Abstract
Introduction
Cow's milk allergy (CMA) is common in infants and children. Clinical presentations may vary, with a range of symptoms affecting the gastrointestinal (GI), skin and respiratory systems. Whilst the primary focus of research to date has been on the management of these symptoms, studies investigating the broader clinical burden of CMA are limited.
Methods
We performed a retrospective matched cohort study examining clinical data, including allergic symptoms and infections, extracted from case records within The Health Improvement Network database. A total of 6998 children (54% male) were included in the study, including 3499 with CMA (mean age at diagnosis 4.04 months) and 3499 matched controls without CMA, observed for a mean period of 4.2 years.
Results
GI, skin and respiratory symptoms affected significantly more children with CMA (p < .001), which recurred more often (p < .001), compared with children without CMA. More children with CMA had symptoms affecting multiple systems (p < .001). CMA was associated with a greater probability of these symptoms requiring hypoallergenic formula (HAF) prescription persisting over time (log‐rank test p < .0001, unadjusted hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.76–0.85, p < .001), with a longer median duration of symptoms and HAF prescription compared with the duration of symptoms in those without CMA (3.48 vs. 2.96 years). GI, skin, respiratory and ear infections affected significantly more children with CMA than those without, increasing by 74% (p < .001), 20% (p < .001), 9% (p < .001), and 30% (p < .001) respectively. These infections also recurred more often among children with CMA, increasing by 62% for GI infections, 37% for skin and respiratory infections, and 44% for ear infections (p < .001).
Conclusions
This real‐world study provides evidence to suggest that CMA presents a significant clinical burden to children, which has implications for the healthcare system. Further research is warranted to understand the health economic impact of this, and the phenotypes, factors and management approaches which may affect clinical outcomes.
Keywords: cow's milk allergy, functional gastrointestinal disorders, infants, infections, pediatrics, primary care
This large, real‐world, restrospective cohort study provides novel evidence of a significant clinical burden of CMA in children. Children with cow's milk allergy (CMA) have significantly more gastrointestinal, skin and respiratory symptoms, lasting significantly longer, than children without CMA. Additionally, children with CMA have significantly more gastrointestinal, skin, respiratory and ear infections.

1. INTRODUCTION
Cow's milk allergy (CMA) is a common food allergy during childhood, typically presenting within the first year of life among 2‐5% of infants in Europe. 1 , 2 , 3 , 4 It involves a hypersensitive immune‐mediated reaction to cow's milk protein (CMP). 1 The avoidance of CMP is therefore the hallmark of CMA management, while ensuring nutritional adequacy. 5 This should ideally include breastmilk, and where not available, hypoallergenic formula (HAF), including extensively hydrolyzed formula (eHF) which are usually suggested as first‐line feeds with amino‐acid formula (AAF) being used for severe CMA or when symptoms remain unresolved with eHF. 1 , 3 , 6 , 7
CMA can be IgE, non‐IgE, or mixed IgE and non‐IgE mediated. 1 , 3 Approximately 44% of CMA cases are thought to be IgE mediated, 2 which is associated with an immediate onset of symptoms typically affecting one or more organ systems within minutes to an hour of exposure. 1 Conversely, up to 56% of CMA cases may be non‐IgE mediated, 2 which may be even greater in the United Kingdom. 8 These cases tend to present with delayed gastrointestinal (GI) with or without skin or respiratory symptoms, which can appear several days after CMP exposure. 1
A combination of upper and lower GI symptoms are usually observed such as reflux, vomiting, soft stool constipation, diarrhea, perianal dermatitis, presence of blood or mucus in stools, abdominal distension, colic and in some cases faltering growth. Skin symptoms such as erythema, urticaria, angio‐edema and eczema are common and respiratory symptoms may also be present, including allergic rhinitis, cough, wheeze, asthma, and anaphylaxis in rare and severe cases. 9
To date, the primary research focus has been on the resolution of the typical CMA associated symptoms, but not on the broader burden of this condition. Increased susceptibility to infections, such as upper respiratory tract 10 and ear infections, 11 has been documented in some observational studies of children with food allergies, along with lower IgA and deviated IgG classes, which may be suggestive of immunodeficiency. 10 , 12 This may have a significant impact on infants, families, and the healthcare system. This real‐world retrospective cohort study aimed to compare the clinical burden, including symptoms and infections, of children with CMA to those without.
2. METHODS AND MATERIALS
2.1. Study design
This retrospective cohort study compared case records from The Health Improvement Network (THIN, A Cegedim Proprietary Database) of children with CMA compared with children without CMA. Similar methodologies using the THIN database have been cited in more than 1000 research publications to date. 13
At the time the study was conducted, the THIN database contained anonymized longitudinal records of 2.9 million active patient records from 365 general practices in the United Kingdom. Demographic and clinical information is documented within the THIN database using read‐codes. These have been used by healthcare professionals since 1985, and provide a coded thesaurus of clinical terms. 14 Information relating to prescriptions is documented using the World Health Organisation index of Anatomical Therapeutic Chemical (ATC) codes. 15 Predefined codes can be extracted from the THIN database to provide full patient histories including their demographics, clinical symptoms, procedures, prescriptions, diagnoses, healthcare professional referrals and contacts, providing a generalizable insight into real‐world clinical practice in the United Kingdom. 16
2.2. Study population
Data were extracted on the 4th November 2020 from 6998 anonymised case records indexed within the last five years. This included 3499 children with confirmed or suspected CMA at ≤12 months of age. Confirmed CMA was defined by a CMA diagnosis read‐code. Suspected CMA, in the absence of a CMA diagnosis read‐code, was defined by the prescription of a HAF for at least three consecutive months. A cohort of 3499 children without CMA (matched for age, sex, and index of multiple deprivation [IMD]) were also included. Exclusion criteria aimed to omit children receiving HAF for documented conditions outside of allergy, and those with conditions which could impact on clinical outcomes. This included:
Children with read‐codes for intestinal failure; necrotizing enterocolitis; cancer, malignancy or tumor; congenital heart disease; cystic fibrosis; cerebral palsy; metabolic conditions; chromosomal anomalies.
