Abstract
Type 2 diabetes mellitus is a heterogeneous disease. Recently introduced new subclassifications promise more efficacious, tailored treatments which could complement current guidelines. In the differentiation of the new diabetes subphenotypes, assessment of insulin secretion is one of the essential components. Based on a large number of insulin secretion measurements, we propose fasting C-peptide/glucose ratio (CGR) as an adequate and practicable estimate of insulin secretion. CGR discriminates insulin deficiency from insulin hypersecretion. We suggest using insulin secretion, determined from CGR, as an essential input for therapeutic decisions at the beginning or modification of diabetes treatment. Furthermore, we propose 3 practical steps to guide decisions in the subtype-specific therapy of diabetes mellitus. The first step consists of detecting insulin deficiency indicated by a low CGR with the need for immediate insulin therapy. The second step is related to high CGR and aims at lowering cardiovascular risk associated with diabetes. The third step is the consideration of a de-escalation of glucose-lowering therapy in individuals with mild diabetes subphenotypes.
Key words: insulin secretion, classification, subphenotype, C-peptide, treatment
Introduction
Significant innovations have recently been made in the treatment of type 2 diabetes. There is now evidence that new antidiabetic drugs not only lower blood glucose but also reduce mortality 1 . This has led to guideline updates by the European Association for the Study of Diabetes/American Diabetes Association 2 3 and also the European Society of Cardiology 4 . The main change in these guidelines is that the decision on treatment strategy is no longer solely based on blood glucose deterioration (i. e. increase in HbA1c), but additionally guided by the cardiovascular risk of the patient. However, these updated guidelines do not address the probable pathologic underpinnings of the diabetes phenotype in the specific patient. Importantly, 20–25% of patients with newly diagnosed diabetes belong to the severe autoimmune diabetes and severe insulin deficient diabetes clusters 5 6 , implicating absolute insulin deficiency. Failure to recognize insulin deficiency at an early time-point leads to delayed glucose control, higher glycemic burden and eventually an increased incidence of glycaemia-related complications such as retinopathy 5 . Therefore, detection of severe insulin deficiency is critically important in an optimal diabetes treatment. We propose an easy and feasible approach which allows a more precise diabetes therapy based on the prevailing pathophysiological disorder.
Classification of Diabetes: New Subphenotypes of Diabetes
The entity of type 2 diabetes is an aggregation of various disorders that are mainly characterized by “elevated blood sugar”. In order to be able to carry out a better and more specific therapy of diabetes, a precise subclassification of diabetes is essential.
In 2018 a new classification of diabetes was introduced by the research group of Leif Groop 5 . Five subphenotypes were derived from cluster-analysis based on the variables BMI, HbA1c, age at diagnosis, presence of GAD antibodies, insulin sensitivity, and insulin secretion.
The identified subtypes of diabetes are described as severe autoimmune diabetes (SAID) which essentially reflects the hitherto known type 1 diabetes. The other 4 subphenotypes are labelled as severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD) and mild age- related diabetes (MARD). It is important to note that these groups show characteristic responses to therapy: for example, time for sustained treatment with insulin was shortest in SAID and SIDD patients reflecting their impaired insulin secretion. Furthermore, some of the new subtypes of type 2 diabetes are strongly associated with secondary complications of diabetes (e. g. SIRD with nephropathy 5 , SIDD with polyneuropathy 7 ). This new classification will hopefully allow a pathophysiologically based, more precise diabetes therapy in the future. However, randomized controlled studies are needed that test different therapies for different subtypes. Additionally, it is important to work with clear and easy criteria that identify subphenotypes with high accuracy. For this purpose, determination of endogenous insulin secretion plays an essential role.
Importance of Insulin Secretion
Insulin secretion, based on fasting C-peptide determination and calculated with the “homoeostasis model assessment 2” estimates of β-cell function (HOMA2-B), is an integral part of the classification of the new subphenotypes of diabetes. For example, the clinically most challenging subphenotypes SIDD and SIRD 5 are mainly characterized by low (mean±SD 48±29, SIDD) or increased (150±47, SIRD) HOMA2-B, respectively. Furthermore, the subphenotype SAID (type 1 diabetes) also features low insulin secretion (57±45). Therefore, the assessment of insulin secretion is an important diagnostic tool for the differentiation between insulin deficient diabetes and hyperinsulinaemic diabetes.
