Abstract
Objectives
To evaluate safety and effectiveness of prophylactic anticoagulation with low molecular weight heparin (LMWH) in individuals hospitalised for COVID-19.
Methods
Using healthcare records from the Capital Region of Denmark (March 2020-February 2021) and Karolinska University Hospital in Sweden (February 2020-September 2021), we conducted an observational cohort study comparing clinical outcomes 30 days after admission among individuals hospitalised for COVID-19 starting prophylactic LMWH during the first 48 hours of hospitalisation with outcomes among those not receiving prophylactic anticoagulation. We used inverse probability weighting to adjust for confounders and bias due to missing information. Risk ratios, risk differences and robust 95% confidence intervals (CI) were estimated using binomial regression. Country-specific risk ratios were pooled using random-effects meta-analysis.
Results
We included 1692 and 1868 individuals in the Danish and Swedish cohorts. Of these, 771 (46%) and 1167 (62%) received prophylactic LMWH up to 48 hours after admission. The combined mortality in Denmark and Sweden was 12% (N = 432) and the pooled risk ratio was 0.91 (CI 0.60-1.38) comparing individuals who received LMWH to those who did not. The relative risk of ICU admission was 1.12 (0.76-1.66), while we observed no increased risk of bleeding 0.63 (0.13-2.94). The relative risk of venous thromboembolism was 0.80 (0.43-1.47).
Conclusion
We found no benefit on mortality with prophylactic LMWH and no increased risk of bleeding among COVID-19 patients receiving prophylactic LMWH.
Keywords: COVID-19, SARS-CoV-2, venous thromboembolism, anticoagulants, pharmacoepidemiology
Introduction
High rates of venous thromboembolism (VTE) were initially reported in individuals hospitalised for coronavirus disease 2019 (COVID-19) [1] and guidelines for prophylactic anticoagulation in COVID-19 were quickly established [2,3]. Newer and population-based studies, however, reported lower rates of VTE [4]. Randomized trials on prophylactic anticoagulation in COVID-19 are ongoing [5], with available results suggesting no benefit on mortality when comparing intermediate to full-dose anticoagulation in critically ill patients [6,7]. While full-dose anticoagulation may be superior to prophylactic-dose in non-critically ill patients [8,9], conflicting results have been reported [10]. An observational study comparing prophylactic anticoagulation to no anticoagulation also indicated a beneficial effect on mortality [11]. We aimed to provide additional evidence by analysing clinical outcomes among COVID-19 patients receiving prophylactic low-molecular weight heparin (LMWH) compared to individuals receiving no anticoagulation.
Methods
We conducted a cohort study using the electronic health records systems from the Capital Region of Denmark and from Karolinska University Hospital, an academic two-site tertiary hospital with 1100 beds, in the Stockholm region in Sweden. Patients were included until 06 February 2021 in Denmark and 31 August 2021 in Sweden. We included all individuals with a positive reverse transcriptase polymerase chain reaction test (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) between 14 days before and 24 hours after admission for COVID-19. Individuals were excluded if they were below 18 years of age, were current users of anticoagulants, had major bleeding during the previous year, were hospitalised for less than 24 hours, or if they within 48 hours of hospitalisation experienced an outcome of interest, received multiple types of anticoagulation or initiated intermediate or therapeutic-dose LMWH. Individuals were classified as receiving prophylactic LMWH (≤5000 IU dalteparin, 4500 IU tinzaparin or 40 mg enoxaparin) or not during the first 48 hours of hospitalisation. In the main analysis, individuals were followed from 48 hours until 30 days after admission, regardless of changes in exposure status (Fig. S1). Outcomes were death, intensive care unit admission, receiving a discharge diagnosis of VTE and bleeding. For covariate adjustment, we obtained information on selected hospital diagnoses during the 10 years prior to admission, prescription drug use during the prior year, clinical measurements, and results of blood tests at admission (Table S1).