Children prescribed any other medical nutrition product not indicated for CMA.
2.3. Study variables and outcome measures
Demographic data was extracted from case records including age, sex, country of residence, IMD (quintiles 1 [least deprived] to 5 [most deprived] calculated from the IMD score distribution), 17 , 18 , 19 , 20 ethnicity, presence of other allergies, and family history of allergies. Data on breastfeeding was not reliably recorded within the data set and therefore could not be included. Clinical outcome data included symptoms, selected from the National Institute for Health and Care Excellence (NICE) Clinical Knowledge Summary for CMA, 9 and infections. Symptom data included overall GI symptoms (reflux, vomiting, diarrhea, constipation, flatulence, blood in stools, mucus in stools, colic, general GI illness, and faltering growth), overall skin symptoms (eczema, urticaria, and erythema), overall respiratory symptoms (asthma and rhinitis of any type) and anaphylaxis. Infection data included GI, skin, respiratory, and ear infections. GI infections included viral gastroenteritis, gastroenteritis of other presumed infectious origin, campylobacter GI infection, and diarrhea and vomiting caused by suspected infection. Skin infections included skin and subcutaneous tissue infections. Respiratory infections included upper respiratory tract infection and acute tonsilitis. Ear infections included otitis media, infective otitis externa, and ear pain.
2.4. Statistical analysis
Outcomes were measured from birth over the duration of available data for each child (referred to as the observation period throughout) (mean: 4.2 years [range: 3.5–5.8] for both cohorts). Results were presented primarily as the number (n) and proportion (%) of children who had the outcome at least once during the observation period. Outcome data were also presented as rates per 5‐person‐years, to estimate the average number of times that each child in the cohort would experience the outcome during a five‐year period. Rates per 5‐person‐years were calculated by dividing the total number of events for a specific outcome by the total number of years over which the children were observed during the study, then multiplying by five.
Statistical analysis was performed using R software, version 4.0.2. 21 Statistical significance was set at p < .05. Between group differences in proportional data were measured using the Fisher's exact or χ 2 test of independence, as appropriate. Between group differences in rates were measured using the Poisson test. A Kaplan–Meier model was used to estimate the probability for the outcome of achieving at least three months of no symptoms (and no HAF prescription). Probability (survival) curves were generated from the model to compare the probability distribution of symptom persistence requiring HAF among the CMA group, and symptom persistence among the non‐CMA group. Their differences were compared using the log‐rank test. Median duration of symptoms (and HAF prescription for the CMA group) was also estimated for each group. A Cox proportional hazard regression model was used to determine crude (unadjusted) hazard ratios (HR) for the persistence of symptoms and HAF among CMA group, compared with the persistence of symptoms among the comparator group of children without CMA.
3. RESULTS
3.1. Characteristics
Cohort characteristics are presented in Table 1. Groups were matched for age, sex and IMD. There were some statistically significant demographic differences between the CMA and non‐CMA groups. This included country of residence, with a higher proportion children in Northern Ireland having CMA than not, and ethnicity, where the majority of the CMA group were white (of note, overall the majority of case records did not contain data on ethnicity, Table 1). As expected, more children with CMA had records of “other” allergies and a family history of allergy, compared with those without.
Table 1.
Cohort characteristics
| Characteristic | CMA (n = 3499) | non‐CMA (n = 3499) | p‐value |
|---|---|---|---|
| Male, n (%) | 1896 (54) | 1896 (54) | >.9 |
| Country of residence, n (%) | <.001 | ||
| England | 968 (28) | 1285 (37) | |
| Northern Ireland | 607 (17) | 385 (11) | |
| Scotland | 978 (28) | 1033 (30) | |
| Wales | 946 (27) | 796 (23) | |
| IMD quintile, n (%) | .071 | ||
| 5th | 776 (23) | 788 (23) | |
| 4th | 916 (27) | 915 (27) | |
| 3rd | 597 (18) | 546 (16) | |
| 2nd | 378 (11) | 449 (13) | |
| 1st | 743 (22) | 726 (21) | |
| Ethnicity, n (%) | <.001 | ||
| White | 1207 (93) | 1265 (87) | |
| Mixed/multiple ethnic groups | 17 (1.3) | 31 (2.1) | |
| Asian/Asian British | 51 (3.9) | 85 (5.8) | |
| Black/Black British | 19 (1.5) | 59 (4.0) | |
| Other | 10 (0.8) | 17 (1.2) | |
| Presence of “other” allergya, n (%) | 547 (16) | 184 (5.3) | <.001 |
| Family history of allergyb, n (%) | 55 (1.6) | 25 (0.7) | .001 |
Abbreviations: CMA, cow's milk allergy; IMD, index of multiple deprivation, 5th, most deprived; 1st, least deprived.