Importantly, a considerably high number of new manifestations of autoimmune diabetes is seen in older people. A study using a polygenic risk score based definition of type 1 diabetes revealed that 42% of all new type 1 manifestations occur after the age of 30 8 . In contrast, only 38% of individuals with newly diagnosed type 1 diabetes receive insulin therapy immediately 9 . This underlines that a detection of insulin deficiency present in late onset autoimmune diabetes is particularly important, since the failure of a timely initiation of insulin therapy and/or an inadequate therapy with ketoacidosis-promoting agents such as SGLT2 inhibitors could have fatal consequences for type 1 diabetes patients 10 .
The C-peptide/Glucose Ratio (CGR)
Measurement of C-peptide from blood serum or plasma is a reliable and well standardized laboratory method to assess endogenous insulin secretion 11 . In contrast, measurement of insulin is still not standardized and thus not well comparable between different laboratories 11 . However, the complex calculation of C-peptide-based endogenous insulin secretion using HOMA2-B which has been applied in the study of Ahlqvist et al is not commonly performed by general practitioners and diabetologists, mainly because this calculation is impractical and time demanding. The determination of fasting C-peptide or C-peptide glucose ratio have been shown to correctly classify insulin deficient type1 diabetes vs. type 2 diabetes 12 . Therefore, a simple determination of C-peptide that is adjusted for the current plasma glucose concentration could be similarly sufficient and more convenient than HOMA2-B to identify insulin deficient patients needing insulin therapy.
We examined 3751 individuals from the Tuebingen Family study and Tuebingen Lifestyle Programme with screen detected diabetes, prediabetes and normal glucose tolerance (age 18–91 years, median 46 years) 13 14 15 . We performed 7349 five-points oral glucose tolerance tests with measurement of glucose and C-peptide. The fasting C-peptide / glucose ratio correlates well with HOMA2-B (r²=0.74, p<0.0001) in the whole population ( Fig. 1a ). In screen detected, newly diagnosed patients with type 2 diabetes, an even stronger correlation is present (r²=0.80, p<0.0001, Fig. 1b ). This correlation is higher than a simple fasting –C-peptide without adjustment for plasma glucose (r²=0.47). Note that not a single individual had a CGR below 2, and the median of CGR was 5.3 in the population with normal glucose tolerance, 6.4 in the prediabetic population and 7.4 in the screen detected, newly diagnosed type 2 diabetic population (who did not take any glucose-lowering therapy or dietary measures).
Fig. 1 a.
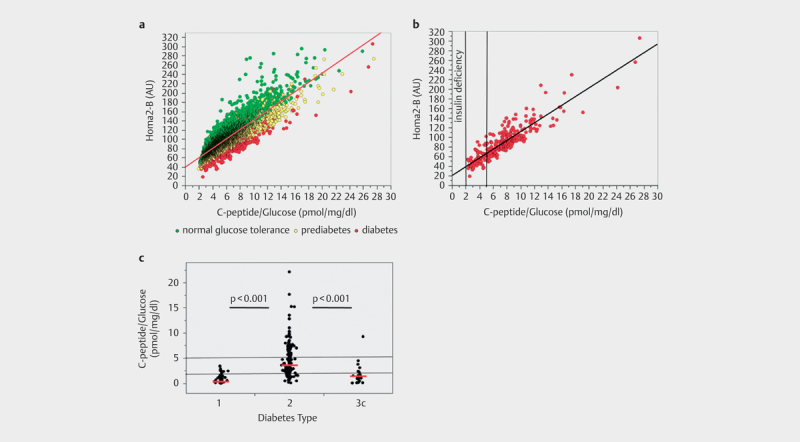
Association between fasting C-peptide / glucose ratio (CGR) and HOMA 2-B in individuals with normal glucose tolerance, prediabetes and newly diagnosed type 2 diabetes. b Association between fasting C-peptide / glucose ratio (CGR) and Homa2-B in newly diagnosed type 2 diabetes. Vertical lines indicate proposed limits for insulin deficiency (CGR<2) and non-insulin based therapy (CGR>5) c CGR on day of admission in 330 individuals with diabetes admitted to hospital for diabetes treatment, red lines indicate median CGR of the respective type of diabetes
We furthermore assessed fasting CGR in a population of 330 patients with known diabetes (type 1 diabetes: n=71, type 2 diabetes: n=238 type 3 diabetes: n=21) admitted to our university hospital for diabetes therapy (Fig. 1c). These patients were between 18–89 years old (median 58 years), had a median HbA1c of 8.7% on admission (range: 5.8 and 16.4%), and the median diabetes duration was 10 years (between 0 and 57 years of duration). In this patient population, patients with a history of type 1 diabetes had a median fasting CGR of 0.4 which was reduced to 0.1 when patients with short duration of diabetes (≤2 years) were excluded. Patients with type 2 diabetes had a median fasting CGR of 3.6 and with pancreatogenic (type 3c) diabetes a median fasting CGR of 1.4 ( Fig. 1c ).
