985 patients, and 19 observational studies that included a total of 2669 patients (the reference list for observational studies [non-RCTs] is available in the appendix pp 6–7).
Figure 1.
Study flow diagram
Among the 29 included studies, the awake prone positioning procedures were variable, and the targeted daily proning duration varied between 1 h and 16 h, or as long as the patients could tolerate. Different types of initial respiratory support were used in the ten RCTs, of which seven studies12, 13, 15, 16, 17, 18, 19 included patients with a lower level of respiratory support (ie, conventional oxygen therapy), two studies11, 14 used advanced respiratory support (ie, high-flow nasal cannula or NIV), and one study (NCT04853979) applied all the types of respiratory support. Patients were exclusively in an ICU setting in two RCTs,16, 17 exclusively in a general ward setting in six RCTs12, 13, 15, 18, 19 (NCT04853979), and in both settings in two RCTs.11, 14 Further demographic details of each RCT are shown in the table and in the appendix for observational studies (appendix pp 8–11).
Table.
Demographic details of the included RCTs
| Country | Study design | Enrolment location | Intervention* | Control* | Primary outcome | Follow-up time | Secondary outcome(s) | |
|---|---|---|---|---|---|---|---|---|
| Ehrmann et al (2021)11 | USA, Mexico, Canada, Ireland, France, and Spain | Multicentre RCT | ICU, intermediate care unit, emergency department, and general wards | 564 participants received awake prone positioning for as long and as frequently as possible; daily median duration 5·0 h (IQR 1·6–8·8) plus usual care | 557 participants received usual care (HFNC) | Treatment failure within 28 days of enrolment, defined as intubation or death | 28 days | Intubation; mortality; use of NIV; length of hospital stay; time to HFNC weaning in patients with treatment success; duration of IMV among intubated patients surviving to day 28; mortality in IMV patients; predefined safety outcomes; physiological response to awake prone positioning, including ROX index |
| Taylor et al (2021)12 | USA | Single-centre RCT | General ward | 27 participants received awake prone positioning plus usual care | 13 received usual care (room air, nasal cannula, HFNC, or NIV) | Outcomes relative to the successful implementation of a future definitive RCT | Until discharge or death | S/F; time on S/F <315; receipt of intensive care; oxygen flow >6 L/min; intubation; hospital length of stay; hospital mortality at 48 h; safety outcomes |
| Johnson et al (2021)13 | USA | Single-centre RCT | General ward | 15 participants received awake prone positioning every 4 h, with a duration of 1–2 h or as long as tolerated; total median duration 1·6 h (IQR 0·2–3·1) plus usual care | 15 participants received usual care (room air or nasal cannula) | The change in P/F at 72 h after admission | 28 days | The change in P/F at 48 h; the need for endotracheal intubation; ICU transfer; escalation in oxygen delivery system; the length of stay; ventilator-free days; in-hospital mortality |
| Rosén et al (2021)14 | Sweden | Multicentre RCT | ICU and general ward | 36 participants received awake prone positioning for at least 16 h/day; daily median duration 9·0 h (IQR 4·4–10·6) plus usual care | 39 participants received usual care (HFNC or NIV) | Intubation within 30 days after enrolment | 30 days | Duration of awake prone positioning; use of NIV; time to NIV for patients included with HFNC; use of vasopressors or inotropes; CRRT; ECMO; ventilator-free days; days free of NIV or HFNC; hospital and ICU length of stay; 30-day mortality; WHO-ordinal scale for clinical improvement at 7 and 30 days; adverse events |
| Kharat et al (2021)15 | Switzerland | Single-centre RCT | General ward | 10 participants received awake prone positioning, self-proning for 12 h/day and alternate body positioning every 4 h; total median duration 4·9 h (SD 3·6) plus usual care | 17 participants received usual care (nasal cannula) | Oxygen needs assessed by nasal cannula oxygen flow at 24 h | 28 days | S/F ratio at 24 h; respiratory and heart rate at 24 h; patient trajectory (transfer to critical care unit) and potential intervention-related adverse effects as defined by neck pain; position-related discomfort and gastro-oesophageal reflux; intubation; death at 28 days |
