Abstract
Brucellae survive acidic pHs in phagolysosomes. Azithromycin, streptomycin, and quinolones were active against Brucella melitensis at pH 7.0 but not at pH 5.0; rifampin and doxycycline retained activity at pH 5.0. Regardless of pH, azithromycin-rifampin and ofloxacin-rifampin showed less synergy than established streptomycin-doxycycline and rifampin-doxycycline combinations.
Brucellosis occurs worldwide but is most frequent in the Mediterranean basin and South America (13). Because the bacteria are intracellular, successful treatment requires antibiotics with good cellular penetration: combinations of doxycycline with either rifampin or an aminoglycoside usually are effective, but administration for 6 weeks is required and relapse is frequent (9). Consequently, new treatments are sought. Fluoroquinolones and newer macrolides have good anti-Brucella activity in vitro (1, 9, 12) and reach high intracellular concentrations, but their in vitro activity may predict efficacy poorly, since brucellae survive in compartments that are inaccessible or hostile to antimicrobial activity. These include the phagolysosomes of macrophages, where the pH may be as low as 5.0 (8). Acidity impairs the activities of quinolones and macrolides.
Moreover, as new antimicrobials may be used in combination, their interactions with established anti-Brucella agents need assessment. We therefore evaluated the in vitro activities of doxycycline, rifampin, streptomycin, quinolones, erythromycin, and azithromycin alone and in combination against Brucella melitensis at pH 5.0 and pH 7.0.
Bacteria.
The 43 B. melitensis isolates were collected between 1991 and 1994 from blood or bone marrow cultures of individual inpatients with acute brucellosis at Hacettepe University Hospital. They were identified to the species level by conventional methods, on the basis of not requiring CO2 and not producing H2S. A class II biological safety cabinet was used.
MICs at different pH values.
In vitro activities of doxycycline (Sigma, St. Louis, Mo.), streptomycin (Sigma), rifampin (Sigma), ofloxacin (Hoechst Marion Roussel, Istanbul, Turkey), ciprofloxacin (Bayer, Istanbul, Turkey), erythromycin (Sigma), and azithromycin (Pfizer, Istanbul, Turkey) were determined by microdilution. Mueller-Hinton broth (Oxoid, Basingstoke, Hants, United Kingdom), supplemented with 1% PoliVitex (BioMèrieux, Marcy l’Etoile, France) and adjusted to pH 7.0 or pH 5.0, was used. The inoculum was 105 to 106 CFU per well, and the trays were incubated at 35°C. MICs were evaluated after 48 h. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 served as controls.
Combination studies.
Twenty of the 43 isolates were chosen randomly for the combination studies. The activities of azithromycin-rifampin, ofloxacin-rifampin, doxycycline-rifampin, and doxycycline-streptomycin were tested by checkerboard titration (5) at pH 7.0 or pH 5.0. The media, inocula, and conditions were the same as those for MIC tests. Fractional inhibitory concentrations (FICs) were calculated as (MIC of antibiotic in combination)/(MIC of antibiotic alone) and summed to give ΣFIC indices, which were classified as follows: ≤0.75, synergistic; 0.75 to 1, additive; 1 to 2, indifferent; ≥2, antagonistic (11).
Activities of individual antibiotics.
All the antibiotics except erythromycin had good activities against most isolates at pH 7.0, with MICs at which 90% of the isolates were inhibited (MIC90s) below standard National Committee for Clinical Laboratory Standards breakpoints (10). The activity of rifampin was increased two- to eightfold at pH 5.0, but the MIC50s of ofloxacin, ciprofloxacin, erythromycin, streptomycin, and azithromycin increased to well above their breakpoints. Doxycycline MICs were increased at pH 5.0, but the MIC90 remained below the breakpoint of 4 μg/ml (Table 1).
TABLE 1.