Statistical analyses
Bias due to missing information was handled by inverse probability (IP) weighting of complete cases [12], while measured confounders were adjusted for by IP of treatment weighting [13] (Table S2). Covariate balance was assessed using standardised mean differences [14]. IP-weights greater than 4 were truncated. Using binomial regression, we obtained crude and IP-weighted risk differences (RD) and risk ratios (RR), with robust 95% confidence intervals, comparing individuals who received LMWH in prophylactic doses to individuals not receiving anticoagulation. Country-specific RRs were pooled using a random effects meta-analysis model.
In sensitivity analyses, we (i) shortened the exposure assessment window to 24 hours, (ii) adjusted for body mass index (omitted from the main analysis due to a high prevalence of missing information in Sweden), (iii) restricted inclusion in Sweden to February 2021 (matching data availability in Denmark), (iv) considered initiation of therapeutic dose LMWH an outcome as a proxy for VTE, and (v) obtained risk estimates among patients who received in-hospital corticosteroid treatment. Statistical analyses were performed using R. The source code is available from https://gitlab.sdu.dk/lclund/lmwh-covid19/.
Ethics
The study was approved by the Danish Patient Safety Authority and the Danish Data Protection Agency. Ethics committee approval and informed consent were not required by Danish law. In Sweden, the study was approved by the Regional Ethical Review Board in Stockholm.
Results
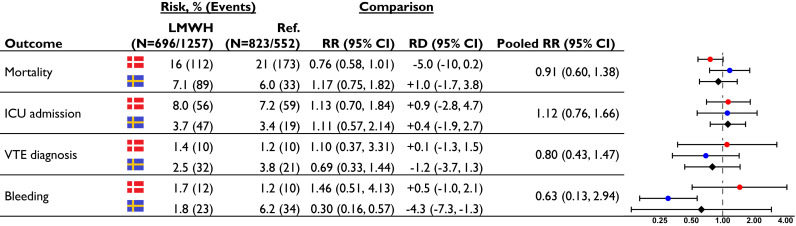
We identified 3483 individuals hospitalised for COVID-19 in Denmark and 3919 individuals in Sweden, of whom 1692 (49%) and 1868 (48%) were included in the final study cohorts (Fig. S2). The median age was 72 and 58 years in the Danish and Swedish cohort. Overall, 1938 individuals (54%) received prophylactic LMWH and 1622 individuals (46%) received no anticoagulation. The proportion of individuals who received prophylactic LMWH in Denmark increased from <10% in March 2020 to about 60% and in Sweden over 80% at the end of the study period (Fig. S3). Individuals receiving prophylactic LMWH more often received oxygen therapy and in-hospital glucocorticoid treatment for COVID-19 (Table 1 ). Individuals with missing information were generally younger, more often female, and more healthy than complete cases (Table S3). After IP-weighting, the abovementioned characteristics were balanced, except for a slight imbalance in in-hospital corticosteroid treatment (Fig. S4, Table S4). In the combined population, we observed 432 deaths within 30 days of hospitalisation for COVID-19 (mortality: 12%) and 70 patients had a discharge diagnosis of VTE (2.0%) (Table S5). We observed 211 deaths (risk 11%) among individuals who received prophylactic LMWH compared to 221 deaths among those who did not (14%; pooled IP-weighted risk ratio [RR] 0.91, 95% CI 0.60-1.38). The relative risk of being admitted to the ICU was 1.12 (0.76-1.66). The risk of receiving a VTE diagnosis was non-significantly lowered among individuals who received prophylactic LMWH (RR 0.80, 0.43-1.47). Finally, we observed no increased risk of receiving a discharge diagnosis related to bleeding (RR 0.63, 0.13-2.94) (Fig. 1 ).
Table 1.