Including read‐codes documented in case records for egg allergy, peanut allergy, food allergy, history of drug allergy, history of nondrug allergy and allergic reaction unspecified.
Including read‐codes documented in case records for family history of allergic disorders, allergy, atopy, eczema and hay fever.
A read‐code for CMA was present for 29% of the CMA group, with the remainder assigned to this group due to having a HAF prescription for at least three consecutive months. Of the CMA group, all were prescribed HAF (mean: 122 [SD: 35.6] g/day, for a mean of 9.5 [SD: 9.1] months) of whom 88% were prescribed eHF and 35% AAF, indicating that some children had both feeds prescribed during the observation period. The mean age of CMA diagnosis (defined as age at recording of a CMA read‐code or first HAF prescription) was 4.04 (SD: 2.79) months.
3.2. Symptoms
3.2.1. GI, skin and respiratory symptoms
During the observation period, GI, skin and respiratory symptoms occurred in both groups, but affected significantly more children in the CMA cohort than those in the non‐CMA cohort (Table 2). These differences were statistically significant for all types of symptoms, except for blood in stools (p = .073), mucus in stools (p= .6) and anaphylaxis (p = .12), which were infrequently recorded in both cohorts. Significantly more infants with CMA had symptoms which affected multiple organ systems, compared with those without CMA (28% vs. 14%, p < .001).
Table 2.
Differences in the proportion of children with GI, skin, respiratory, and multisystem symptoms in the CMA versus non‐CMA cohort
| CMA (n = 3499) | Non‐CMA (n = 3499) | p‐value | |
|---|---|---|---|
| Overall GI symptoms, n (%) | 2262 (65) | 1463 (42) | <.001 |
| Reflux | 534 (15) | 116 (3.3) | <.001 |
| Vomiting | 907 (26) | 519 (15) | <.001 |
| Diarrhea | 977 (28) | 609 (17) | <0001 |
| Constipation | 843 (24) | 545 (16) | <.001 |
| Flatulence | 29 (0.8) | 9 (0.3) | .002 |
| Blood in stools | 29 (0.8) | 16 (0.5) | .073 |
| Mucus in stools | 3 (<0.1) | 1 (<0.1) | .6 |
| Colic | 317 (9.1) | 95 (2.7) | <.001 |
| General GI illness | 530 (15) | 238 (6.8) | <.001 |
| Faltering growth | 75 (2.1) | 27 (0.8) | <.001 |
| Overall skin symptoms, n (%) | 1286 (37) | 841 (24) | <.001 |
| Eczema | 1215 (35) | 776 (22) | <.001 |
| Urticaria and erythema | 140 (4) | 92 (2.6) | .002 |
| Overall respiratory symptoms, n (%) | 274 (7.8) | 140 (4.0) | <.001 |
| Asthma | 250 (7.1) | 132 (3.8) | <.001 |
| Rhinitis | 25 (0.7) | 12 (0.3) | .048 |
| Anaphylaxis | 6 (0.2) | 1 (<0.1) | .12 |
| Proportion of infants with multiple systems affected (GI/respiratory/skin), n (%) | |||
| <2 systems affected | 2522 (72%) | 3011 (86%) | <.001 |
| ≥2 systems affected | 977 (28%) | 488 (14%) |
Abbreviations: CMA, cow's milk allergy; GI, gastrointestinal.
Additionally, the rate of overall GI, skin and respiratory symptoms per 5‐person‐years was significantly higher among children with CMA compared with those without CMA (Table 3). GI symptoms were the most common in both cohorts, and children with CMA had twice as many episodes of GI, skin and respiratory symptoms than those without.
Table 3.
Differences in the rate of GI, skin, respiratory and anaphylaxis symptoms in the CMA versus non‐CMA cohort
| Symptom rate per 5 person‐years | CMA (n = 3499) | Non‐CMA (n = 3499) | p‐value |
|---|---|---|---|
| Overall GI symptoms | 2.15 | 1.00 | <.001 |
| Reflux | 0.27 | 0.06 | <.001 |
| Vomiting | 0.48 | 0.24 | <.001 |
| Diarrhea | 0.50 | 0.28 | <.001 |
| Constipation | 0.49 | 0.29 | <.001 |
| Flatulence | 0.01 | 0.005 | .004 |
| Blood in stools | 0.01 | 0.005 | .041 |
| Mucus in stools | <0.000 | <0.000 | .625 |
| Colic | 0.13 | 0.04 | <.001 |
| General GI illness | 0.23 | 0.10 | <.001 |
| Overall skin symptoms | 1.05 | 0.55 | <.001 |
| Eczema | 0.98 | 0.51 | <.001 |
| Urticaria and erythema | 0.06 | 0.04 | <.001 |
| Overall respiratory symptoms | 0.20 | 0.10 | <.001 |
| Asthma | 0.16 | 0.08 | <.001 |
| Rhinitis | 0.01 | 0.005 | .023 |
| Anaphylaxis | <0.000 | <0.000 | .070 |
Abbreviations: CMA, cow's milk allergy; GI, gastrointestinal.