In vitro activities of antibiotics against B. melitensis isolates (n = 43) in relation to pH
| Antibiotic | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Range
|
50%
|
90%
|
||||
| pH 7.0 | pH 5.0 | pH 7.0 | pH 5.0 | pH 7.0 | pH 5.0 | |
| Erythromycin | 0.5–256 | 32–>256 | 8 | >256 | 128 | >256 |
| Azithromycin | <0.125–4 | 16–>256 | 1 | 256 | 1 | >256 |
| Streptomycin | 0.25–8 | 8–256 | 1 | 16 | 2 | 128 |
| Doxycycline | <0.125–8 | <0.125–8 | <0.125 | 1 | <0.125 | 2 |
| Rifampin | 1–32 | <0.125–1 | 2 | 0.25 | 2 | 1 |
| Ciprofloxacin | <0.125–8 | 2–>16 | 0.50 | 16 | 2 | >16 |
| Ofloxacin | <0.125–4 | 4–>16 | 1 | 16 | 1 | >16 |
Activities of antimicrobial combinations.
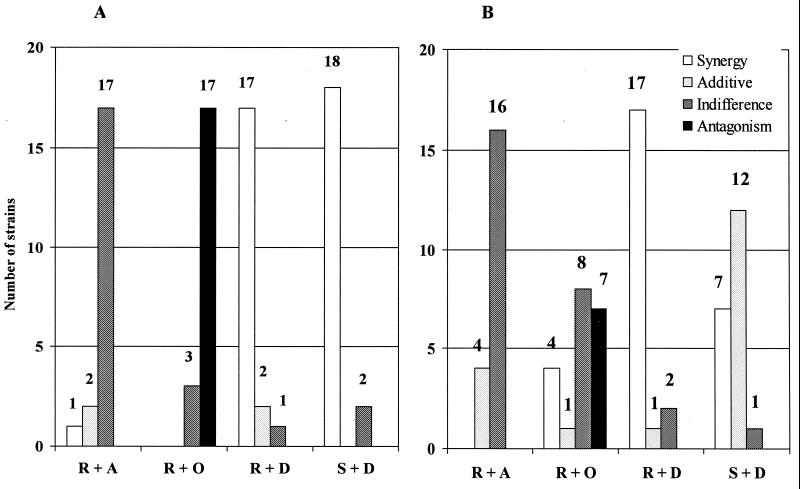
The activities of combinations at pH 7.0 and pH 5.0 are summarized in Fig. 1A and B, respectively. The established rifampin-doxycycline and streptomycin-doxycycline combinations were synergistic against almost all (17 or 18 of 20) the isolates at pH 7.0. Synergism was observed with rifampin-doxycycline for 17 of 20 isolates at pH 5.0, whereas the streptomycin-doxycycline combination was synergistic for 7, additive for 12, and indifferent for 1. The rifampin-azithromycin combination was indifferent for activity against most (16 to 17 of 20) isolates at both pH levels, whereas the rifampin-ofloxacin combination was antagonistic for activity against 17 isolates at pH 7.0 but only for activity against 7 isolates at pH 5.0, with synergy apparent for four organisms.
FIG. 1.
Interactions of different antibiotic combinations at pH 7.0 (A) and at pH 5.0 (B) against 20 B. melitensis isolates. R, rifampin; A, azithromycin; O, ofloxacin; D, doxycycline; S, streptomycin.
Brucellae grow and replicate in the phagolysosomes of macrophages, where the pH is 5.0 (8). Antimicrobials that penetrate this compartment and act under acidic conditions might be used as monotherapy. Only doxycycline and rifampin met these criteria, and neither is effective as monotherapy for brucellosis (9, 13). The fluoroquinolones and azithromycin had good activity at pH 7.0 but not at pH 5.0. Garcia-Rodriguez et al. (7) also found that the activity of fluoroquinolones against brucellae was two to fourfold lower at pH 5.0 than at pH 7.0. These data are supported by results of animal and clinical studies: azithromycin was less effective than doxycycline alone or with streptomycin for experimental brucellosis in mice (6), and Al-Sibai et al. (3) reported a 25% relapse rate with oral ciprofloxacin given for 6 to 8 weeks. We obtained somewhat better clinical results with oral ofloxacin, but the relapse rate was still 15% after 4 weeks (1). No relationship between in vitro susceptibility and relapse was apparent. Quinolones and macrolides might still be useful as components in multidrug therapy for brucellosis, since they show good anti-Brucella activity in compartments where the pH is ≥7.0, such as the serum. Streptomycin likewise loses activity against brucellae at pH 5.0 (Table 1) but still is useful in combination therapy for brucellosis (4). We therefore tested azithromycin and ofloxacin combined with rifampin, which has good intracellular penetration and is active at acidic pHs. Rifampin-doxycycline and streptomycin-doxycycline were tested for comparison. Acidic pHs did not compromise the synergy between rifampin and doxycycline, and although the synergy between streptomycin and doxycycline was reduced at pH 5.0, the combination remained synergistic or additive. No antagonism was seen with these established combinations. By contrast, virtually no synergy was seen with the new combinations.
