Abstract
COVID-19 impacted hospital systems across the globe. Focus shifted to responding to increased healthcare demand while mitigating COVID-19 spread on their campuses. Mitigation efforts limited medical professional-patient interactions, including patient access to preventive cancer screenings. Data were gleaned from a health information exchange containing records on over 2 million patients in southeastern North Carolina, USA. This study tested five hypotheses: H1: Weekly cancer screenings significantly decreased during North Carolina's (NC) Stay-At-Home (SAH) orders; H2: Weekly cancer diagnoses significantly decreased during NC's SAH orders; H3: Weekly cancer screenings significantly increased after the end of NC's SAH orders; H4: Weekly cancer diagnoses significantly increased after the end of NC's SAH orders; and H5: Weekly advanced cancer diagnoses significantly increased after the end of NC's SAH orders. Time series regression analysis was employed to quantify trends. Results suggested strong support of H1 and H3, moderate support of H4, mixed support of H5, and no support of H2. For example, compared to before the SAH orders, we estimated 662.3 fewer weekly breast cancer screenings during the SAH orders (H1). After the SAH orders (H3), we estimated 232.5 more breast cancer screenings and 10.6 more breast cancer diagnoses. This work quantifies the impact of COVID-19 associated SAH orders on cancer screenings and diagnoses and suggests the potential for delayed or missed cancer diagnoses. This evident disruption in providing routine medical care also highlights the importance of strengthening health systems (or organizations) and improving resilience to natural disasters and infectious disease outbreaks.
Keywords: Cancer, COVID-19, Diagnosis, Screening, Time series analysis
1. Introduction
The coronavirus 2019 (COVID-19) pandemic influenced all aspects of life beginning in early 2020 with the world's hospital systems at the forefront (Blumenthal et al., 2020). Nearly immediately, hospitals around the world were required to shift focus, control the numbers of individuals on campuses, and limit interactions between medical professionals and patients (Blumenthal et al., 2020). In an effort to minimize interactions, one of the major changes in focus involved delaying and limiting elective procedures, including cancer screenings (American Cancer Society, 2020). Individuals considered low risk and with a record of normal screenings were recommended to delay screenings for three regularly screened cancers: breast, cervical, and colorectal (American Cancer Society, 2020).
Whereas several reports suggest a decrease in screenings during state-issued stay-at-home (SAH) orders, none have employed robust statistical methods to quantify the trends in screenings or diagnoses (Miller et al., 2021; Bakouny et al., 2021; Mitchell, 2020). Understanding the impact of COVID-19 on routine cancer screenings and diagnoses is crucial to preparing for the potential short-term implications of this disruption and for improving health care system resilience to avoid such disruptions in the future.
Given the pressures to reduce interactions and limit elective procedures, we anticipate significant disruption in the rates of cancer screenings and diagnoses. We expect to observe reductions in screenings and diagnoses during state-imposed SAH orders, followed by a “catch-up” period with greater than typical cancer screenings and diagnoses after those orders ended. We also expect that the delay in screenings will lead to higher-than-normal levels of advanced cases of cancer during the “catch-up” period. This study tests the following hypotheses:
H1
Weekly cancer screenings significantly decreased during North Carolina's SAH orders.
H2
Weekly cancer diagnoses significantly decreased during North Carolina's SAH orders.
H3
Weekly cancer screenings significantly increased after the end of North Carolina's SAH orders.
H4
Weekly cancer diagnoses significantly increased after the end of North Carolina's SAH orders.
H5
Weekly advanced cancer diagnoses significantly increased after the end of North Carolina's SAH orders.
2. Materials and methods
Time series regression analysis was employed to examine these hypotheses in the following cancers: breast, cervical, colorectal, leukemia, lung and bronchus, and prostate. These cancers were selected for their range in screening regimen, etiology, rarity, and risk factors (American Cancer Society, 2021). Data were gleaned from a health information exchange containing records on over 2 million patients in our region of southeastern North Carolina, USA. Personal medical records were aggregated and modeled as weekly counts of specific cancer screenings or diagnoses. The relationships between those counts and the following variables were then assessed: an indicator if the week contained a holiday, the number of new patient identifiers in the database, and North Carolina state-issued SAH orders which extended from March 30, 2020 to May 7, 2020.