Kaplan–Meier analysis showed that the CMA cohort had a significantly greater probability of these symptoms requiring HAF prescription persisting over time (log‐rank test, p < .0001, unadjusted HR: 0.81, 95% confidence intervals: 0.76–0.85, p < .001). For children with CMA, this was associated with a longer median duration of symptoms and HAF prescription, compared with the median duration of symptoms among those without CMA (3.48 vs. 2.96 years for the non‐CMA cohort, Figure 1).
Figure 1.
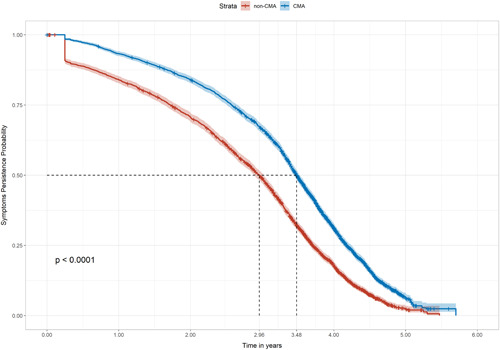
Kaplan–Meier analysis of the probability of symptom persistence over the observation period (log rank: p < .0001). CMA, cow's milk allergy
3.2.2. Infections
During the observation period, GI, skin, respiratory and ear infections occurred in both groups. Respiratory infections were the most common, followed by skin infections. Table 4 illustrates that all categories of infections significantly affected more children with CMA and at a greater frequency per 5 person‐years, compared with children without CMA (p < .001). Overall, 92% (n = 3208) of children with CMA suffered from an infection compared with 86% (n = 3001) in the non‐CMA cohort (p < .001).
Table 4.
Differences in occurrence of infections in the CMA versus non‐CMA cohort
| CMA (n = 3499) | Non‐CMA (n = 3499) | p‐value | |
|---|---|---|---|
| GI infections | |||
| n (%)a | 282 (8.1) | 162 (4.6) | <.001 |
| Infection rateb | 0.105 | 0.065 | <.001 |
| Skin infections | |||
| n (%)a | 1898 (54) | 1584 (45) | <.001 |
| Infection rateb | 1.305 | 0.955 | <.001 |
| Respiratory infections | |||
| n (%)a | 3098 (89) | 2854 (82) | <.001 |
| Infection rateb | 6.88 | 5.03 | <.001 |
| Ear infections | |||
| n (%)a | 875 (25) | 673 (19) | <.001 |
| Infection rateb | 0.51 | 0.355 | <.001 |
Abbreviations: CMA, cow's milk allergy; GI, gastrointestinal.
Proportion of children with at least one infection during observation period.
Per 5‐person‐years.
4. DISCUSSION
To our knowledge, this is the first large real‐world cohort study to compare the clinical burden of children with CMA to those without. This data, from nearly 7000 children who were observed for more than 4 years on average, shows that children with CMA not only suffer from more symptoms, but also face a significantly greater infectious burden than children without CMA, providing valuable insights into the clinical experiences of children with CMA in the United Kingdom.
Interesting differences in the demographic distribution of cohorts were present. A higher proportion of the CMA cohort resided in Northern Ireland, compared with the non‐CMA cohort. This may be a reflection of local training programs in recent years, leading to increased awareness of CMA and changes to prescribing practice among GPs. “Other” allergies and family history of allergy were also more prevalent among the CMA cohort, which is consistent with previous studies which have identified these as risk factors for CMA. 22 , 23 , 24
As expected, GI, skin and respiratory symptoms were present in both cohorts, with such symptoms commonly experienced during infancy as part of immune development. 25 Recent research suggests that 5‐20% of infants have colic, 26 with several possible aetiologies hypothesized in addition to CMA. 27 , 28 Similarly, reflux and functional constipation affect 30%–67% and 3%–27% of infants respectively and may be behavioral, physiological or atopic in etiology. 26 Skin rashes are also common in infants, accounting for 20%–30% of primary care visits. 29 Whilst a proportion of eczema cases may be non‐atopic, 30 there is a greater prevalence of CMA among children with infantile eczema. 31 Similarly, atopic dermatitis may present in 11%–20% of children in the United Kingdom, 32 and has been found to be more common in children with CMA than those without. 33 The presentation of these symptoms in children with and without CMA may lead to challenges with differential diagnosis in clinical practice. This invites future research about the prognostic impact of the timing of CMA diagnosis and management.
In the present study, whilst symptoms were common to both cohorts, those with CMA had significantly more symptoms compared with children without CMA. GI symptoms affected 55% more children with CMA, occurring 115% more often. The greatest increases in prevalence were seen in reflux and colic. Skin symptoms affected more children with CMA, with increases of 57% in eczema and 52% in urticaria and erythema, recurring 91% more often overall. Respiratory symptoms affected 96% more children with CMA, increasing by 108% for rhinitis and 89% for asthma, recurring 100% more frequently overall. Moreover, the proportion of children with symptoms affecting multiple organ systems was also significantly higher among the CMA cohort, indicating a greater cumulative burden of symptoms, compared with those without.
