Abstract
This study investigated the impact of the COVID-19 pandemic on antimicrobial use (AU) trends in Japan in 2020 and explored its potential effects on appropriate AU. Using nationwide antimicrobial sales data, we examined the annual and monthly trends in AU from 2016–2020 according to the AWaRe classification (Access and Watch categories) and administration route (oral and injectable). To analyze the possible impact of the COVID-19 pandemic on AU, seasonal autoregressive integrated moving average (SARIMA) models were used to predict AU in 2020 (based on the trends from 2016–2019) under the assumption that the pandemic did not occur. We observed a substantial reduction in AU in 2020 compared with preceding years. In addition, the reductions in AU for total antimicrobials and Watch category antimicrobials were greater than predicted regardless of administration route. These results suggest that the COVID-19 pandemic contributed to the observed reductions in AU, but it is also possible that the changes reflect recent efforts to improve AU. Continued AU surveillance and research are needed to optimize prescribing practices through appropriate antimicrobial stewardship.
Keywords: antimicrobial stewardship, COVID-19, AWaRe classification, appropriate antimicrobial use
Introduction
The COVID-19 outbreak in China at the end of 2019 had spread to Japan by early 2020. The surge in infected cases may have precipitated behavioral changes among physicians and patients, including increased unnecessary antimicrobial use (AU) that could promote antimicrobial resistance (AMR).
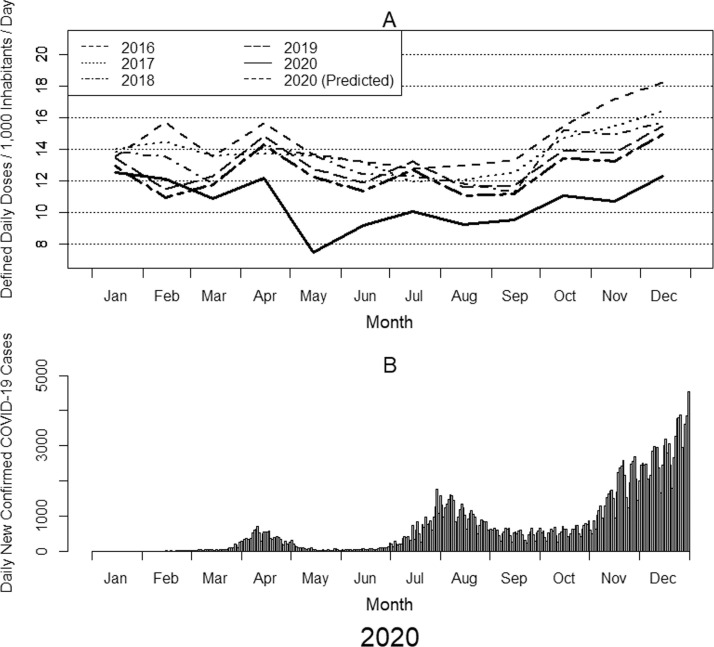
The number of new COVID-19 cases per day increased to over 1,600 at the end of July, plateaued for a few months, and then began to increase again until it reached a maximum of approximately 8,000 at the end of 2020 (Figure 1 ) (Ministry of Health, 2021; Global Change Data Lab, 2021). During 2020, there was a notable reduction in the incidences of other infectious diseases (Ministry of Health and National Institute of Infectious Diseases, 2020; National Institute of Infectious Diseases, 2021a; National Institute of Infectious Diseases, 2021b), which may be attributable to COVID-19-related behavioral restrictions, widespread implementation of infection control measures (eg, improved hand hygiene and increased mask usage), fewer visits to hospitals and clinics, and a decrease in the number of surgeries (Shin et al., 2020). This resulted in an expected decline in AU. However, it is also possible that the fear of bacterial complications in patients with COVID-19 could have induced an increase in indiscriminate broad-spectrum AU. AU surveillance has been conducted since 2013, and the use of antimicrobial agents in Japan overall had been decreasing until 2020 (particularly from 2016) (Government of Japan, 2016; Kusama et al., 2021), although there was a slight increase until 2016. In 2016, the National Action Plan on AMR and the Manual of Antimicrobial Stewardship were published, which may have changed the AU trend. Thus, although AU in Japan decreased from 2013–2019 owing to the widespread implementation of AMR measures, the COVID-19 pandemic may have altered this trend.