2.1. Data
Electronic health record data were extracted and compiled from a health information exchange. We limited our sample to records from 2019 and 2020; 2019 records were used to establish a baseline and reference for examining the 2020 data. These data mostly cover the southeastern region of . Our university's policies on human subjects research require Institutional Review Board (IRB) approval before commencing data collection and analysis. Hence, we sought and gained IRB approval for our research proposal in December 2020.
2.1.1. Obtaining cancer outcomes
Entries of cancer screenings or diagnoses were identified by searching for the appropriate ICD9 and ICD10 encounter codes: breast (ICD9: 174, 233, ICD10: C50, D50, Z12.31, Z12.39), cervical (ICD9: 180.0, 180.1, 180.8, 180.9, 233.1; ICD10: C53.0, C53.1, C53.8, C53.9, D06.0, D06.1. D06.7, D06.9, V76.2, Z12.4), colorectal (ICD9: 152–154.9; ICD10: C17-C21.9, Z12.11), leukemia (ICD9: 203.1, 204.00–208.92 but not remissions; ICD10: C91.1, C92.00-C95.90 but not remissions, V76.89, Z12.89), lung and bronchus (ICD9: 162; ICD10: C7A.090, C34, Z212.2 and G0297), and prostate (ICD9: 185, 233.4, 236.5; ICD10: C61, D07.5, V76.44, Z12.5). We used the date associated with each record to determine a weekly count of screenings and diagnoses for each type of cancer. Advanced cases of cancer were identified by searching for codes indicating lymph node involvement or secondary neoplasms (ICD9: 190–199, ICD10: C76-C80) among individuals with one of the cancers of interest. Cases that had lymph node involvement or a secondary neoplasm indicated a stage II-IV diagnosis (from regionalized to distant metastases (Simu et al., 2002). We were unable to determine curable versus incurable cancers based on the diagnoses and for coding purposes only, these diagnoses were coded as advanced in our data.
2.1.2. Independent variables
Several independent, time series variables were constructed for model fitting purposes. The main variable of interest involved the North Carolina state-issued SAH orders. The mandate initiated on March 30, 2020 and the phased reopening began on May 7, 2020 (Cooper and Maddox, 2020). The variable created to represent this mandate was categorical and coded to indicate whether each week in our data fell before, during, or after the SAH period. The categories were defined as follows: Before - January 1, 2019 to March 29, 2020 or weeks 1 to 65, during - March 30, 2020 to May 7, 2020 or weeks 66 to 72, and after - May 7, 2020 to December 31, 2020 or weeks 73–106. As constructed, the variable allowed us to quantify the effects of SAH orders on changes in screenings and diagnoses. Additional variables used for adjustment comprised an indicator for whether the week included a holiday (which would presumably reduce the rate of patient screenings and diagnoses) and the number of new patient identities in the database (to control for increases in the number of and trends with patients in our sample, see Supplemental Fig. 1).
2.2. Statistical methods
Time series regression is a powerful statistical tool for examining relationships between a time-dependent outcome and one or more independent time series variables. A time series linear regression model is written as follows for temporal unit t = 1, …, T:
where y t is the time series outcome, β 0 is the intercept, β 1, β 2, …, β k are the parameter estimates associated with independent time series variables x 1t, x 2t, …, x kt, and ϵ t is the random error term. Time series regression was performed using R statistical software and the tslm function within the R package forecast (Hyndman et al., 2019; Hyndman and Khandakar, 2008; R Core Team. R, 2015). Statistical significance was determined from p-values included in and confidence intervals calculated with (confint function) the model summary results. Adjusted R 2 values were also examined. All R code for data processing, preparation, visualization, and analysis is included in the Supplemental Materials.
3. Results
Table 1 presents the counts for screenings, diagnoses, and advanced case diagnoses associated with each cancer in terms of total counts as well as counts for before (January 1, 2019 to March 29, 2020 or weeks 1 to 65), during (March 30, 2020 to May 7, 2020 or weeks 66 to 72), and after (May 7, 2020 to December 31, 2020 or weeks 73–106) the SAH orders. As expected, breast, cervical, colorectal, and prostate had the highest rates of screenings. Incidence of lung cancer was lower than expected and not in the same range as prostate cancer, as national statistics suggest. Supplemental Table 1 states the percentages of cancer diagnoses from screenings and those considered advanced. The percent of diagnosed cases indicated as advanced (i.e., cases that had lymph node involvement or a secondary neoplasm) differed in reliability across cancers.