Despite in vitro antagonism, we have obtained successful cures of human brucellosis with ofloxacin-rifampin, at rates comparable to those with doxycycline-rifampin (2). Ofloxacin and rifampin may achieve their highest anti-Brucella activities at different sites in vivo, perhaps evading the antagonism seen in vitro. Moreover, intracellular killing of brucellae by lysosome-tropic antibiotics such as macrolides may be augmented by host factors, and National Committee for Clinical Laboratory Standards breakpoints may not be appropriate to intracellular pathogens. The azithromycin-plus-rifampin combination has not been evaluated in vivo but may deserve study in animals, considering the poor correlation between in vivo and in vitro data for other combinations. Nevertheless, based on the present results, there is little reason to discard established regimens.
Acknowledgments
D.M.L. is grateful to the British Council for supporting a Link Program between Hacettepe University and The London Hospital Medical College, where he worked at the time of this study. This program financed the visits during which this study was partly undertaken.
REFERENCES
- 1.Akalin H, Ünal S, Gür D, Baykal M. Ofloxacin in the treatment of brucellosis. Eur J Clin Microbiol Infect Dis. 1990;1990:326–328. . (Special issue: Proceedings of the Third International Symposium on Quinolones, Vancouver, Canada, 1990.) [Google Scholar]
- 2.Akova M, Uzun Ö, Akalin H E, Hayran M, Ünal S, Gür D. Quinolones in treatment of human brucellosis: comparative trial of ofloxacin-rifampin versus doxycycline-rifampin. Antimicrob Agents Chemother. 1993;37:1831–1834. doi: 10.1128/aac.37.9.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sibai M B, Halim M A, El-Shaker M M, Khan B A, Quadri S M H. Efficacy of ciprofloxacin for treatment of Brucella melitensis infections. Antimicrob Agents Chemother. 1992;36:150–152. doi: 10.1128/aac.36.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariza J, Gudiol F, Pallares R, Viladrich P F, Rufi G, Corredoira J, Miravitlles M R. Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized double-blind study. Ann Intern Med. 1992;117:25–30. doi: 10.7326/0003-4819-117-1-25. [DOI] [PubMed] [Google Scholar]
- 5.Chow A W, Wong J, Bartlett K H. Synergistic interactions of ciprofloxacin and extended-spectrum beta-lactams or aminoglycosides against multiply drug-resistant Pseudomonas maltophilia. Antimicrob Agents Chemother. 1988;32:782–784. doi: 10.1128/aac.32.5.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domingo S, Gastearena I, Vitas A I, Lopez-Goni I, Dios-Vieitez C, Diaz R. Comparative activity of azithromycin and doxycycline against Brucella spp. infection in mice. J Antimicrob Chemother. 1995;36:647–656. doi: 10.1093/jac/36.4.647. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Rodriguez J A, Sanchez J E G, Trujillano I. Lack of effective bactericidal activity of new quinolones against Brucella spp. Antimicrob Agents Chemother. 1991;35:756–759. doi: 10.1128/aac.35.4.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 9.Lang R, Rubinstein E. Quinolones for the treatment of brucellosis. J Antimicrob Chemother. 1992;29:357–363. doi: 10.1093/jac/29.4.357. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Eighth informational supplement. NCCLS document M100-S8. Vol. 18. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 11.Peer E J, Clavijo E, Nogales M C, Garcia Luque I G. Interaction of aminoglycosides and cephalosporins against Pseudomonas aeruginosa. Correlation between interaction index and killing curve. J Antimicrob Chemother. 1988;22:175–183. doi: 10.1093/jac/22.2.175. [DOI] [PubMed] [Google Scholar]
- 12.Quadri S M, Halim M A, Ueno Y, Abumustafa F M, Postle A G. Antibacterial activity of azithromycin against Brucella melitensis. Chemotherapy (Basel) 1995;41:253–256. doi: 10.1159/000239353. [DOI] [PubMed] [Google Scholar]
- 13.Young E J. An overview of human brucellosis. Clin Infect Dis. 1995;21:283–290. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]