| Jayakumar et al (2021)16 | India | Multicentre RCT | ICU | 30 participants received awake prone positioning for at least 6 h/day plus usual care | 30 participants received usual care (nasal cannula, face mask, non-rebreather mask, HFNC, or NIV) | The proportion of patients adhering to the protocol | Until discharge or death | Proportion of patients requiring escalation of respiratory support; number of h prone and maximum h of continuous prone positioning in a day; the length of stay in ICU; ICU mortality; adverse events |
| Gad et al (2021)17 | Egypt | Single-centre RCT | ICU | 15 participants received awake prone positioning for 1–2 h each session 3 h apart during waking h for the first 3 days plus usual care | 15 participants received usual care (non-rebreather mask) | Improvement of oxygenation and avoidance of intubation within the first 3 days of critical care admission | .. | ICU stay and hospital stay |
| Fralick et al (2021)18 | Canada, USA | Multicentre RCT | General ward | 126 participants received awake prone positioning four times per day (up to 2 h for each session) and they were encouraged to sleep in the prone position overnight; total median duration 6 h (IQR 1·5–12·8) in the first 72 h and 0 h (IQR 0–12) from 72 h to 7 days; plus usual care | 122 participants received usual care (nasal cannula, venturi mask, HFNC) | A composite of in-hospital death, mechanical ventilation, or worsening respiratory failure defined as requiring at least 60% fraction of inspired oxygen for more than 24 h | 30 days | The components of the composite analysed individually; time spent in the prone position; change in S/F; time to recovery (defined as being on room air for at least 24 h); time to discharge from hospital; and the rate of serious adverse events |
| Garcia et al (2021)19 | USA | Multicentre RCT | General ward | 159 participants received awake prone positioning in up to four 1–2 h daily sessions, and up to 12 h nightly plus usual care | 134 participants received usual care (room air, nasal cannula, mask, or HFNC) | Progression of acute respiratory failure, composite outcome of either respiratory deterioration (ie, progression to non-rebreather mask, HFNC, NIV, IMV, or requiring an increase in oxygen ≥2 L/min compared with their baseline) or admission to the ICU | 14 days (or until discharge or death) | Respiratory deterioration; admission to the ICU; receipt of IMV; hospital mortality; diagnosis of ARDS; median self-reported dyspnoea (Borg Score); safety outcomes; and compliance with awake prone positioning |
| Harris et al (NCT04853979) | Qatar | Multicentre RCT | General ward | 31 participants received awake prone positioning for at least 3 h/day and up to 16 h/day plus usual care | 30 participants received usual care (nasal cannula, non-rebreather mask, HFNC, or NIV) | Escalation of respiratory support within the 30 days of the study | 30 days | Incidence of intubation within 30 days of enrolment; use of nasal prongs, Hudson mask, non-rebreather mask, NIV, and IMV in each group in the first 3 days of the study; physiological response to prone averaged over days 1–3; P/F or S/F ratio and ROX index at baseline, 1 h after the first prone and daily for 4 days; length of time tolerating proning; 28-day mortality; length of stay in ICU and hospital; duration of IMV; displacement of devices; adverse events |
RCT=randomised controlled trial. ICU=intensive care unit. HFNC=high flow nasal cannula. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. S/F=ratio of pulse oxygen saturation to fraction of inhaled oxygen. S/F=ratio of pulse oximetric saturation to fraction of inhaled oxygen. P/F=ratio of partial pressure of arterial oxygen to fraction of inhaled oxygen. ROX index= ratio of S/F to respiratory rate. CRRT=continuous renal replacement therapy. ECMO=extracorporeal membrane oxygenation. ARDS=acute respiratory distress syndrome.
For further baseline characteristics of the participants please see the full table in the appendix (appendix pp 46–47)
None of the included RCTs had incomplete or selective outcome data reporting. All RCTs except for one,13 had a clear description of random sequence generation, six RCTs11, 14, 16, 18, 19 (NCT04853979) also explained the concealment of allocations (appendix p 12). Due to the nature of prone positioning in non-intubated patients, masking participants or the treating clinicians was not possible. No obvious publication bias was observed among the RCTs in terms of the primary outcome (appendix pp 13–18). The summaries of the bias assessment of observational studies indicated potential reporting bias on mortality (appendix pp 19–21). The GRADE assessment for the certainty of evidence for primary and secondary outcomes is summarised in the appendix (appendix p 22).