Table 1.
Total counts of cancer screenings and diagnoses as well as percent of diagnoses considered advanced. Total counts are for 2019 and 2020, ‘Before’ counts are for before the SAH orders (January 1, 2019 to March 29, 2020 or weeks 1 to 65), ‘During’ counts are for during the SAH orders (March 30, 2020 to May 7, 2020 or weeks 66 to 72), and ‘After’ counts are for after the SAH orders (May 7, 2020 to December 31, 2020 or weeks 73–106).
| Cancer |
Screenings |
New diagnoses |
Advanced cases |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Before | During | After | Total | Before | During | After | Total | Before | During | After | |
| Breast | 101,774 | 61,647 | 1424 | 38,703 | 5154 | 3270 | 100 | 1784 | 1726 | 1140 | 19 | 567 |
| Cervical | 10,305 | 6099 | 275 | 3931 | 3010 | 1799 | 97 | 1114 | 297 | 156 | 8 | 133 |
| Colorectal | 28,260 | 15,794 | 526 | 11,940 | 2515 | 1789 | 53 | 673 | 709 | 533 | 16 | 160 |
| Leukemia | 25 | 16 | 1 | 8 | 3094 | 1542 | 40 | 1512 | 359 | 158 | 7 | 194 |
| Lung and bronchus | 1193 | 660 | 19 | 514 | 3018 | 2078 | 80 | 860 | 1324 | 915 | 29 | 380 |
| Prostate | 7572 | 3166 | 190 | 4216 | 5301 | 3661 | 92 | 1548 | 797 | 587 | 17 | 193 |
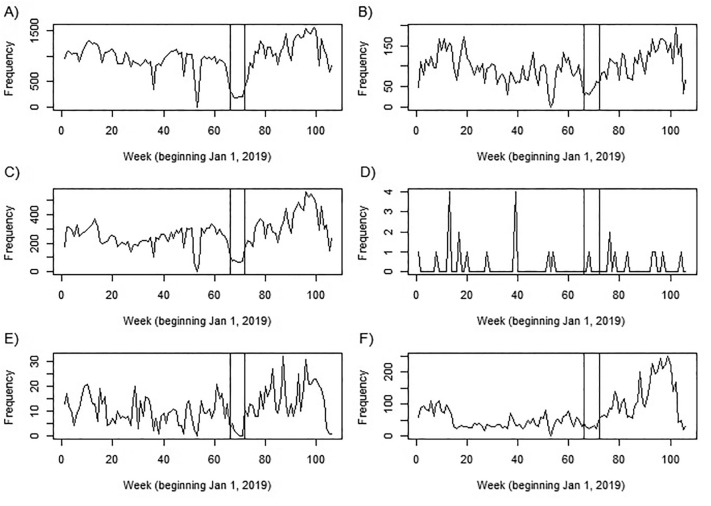
Fig. 1 displays the time series for weekly cancer specific screenings across all cancers considered. The vertical lines in all plots indicate the period when North Carolina was under full SAH orders (March 30, 2020 – May 7, 2020). Figures for new diagnoses and advanced cases can be found in the supplement. The plots in Fig. 1 show a clear decrease in weekly screenings and diagnoses across all cancers, particularly for regularly screened cancers (breast, cervical, colorectal, and prostate). Other trends indicate: a potential “catch up” period after the SAH orders that commenced around week 90 (mid-September), a sharp drop at the turn of the new year (the last week in 2019 had 3 days – Sunday December 29, a single work day of Monday December 30, and New Year's Eve on December 31), and a general increase in screenings over time. As expected, leukemia's plot is different from the others given the relative infrequency for which it is screened and the lack of standardized screening methods for testing the general population. Supplemental Figs. 2 and 3 display cancer diagnoses and advanced case diagnoses over time. These show similar trends to screenings.
Fig. 1.
Weekly cancer screenings over time and by cancer: A) breast cancer, B) cervical cancer, C) colorectal cancer, D) leukemia, E) lung cancer, and F) prostate cancer. The vertical lines in all plots indicate the period when North Carolina was under full SAH orders (March 30, 2020 – May 7, 2020 or weeks 66–72).