Importantly, these symptoms persisted over a significantly longer period in children with CMA, compared with those without, equating to an additional 6.2 months (+18%) median duration of symptoms. This persistence of allergic symptoms in those with CMA, beyond what might be expected and observed in those without CMA, supports previous findings in the literature. Indeed, the concept of an “allergic march” has been described extensively, relating in particular to the role of atopic dermatitis as a trigger for the later development of respiratory conditions such as asthma and rhinitis 34 , 35 , 36 , 37 and of gastrointestinal conditions including irritable bowel syndrome. 38 Studies suggest that 50%–75% of children with CMA will outgrow their allergy by 2 years of age, although this may vary with the type of CMA. 39 , 40 , 41 , 42 Whilst it is not possible to determine CMA outgrowth from the THIN database, the findings of this study may support the hypothesis of an early role of CMA in the allergic march, 35 with an ongoing clinical impact beyond its outgrowth.
These findings have implications for allergic children, their families and the healthcare system. Functional gastrointestinal symptoms (FGIDs) such as colic, constipation and regurgitation may have a personal burden and lead to economic costs for families and healthcare systems. 26 Furthermore, allergic symptoms are correlated with lower quality of life 43 and psychosocial burden, 44 which may be exacerbated by delayed diagnosis. Indeed, there have been reports of dissonance between GP and parental experiences of allergic conditions within the literature, with parents of children with eczema feeling that the psychosocial impact of symptoms is overlooked by GPs, 45 and over half of parents of children with CMA stating that they were made to feel like they were over‐reacting or “worrying too much about nothing.” 44 These findings support the notion that the clinical experiences of children with CMA are significantly different from those of children without CMA, and may therefore help to reconcile discrepant perspectives between parent and physician of the clinical burden of CMA.
A novel and important finding of this study was that a range of infections occurred in significantly more children with CMA, more often, compared with children without CMA. The greatest increases were found in GI infections, occurring in 74% more children, followed by ear, skin and respiratory infections (30%, 20%, and 9% increases, respectively). Respiratory infections are the most common infection in early childhood, 46 which may explain the smaller differences between groups. However, children with CMA also had more frequent episodes of infection, with 62% more GI infections, 44% more ear infections and 37% more skin and respiratory infections respectively.
Several studies have recognized the association between atopic conditions and infection, finding an increased susceptibility to infections of bacterial, fungal and viral origin in children. 47 Ear infections have previously been reported in children with allergic conditions 11 , 48 , 49 and, among those with food protein‐induced gastrointestinal allergy, 68% have been reported to have frequent upper respiratory tract infections occurring more than once a month and lasting longer than their siblings. 10
The dualistic mechanisms between allergic and infectious illnesses are not completely understood. 50 Ear infections are a common manifestation of reflux in young infants, 51 which was more common in the CMA cohort and may suggest a non‐atopic mechanism. There is also speculation of an inhibitive interaction between allergic inflammation and antiviral cytokines, which may account for the concurrence of chronic otitis media in allergic rhinitis 52 and viral infections in atopic asthma. 50 Indicators of immunodeficiencies including deviated levels of serum immunoglobulin‐A, immunoglobulin‐G subclasses and lymphocytes among children with allergy 10 , 12 may suggest an inadequate immune response. This may be further affected by gut bacteria 12 which influences the development and maintenance of immune homeostasis, and defends against pathogenic colonization. 53 , 54 , 55 Gut dysbiosis is prevalent among infants with CMA 56 , 57 , 58 , 59 , 60 and may contribute to their increased incidence of infections. 53 Studies have shown reductions in infections associated with the modification of the dysbiotic gut microbiota, with the use of an AAF containing pre‐ and probiotics (synbiotics). 57 , 61 This invites further research into this potential therapeutic target for immune health in allergic disease.
This study has a number of limitations. Whilst the prevalence and frequency of symptoms may vary in the first 3 years of life, 8 changes in specific symptoms over time were not investigated, causing difficulty in distinguishing symptoms associated with allergy from those of common FGIDs which may be outgrown in early life. There was also no differentiation between symptoms before or following CMA diagnosis, with no investigation of the impact of the timing of diagnosis on clinical outcomes, which warrants further research. Furthermore, read‐codes may only be recorded when the patient presents to the clinician, such as for a repeat prescriptions, leading to potential underreporting of recurrent episodes.
Indeed, variations in recording practices may have contributed to differences between the data generated in this study, and that reported elsewhere. “Other” allergies were recorded in a small proportion of the CMA cohort, whereas they have been observed in 91% of patients with IgE‐mediated CMA. 24 Similarly, whilst there is up to 72% chance of atopy in infants when present in both parents, family history of allergy was infrequently recorded, suggesting this information is not routinely documented in primary care. Emergency attendance for anaphylaxis may not be transferred to GP records, which may explain why incidence was low and similar between groups. Blood and mucus in stools are common in children with CMA, 62 but were infrequently recorded in the present study, possibly due to limited read‐codes. Whilst asthma is a recognized comorbidity of CMA 9 and present in 13.5% of the general population of children in England at four years of age, 63 overall prevalence was low in the present study. This may reflect challenges with diagnosis in young children, 63 warranting the inclusion of alternative read‐codes, such as cough or wheeze, to increase sensitivity of data analysis. However, this would reduce the specificity of the results, as such symptoms could have allergic or infectious causes. Therefore, where possible, read‐codes for comorbidities (such as asthma and eczema) were used instead of generic symptoms (such as wheeze and rash) to differentiate between manifestations of CMA and infection, which itself presents further challenges in clinical practice. However, a similar margin of error may apply across the cohort, affecting absolute values but not necessarily differences between groups.