Figure 1.
Monthly changes in total antimicrobial use from 2016 to 2020 and daily new confirmed COVID-19 cases in Japan in 2020. (A) The line graph shows the monthly antimicrobial use for each of the 5 years from 2016 to 2020. The lines indicate drug utilization standardized by defined daily dose (DDD) per population and drug or by DID (DDDs/1,000 inhabitants/day). DDD values from January 1, 2021, were used. Population data were based on estimates published every year on October 1 by the Statistics Bureau of the Japanese Ministry of Internal Affairs and Communications. (B) The bar graph shows the daily numbers of new confirmed COVID-19 cases in Japan in 2020, which were obtained from the Our World in Data website (Global Change Data Lab, 2021).
This study aimed to descriptively assess the impact of the COVID-19 pandemic on AU trends in Japan and to explore its potential effects on appropriate AU.
Methods
Data source
Systemic antimicrobial sales data for oral and injectable antibiotics were acquired from IQVIA Japan K.K. (Tokyo, Japan). These data encompass over 99% of wholesale pharmaceutical sales throughout Japan. Data from 2016–2020 were obtained for analysis. IQVIA is a multinational company that collects sales data on a national level, covering the ethical pharmaceuticals market with high accuracy since 2016. The data include all of the basic information required for market analysis, from the value of pharmaceutical sales to the number of units, market share, and percentage change. We have previously used these sales data to estimate AU because of their wide coverage and ease of acquisition (Kusama et al., 2019). Although sales data do not always reflect actual AU practices in healthcare settings and, therefore, do not distinguish between inpatient and outpatient settings, their accessibility and simplicity have led to their widespread use in estimating AU in high-income and low-income countries alike (Jackson et al., 2019).
AU estimation
Antimicrobial drugs were identified using the J01 code of the Anatomical Therapeutic Chemical classification system established by the World Health Organization (WHO Collaborating Centre for Drug Statistics Methodology, 2019). Population-standardized AU was estimated using the defined daily dose (DDD) per 1000 inhabitants per day (DID). The DDD for each drug was obtained from data published on January 1st, 2021 (WHO Collaborating Centre for Drug Statistics, 2021). Population data were based on estimates published every year on October 1 by the Statistics Bureau of the Japanese Ministry of Internal Affairs and Communications (Statistics Bureau of Japan and National Statistics Center, 2020; Statistics Bureau of Japan, 2021).
Analysis
We evaluated the temporal trends in AU in 2020 and compared these trends with those from 2016–2019. Next, we assessed the impact of the COVID-19 pandemic by comparing the actual AU in 2020 with the corresponding predicted AU under the hypothetical condition that the pandemic did not occur. Predicted values were obtained using seasonal autoregressive integrated moving average (SARIMA) models based on data from 2016–2019. We calculated 80% and 95% confidence intervals for each month of the predicted value for 2020. In the monthly data, we compared the empirical value with the lower limit of the 95% confidence interval of the predicted value. An empirical value lower than the lower limit of the confidence interval derived from the model was regarded as a substantial difference. AU was evaluated according to the AWaRe classification system (Hsia et al., 2019) and administration route (ie, injection and oral) to assess the appropriateness of use. Because the Access and Watch categories of the AWaRe system account for more than 95% of the total AU in Japan (AMR Clinical Reference Center, 2021), we analyzed antimicrobials only within these 2 categories and did not include those from other categories (ie, Reserve, Not Recommended, and Unclassified). All data management and statistical analyses were conducted using R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical considerations
This study did not require an ethical review owing to the use of anonymized sales volume data and the absence of human subjects.