Table 2 includes time series regression results (point estimate and 95% confidence interval) across models and cancers considered. Levels of significance as determined by p-values are indicated and associated codes for these symbols are included below the table. A negative estimate indicates fewer screenings, diagnoses, or advanced case diagnoses during weeks with the given characteristic. Alternatively, a positive estimate indicates more screenings, diagnoses, or advanced case diagnoses during weeks with the given characteristic. Using breast cancer screenings and the SAH orders variable as an example, we observed an average of 662.3 fewer in the weeks during and 232.5 more in the weeks after the SAH orders than the weeks before the SAH orders were initiated. H1 was mostly supported as four of the six observed cancer screenings significantly decreased in weeks during SAH orders. The null results for leukemia were expected given the relative infrequency for which it is screened and the lack of standardized screening methods for testing the general population. Despite the significant decline in screenings, cancer diagnoses did not significantly decline suggesting H2 is not supported.
Table 2.
Time series regression results for screenings, diagnoses, and advanced cases by cancer. Results include point estimates, 95% confidence intervals, and significance level as determined by p-value.
| Breast |
Cervical |
Colorectal |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Screenings | Diagnoses | Advanced | Screenings | Diagnoses | Advanced | Screenings | Diagnoses | Advanced |
| New Patient IDs | 0.02*** (0.01, 0.03) |
0.01*** (0.01, 0.01) |
0.004*** (0.003, 0.004) |
0.002** (0.001, 0.003) |
0.002*** (0.001, 0.002) |
0.001*** (0.001, 0.001) |
0.01*** (0.01, 0.01) |
0.004*** (0.003, 0.005) |
0.001*** (0.001, 0.002) |
| Holiday | |||||||||
| No (Ref) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Yes | -245.6*** (−341.8, −149.4) | -0.3 (−7.8, 7.2) | 1.1 (−3.8, 6.0) | −38.2*** (−53.6, −22.9) | −5.5* (−10.6, −0.3) | −0.3 (−1.44, 0.90) | −87.8***(−121.2, −54.3) | −1.5 (−4.22, 1.17) | 0.7 (−1.15, 2.52) |
| Stay Home | |||||||||
| Before (Ref) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| During | −662.3*** (−832.7, −491.9) |
0.8 (−12.5, 14.1) |
3.3 (−5.4, 12.0) |
−45.3** (−72.5, −18.1) |
−4.0 (−13.2, 5.2) |
0.4 (−1.63, 2.50) |
−134.2*** (−193.5, −74.9) |
−2.9 (−7.71, 1.82) |
1.3 (−1.93, 4.56) |
| After | 232.5*** (149.7, 315.3) | 10.6* (4.1, 17.0) | 3.2 (−1.0, 7.5) | 26.6*** (13.5, 39.9) | 7.6** (3.1, 12.0) | 123.1*** (0.90, 2.91) | 123.1*** (94.3, 151.9) | −3.7** (−6.02, −1.39) | −1.9* (−3.48, −0.32) |
| Leukemia |
Lung and Bronchus |
Prostate |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Screenings | Diagnoses | Advanced | Screenings | Diagnoses | Advanced | Screenings | Diagnoses | Advanced |
| New Patient IDs | 0.000 (0.000, 0.000) |
0.003*** (0.002, 0.004) |
0.001*** (0.000, 0.001) |
0.001*** (0.000, 0.001) |
0.005*** (0.004, 0.005) |
0.003*** (0.002, 0.003) |
0.004*** (0.002, 0.005) |
0.01*** (0.008, 0.009) |
0.002*** (0.002, 0.002) |
| Holiday | |||||||||
| No (Ref) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Yes | −0.06 (−0.39, 0.28) | −4.0 (−13.83,5.90) | −0.8 (−2.29, 0.70) | −2.9* (−5.83, 0.004) | −1.7 (−5.37, 1.91) | −0.4 (−3.16, 2.38) | −18.7* (−37.6, 0.2) | −3.8 (−10.0, 2.3) | −0.9 (−2.9, 1.15) |
| Stay Home | |||||||||
| Before (Ref) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| During | −0.1 (−0.70, 0.48) |
−4.1 (−21.52, 13.40) |
0.6 (−2.06, 3.24) |
−6.2* (−11.33, −0.99) |
1.7 (−4.71, 8.17) |
2.1 (−2.78, 7.03) |
−2.5 (−36.0, 30.9) |
−2.7 (−13.6, 8.1) |
2.1 (−1.5, 5.7) |
| After | −0.01 (−0.30, 0.28) | 24.3*** (15.82, 32.79) | 3.8*** (2.50, 5.08) | 5.5*** (3.00, 8.02) | −1.5 (−4.67, 1.60) | −0.1 (−2.51, 2.26) | 80.7*** (64.5, 97.0) | −1.1 (−6.4, 4.2) | −1.3 (−3.0, 0.5) |
1. Significance codes: *** p < 0.001, ** p < 0.01, * p < 0.05, '. < p < 0.1, ' ' p > 0.1
The positive value for the after SAH orders category could represent a “catch up” period, and we observed that trend with breast, cervical, colorectal, lung and bronchus, and prostate cancer screenings as well as breast, cervical, and leukemia diagnoses. These findings indicate that H3 is mostly supported with weekly cancer screenings increasingly significantly in five of the six observed cancers. H4 is partially supported as cancer diagnoses increased significantly in three of the six observed cancers but decreased significantly for colorectal diagnoses. We hypothesized that advanced cancer diagnoses would also significantly increase after the end of SAH orders (H5), but those results are mixed with only three achieving statistical significance. Two of the six observed cancers – cervical and leukemia – had significantly higher rates of advanced diagnoses, while advanced colorectal cancer diagnoses were significantly lower.
