Abstract
The COVID-19 pandemic has witnessed highly successful efforts to produce effective vaccines and treatments at an unprecedented pace. This perspective discusses factors that made this possible, from long-term investments in research infrastructure to major government interventions that absorbed much of the risk from research and development. We discuss key economic obstacles in the discovery of new drugs for infectious diseases, from novel antibiotics to diseases that primarily affect the poor. The world's collective experience of the pandemic may present an opportunity to reform traditional economic models of drug discovery to better address unmet needs. A tax-funded global institution could provide incentives for drug discovery based on their global health impact. International co-operation would be needed to agree and commit to adequate funding mechanisms, and the necessary political will would require strong public support. With the current heightened appreciation of the need for global health system resilience, there may be no better opportunity than now.
Graphical abstract
1. The accelerated drug discovery of the pandemic
The COVID-19 pandemic has seen unprecedented and highly successful efforts to produce effective vaccines and treatments at lightning speed. Much like the anecdotal professor writing their keynote speech in half an hour, en route to the conference, this has only been possible thanks to years of prior investments in cognate research [1,2]. These include, among others, the considerable investments into mRNA vaccines that acted as a springboard for the Pfizer and Moderna vaccines; the research infrastructure employed to tackle coronaviruses that underpinned the Oxford-AstraZeneca vaccine; and the research infrastructure underpinning the RECOVERY trial, which was set up in record time, taking just nine days from conception to launch.
This research infrastructure, together with unprecedented amounts of funding, made available at short notice because of the global emergency, allowed drug discovery to occur at a phenomenal pace. To a vastly greater extent than in normal times, pharmaceutical companies were able to gamble and risk failure as governments took on liabilities by providing huge development support and committing to pre-purchasing candidate vaccines [3]. This enabled multiple large trials to be conducted in parallel [1] and trial timelines to be compressed by running the usually distinct phases I to III of clinical trials, as well as licensure processes, partly in tandem [3]. Meanwhile, manufacturing of multiple candidates began at scale so that large stockpiles of vaccines were available immediately upon licensure to begin Phase IV and national vaccination programmes immediately [3]. In sum, the fast success was a combination of sustained long-term investment in research infrastructure, large sums of newly available funding, government intervention, and some good fortune that, as a coronavirus, the pandemic came from a family of viruses that was being studied [1,2].
In this perspective, we reflect on some key economic obstacles in drug development. We argue that the world's collective experience of the pandemic may present an opportunity to reform traditional economic models of drug discovery to help address present and future unmet needs.
2. The unmet needs from diseases with high global burden but low margins
From the perspective of a drug company, most infectious diseases are low margin businesses. In the traditional economic model, remuneration for drug companies is based on prices times volumes. This means that there may only be sufficient financial incentives to innovate if anticipated volumes, at an above break-even price, are high.
While there is certainly such potential once a disease becomes a pandemic, at which point sales volumes are extremely high and high-income countries are prepared to spend heavily to acquire them, it can be a major barrier beforehand. Even relatively high anticipated sales volumes may provide insufficient incentives if the expected pricing is low. In large part, this explains the failure of the traditional model to eradicate diseases that mainly affect poor people in poor countries, even when the numbers are high – such as malaria and tuberculosis which, respectively, kill 500,000 and 1.5m people annually [4,5]. At pricing levels low enough to ensure high sales volumes and good access to a new drug, the resulting revenue may be insufficient to offset the costs of research and development (R&D).
What of the patent, a cornerstone of the traditional economic model that aims to improve incentives for innovation? A patent affords a company exclusivity for a period of time to manufacture, market, and sell a new drug. (In the US, this is typically 20 years from filing date though extensions are possible). By eradicating competition, this enables the drug innovator to charge higher prices. The intent from a societal perspective is to accept reduced access to drugs in the short-term as the price of providing better incentives for future innovation. However, in the case of diseases that mainly affect poor people, exclusivity may not equate to the ability to make a profit, as sales volumes at a price likely to recoup R&D costs may not be widely affordable in poor countries. Thus, patents do not necessarily unblock the pipeline for discovery of such drugs, and unmet needs in poor countries continue.
Alongside the COVID-19 pandemic, the world also faces the slow-burning crisis of increasing resistance of bacteria to our stock of antibiotics. Antibiotic consumption continues to grow, increasing selection pressure on bacteria to develop resistance to treatment. This poses a grave threat to modern health care, much of which is dependent on effective antibiotics to prevent and treat infections associated with routine medical procedures [6].
An unusual feature of antibiotics is that there can be considerable value in having access to several types of antibiotics, with different mechanisms of action, to fight the same pathogen. This contrasts with other drugs, for which a new drug is generally only advantageous if it is more cost-effective – that is, some combination of being more effective, cheaper, or both. The value of having multiple antibiotics stems from the ability to continue to use a new antibiotic once bacteria develop resistance to an older antibiotic that was once equally effective [6]. Without innovation and a diverse set of antibiotics, over time, infections become difficult or impossible to treat due to the inevitable onset of resistance [7].