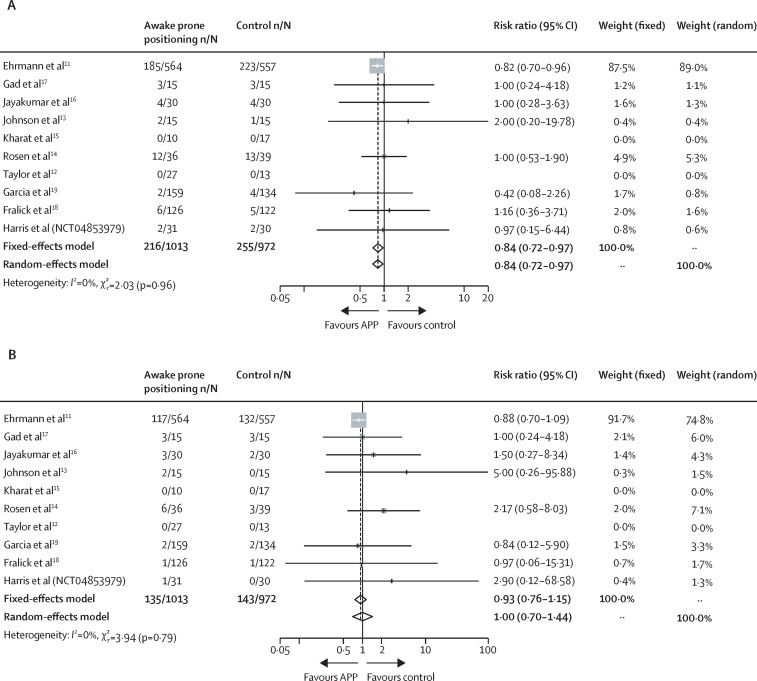
All the RCTs reported patients requiring intubation as an outcome, two RCTs12, 15 did not report any patients requiring intubation in either the awake prone positioning group or the supine position (control) group; the remaining studies11, 13, 14, 16, 17, 18, 19 (NCT04853979) showed that awake prone positioning significantly reduced the need for intubation (RR 0·84 [95% CI 0·72–0·97]; figure 2 ).
Figure 2.
Intubation (A) and mortality (B) for included randomised controlled trials
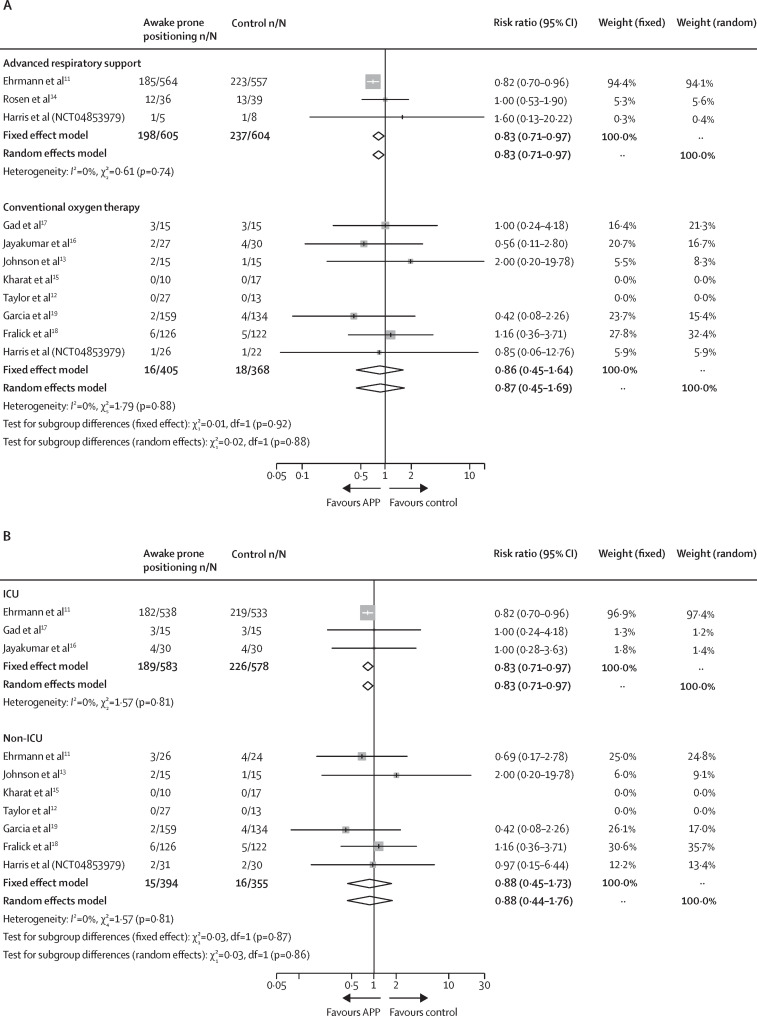
In the subgroup analysis, the significant reduction in intubation requirement was observed in RCTs11, 14 (NCT04853979) that included patients on advanced respiratory support at enrolment (RR 0·83 [95% CI 0·71–0·97]), and RCTs11, 16, 17 that included patients who were in ICU settings at enrolment (RR 0·83 [0·71–0·97]), but not for RCTs12, 13, 15, 16, 17, 18, 19 (NCT04853979) that included patients on conventional oxygen therapy at enrolment (RR 0·87 [0·45–1·69]) or RCTs11, 12, 13, 15, 18, 19 (NCT04853979) that included patients in non-ICU settings at enrolment (RR 0·88 [0·44–1·76]; figure 3 ). The test for subgroup differences did not demonstrate any statistically significant interaction between intubation risk and enrolment location (ICU vs non-ICU χ2=0·03; p=0·86; figure 3) or the type of respiratory support (advanced vs conventional respiratory support χ2=0·02; p=0·88; figure 3). TSA confirmed these findings by showing that the optimal information size was reached (close to the optimal information size for advanced respiratory support and ICU subgroups) and the crossing of the significance boundaries (appendix p 23–25). The 18 observational studies with data on intubation also reported a lower risk of intubation in the awake prone positioning group (RR 0·62 [95% CI 0·47–0·83]) but with significant statistical heterogeneity (I 2 =65%; χ2= 48·51; p<0·0001; appendix p 26).
Figure 3.
Subgroup analysis of intubation
(A) Advanced versus conventional respiratory support. (B) ICU versus non-ICU. ICU=intensive care unit.
In the ten RCTs with data on mortality, two RCTs12, 15 reported no deaths in either the awake prone positioning group or the supine position group. The pooled estimates demonstrated that awake prone positioning did not significantly reduce mortality (RR 1·00 [95% CI 0·70–1·44]; figure 2). Moreover, no significant differences in mortality were found in the subgroup analyses, which included types of respiratory support at enrolment (advanced respiratory support RR 1·23 [95% CI 0·54–2·80]; conventional oxygen therapy RR 1·14 [0·47–2·75]) or enrolment location (ICU RR 0·90 [0·72–1·13]; non-ICU RR 0·81 [0·41–1·59]; appendix p 27). No significant correlation was found in treatment interaction between types of respiratory support or enrolment locations and mortality (advanced vs conventional respiratory support χ2=0·02, p=0·90; ICU vs non-ICU χ2=0·08, p=0·77; appendix p 27). However, TSA showed that the optimal information size was not reached, except for the non-ICU subgroup (appendix pp 28–30). On the contrary, a significant reduction in mortality was found in the 17 observational studies that reported this outcome (RR 0·56 [95% CI 0·48–0·65]; appendix p 26). Due to the observation of significant publication biases for mortality in RCTs and observational studies, further pooled estimates were calculated after exclusion of the studies identified as small-study effects by trim and fill (including five RCTs13, 14, 16, 17 [NCT04853979] and five observational studies [references 6,12,14,15,17 in appendix pp 6–7]); and the results were similar to the results presented before the exclusion of these studies (RCTs RR 0·88 [95% CI 0·70–1·09]; observational studies RR 0·58 [0·50–0·68]; appendix pp 31–32)