The confidence intervals included in this table allow for an examination of the appropriateness of the point estimates. The lower bound for breast cancer screenings is 832.7 which corresponds to about 5000 missed screenings over the 6-week SAH orders. A corresponding 32-week catch-up period would expect about 156 per week, which does fall in the given confidence interval for after SAH. This same lower bound examination confirms appropriate estimates for cervical cancer. Lung and bronchus and colorectal are still over the expected, however there could be many other reasons for this since COVID symptoms overlap with these cancers – respiratory and gastrointestinal issues respectively.
The control variables performed as expected. The holiday variable is negative and significant for breast, cervical, colorectal, lung and bronchus, and prostate cancer screenings, as well as for cervical cancer diagnoses, suggesting fewer observations for weeks with holidays compared to weeks without holidays. The new patient identity variable was positive and significant for most categories, suggesting as one would expect that having more patients in the dataset increased screenings, total diagnoses, and advanced diagnoses. Supplemental Table 1 reports the adjusted R 2 values for all models. These statistics demonstrate that most models fit well.
4. Discussion
This manuscript displays trends in cancer screenings, diagnoses, and advanced case diagnoses for the years 2019 and 2020. The time series plots and statistical model results offer compelling evidence that the COVID-19 pandemic and associated SAH orders affected cancer screenings, total diagnoses, and advanced case diagnoses. Notably, we observed a decrease in most cancer screenings in the weeks during North Carolina's SAH orders for nearly all cancers. Additionally, we observed a potential “catch up” in screenings and diagnoses for many of the cancers under observation in the weeks after the SAH orders were lifted. Not as evident, but still concerning was the uptick in advanced cervical and leukemia diagnoses in the weeks after the SAH orders were lifted. Together these observations suggest the potential for delayed or missed cancer diagnoses during the pandemic.
Our hypotheses were largely supported; however, three findings raise additional questions for future inquiry. First, weekly cancer diagnoses did not significantly decrease during North Carolina's SAH orders (H2), suggesting the disruption to screenings was mitigated for at least a portion of the population. It is possible that doctors and patients with higher risk worked together to ensure screenings were conducted, while lower-risk patients less likely to receive a positive diagnosis opted to pass on screenings. This finding runs opposite to what was observed in a study from the Netherlands (Dinmohamed et al., 2020). Second, the somewhat counterintuitive findings for advanced cancer diagnoses (H5) may be influenced by disease etiology. Although we are not able to empirically test it here, it is possible that colorectal diagnoses and advanced case diagnoses remained significantly lower after SAH orders because this disease is largely found in older individuals (median diagnosis age of 68 in men and 72 in women (American Cancer Society, 2022)). This older group is more vulnerable to COVID-19 and may not have felt comfortable seeking care once SAH orders were lifted and those diagnoses are yet to be made. Conversely, we observed an increase in advanced case diagnoses for cervical cancer, a disease that is most frequently diagnosed in women between the ages of 35 and 44 (Fontham et al., 2020) – a group that may have been willing to venture out to obtain screenings following the end of the SAH orders. Finally, it is too soon to evaluate the impact of this observed decrease in screenings on cancer survival; however, prior research examining cancer mortality following events that caused a delay in screening suggests a reduction in cancer-specific survival (Carroll et al., 2017; Carroll et al., 2019a; Carroll et al., 2019b; Huse et al., 2020) . Some scholarship has considered these possibilities, but further research is needed (Dinmohamed et al., 2020).