Results
Annual and monthly trends in AU
Table 1 shows the annual AU in Japan from 2016–2020. We observed a general decline in the use of oral antimicrobials, especially those classified in the Watch category (9.69 DID in 2019 to 7.22 DID in 2020). Overall, AU was substantially lower in 2020 than in the previous 4 years. In addition to these annual trends, we also analyzed the monthly trends in AU (Figure 1). Notably, there was a sudden drop in AU in May 2020 that was not observed during the same month in previous years.
Table 1.
Annual antimicrobial use from 2016 to 2020 according to AWaRe classification and administration route
| 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|
| Injectable | |||||
| Access category | 0.29 | 0.30 | 0.31 | 0.28 | 0.26 |
| Watch category | 0.61 | 0.61 | 0.62 | 0.67 | 0.56 |
| Oral | |||||
| Access category | 1.52 | 1.57 | 1.72 | 1.94 | 1.88 |
| Watch category | 11.53 | 10.69 | 10.08 | 9.69 | 7.22 |
The values are drug utilization standardized by defined daily dose (DDD) per population and drug or by DID (DDDs/1,000 inhabitants/day). The Access and Watch categories of the AWaRe classification system were analyzed. DDD values from January 1, 2021, were used. Population data were based on estimates published every year on October 1 by the Statistics Bureau of the Japanese Ministry of Internal Affairs and Communications.
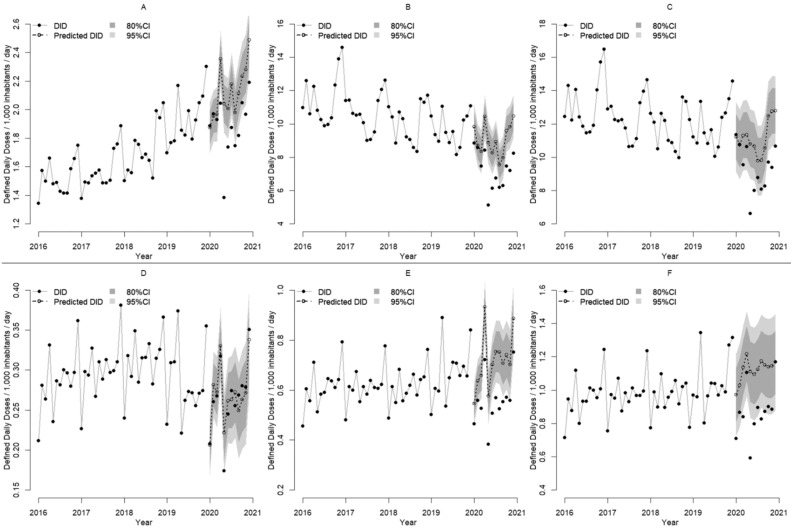
The AU trends according to the AWaRe classification and administration route are presented in Figure 2, Figure 3 . There were decreases in AU for both injectable and oral antimicrobials in 2020: injectable antimicrobials decreased from 1.10 DID in April to 0.59 DID in May, and oral antimicrobials decreased from 10.6 DID in April to 6.62 DID in May. Similarly, there were decreases in AU for both Access and Watch category antimicrobials in 2020: Access category antimicrobials decreased from 2.36 DID in April to 1.56 DID in May, and Watch category antimicrobials decreased from 9.14 DID in April to 5.52 DID in May.
Figure 2.
Monthly changes in antimicrobial use from 2016 to 2020 and predicted antimicrobial use in 2020 according to the AWaRe classification and administration route. Solid lines and black circles show the actual monthly antimicrobial use from 2016 to 2019, while dashed lines and white circles show the predicted monthly antimicrobial use for 2020. The 95% and 80% confidence intervals (CIs) of the predicted values are provided. For 2020, black circles show the actual monthly antimicrobial use. The lines indicate drug utilization standardized by defined daily dose (DDD) per population and drug or by DID (DDDs/1,000 inhabitants/day). The Access and Watch categories of the AWaRe classification system were analyzed. (A) Access category oral antimicrobials, (B) Watch category oral antimicrobials, (C) Total oral antimicrobials, (D) Access category injectable antimicrobials, (E) Watch category injectable antimicrobials, and (F) Total injectable antimicrobials. DDD values from January 1, 2021, were used. Population data were based on estimates published every year on October 1 by the Statistics Bureau of the Japanese Ministry of Internal Affairs and Communications.