Absence of read‐codes prevented the analysis of other symptoms of interest (such as angioedema, perianal dermatitis and specific classifications of rhinitis) and IgE status, which may be correlated with the timing of CMA outgrowth. 39 CMA outgrowth itself was also not recorded, leading to uncertainty as to whether persistent symptoms related to ongoing CMA or manifestations of the allergic march. CMA diagnosis read‐codes were also infrequently recorded throughout the THIN database. HAF prescription for at least three months was therefore used as a diagnostic proxy for the CMA cohort, in the absence of a read‐code. Chronic conditions were excluded as described in the methodology, which is expected to have omitted children requiring HAF for chronic conditions outside of allergy 64 and for a 2–4 week diagnostic elimination diets. 9 However, this pragmatic approach meant that all of the CMA cohort had a HAF prescription. As breastfeeding is recommended first‐line in allergic infants, this may not reflect actual clinical practice, and could highlight potentially confounding differences between groups. However, HAF prescription may have been exclusive or additive to breastfeeding, and exclusive breastfeeding may have been practiced before or after HAF prescription. Breastfeeding is not commonly coded in GP databases, and published statistics on breastfeeding in CMA are lacking. In the general UK population, by four months of age (comparable with the mean age of CMA diagnosis and/or first HAF prescription in the CMA cohort) 12% of infants are exclusively breastfed, 65 suggesting many children in the non‐CMA group may have been formula‐fed. Therefore, whilst different distributions of ethnic groups 66 , 67 and country of residence 65 may lead to some variations between groups, similar rates of exclusive breastfeeding may be assumed across the cohorts. Further research to better understand breastfeeding practices in CMA is warranted.
5. CONCLUSIONS
Overall, the findings of this study are suggestive of a heightened clinical burden among children with CMA that may have additional implications for the healthcare system. Previous research has demonstrated an extensive impact of allergic conditions on UK healthcare services, with substantial associated costs. This large real‐world cohort study provides novel evidence of a significant clinical burden of CMA in children. Additional research is required to further quantify the healthcare burden of CMA with comprehensive cost‐effectiveness modeling, and to investigate the impact of clinical phenotypes and factors such as the timing of diagnosis and approaches to management, which may impact the broader clinical outcomes of children with CMA.
CONFLICT OF INTERESTS
R. M. and K. E. G. have previously received honoraria from Nutricia, Nestle Health Science, Mead Johnson and Abbott. D.A.‐M. is an honorary Associate Professor at the Institute of Health Informatics, University College London, UK, and an employee of Cegedim Rx, who was funded by Nutricia. to undertake the research. K. S. is employed by Nutricia. A. L. C. and R. J. S., both of whom hold honorary research posts with the University of Southampton, are also employed part‐time by Nutricia.
ETHICS STATEMENT
Ethical approval for this study was granted by the Scientific Review Committee which reviews all research involving the THIN database (protocol reference number: 20‐009).
AUTHOR CONTRIBUTIONS
Conceptualization: Katy Sorensen, Abbie L. Cawood, and Rebecca J. Stratton. Methodology: Katy Sorensen, Abbie L. Cawood, and Dionisio Acosta‐Mena. Formal analysis: Dionisio Acosta‐Mena. Data curation: Katy Sorensen, Abbie L. Cawood, and Dionisio Acosta‐Mena. Writing—original draft preparation: Katy Sorensen. Writing—review and editing: Katy Sorensen, Abbie L. Cawood, Rosan Meyer, Kate E. Grimshaw, Dionisio Acosta‐Mena, and Rebecca J. Stratton. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENT
This study was funded by Nutricia Ltd., Trowbridge, UK.
Sorensen K, Meyer R, Grimshaw KE, Cawood AL, Acosta‐Mena D, Stratton RJ. The clinical burden of cow's milk allergy in early childhood: a retrospective cohort study. Immun Inflamm Dis. 2022;10:e572. 10.1002/iid3.572
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow's milk allergy (DRACMA) guidelines. World Allergy Organ J. 2010;3(4):57‐125. 10.1111/j.1399-3038.2010.01068.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schoemaker AA, Sprikkelman AB, Grimshaw KE, et al. Incidence and natural history of challenge‐proven cow's milk allergy in European children—EuroPrevall birth cohort. Allergy. 2015;70(8):963‐972. 10.1111/all.12630 [DOI] [PubMed] [Google Scholar]
- 3. Luyt D, Ball H, Makwana N, et al. BSACI guideline for the diagnosis and management of cow's milk allergy. Clin Exp Allergy. 2014;44(5):642‐672. 10.1111/cea.12302 [DOI] [PubMed] [Google Scholar]
- 4. Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta‐analysis. Allergy. 2014;69(8):992‐1007. 10.1111/all.12423 [DOI] [PubMed] [Google Scholar]
- 5. Ludman S, Shah N, Fox AT. Managing cows' milk allergy in children. Br Med J. 2013;347:f5424. 10.1136/bmj.f5424 [DOI] [PubMed] [Google Scholar]
- 6. Koletzko S, Niggemann B, Arato A, et al. Diagnosis approach and management of cow's milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55:221‐229. 10.1097/MPG.0b013e31825c9482 [DOI] [PubMed] [Google Scholar]
- 7. Meyer R, Groetch M, Venter C. When should infants with cow's milk protein allergy use an amino acid formula? A practical guide. J Allergy Clin Immunol. 2017;6(2):383‐399. 10.1016/j.jaip.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 8. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63(3):354‐359. 10.1111/j.1398-9995.2007.01570.x [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence . Clinical knowledge summaries: cow's milk allergy in children. https://cks.nice.org.uk/topics/cows-milk-allergy-in-children/. Accessed 7 April 2021.