These findings also have implications for health system (or organization) resilience to future natural disasters and infectious disease outbreaks. A strong health system is better positioned to respond to and recover from acute shocks without adverse impact to their ability to perform routine functions. In the context of this article, we considered the impact of the COVID-19 pandemic and associated SAH orders on preventive cancer screenings and diagnoses. Employing robust statistical methods to quantify the changes in screenings and diagnoses during periods of system disruption due to disasters may inform strategies that strengthen “health care system capacity and capability needed to respond to emergencies while also maintaining routine services, that, if neglected, could lead to increased morbidity and mortality” (Puricelli Perin et al., 2021).
These methods were not without limitation. First, we were only able to include a single year to represent our baseline. Hurricane Florence greatly impacted our region in September 2018, which distorted “typical” counts in that year. However, our data indicate that this limitation may not be that consequential. The health information exchange from which we sourced our data experienced a large influx of records for the years under study compared to previous years, presumably given the increase in their number of contributing providers. The more apparent limitation pertains to using aggregated electronic health records for scientific inquiry, as previous literature indicates (Meyer et al., 2020; Farmer et al., 2018). For this research, we reduced the noise to the extent possible by ensuring proper encounter codes and dates, using historical records to identify only new diagnoses, and avoiding possible duplicates by focusing on a single patient identifier per patient. Another limitation that stems from the use of electronic health records data includes the advanced case diagnosis outcome and its varied reliability across cancers. For example, the American Cancer Society reports roughly 27% and 6% of breast cancer cases as regional (i.e., cancer spread to nearby lymph nodes) or distant (i.e., cancer spread to distant parts) and we determined 33.5% of ours in those categories (American Cancer Society, 2021) . However, the American Cancer Society reports roughly 35% and 21% of colorectal cancer cases as regional or distant and we determined only 28.2% from our data (American Cancer Society, 2021). Finally, we did not incorporate socio-demographic information in the analysis given the inherent reliability issues with these data in electronic health records.
There are several directions for future inquiry that can build on this work by applying different models or exploring other outcomes within these or similar data. For example, one could use change point detection modeling to aid in identifying the time when the effect of the SAH orders ended, since North Carolina (and surely other states) experienced a phased reopening (Carroll et al., 2019a; Carroll et al., 2019b) . Revisiting this research question after more time has passed might also lead to a better picture of the relevant “catch up” period and potential implications of this disruption on cancer mortality.
5. Conclusion
This work quantifies the impact of the COVID-19 pandemic and associated SAH orders on cancer screenings and diagnoses and suggests the potential for delayed or missed cancer diagnoses, particularly during the state-issued SAH orders. This evident disruption in providing routine medical care also highlights the importance of strengthening health systems (or organizations) and improving resilience to natural disasters and infectious disease outbreaks. Although one should exert caution in generalizing findings beyond this region, we suspect other states that issued SAH orders may have experienced similar trends. We urge other researchers to explore these claims in similar and disparate data sets to improve our understanding of how the global pandemic disrupted health care utilization patterns and the potential downstream consequences of those changes.
CRediT authorship contribution statement
Rachel Carroll: Data curation, Conceptualization, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Stephanie R. Duea: Conceptualization, Writing – original draft, Writing – review & editing. Christopher Prentice: Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported in part by the University of North Carolina's Center for Social Impact with special thanks to Cape Fear Collective.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2022.107010.
Appendix A. Supplementary data
Supplementary material: Additional Tables, Figures, and R code.
References
- American Cancer Society Cancer Screening During the COVID-19 Pandemic [Internet]. cancer.org. 2020. https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/cancer-screening-during-covid-19-pandemic.html [cited 2021 Mar 8]. Available from.