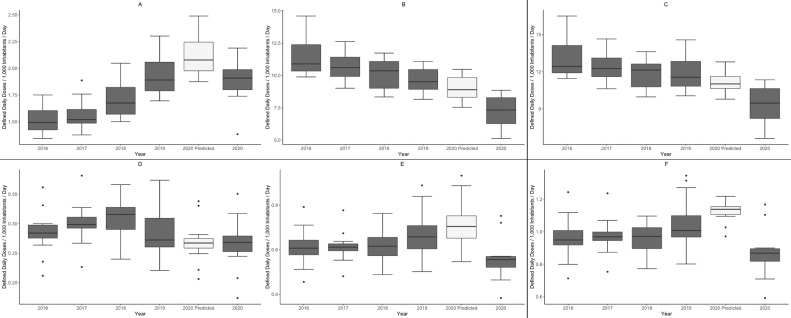
Figure 3.
Annual changes in antimicrobial use from 2016 to 2020 and predicted antimicrobial use in 2020 according to the AWaRe classification and administration route. The gray boxplots show the actual antimicrobial use for each of the 5 years from 2016 to 2020, and the white boxplots show the predicted antimicrobial use for 2020 under the assumption that the COVID-19 pandemic did not occur. The horizontal line inside each box indicates the median value, and the upper and lower edges indicate the quartile ranges. The black dots are outliers. The Access and Watch categories of the AWaRe classification system were analyzed. (A) Access category oral antimicrobials, (B) Watch category oral antimicrobials, (C) Total oral antimicrobials, (D) Access category injectable antimicrobials, (E) Watch category injectable antimicrobials, and (F) Total injectable antimicrobials.
Comparison of actual AU and predicted AU
We compared the actual values with the lower limits of the 95% confidence intervals of the predicted values in the monthly data on the line graph (Figure 2). In 8, 9, and 8 months, the actual values were below the lower limit of the predicted values for Access and Watch category antimicrobials among oral antimicrobials (Figure 2A and B) and Watch category antimicrobials among injectable antimicrobials (Figure 2E), respectively. In contrast, Access category antimicrobials for injectable antimicrobials (Figure 2D) showed a close match between the predicted and measured values in all months.
We analyzed the temporal trends in AU using boxplots of actual values and predicted values from the SARIMA models (Figure 3). Among the oral antimicrobials, the median AU for Access category antimicrobials in 2020 did not increase as much as predicted and was similar to 2019 values (Figure 3A); however, the median AU for Watch category antimicrobials in 2020 decreased more than predicted (Figure 3B). Among the injectable antimicrobials, the median AU for Access category antimicrobials in 2020 was in line with the predicted values (Figure 3D), but the median AU for Watch category antimicrobials in 2020 was lower than predicted (Figure 3E). Overall, the median AU for total antimicrobials in 2020 decreased more than predicted (Figure 3C and F).
Discussion
To assess the possible impact of COVID-19 on AU in Japan, we used SARIMA models to predict the AU values in 2020 under the assumption that the pandemic did not occur. By comparing these predicted values with their corresponding measured values, we found that the AU for total antimicrobials and Watch category antimicrobials was lower than predicted. Moreover, the rate of reduction in AU in 2020 exceeded the downward trend that had occurred until 2019 and closely approached the target set by the National Action Plan on AMR in 2016 (Government of Japan, 2016).