As C-peptide is cleared by the kidney, fasting CGR could be inaccurate in renal insufficeincy. Therefore, CGR should not be used in patients with a glomerular filtration rate below 50 mL/min/1.73 m². Furthermore, CGR should not be calculated in a state of severe metabolic decompensation, such as a fasting plasma glucose above ~250 mg/dl, as glucotoxicity may acutely but reversibly impair insulin secretion. Finally, there might be minor differences between different C-peptide essays 11 which may affect generalization of limits for treatment decisions.
Three Practical Steps for a Pathophysiologically Justified, More Precise Diabetes Therapy (see Fig. 2 )
Fig. 2.
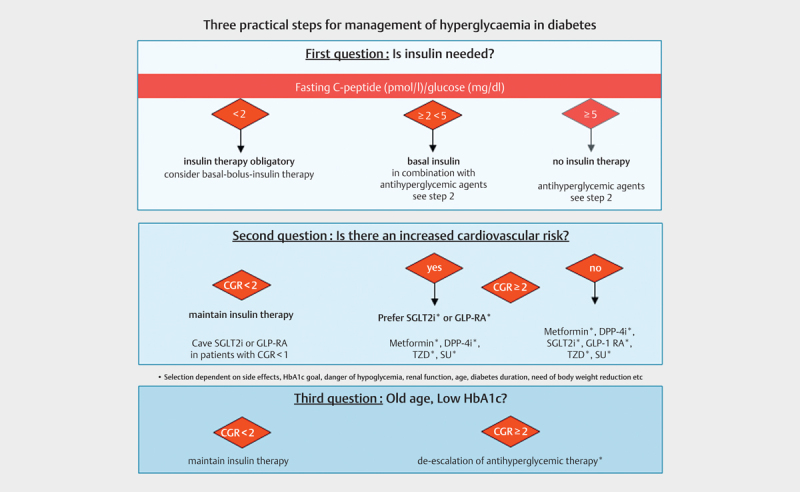
Use of CGR for diabetes therapy. DPP4i=Dipeptidylpeptidase-4 inhibitor; SGLT2i=Sodium dependent glucose co-transporter-2 inhibitor; GLP1-RA=glucagon like peptide 1 receptor agonist, TZD=thiazolidinedione, SU=sulfonylurea.
Currently, guidelines for the treatment of diabetes mellitus do not adequately address the various new subphenotypes of diabetes. Below we propose a simple concept for a more precise subphenotype oriented diabetes therapy. The importance of lifestyle intervention as a base for diabetes therapy is taken for granted.
Question 1: Is insulin needed?
This decision must be made at the beginning of a pharmacological diabetes therapy or, even more important, when modifying therapy in a patient with inadequate glycaemic control. There are two forms of insulin deficiency, autoimmune diabetes (type 1 diabetes, SIAD) and severe insulin deficient diabetes (type 2 diabetes, SIDD). Both need to be treated with insulin based on a simple rule that a specific hormone deficiency must be treated with the replacement of this specific hormone to restore normal physiology. Here, this concept is desperately needed because failure to treat insulin deficiency in diabetes can lead to ketoacidosis, coma and death.