Unfortunately, the societal value that would be reaped from innovation and a diverse pool of antibiotics is not being realised. Though the details differ, fundamentally the problem boils down to a similar lack of incentives as in malaria and tuberculosis. In the case of antibiotics, a particular issue is that, once an effective new antibiotic is developed, there is likely to be considerable pressure to restrict its use, to reduce the selection pressure for resistance to develop. This, together with the fact that effectiveness of the new antibiotic is nevertheless likely to decline over time as bacteria inevitably develop some resistance, reduces the volumes that are likely to be sold over the time horizon during which companies will have a patent.
As with drugs that mostly affect poor people, high costs of drug development together with insufficient anticipated revenues have resulted in a colossal market failure, whereby a misalignment of incentives between pharmaceutical firms and those of society results in considerable unmet need.
3. Reforming drug discovery to meet unmet needs from diseases with high global burden but low margins
In recent years it has become widely recognized that incentivising novel antibiotic development requires a combination of so-called “push” and “pull incentives”, and this perspective argues that the same is needed to address unmet needs from diseases such as malaria and tuberculosis. “Push” incentives refer to government or regulatory interventions that bring down R&D costs, reducing their financial risks, and increasing the probability of success. Beyond industry, push incentives in the form of research grants for targeted basic research could also help to address the problem that high-burden diseases common in low-income countries have been relatively under-studied in academia [8,9] (Fig. 1 ).
Fig. 1.
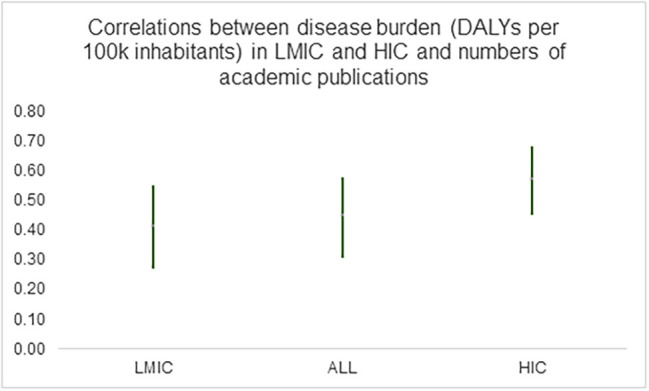
1. Author's estimates based on data reported in [10] (Yegros-Yegros et al.'s Additional File 2, worksheet “Diseases Included in study”); 2. Vertical bars denote 95% confidence intervals of Pearson’s product-moment correlations; 3. LMIC denotes “low and middle income countries”; HIC denotes “high income countries”; ALL denotes full sample of countries; 3. DALY denotes “disability adjusted life years” and is a measure of the burden of a disease in terms of mortality and disability. One DALY represents the loss of the equivalent of one year of full health.
In addition, “pull” incentives are intended to ensure that, once a safe and effective drug is developed, it will provide sufficient revenue to ensure an attractive return on investment for its developers. In the context of diseases with high global burden but low margins, this means that pull incentives need to be designed in such a way that there are sufficient incentives for innovation, even with low prices and/or sales volumes [[11], [12], [13], [14]]. This requires “delinking” the profits from drug discovery from prices and volumes.
In the case of antibiotics, there have been tentative steps towards subscription-based models. In the UK, the government is currently testing a subscription model, with annual lump-sum payments being made for two antibiotics, the amount being based on their value to the National Health Service rather than the number of doses that are sold. However, progress remains slow. A critical question that needs to be addressed is how a reasonable fixed price can be determined [15]. While the price must incentivize innovation, it must also, in a single-payer healthcare system, be good value to the taxpayer [15]. While this value should undoubtedly account in some way for the wider costs of antibiotic resistance, estimating such a value is immensely challenging; it may be more pragmatic to adopt an insurance-based valuation approach, where the new antibiotic is seen as contributing to insuring against the societal costs from a substantial reduction in the effectiveness of current antibiotics [6].
Critical to the design of sufficient pull incentives, whether for antibiotics or for diseases like malaria and tuberculosis, is the problem of finding and co-ordinating countries or institutions that are willing to pay for them. It has recently been estimated that the sum needed globally to return a positive net present value for discovery of a novel antibiotic ranges from US$2.2–4.8bn, with a best estimate of $3.1bn [16]. Considering that the current subscription scheme in the UK, the first of its kind in the world, contributes £100m over a 10-year period, it is clear that global co-ordination will be needed to make the provision of sufficient pull incentives a reality.