In the United States, which has shown a substantially higher number of COVID-19 cases per population than Japan, a study reported a similar decrease in AU during the pandemic; this might have been owing to changes associated with COVID-19, including fewer outpatient visits and increased infection prevention measures (eg, social distancing and mask wearing) (Chua, Volerman, and Conti, 2021). The same may be true for Japan. Indeed, the Japanese government reported reductions in the incidences of other infections, especially viral infections caused by influenza viruses and respiratory syncytial virus (Ministry of Health and National Institute of Infectious Diseases, 2020; National Institute of Infectious Diseases, 2021a; National Institute of Infectious Diseases, 2021b). These lower incidences may have reduced the need for outpatient consultations and hospital visits. If such viral infections had occurred in previous years and antimicrobials had been erroneously prescribed for their treatment, this would have resulted in higher levels of inappropriate AU. Therefore, the COVID-19 pandemic may have indirectly reduced the number of unnecessary consultations and antimicrobial prescriptions, thereby leading to a general decrease in AU. In this way, the observed reduction in AU would not necessarily reflect an actual improvement in appropriate AU. If this is indeed the case, the National Action Plan on AMR's targeted reduction in AU might be achieved by further reducing inappropriate use (ie, promoting appropriate use) as well as by reducing the number of unnecessary consultations.
A strength of this study is that we did not simply observe annual trends in AU but instead, we predicted AU based on previous annual trends and compared it with the actual AU. Furthermore, the study used recent data that included AU trends up to the end of 2020. In addition, the analysis was conducted using national-level data that covered almost all antimicrobials sold in Japan.
Our results showed that the actual AU in 2020 was lower than the predicted values calculated using the SARIMA models. Among the AWaRe categories, the use of Access category antimicrobials, which are less commonly prescribed in Japan than in Europe (Muraki et al., 2016), had increased. In contrast, the use of Watch category antimicrobials had decreased. These are desirable changes, and it is hoped that such improvements will be maintained.
The observed reduction in AU in 2020 was likely owing to the COVID-19 pandemic, which led to a decline in hospital visits and lower incidences of other infectious diseases. However, AU had been decreasing every year before 2020, which might reflect an increase in antimicrobial stewardship measures following the National Action Plan on AMR. Antimicrobial stewardship efforts should be supported through the continuous evaluation of appropriate AU by comparing predicted values (calculated using SARIMA models) with actual values. Events with immense social impact, such as the COVID-19 pandemic, can bring about unexpected situations. To promote appropriate AU in the future, it is necessary to understand the reasons for the observed decline in AU, including initiatives to increase appropriate AU or other underlying factors with unintended consequences. In addition to the use of predictive models, the consistent monitoring of AU is also essential for improving prescribing practices.
This work has several limitations. First, the study was conducted using only antimicrobial sales data without any patient-level clinical information. Therefore, the search for AU-related factors was limited to descriptive analyses. For instance, telehealth can be regarded as one of the factors with a substantial impact on antibiotic prescription, but it was not implemented until 2020 in Japan. Therefore, its impact on antimicrobial sales is not known. The predictions for 2020 are based on data up to 2019, which did not include telehealth. Accordingly, its impact was not considered in our model. Second, we were unable to assess the appropriateness of AU with our dataset. Further studies may resolve these issues by merging sales data with clinical data or by incorporating indicators such as trends in mortality and serious diseases. Third, the analysis may be strengthened by a longer study period, which might provide greater insight into AU trends before 2020. Despite these limitations, this study benefitted from being able to assess nationwide AU in Japan using recent data that included the entirety of 2020.
In summary, the fact that AU has declined is, in itself, a promising change because it shows that there is potential for further reductions. It will be important to keep a close eye on whether this reduction is only temporary or if it can be sustained. Further research is needed to clarify the factors behind this reduction and to ensure that it leads to more appropriate AU.
Acknowledgments
Conflict of Interest
The authors have no conflicts of interest to declare.
Funding Source
This study was funded by the Ministry of Health, Labour and Welfare Research Grant (20HA2003).
Ethical Approval statement
This study did not require an ethical review owing to the use of anonymized sales volume data and the absence of human subjects.
References
- AMR Clinical Reference Center (2021) Surveillance of antibiotic sales in Japan. Available at: https://amrcrc.ncgm.go.jp/surveillance/020/20200813162318.html (Accessed: 14 September 2021).