Seven RCTs11, 13, 15, 16, 18, 19 (NCT04853979) reported the need for escalation of respiratory support as an outcome; there was no significant difference between the awake prone positioning group and the control groups on the need for escalation of respiratory support (RR 1·03 [95% CI 0·77–1·37]; appendix p 33), regardless of the subgroups of respiratory support (advanced respiratory support RR 1·06 [95% CI 0·39–2·89]; conventional oxygen therapy RR 0·77 [0·43–1·39]) or enrolment location (ICU RR 1·08 [0·60–1·96]; non-ICU RR 1·0 [0·69–1·44]; appendix p 34). From the six RCTs11, 12, 13, 15, 19 (NCT04853979) that reported the need for ICU admission as an outcome, no significant difference was found between the awake prone positioning group and the control groups (RR 0·75 [95% CI 0·51–1·10]; appendix p 33) or in the subgroup analysis of respiratory support (advanced respiratory support RR 0·77 [95% CI 0·53–1·11]; conventional oxygen therapy RR 0·91 [0·56–1·50]; appendix p 35), and a similar result was shown after exclusion of a small study13 identified by trim and fill (RR 0·69 [0·47–1·03]; appendix p 36). Furthermore, awake prone positioning did not significantly reduce the ICU length of stay in five RCTs11, 14, 16, 17 (NCT04853979),(mean difference 0·08 day [95% CI –0·89 to 1·05]) or hospital length of stay in eight RCTs11, 12, 13, 14, 15, 17, 19 (NCT04853979),(mean difference 0·57 day [–0·35 to 1·49]; appendix p 33). However, in the subgroups of conventional oxygen therapy and non-ICU, a small but significant difference in length of hospital length of stay was found in the awake prone positioning group (conventional oxygen therapy mean difference 1·15 days [95% CI 0·26–2·05]; non-ICU mean difference 1·16 days [0·27–2·05]; appendix pp 37–38). TSA showed that the optimal information size was not reached for any of these secondary outcomes but the need for ICU admission crossed the futility boundary (appendix pp 39–42). Similar results of secondary outcomes were shown in observational studies (appendix p 43).
Adverse events were reported in seven RCTs11, 12, 14, 15, 16, 19 (NCT04853979) with one RCT12 showing no complications in either group, and six RCTs reporting mild complications, such as skin breakdown, central venous or arterial line dislodgement, vomiting, back pain, bloating sensation, and general discomfort (appendix pp 44–45).
Discussion
In this systematic review and meta-analysis, we found that awake prone positioning significantly reduced the need for intubation of patients with COVID-19-related acute hypoxaemic respiratory failure, in particular among patients who required advanced respiratory support including high-flow nasal cannula or NIV and those who were in an ICU setting at enrolment. This aligns with the findings in the recently published meta-trial,11 which enrolled 1121 patients with COVID-19-related acute hypoxaemic respiratory failure who were supported with high-flow nasal cannula; 94% of these patients were in an ICU setting at enrolment. In contrast, none of the seven RCTs, which included a total of 688 patients,12, 13, 15, 16, 17, 18, 19 that enrolled patients supported with conventional oxygen therapy demonstrated a difference in the need for intubation between awake prone positioning and the supine position.