- 10. Meyer R, Fleming C, Dominguez‐Ortega G, et al. Manifestations of food protein induced gastrointestinal allergies presenting to a single tertiary paediatric gastroenterology unit. World Allergy Organ J. 2013;6(1):1‐9. 10.1186/1939-4551-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Juntti H, Tikkanen S, Kokkonen J, Alho OP, Niinimäki A. Cow's milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol. 1999;119(8):867‐873. 10.1080/00016489950180199 [DOI] [PubMed] [Google Scholar]
- 12. Latcham F, Merino F, Lang A, et al. A consistent pattern of minor immunodeficiency and subtle enteropathy in children with multiple food allergy. J Pediatr. 2003;143(1):39‐47. 10.1016/s0022-3476(03)00193-8 [DOI] [PubMed] [Google Scholar]
- 13. The Health Improvement Network . THIN research. https://www.the-health-improvement-network.com/en/#thin-research. Accessed 8 June 2021.
- 14. NHS Digital . Read codes. 2020. https://digital.nhs.uk/services/terminology-and-classifications/read-codes. Accessed 11 January 2021.
- 15. World Health Organization Collaborating Centre for Drug Statistics Methodology . International language for drug utilization research—ATC/DDD. https://www.whocc.no/. Accessed 11 January 2021.
- 16. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251‐255. 10.14236/jhi.v19i4.820 [DOI] [PubMed] [Google Scholar]
- 17. Ministry of Housing CLG . English indices of deprivation. 2019. https://imd-by-postcode.opendatacommunities.org/imd/2019. Accessed 13 July 2021.
- 18. Northern Ireland Statistics and Research Agency . Northern Ireland Multiple Deprivation Measure 2017 (NIMDM2017). https://www.nisra.gov.uk/statistics/deprivation/northern-ireland-multiple-deprivation-measure-2017-nimdm2017#toc-1. Accessed 13 July 2021.
- 19. Scottish Government . Scottish Index of Multiple Deprivation 2020. https://www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020/. Accessed 13 July 2021.
- 20. StatsWales . WIMD. 2019. https://statswales.gov.wales/Catalogue/Community-Safety-and-Social-Inclusion/Welsh-Index-of-Multiple-Deprivation/WIMD-2019. Accessed 13 July 2021.
- 21. R Core Team . R: a language and environment for statistical computing. 2020. https://www.R-project.org/. Accessed 17 March 2021.
- 22. Flom JD, Sicherer SH. Epidemiology of cow's milk allergy. Nutrients. 2019;11(5):1051. 10.3390/nu11051051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsai HJ, Kumar R, Pongracic J, et al. Familial aggregation of food allergy and sensitization to food allergens: a family‐based study. Clin Exp Allergy. 2009;39(1):101‐109. 10.1111/j.1365-2222.2008.03111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE‐mediated cow's milk allergy. J Allergy Clin Immunol. 2007;120(5):1172‐1177. 10.1016/j.jaci.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 25. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Royal Soc B. 2015;282:20143085. 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salvatore S, Abkari A, Cai W, et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants. Acta Paediatr. 2018;107(9):1512‐1520. 10.1111/apa.14378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts DM, Ostapchuk M, O'Brien JG. Infantile colic. Am Fam Physician. 2004;70(4):735‐740. [PubMed] [Google Scholar]
- 28. Mai T, Fatheree NY, Gleason W, Liu Y, Rhoads JM. Infantile colic: new insights into an old problem. Gastroenterol Clin North Am. 2018;47(4):829‐844. 10.1016/j.gtc.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singleton JK. Pediatric dermatoses: three common skin disruptions in infancy. Nurse Pract. 1997;22(6):32‐3, 37 43‐44. [PubMed] [Google Scholar]
- 30. Rożalski M, Rudnicka L, Samochocki Z. Atopic and non‐atopic eczema. Acta Dermatovenerol Croat. 2016;24(2):110‐115. [PubMed] [Google Scholar]
- 31. Kawada S, Futamura M, Hashimoto H, et al. Association between sites and severity of eczema and the onset of cow's milk and egg allergy in children. PLoS One. 2020;15(10):e0240980. 10.1371/journal.pone.0240980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cork MJ, Danby SG, Ogg GS. Atopic dermatitis epidemiology and unmet need in the United Kingdom. J Dermatolog Treat. 2020;31(8):801‐809. 10.1080/09546634.2019.1655137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hansen MM, Nissen SP, Halken S, Høst A. The natural course of cow's milk allergy and the development of atopic diseases into adulthood. Pediatr Allergy Immunol. 2021;32(4):727‐733. 10.1111/pai.13440 [DOI] [PubMed] [Google Scholar]