- Chua K.-P., Volerman A., Conti R.M. Prescription Drug Dispensing to US Children During the COVID-19 Pandemic. Pediatrics. 2021;148(2) doi: 10.1542/peds.2021-049972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Change Data Lab (2021) Daily new confirmed COVID-19 cases per million people, the COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University.Available at: https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&pickerSort=asc&pickerMetric=location&Metric=Confirmed+cases&Interval=7-day+rolling+average&Relative+to+Population=true&Align+outbreaks=false&country=∼JPN (Accessed: 11 October 2021).
- Government of Japan (2016) National Action Plan on Antimicrobial Resistance (AMR) 2016-2020. Available at: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000138942.pdf (Accessed: 25 May 2020).
- Hsia Y., et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. The Lancet Global Health. 2019;7(7):e861–e871. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- Jackson C., et al. Estimating global trends in total and childhood antibiotic consumption. BMJ Glob Health. 2019;4:1241. doi: 10.1136/bmjgh-2018-001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama Y., et al. Regional Variation of Antimicrobial Use in Japan from 2013–2016, as Estimated by the Sales Data’. Japanese Journal of Infectious Diseases. 2019;72(5):326–329. doi: 10.7883/YOKEN.JJID.2018.417. [DOI] [PubMed] [Google Scholar]
- Kusama Y., et al. The effects of Japan's National Action Plan on Antimicrobial Resistance on antimicrobial use. International Journal of Infectious Diseases. 2021;103:154–156. doi: 10.1016/j.ijid.2020.11.158. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, L. and W. (2021) Novel Coronavirus (COVID-19), Current Situation Report in Japan, Ministry of Health, Labour and Welfare. Available at: https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou_00006.html (Accessed: 11 October 2021).
- Ministry of Health, L. and W. and National Institute of Infectious Diseases (2020) Infectious Diseases Weekly Report Japan. Available at: https://www.niid.go.jp/niid/images/idsc/idwr/IDWR2020/idwr2020-52-53.pdf (Accessed: 11 October 2021).
- Muraki Y., et al. ‘Japanese antimicrobial consumption surveillance: First report on oral and parenteral antimicrobial consumption in Japan (2009–2013)’. Journal of Global Antimicrobial Resistance. 2016;7:19–23. doi: 10.1016/j.jgar.2016.07.002. [DOI] [PubMed] [Google Scholar]
- National Institute of Infectious Diseases (2021a) IDWR Surveillance Data Table 2020 week 52. Available at: https://www.niid.go.jp/niid/en/survaillance-data-table-english/10100-idwr-sokuho-data-e-2052.html (Accessed: 12 October 2021).
- National Institute of Infectious Diseases (2021b) IDWR Surveillance Data Table 2020 week 53. Available at: https://www.niid.go.jp/niid/en/survaillance-data-table-english/10104-idwr-sokuho-data-e-2053.html (Accessed: 12 October 2021).
- Shin J.H., et al. Economic impact of the first wave of the COVID-19 pandemic on acute care hospitals in Japan. PLoS ONE. 2020;15(12 December) doi: 10.1371/journal.pone.0244852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Bureau of Japan (2021) Statistics, Population Estimates, Monthly Report. Available at: https://www.stat.go.jp/english/data/jinsui/tsuki/index.html (Accessed: 12 October 2021).
- Statistics Bureau of Japan and National Statistics Center (2020) Statistics of Japan, Population Estimates, Annual Report. Available at: https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200524&tstat=000000090001&cycle=7&tclass1=000001011679&cycle_facet=tclass1%3Acycle&tclass2val=0 (Accessed: 12 October 2021).
- WHO Collaborating Centre for Drug Statistics (2021) ATC/DDD Index 2021. Available at: https://www.whocc.no/atc_ddd_index/(Accessed: 8 October 2021).
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2020. 23rd edn Oslo, Norway: WHO; 2019 Edited by WHO Collaborating Centre for Drug Statistics Methodology Norwegian Institute of Public HealthAvailable at: https://www.whocc.no/filearchive/publications/2020_guidelines_web.pdf Accessed: 8 April 2020..