The findings in the subgroup analysis of patients with COVID-19 requiring different levels of respiratory support and treatment in different locations (ie, ICU vs non-ICU) might have several explanations. The provision of advanced respiratory support and an ICU setting are directly correlated. Taken together, the apparent lack of efficacy of awake prone positioning in less severely ill patients (non-ICU or receiving conventional oxygen therapy) could be related to a lower event rate, less intensive monitoring, lower nursing to patient ratios, lower adherence to awake prone positioning, and differences in the patient's disease severity. First, patients who require advanced respiratory support or ICU admission are sicker, and they are more likely to progress to invasive mechanical ventilation than patients supported with conventional oxygen therapy. When the event rate is low with large variances, precision is lower. Although the point estimates of relative risk were similar in higher and lower-acuity patients (ie, patients in non-ICU settings or receiving low respiratory support), the confidence intervals were wider in lower-acuity patients. In addition, invasive ventilation was more commonly implemented during the early phase of the pandemic,28 and study sample sizes calculated on the basis of the intubation rate during the early pandemic can result in underpowered studies even after completion. Indeed, some of the studies were terminated early owing to the inability to recruit enough patients because of the declining COVID-19 pandemic (NCT04853979).14, 18, 19 Second, the finding that awake prone positioning resulted in a lower risk of intubation in patients with COVID-19 in an ICU setting but not for non-ICU patients could be explained by the higher ratio of health-care workers to patients in ICU. ICU patients might have more opportunities to receive encouragement or assistance to help them prone. However, even in ICU settings, patient adherence to awake prone positioning varied, with daily awake prone positioning duration ranging from 1–2 h/day to 8–10 h/day.11 Besides patient factors, clinician-driven awake prone positioning 29 rather than patient-driven awake prone positioning12, 13, 17, 18, 19 was probably the key to improving compliance with awake prone positioning. Third, the use of prone positioning in patients with advanced respiratory support might be associated with an increase in end-expiratory lung volume and a more homogeneous distribution in lung aeration,30 potentially decreasing tidal hyperinflation of the ventral regions and promoting the recruitment of the dorsal regions of the lung, which leads to better ventilation–perfusion matching. Moreover, in intubated patients with ARDS, allowing spontaneous breathing during prone positioning improved gas exchange, decreased inspiratory effort and lung stress, and attenuated systemic inflammatory response.31 These physiological benefits might have enabled some patients to stay in a prone position for a longer period; and a longer duration of awake prone positioning was found to be associated with treatment success.11, 32 However, the results from the conventional oxygen therapy and non-ICU subgroups are inconclusive since the optimal information size was not reached. Therefore, further studies on these subpopulations are warranted.