- American Cancer Society . American Cancer Society; Atlanta, GA: 2021. Cancer Facts & Figures 2021. [Internet]https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf Available from. [Google Scholar]
- American Cancer Society . American Cancer Society; Atlanta, GA: 2022. Colorectal Cancer Facts & Figures. [Internet]. Vol. 2015.http://www.cancer.org/cancer/colonandrectumcancer/index Available from. [Google Scholar]
- Bakouny Z., Paciotti M., Schmidt A.L., Lipsitz S.R., Choueiri T.K., Trinh Q.-D. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458–460. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal D., Fowler E., Abrams M., Collins S. Covid-19 — implications for the health care system. N. Engl. J. Med. 2020;383(Oct):1483–1488. doi: 10.1056/NEJMsb2021088. [DOI] [PubMed] [Google Scholar]
- Carroll R., Lawson A.B., Jackson C.L., Zhao S. Assessment of spatial variation in breast cancer-specific mortality using Louisiana SEER data. Soc. Sci. Med. 2017;193(11):1–7. doi: 10.1016/j.socscimed.2017.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R., Lawson A.B., Zhao S. A Data-Driven Approach for Estimating the Change-Points and Impact of Major Events on Disease Risk. Spat. Spatiotemporal Epidemiol. 2019;29:111–118. doi: 10.1016/j.sste.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R., Lawson A.B., Zhao S. Temporally dependent accelerated failure time model for capturing the impact of events that alter survival in disease mapping. Biostatistics. 2019;20(4):666–680. doi: 10.1093/biostatistics/kxy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R., Maddox R. The State of North Carolina; Raleigh, NC: 2020. Stay at Home Order and Strategic Directions for North Carolina in Response to Increasing COVID-19 Cases; pp. 1–13. [Google Scholar]
- Dinmohamed A.G., Cellamare M., Visser O., de Munck L., Elferink M.A.G., Westenend P.J., et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J. Hematol. Oncol. 2020;13(1):147. doi: 10.1186/s13045-020-00984-1. Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer R., Mathur R., Bhaskaran K., Eastwood S.V., Chaturvedi N., Smeeth L. Promises and pitfalls of electronic health record analysis. Diabetologia. 2018;61(6):1241–1248. doi: 10.1007/s00125-017-4518-6. Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontham E.T.H., Wolf A.M.D., Church T.R., Etzioni R., Flowers C.R., Herzig A., et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020;70(5):321–346. doi: 10.3322/caac.21628. Sep 30. [DOI] [PubMed] [Google Scholar]
- Huse E., Malone J., Ruesch E., Sulak T., Carroll R. An analysis of hurricane impact across multiple cancers: accessing spatio-temporal variation in cancer-specific survival with Hurricane Katrina and Louisiana SEER data. Health Place. 2020;63 doi: 10.1016/j.healthplace.2020.102326. May. 102326. [DOI] [PubMed] [Google Scholar]
- Hyndman R., Khandakar Y. Automatic time series forecasting: the forecast package for R. J. Stat. Softw. 2008;26(3):1–22. [Google Scholar]
- Hyndman R., Athanasopoulos G. Bergmeir C., Caceres G., Chhay L., O’Hara-Wild M., Petropoulos F., et al. Forecast: forecasting functions for time series and linear models. CRAN. 2019 [Google Scholar]
- Meyer D., Bishai D., Ravi S.J., Rashid H., Mahmood S.S., Toner E., et al. A checklist to improve health system resilience to infectious disease outbreaks and natural hazards. BMJ Glob. Health. 2020;5(8) doi: 10.1136/bmjgh-2020-002429. Aug 5. e002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Xu L., Qin J., Hahn E.E., Ngo-Metzger Q., Mittman B., et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system — Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70(4):109–113. doi: 10.15585/mmwr.mm7004a1. Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E.P. Declines in cancer screening during COVID-19 pandemic. J. Natl. Med. Assoc. 2020;112(6):563–564. doi: 10.1016/j.jnma.2020.12.004. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puricelli Perin D.M., Elfström K.M., Bulliard J.-L., Burón A., Campbell C., Flugelman A.A., et al. Early assessment of the first wave of the COVID-19 pandemic on cancer screening services: the international cancer screening network COVID-19 survey. Prev. Med. 2021;151 doi: 10.1016/j.ypmed.2021.106642. Oct. 106642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R . R Foundation for Statistical Computing; Vienna, Austria: 2015. A Language and Environment for Statistical Computing. [Google Scholar]
- Simu K.T., Sandra E.B., C Daniel M., Claudia R.B., Sanjay M. Use of ICD-9 coding as a proxy for stage of disease in lung cancer. Pharmacoepidemiol. Drug Saf. 2002;11(8):709–713. doi: 10.1002/pds.759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Additional Tables, Figures, and R code.