Baseline characteristics of individuals receiving prophylactic LMWH and those not receiving prophylactic anticoagulation for the capital regions of Denmark and Sweden
| Denmark |
Sweden |
|||||
|---|---|---|---|---|---|---|
| Prophylactic LMWH, n (%) |
No anticoagulation, n (%) |
Missing, % | Prophylactic LMWH, n (%) |
No anticoagulation, n (%) |
Missing, % | |
| (N = 771) | (N = 921) | (N = 1167) | (N = 701) | |||
| Demographics | ||||||
| Age, median [IQR] | 72 [59, 82] | 72 [56, 81] | — | 60 [47, 73] | 55 [36, 70] | — |
| Male sex | 412 (53) | 465 (50) | — | 658 (56) | 349 (50) | — |
| Time period | — | — | ||||
| Before June 2020 | 94 (12) | 525 (57) | 414 (36) | 363 (52) | ||
| June to October 2020 | 119 (15) | 74 (8) | 122 (11) | 94 (13) | ||
| November 2020 to February 2021 | 558 (72) | 322 (35) | 383 (33) | 149 (21) | ||
| March to June 2021 | — | — | 216 (19) | 90 (13) | ||
| July 2021 to August 2021 | — | — | 32 (3) | 5 (1) | ||
| Clinical measurements | ||||||
| Body mass index | 16 | 50 | ||||
| <18.5 | 34 (5) | 31 (4) | 22 (4) | 8 (3) | ||
| 18.5-24 | 230 (34) | 256 (35) | 203 (33) | 133 (41) | ||
| 25-34 | 343 (51) | 398 (54) | 366 (59) | 163 (51) | ||
| 35+ | 69 (10) | 56 (8) | 30 (5) | 17 (5) | ||
| Smoking history | 29 | 100 | ||||
| Ex-smoker | 287 (53) | 299 (45) | — | — | ||
| Current smoker | 55 (10) | 70 (10) | — | — | ||
| Body temperature, C | <1 | 9 | ||||
| 37.5-38.4 | 219 (28) | 266 (29) | 353 (31) | 165 (29) | ||
| 38.5+ | 194 (25) | 192 (21) | 358 (32) | 115 (20) | ||
| Respiratory frequency/min >22 | 305 (40) | 280 (31) | <1 | 526 (47) | 215 (38) | 9 |
| Systolic blood pressure <100 mmHg | 26 (3) | 42 (5) | <1 | 41 (4) | 22 (4) | 9 |
| Reduced peripheral oxygen saturation, % | 7 | 16 | ||||
| <88 | 40 (5) | 15 (2) | 56 (5) | 27 (5) | ||
| 88-92 | 122 (17) | 100 (12) | 199 (19) | 65 (13) | ||
| Oxygen therapy, l/min | 3 | 11 | ||||
| 1-4 | 280 (37) | 200 (23) | 443 (40) | 139 (25) | ||
| 5+ | 76 (10) | 51 (6) | 104 (9) | 33 (6) | ||
| Biochemical measurements | ||||||
| Estimated GFR l/min/1.73m2 | 3 | 10 | ||||
| 30-59 | 101 (13) | 119 (13) | 192 (17) | 115 (20) | ||
| 15-29 | 37 (5) | 36 (4) | 51 (5) | 35 (6) | ||
| <15 | 11 (1) | 8 (1) | 21 (2) | 8 (1) | ||
| Haemoglobin below reference | 289 (38) | 335 (37) | 2 | 333 (30) | 186 (32) | 9 |
| Leukocyte levels | 3 | 8 | ||||
| Below reference | 183 (24) | 226 (25) | 81 (7) | 51 (9) | ||
| Above reference | 49 (6) | 52 (6) | 214 (19) | 174 (29) | ||
| Thrombocyte levels | 3 | 9 | ||||
| Below reference | 100 (13) | 130 (15) | 175 (16) | 93 (16) | ||
| Above reference | 76 (10) | 84 (10) | 54 (5) | 22 (4) | ||
| Elevated D-dimer∗ | 355 (66) | 323 (67) | 40 | 704 (72) | 234 (70) | 30 |
| Prescription drug use prior to hospitalisation | ||||||
| Platelet inhibitors | 193 (25) | 226 (25) | — | 125 (11) | 71 (10) | — |
| Antihypertensives | 346 (45) | 400 (43) | — | 312 (27) | 175 (25) | — |
| Loop diuretics | 115 (15) | 116 (13) | — | 89 (8) | 69 (10) | — |
| Glucose lowering therapy | 176 (23) | 171 (19) | — | 206 (18) | 111 (16) | — |
| Lipid lowering therapy | 235 (30) | 273 (30) | — | 189 (16) | 84 (12) | — |
| Glucocorticoids | 191 (25) | 91 (10) | — | 277 (24) | 122 (17) | — |