No significant difference in mortality between awake prone positioning and the supine position was found in this meta-analysis, which might be attributed to the low power for mortality outcome, which was investigated as a secondary outcome in all the RCTs, and the short follow-up time of the studies (most reported mortality as a 28-day outcome or as an in-hospital outcome). In contrast, mortality was found to be significantly reduced in observational studies, possibly due to the use of historical control groups or selection bias, including patients unable to prone in the control groups. Similar explanations apply to the absence of a difference between awake prone positioning and the supine position in the duration of ICU stay and duration of hospital stay, in addition to no difference between the two groups in escalation of respiratory support.
A strength of our meta-analysis is that we systematically reviewed the current literature on the basis of previous meta-analyses,10, 33, 34, 35 which did not include any RCTs. Since 2021, several RCTs and meta-analyses have been published. Only two meta-analyses20, 21 included RCTs, and one reported that awake prone positioning reduced mortality;21 however, we suspect this benefit might have primarily been driven by observational data. Our analysis of observational studies also showed that awake prone positioning reduced mortality, confirming that this benefit is probably driven by observational data. To our knowledge, this is the first study that comprehensively includes all the published RCTs and observational studies of awake prone positioning with a control group. We separated the analysis of RCTs and observational studies, using observational studies as a sensitivity analysis. The results generated from the RCT analysis are convincing. Additionally, we contacted all the authors of RCTs with completed but unpublished studies for their aggregate data, with the aim of providing the strongest evidence on awake prone positioning to guide clinical practice in the ongoing pandemic.
There are several limitations in this study. First, the results are mainly driven by the three large sample size RCTs.11, 18, 19 Second, only a few studies reported the actual awake prone positioning duration; and this value might not have been recorded very precisely since it was observed and recorded unsystematically and with unknown accuracy by bedside clinicians. Therefore, we were not able to assess the impact of awake prone positioning duration on patient outcome. Future studies need to find ways to accurately document the start and stop time for each position, including prone, lateral, and supine positions, and more efforts are required to improve patient adherence to awake prone positioning. Third, only patients with COVID-19 were included in this meta-analysis, thus the results might not be generalisable to non-COVID-related acute hypoxaemic respiratory failure. Confirmatory trials in patients with acute hypoxaemic respiratory failure unrelated to COVID-19 remain warranted.
In conclusion, this systematic review and meta-analysis found that in patients with COVID-19-related acute hypoxaemic respiratory failure, awake prone positioning reduced the need for intubation, particularly in those patients requiring advanced respiratory support and in those in the ICU setting at enrolment. The study did not demonstrate a benefit on mortality, the need for escalation of respiratory support, ICU admission, ICU length of stay, or hospital length of stay.
Data sharing
Data will be available for 36 months after Article publication for any purpose of analysis to researchers who provide a methodologically sound and ethically approved proposal.
Declaration of interests
SE discloses consultancy fees from Aerogen, research support from Aerogen and Fisher & Paykel Healthcare, and travel reimbursements from Aerogen and Fisher & Paykel Healthcare. JL discloses research funding from Fisher & Paykel Healthcare, Aerogen, and Rice Foundation, and speaker fees from American Association for Respiratory Care, Aerogen, Heyer, and Fisher & Paykel Healthcare. IP discloses a research grant and speaker fees from Fisher & Paykel Healthcare. YP discloses research support from Fisher & Paykel Healthcare. OR discloses a research grant from Hamilton Medical and speaker fees from Hamilton Medical, Ambu, Fisher & Paykel and Aerogen, and non-financial research support from Timpel and Masimo. DV discloses research funding from Teleflex Medical and Rice Foundation, and speaker fees from Theravance Biopharma. MT discloses consulting fees from Fisher & Paykel Healthcare. JRM discloses research support from Fisher & Paykel Healthcare, and speaker fees from Fisher & Paykel Healthcare, Gilead, Dextro and Linet. JGL discloses consulting fees from Baxter Healthcare and GlaxoSmithKline. KM is the primary investigator for the industry funded trial NCT03808922. MF is a consultant for a start-up company (ProofDx) developing a CRISPR based diagnostic test for COVID-19. All other authors declare no competing interests.