The initial question of whether a patient with diabetes requires insulin can easily be answered by calculating the fasting CGR. A patient with HbA1c above target and a CGR of less than 2 exhibits insulin deficiency and should therefore be treated with insulin. No individual with normal glucose tolerance, prediabetes or screen detected, newly diagnosed type 2 diabetes exhibits a CGR below 2, as shown in ( Fig. 1a,b ). Patients with type 1 diabetes (among them newly diagnosed patients in remission) show a median CGR below 1 ( Fig. 1c ). Furthermore, a CGR below 2 equates to a HOMA2-B of 50 which is well in the range of SAID and SIDD in the classification of Ahlqvist et al 5 . The lower the CGR, the more likely a basal bolus insulin therapy is required. Furthermore, it has to be added that an auto-antibody determination is clinically not helpful in answering the question of whether or not to treat with insulin 8 16 .
Another purpose of calculating CGR is the identification of patients who will not necessarily require insulin treatment, because they exhibit a high insulin secretion or insulin hypersecretion. However, it is challenging to locate a threshold for this. One suitable approach may be to define a fasting CGR 5 as a threshold. An argument for such a limit is that the median fasting CGR in a large middle-aged population of healthy individuals is 5.3. Therefore, a fasting CGR of >5 presumably indicates that there is enough endogenous insulin secreted. Another argument for such a threshold comes from the study from Sweden, where the non-insulin-treated diabetes group SIRD, that is characterized by insulin hypersecretion and insulin resistance, had a mean HOMA2-B index of ~150 5 . This corresponds to a fasting CGR of 10 (Fig 1a/b).
Question 2: Is there a cardiovascular risk?
If it has been decided that an insulin therapy is absolutely necessary (CGR <2), possible (fasting CGR 2–5) or to be avoided (fasting CGR> 5), then it should be further decided whether a cardiovascular risk exists to guide the appropriate oral antihyperglycaemic therapy. If such a risk is present, SGLT2 inhibitors or GLP-1 agonists should be primarily used, as studies show that they cause a reduction in cardiovascular mortality and morbidity 1 . This is undisputed unequivocally supported in both the current ADA/EASD and the ESC guidelines 2 3 . Such a therapy is recommended regardless of the level of HbA1c. However, it is also important in this therapeutic decision to pay attention to the CGR, which indicates whether an insulin deficient diabetes is present (CGR <2). Currently GLP-1 agonists are not approved in type 1 diabetes, and only some SGLT2 inhibitors are approved for this indication in Europe. If the CGR is less than 1 (= absolute insulin deficiency, risk of ketoacidosis), SGLT2 therapy should be initiated very carefully and never without insulin therapy.
Question 3: Old age and low HbA1c?
Milder forms of type 2 diabetes (MARD and MOD) are also described in the new classification 5 . These are characterized by slightly elevated HbA1c, higher age at onset and lower incidence of complications. A de-escalation of pharmacological glucose-lowering therapy could be considered for already initiated therapies, when there is a sufficient endogenous insulin secretion (e. g. a CGR of more than 2) to prevent decompensation of glucose metabolism. The possibility of de-escalation should also depend from the cardiovascular risk and the HbA1c level. An HbA1c of anything above 8% should only be accepted in exceptional cases. However, HbA1c goals for different types of diabetes patients are highly individual, and there is no broad consensus regarding the limits 17 .
Again, fasting CGR also plays an important role in therapeutic decisions in favour or against insulin therapy. If insulin deficiency exists (CGR <2), discontinuation of insulin is not recommended in any patient including elderly patients, irrespective of age and HbA1c. When the CGR is lower than 1, the discontinuation of insulin is potentially life-threatening. Such values are not only common in type 1 diabetes but can also occur in older type 2 diabetes patients with a disease duration of decades. If the CGR is below 1, SGLT2 and/or GLP-1 therapy which could be indicated by cardiovascular risk should be used carefully and never without insulin therapy.