| In-hospital dexa-/betamethasone treatment | 505 (65) | 158 (17) | — | 381 (33) | 108 (15) | — |
| Medical history | ||||||
| VTE | 6 (1) | 11 (1) | — | — | — | — |
| Atrial fibrillation | 15 (2) | 31 (3) | — | 12 (1) | 15 (2) | — |
| Heart valve disease | 34 (4) | 39 (4) | — | 15 (1) | 16 (2) | — |
| Cardiovascular disease | 188 (24) | 204 (22) | — | 165 (14) | 95 (14) | — |
| Heart failure | 57 (7) | 55 (6) | — | 56 (5) | 38 (5) | — |
| Ischaemic stroke | 58 (8) | 66 (7) | — | 28 (2) | 21 (3) | — |
| Current cancer | 76 (10) | 81 (9) | — | 90 (8) | 73 (10) | — |
| Pulmonary disease | 172 (22) | 185 (20) | — | 140 (12) | 71 (10) | — |
| Liver disease | 15 (2) | 20 (2) | — | 39 (3) | 27 (4) | — |
LMWH: Low molecular weight heparin; GFR: Glomerular filtration rate; VTE: Venous thromboembolism; IQR: Interquartile range.
Age-specific cut-offs between 0.5 and 0.8 FEU/l.
Fig. 1.
Inverse probability weighted number of events, risks and risk estimates for effectiveness and safety outcomes in Denmark, Sweden and combined. Red dots represent Danish point estimates, blue dots Swedish point estimates, and black diamonds the pooled point estimates. LMWH: Low molecular weight heparin; Ref.: Reference cohort not receiving anticoagulation; RR: Risk ratio; RD: Risk difference; ICU: Intensive care unit; VTE: Venous thromboembolism  Capital region of Denmark,
Capital region of Denmark,  Stockholm region of Sweden.
Stockholm region of Sweden.
In sensitivity analyses, we observed comparable risk estimates when shortening the exposure assessment window to 24 hours, restricting the inclusion period in Sweden, when adjusting for body mass index or stratifying on in-hospital corticosteroid treatment (Table S6). In accordance with the other outcomes, the RR for initiating therapeutic LMWH was not increased (RRDenmark 0.99, 0.63-1.57; RRSweden 1.52, 0.87-2.69).
Discussion
We report no beneficial effect on mortality and the risk of ICU admission with use of LMWH thromboprophylaxis in patients admitted for COVID-19. The risk of receiving a VTE diagnosis was lower when receiving LMWH, albeit with imprecise risk estimates, and the risk of bleeding was not increased.
The main strength of our study is the ability to include rich information on clinical and biochemical measurements using electronic health records-based data sources from multiple hospitals, spanning two countries. The major limitation of our study is its non-randomised nature. Even though Danish and Swedish guidelines recommend prophylactic anticoagulation for almost all patients admitted for COVID-19, physicians target treatment to patients at particular risk of VTE. This introduces confounding, as the higher risk patients will be treated, while the lower risk patients remain untreated. Although this potential bias was addressed in our statistical analysis, we cannot rule out some residual confounding, e.g., by suboptimal model specification and measurement of covariates. Finally, we included as reference not only individuals not receiving anticoagulation but also late initiators (>48 hours post-admission). We made this choice, as censoring unexposed individuals upon initiation of LMWH could introduce informative censoring, as late initiation may be a sign of adverse clinical outcomes.