If achieving this seems challenging in the context of antibiotics, where all countries are likely to see clear benefits, how could such a model possibly work in the context of diseases that mainly affect people in poor countries? One possibility lies in a system such as the Health Impact Fund (HIF) [17]. The basic idea of the HIF is to create a global government-funded agency that offers yearly reward pools from which new drugs can receive a share for a fixed period of time (e.g. 10 years), corresponding to the drug's contribution to the global health impact of all HIF-registered drugs. Countries could pay some percentage share of GDP into the fund. While the HIF has been envisaged primarily as being funded by countries via taxes, it is also possible to envisage hypothecated taxes agreed globally and applied internationally. These could take the form, for example, of taxes on destabilising financial transactions, carbon emissions [18] or veterinary antimicrobials, with the added benefit of reducing activities that are damaging [6,18]. Either way, the HIF would be a global public good that could benefit patients and taxpayers around the world as well as pharmaceutical companies.
4. Perspective: the positive legacy of the pandemic for future drug development
It is not easy to take positives from the COVID-19 pandemic, which has devastated lives, livelihoods, economies, and health care systems round the world. There may yet, however, be some positive legacies for the future of drug development. One obvious legacy is the successful development and deployment of the Pfizer and Moderna vaccines, which has provided proof of the immense potential of mRNA vaccine technology. Another may be a greater recognition of the benefits of more efficient clinical trial designs. Important evidence on the effectiveness of multiple candidate COVID-19 treatments from the RECOVERY and REMAP-CAP trials highlights the potential efficiencies from adaptive platform trials with a common control group [19,20]. However, many COVID-19 trials have competed against each other for participants, been too small and had insufficient statistical power to detect meaningful treatment effects [20]. This emphasises the potential benefits from consolidating funding and developing large-scale clinical trials structured according to a master protocol in a coordinated and collaborative manner [20].
More broadly, there is evidence of greater recognition among the public and policy makers of the need to build resilience into health care systems, and the greater investment that this will require [[21], [22], [23]]. There have also been encouraging signs of broad support among the general public in many high-income countries of willingness to donate COVID-19 vaccines to low-income countries [24,25]. While this may reflect altruistic preferences, there appears to be a growing realization that we are all connected and that eradicating diseases in distant locations could bring local benefits by reducing their potential to spread worldwide.
The creation of a global institution, such as the HIF, could provide the incentives that are needed, and for too long have been absent, for the development both of novel antibiotics and for the diseases of the poor. Such a creation will require international co-operation to agree and commit to funding mechanisms that would adequately underwrite it. As international negotiations to tackle climate change have demonstrated, the political will necessary to reach such agreements is contingent on public support. With the current heightened appreciation of the need for global health system resilience, there may be no better opportunity than now.
Acknowledgements
The author is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC).
Footnotes
When confronted with the global threat of a modern plague, the world community of scientists, pharmaceutical companies, and governments came together to meet the challenge in an unprecedented way. Here, a leading thinker in health economics outlines how this occurred, providing a perspective on how the COVID-19 pandemic has provided valuable learnings for the future.
References
- 1.Cleve M. What the lightning-fast quest for Covid vaccines means for other diseases. Nature. 2021 Jan 7:589. doi: 10.1038/d41586-020-03626-1. [DOI] [PubMed] [Google Scholar]
- 2.Roope L.S.J., Candio P., Kiparoglou V., McShane H., Duch R., Clarke P.M. Lessons from the pandemic on the value of research infrastructure. Health Res. Pol. Syst. 2021;19:1–4. doi: 10.1186/s12961-021-00704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winch G.M., Cao D., Maytorena-Sanchez E., Pinto J., Sergeeva N., Zhang S. Operation warp speed: projects responding to the COVID-19 pandemic. Project Leadership Soc. 2021;2 [Google Scholar]
- 4.Ashley E.A., Pyae Phyo A., Woodrow C.J. Malaria. Lancet. 2018;391:1608–1621. doi: 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 5.Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020;8:19. doi: 10.1016/S2213-2600(19)30418-7. [DOI] [PubMed] [Google Scholar]