Acknowledgments
Acknowledgment
The awake prone positioning Meta-analysis group thanks Ms Afsaneh Raissi from the Sinai Health System for her assistance in providing subgroup data from the COVID-PRONE trial, and Dr Stacy A Johnson, Dr Stephanie P Taylor, and Dr Devachandran Jayakumar for their helpful clarifications regarding their studies.
Acknowledgments
Contributors
JLi, JGL, CG, and SE designed the meta-analysis project. Two independent groups of investigators (JLi, WT, and JLu in one group; YP and IP in the other group) performed the literature search, screening, and data extraction. JLi, IP, SE, and CG contacted trialists for clarification on their data and invited researchers to provide unpublished data. MI-E, DV, AK, and BM participated in the resolution of discrepancies on data extraction. All the authors had full access to the data and JLi, JLu, YP, IP, WT, and ET verified the data. JLu and ET conducted data analysis. All authors significantly contributed to the conduct of the meta-analysis. JLi, JLu, YP, IP, WT, MI-E, DV, AK, BM, ET, JGL, CG, and SE attended bimonthly web meetings. JLi drafted the manuscript. All authors reviewed the manuscript for important intellectual content and approved the final manuscript. JLi, JLu, YP, IP, and WT contributed equally to the overall project described in this article. SE and JLi were responsible for the decision to submit the manuscript.
Contributor Information
Awake Prone Positioning Meta-Analysis Group:
Jie Li, Jian Luo, Ivan Pavlov, Yonatan Perez, Wei Tan, Oriol Roca, Elsa Tavernier, Aileen Kharat, Bairbre McNicholas, Miguel Ibarra-Estrada, David Vines, Nicholas A Bosch, Garrett Rampon, Steven Q Simpson, Allan J Walkey, Michael Fralick, Amol Verma, Fahad Razak, Tim Harris, John G Laffey, Claude Guerin, Stephan Ehrmann, Sara Mirza, Luzheng Xue, Ian D Pavord, Patrice Plamondon, Dev Jayaraman, Jason Shahin, Joseph Dahine, Anne Kulenkamp, and Andrés Pacheco
Supplementary Material
References
- 1.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 2.Guérin C, Albert RK, Beitler J, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30:1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24:28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoof S, Nava S, Carpati C, Hill NS. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158:1992–2002. doi: 10.1016/j.chest.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institutes of Health COVID-19 treatment guidelines: care of critically ill patients with COVID-19. 2021. https://www.covid19treatmentguidelines.nih.gov/management/critical-care/oxygenation-and-ventilation [PubMed]
- 8.Nasa P, Azoulay E, Khanna AK, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021;25:106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serpa Neto A, Checkley W, Sivakorn C, Hashmi M, Papali A, Schultz MJ. Pragmatic recommendations for the management of acute respiratory failure and mechanical ventilation in patients with COVID-19 in low and middle-income countries. Am J Trop Med Hyg. 2021;104:60–71. doi: 10.4269/ajtmh.20-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlov I, He H, McNicholas B, et al. Awake prone positioning in non-intubated patients with acute hypoxemic respiratory failure due to COVID-19: a systematic review of proportional outcomes comparing observational studies with and without awake prone positioning in the setting of COVID-19. Respir Care. 2022;67:102–114. doi: 10.4187/respcare.09191. [DOI] [PubMed] [Google Scholar]
- 11.Ehrmann S, Li J, Ibarra-Estrada M, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9:1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor SP, Bundy H, Smith WM, Skavroneck S, Taylor B, Kowalkowski MA. Awake-prone positioning strategy for non-intubated hypoxic patients with COVID-19: a pilot trial with embedded implementation evaluation. Ann Am Thorac Soc. 2021;18:1360–1368. doi: 10.1513/AnnalsATS.202009-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson SA, Horton DJ, Fuller MJ, et al. Patient-directed prone positioning in awake patients with COVID-19 requiring hospitalization (PAPR) Ann Am Thorac Soc. 2021;18:1424–1426. doi: 10.1513/AnnalsATS.202011-1466RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosén J, von Oelreich E, Fors D, et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit Care. 2021;25:209. doi: 10.1186/s13054-021-03602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharat A, Dupuis-Lozeron E, Cantero C, et al. Self-proning in COVID-19 patients on low-flow oxygen therapy: a cluster randomised controlled trial. ERJ Open Res. 2021;7:00692–02020. doi: 10.1183/23120541.00692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayakumar D, Ramachandran Dnb P, Rabindrarajan Dnb E, Vijayaraghavan Md BKT, Ramakrishnan Ab N, Venkataraman Ab R. Standard care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection—a multicenter feasibility randomized controlled trial. J Intensive Care Med. 2021;36:918–924. doi: 10.1177/08850666211014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gad S. Awake prone positioning versus non invasive ventilation for COVID-19 patients with acute hypoxemic respiratory failure. Egypt J Anaesth. 2021;37:85–90. [Google Scholar]
- 18.Fralick M, Colacci M, Munshi L, et al. Prone positioning of patients with moderate hypoxia due to COVID-19: a multicenter pragmatic randomized trial [COVID PRONE] medRxiv. 2021 doi: 10.1101/2021.11.05.21264590. published online Nov 8, 2021. (preprint). [DOI] [Google Scholar]
- 19.Garcia MA, Rampon GL, Doros G, et al. Rationale and design of the awake prone position for early hypoxemia in COVID-19 (APPEX-19) study protocol. Ann Am Thorac Soc. 2021;18:1560–1566. doi: 10.1513/AnnalsATS.202009-1124SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazzini B, Page A, Pearse R, Puthucheary Z. Prone positioning for non-intubated spontaneously breathing patients with acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Br J Anaes. 2022;128:352–362. doi: 10.1016/j.bja.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beran A, Mhanna M, Srour O, et al. Effect of prone positioning on clinical outcomes of non-intubated subjects with COVID-19: A comparative systematic review and meta-analysis. Respiratory Care. Nov 2021 doi: 10.4187/respcare.09362. published online Nov 9, 2021. [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6·2. 2021. Cochrane. https://training.cochrane.org/handbook/PDF/v6.2
- 24.McGrath S, Zhao X, Steele R, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29 doi: 10.1177/0962280219889080. 962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 26.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98. doi: 10.1002/jrsm.1316. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Romieu AC, Adelman MW, Hockstein MA, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48:e1045–e1053. doi: 10.1097/CCM.0000000000004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibarra-Estrada MA, Marín-Rosales M, García-Salcido R, et al. Prone positioning in non-intubated patients with COVID-19 associated acute respiratory failure, the PRO-CARF trial: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:940. doi: 10.1186/s13063-020-04882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58:589–596. doi: 10.4187/respcare.02086. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, Tanaka A, Roldan R, et al. Prone position reduces spontaneous inspiratory effort in patients with acute respiratory distress syndrome: a bicenter study. Am J Respir Crit Care Med. 2021;203:1437–1440. doi: 10.1164/rccm.202012-4509LE. [DOI] [PubMed] [Google Scholar]
- 32.Esperatti M, Busico M, Fuentes NA, et al. Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19-related acute respiratory failure treated with high-flow nasal oxygen: a multicenter cohort study. Crit Care. 2022;26:16. doi: 10.1186/s13054-021-03881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua EX, Zahir SMISM, Ng KT, et al. Effect of prone versus supine position in COVID-19 patients: a systematic review and meta-analysis. J Clin Anesth. 2021;74 doi: 10.1016/j.jclinane.2021.110406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardona S, Downing J, Alfalasi R, et al. Intubation rate of patients with hypoxia due to COVID-19 treated with awake proning: a meta-analysis. Am J Emerg Med. 2021;43:88–96. doi: 10.1016/j.ajem.2021.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponnapa Reddy M, Subramaniam A, Afroz A, et al. Prone positioning of nonintubated patients with coronavirus disease 2019—a systematic review and meta-analysis. Crit Care Med. 2021;49:e1001–e1014. doi: 10.1097/CCM.0000000000005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available for 36 months after Article publication for any purpose of analysis to researchers who provide a methodologically sound and ethically approved proposal.