The finding that prophylactic anticoagulation with LMWH does not reduce mortality is not in alignment with results from a similar observational study [11]. This could be attributed to lower statistical precision or residual confounding in our study but may also be related to the different populations and baseline risk of VTE. Comparison of our risk estimates with the published randomised controlled trials conducted in non-critically ill patients is difficult, as these lacked a comparison group not receiving anticoagulants. One of the three trials in non-critically ill patients reported null-findings in accordance with our results [10].
Conclusion
In these cohort studies, we found no beneficial effect of prophylactic LMWH on mortality or the risk of ICU admission in patients hospitalised for COVID-19. The risk of VTE was reduced among individuals receiving prophylactic anticoagulation, albeit with low statistical precision, while patients receiving prophylactic anticoagulation were not at an increased risk of bleeding events.
Author contributions
Conceptualization: LCL and JH. Methodology: All authors. Data curation: AHA, PH. Software: AHA, PH, LCL. Formal analysis: PH, LCL. Resources: JP, EJS, JH. Writing – original draft: LCL, JH. Writing – Review & Editing: All authors.
Transparency declaration
LCL reports participation in research projects funded by Menarini Pharmaceuticals and LEO Pharma, all with funds paid to the institution where he was employed (no personal fees) and with no relation to the current work. AP reports participation in research projects funded by Alcon, Almirall, Astellas, Astra-Zeneca, Boehringer-Ingelheim, Novo Nordisk, Servier and LEO Pharma, all regulator-mandated phase IV-studies, all with funds paid to the institution where he was employed (no personal fees) and with no relation to the work reported in this paper. JP and EJS report participation in research projects funded by Eli Lilly, Johnson & Johnson, Amgen AB, Novartis and Vertex Pharmaceuticals all with funds paid to the institution where she/he was employed (no personal fees) and with no relation to the current work. TSP reports participation in research projects funded by Amgen AB with funds paid to the institution where he was employed (no personal fees) and with no relation to the current work. AHA, JH, JB, PN, PH report no conflicts of interest.
Funding
None.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.03.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dansk Selskab for Trombose og Hæmostase . 2021. Forebyggelse og behandling af trombose og blødning hos COVID-19 patienter. https://dsth.dk/pdf/COVID-19-retningslinje-web.pdf. [Google Scholar]
- 3.Riktlinjer för profylax och behandling av venös tromboembolism hos patienter med covid-19. https://janusinfo.se/behandling/expertgruppsutlatanden/koagulationssjukdomarochplasmaprodukter/koagulationssjukdomarochplasmaprodukter/riktlinjerforprofylaxochbehandlingavvenostromboembolismhospatientermedcovid19.5.735b5f221714e865c978301e.html
- 4.Dalager-Pedersen M., Lund L.C., Mariager T., Winther R., Hellfritzsch M., Larsen T.B., et al. Venous thromboembolism and major bleeding in patients with COVID-19: a nationwide population-based cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021 doi: 10.1093/cid/ciab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flumignan R.L., Tinôco JD. de S., Pascoal P.I., Areias L.L., Cossi M.S., Fernandes M.I., et al. Prophylactic anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst Rev. 2020;2020:CD013739. doi: 10.1002/14651858.CD013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.INSPIRATION Investigators Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The REMAP-CAP. ACTIV-4a. ATTACC Investigators Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sholzberg M., Tang G.H., Rahhal H., AlHamzah M., Kreuziger L.B., Áinle F.N., et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The ATTACC, ACTIV-4A, and REMAP-CAP investigators Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes R.D., de Barros E Silva P.G.M., Furtado R.H.M., Macedo A.V.S., Bronhara B., Damiani L.P., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet Lond Engl. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rentsch C.T., Beckman J.A., Tomlinson L., Gellad W.F., Alcorn C., Kidwai-Khan F., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021:n311. doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins N.J., Cole S.R., Harel O., Tchetgen Tchetgen E.J., Sun B., Mitchell E.M., et al. Principled approaches to missing data in epidemiologic studies. Am J Epidemiol. 2018;187:568–575. doi: 10.1093/aje/kwx348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robins J.M., Hernán M.A., Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol Camb Mass. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.