Summary
The application of fasting CGR in patients with diabetes provides a practical way for selecting a more precise diabetes therapy according to the concept of the newly proposed diabetes subphenotypes. Three simple steps can help choosing a pathophysiologically justified therapy. However, prospective randomized clinical studies in precision diabetes therapy are still missing, and regulatory considerations have to be taken into account 18 . Insulin deficiency is an essential factor determining antidiabetic therapy. Therefore, endogenous insulin secretion should always be assessed when starting a new therapy or changing the treatment regimen.
Author Contributions Statement
A.F. analysed the data and wrote the manuscript. A.F., M.H., A.P., H-U.H and R.W. contributed to data acquisition. All authors contributed to the interpretation of data and edited the manuscript. All authors have reviewed the manuscript.
Acknowledgments
We thank all the research volunteers for their participation. We gratefully acknowledge the excellent technical assistance of the Diabetes Research Unit Diabetes Research and Metabolic Diseases of the Helmholtz Center Munich at the University of Tübingen, Germany.
Funding Statement
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors’.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Zheng S L, Roddick A J, Aghar-Jaffar R et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580–1591. doi: 10.1001/jama.2018.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies M J, D'Alessio D A, Fradkin J et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61:2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 3.Buse J B, Wexler D J, Tsapas A et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63:221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 4.Cosentino F, Grant P J, Aboyans V et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 5.Ahlqvist E, Storm P, Käräjämäki A et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 6.Dennis J M, Shields B M, Henley W E et al. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019;7:442–451. doi: 10.1016/S2213-8587(19)30087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaharia O P, Strassburger K, Strom A et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 2019;7:684–694. doi: 10.1016/S2213-8587(19)30187-1. [DOI] [PubMed] [Google Scholar]
- 8.Thomas N J, Jones S E, Weedon M N et al. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6:122–129. doi: 10.1016/S2213-8587(17)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas N J, Lynam A L, Hill A V et al. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia. 2019;62:1167–1172. doi: 10.1007/s00125-019-4863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeder S, Edelman S V.Sodium-glucose co-transporter inhibitors as adjunctive treatment to insulin in type 1 diabetes: A review of randomized controlled trials Diabetes Obes Metab 20192162–77.doi:10.1111/dom.13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hörber S, Achenbach P, Schleicher E et al. Harmonization of immunoassays for biomarkers in diabetes mellitus. Biotechnol Adv. 2020;39:107359. doi: 10.1016/j.biotechadv.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Becht F S, Walther K, Martin E et al. Fasting C-peptide and Related Parameters Characterizing Insulin Secretory Capacity for Correctly Classifying Diabetes Type and for Predicting Insulin Requirement in Patients with Type 2 Diabetes. Exp Clin Endocrinol Diabetes. 2016;124:148–156. doi: 10.1055/s-0035-1565177. [DOI] [PubMed] [Google Scholar]
- 13.Herzberg-Schäfer S A, Staiger H, Heni M et al. Evaluation of fasting state-/oral glucose tolerance test-derived measures of insulin release for the detection of genetically impaired β-cell function. PLoS One. 2010;5:e14194. doi: 10.1371/journal.pone.0014194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schäfer S A, Tschritter O, Machicao F et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia. 2007;50:2443–2450. doi: 10.1007/s00125-007-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid V, Wagner R, Sailer C et al. Non-alcoholic fatty liver disease and impaired proinsulin conversion as newly identified predictors of the long-term non-response to a lifestyle intervention for diabetes prevention: results from the TULIP study. Diabetologia. 2017;60:2341–2351. doi: 10.1007/s00125-017-4407-z. [DOI] [PubMed] [Google Scholar]
- 16.Bingley P J.Clinical applications of diabetes antibody testing J Clin Endocrinol Metab 20109525–33.doi:10.1210/jc.2009-1365 [DOI] [PubMed] [Google Scholar]
- 17.Cahn A, Raz I, Kleinman Y et al. Clinical assessment of individualized glycemic goals in patients with type 2 diabetes: Formulation of an algorithm based on a survey among leading worldwide diabetologists. Diabetes Care. 2015;38:2293–2300. doi: 10.2337/dc15-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mol PG M, Thompson A, Heerspink HJ L et al. Precision medicine in diabetes and diabetic kidney disease: Regulatory considerations. Diabetes Obes Metab. 2018;20:19–23. doi: 10.1111/dom.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]