- 6.Roope L.S.J., Smith R.D., Pouwels K.B., Buchanan J., Abel L., Eibich P., Butler C.C., Tan P.S., Walker A.S., Robotham J.V., Wordsworth S. The challenge of antimicrobial resistance: what economics can contribute. Science. 2019;364:eaau4679. doi: 10.1126/science.aau4679. [DOI] [PubMed] [Google Scholar]
- 7.French G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents. 2010;36:S3–S7. doi: 10.1016/S0924-8579(10)70003-0. [DOI] [PubMed] [Google Scholar]
- 8.Evans J.A., Shim J.M., Ioannidis J.P. Attention to local health burden and the global disparity of health research. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller-Langer F. Neglected infectious diseases: are push and pull incentive mechanisms suitable for promoting drug development research? Health Econom. Pol. Law. 2013;8:185–208. doi: 10.1017/S1744133112000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yegros-Yegros A., Van de Klippe W., Abad-Garcia M.F., Rafols I. Exploring why global health needs are unmet by research efforts: the potential influences of geography, industry and publication incentives. Health Res. Pol. Syst. 2020;18:1–14. doi: 10.1186/s12961-020-00560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luepke K.H., Suda K.J., Boucher H., Russo R.L., Bonney M.W., Hunt T.D., J.F. Mohr III, past, present, and future of antibacterial economics: increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017;37:71–84. doi: 10.1002/phar.1868. [DOI] [PubMed] [Google Scholar]
- 12.Morel C.M., Edwards S.E., Harbarth S. Preserving the ‘commons’: addressing the sustainable use of antibiotics through an economic lens. Clin. Microbiol. Infect. 2017;23:718–722. doi: 10.1016/j.cmi.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Outterson K., Powers J.H., Daniel G.W., McClellan M.B. Repairing the broken market for antibiotic innovation. Health Aff. 2015;34:277–285. doi: 10.1377/hlthaff.2014.1003. [DOI] [PubMed] [Google Scholar]
- 14.Outterson K., Gopinathan U., Clift C., So A.D., Morel C.M., Røttingen J.A. Delinking investment in antibiotic research and development from sales revenues: the challenges of transforming a promising idea into reality. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colson A.R., Morton A., Årdal C., Chalkidou K., Davies S.C., Garrison L.P., Jit M., Laxminarayan R., Megiddo I., Morel C., Nonvignon J. Antimicrobial resistance: is health technology assessment part of the solution or part of the problem? Value Health. 2021;24:1828–1834. doi: 10.1016/j.jval.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Outterson K. Estimating the appropriate size of global pull incentives for antibacterial medicines: study examines global antibacterial pull incentives. Health Aff. 2021;40:1758–1765. doi: 10.1377/hlthaff.2021.00688. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee A., Hollis A., Pogge T. The health impact fund: incentives for improving access to medicines. Lancet. 2010;375:166–169. doi: 10.1016/S0140-6736(09)61296-4. [DOI] [PubMed] [Google Scholar]
- 18.The Health Impact Fund 2022. https://healthimpactfund.org/en/faq/ [Accessed, 7th January 2022]
- 19.Park J.J., Ford N., Xavier D., Ashorn P., Grais R.F., Bhutta Z.A., Goossens H., Thorlund K., Socias M.E., Mills E.J. Randomised trials at the level of the individual. Lancet Glob. Health. 2021;9:e691–e700. doi: 10.1016/S2214-109X(20)30540-4. [DOI] [PubMed] [Google Scholar]
- 20.Park J.J., Mogg R., Smith G.E., Nakimuli-Mpungu E., Jehan F., Rayner C.R., Condo J., Decloedt E.H., Nachega J.B., Reis G., Mills E.J. How COVID-19 has fundamentally changed clinical research in global health. Lancet Glob. Health. 2021;9:e711–e720. doi: 10.1016/S2214-109X(20)30542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization - Regional Office for Africa Push for Stronger Health Systems as Africa Battles COVID-19. 2022. https://www.afro.who.int/news/push-stronger-health-systems-africa-battles-covid-19 [Accessed, 8th January 2022]
- 22.Organisation for Economic Co-operation and Development OECD Policy Responses to Coronavirus (COVID-19). Strengthening Health Systems during a Pandemic: The Role of Development Finance. 2022. https://www.oecd.org/coronavirus/policy-responses/strengthening-health-systems-during-a-pandemic-the-role-of-development-finance-f762bf1c/ [Accessed, 8th January 2022]
- 23.Roope L.S.J., Candio P., Kiparoglou V., McShane H., Duch R., Clarke P.M. ePoster Presented at iHEA Conference “Health economics in a time of global change,”. 12–15 July, 2021. Measuring the option value of capacity to tackle future crises: evidence from the COVID-19 pandemic. [Google Scholar]
- 24.Clarke P.M., Roope L.S.J., Loewen P.J., Bonnefon J.F., Melegaro A., Friedman J., Violato M., Barnett A., Duch R. Public opinion on global rollout of COVID-19 vaccines. Nat. Med. 2021;27:935–936. doi: 10.1038/s41591-021-01322-9. [DOI] [PubMed] [Google Scholar]
- 25.Roope L.S.J., Barnett A., Candio P., Violato M., Duch R., Clarke P.M. Is there broad-based support in high-income countries for COVID-19 vaccine donation? Evidence from seven countries. Appl. Health Econ. Health Pol. 2021;20:55–65. doi: 10.1007/s40258-021-00696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]