- 34. Bantz SK, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5(2). 10.4172/2155-9899.1000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carucci L, Nocerino R, Paparo L, Di Scala C, Berni Canani R. Dietary prevention of atopic march in pediatric subjects with cow's milk allergy. Front Pediatr. 2020;8:440. 10.3389/fped.2020.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010;105(2):99‐106; quiz 07‐9, 17. 10.1016/j.anai.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 37. Burgess JA, Lowe AJ, Matheson MC, Varigos G, Abramson MJ, Dharmage SC. Does eczema lead to asthma? J Asthma. 2009;46(5):429‐436. 10.1080/02770900902846356 [DOI] [PubMed] [Google Scholar]
- 38. Loo EXL, Wang DY, Siah KTH. Association between irritable bowel syndrome and allergic diseases: to make a case for aeroallergen. Int Arch Allergy Immunol. 2020;181(1):31‐42. 10.1159/000503629 [DOI] [PubMed] [Google Scholar]
- 39. Høst A. Frequency of cow's milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89(6 suppl 1):33‐37. 10.1016/s1081-1206(10)62120-5 [DOI] [PubMed] [Google Scholar]
- 40. Fiocchi A, Terracciano L, Bouygue GR, et al. Incremental prognostic factors associated with cow's milk allergy outcomes in infant and child referrals: the Milan Cow's Milk Allergy Cohort study. Ann Allergy Asthma Immunol. 2008;101(2):166‐173. 10.1016/s1081-1206(10)60205-0 [DOI] [PubMed] [Google Scholar]
- 41. Hill DJ, Firer MA, Ball G, Hosking CS. Natural history of cows' milk allergy in children: immunological outcome over 2 years. Clin Exp Allergy. 1993;23(2):124‐131. 10.1111/j.1365-2222.1993.tb00307.x [DOI] [PubMed] [Google Scholar]
- 42. Chatchatee P, Nowak‐Wegrzyn A, Lange L, et al. Tolerance development in cow's milk‐allergic infants receiving amino acid‐based formula: a randomized controlled trial. J Allergy Clin Immunol. 2021. 10.1016/j.jaci.2021.06.025 [DOI] [PubMed] [Google Scholar]
- 43. Meyer R, Godwin H, Dziubak R, et al. The impact on quality of life on families of children on an elimination diet for non‐immunoglobulin E mediated gastrointestinal food allergies. World Allergy Organ J. 2017;10(1):8. 10.1186/s40413-016-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lozinsky AC, Meyer R, Anagnostou K, et al. Cow's milk protein allergy from diagnosis to management: a very different journey for general practitioners and parents. Children. 2(3), 2015:317‐329. 10.3390/children2030317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Powell K, Le Roux E, Banks J, Ridd MJ. GP and parent dissonance about the assessment and treatment of childhood eczema in primary care: a qualitative study. BMJ Open. 2018;8(2):e019633. 10.1136/bmjopen-2017-019633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vissing NH, Chawes BL, Rasmussen MA, Bisgaard H. Epidemiology and risk factors of infection in early childhood. Pediatrics. 2018;141(6). 10.1542/peds.2017-0933 [DOI] [PubMed] [Google Scholar]
- 47. Ciprandi G, Tosca MA, Fasce L. Allergic children have more numerous and severe respiratory infections than non‐allergic children. Pediatr Allergy Immunol. 2006;17(5):389‐391. 10.1111/j.1399-3038.2006.00413.x [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Xu M, Zhang J, Zeng L, Wang Y, Zheng QY. Risk factors for chronic and recurrent otitis media—a meta‐analysis. PLoS One. 2014;9(1):e86397. 10.1371/journal.pone.0086397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zernotti ME, Pawankar R, Ansotegui I, et al. Otitis media with effusion and atopy: is there a causal relationship? World Allergy Organ J. 2017;10(1):37. 10.1186/s40413-017-0168-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, Johnston SL. Viral infections in allergy and immunology: how allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017;140(4):909‐920. 10.1016/j.jaci.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caruso G, Passali FM. ENT manifestations of gastro‐oesophageal reflux in children. Acta Otorhinolaryngol Ital. 2006;26(5):252‐255. [PMC free article] [PubMed] [Google Scholar]
- 52. Luong A, Roland PS. The link between allergic rhinitis and chronic otitis media with effusion in atopic patients. Otolaryngol Clin North Am. 2008;41(2):311‐323. 10.1016/j.otc.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 53. Agostoni C, Axelsson I, Braegger C, et al. Probiotic bacteria in dietetic products for infants: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2004;38(4):365‐374. 10.1097/00005176-200404000-00001 [DOI] [PubMed] [Google Scholar]
- 54. Bik EM. Composition and function of the human‐associated microbiota. Nutr Rev. 2009;67(suppl 2):S164‐S171. 10.1111/j.1753-4887.2009.00237.x [DOI] [PubMed] [Google Scholar]
- 55. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859‐904. 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- 56. Thompson‐Chagoyan OC, Vieites JM, Maldonado J, Edwards C, Gil A. Changes in faecal microbiota of infants with cow's milk protein allergy—a Spanish prospective case‐control 6‐month follow‐up study. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e394‐e400. 10.1111/j.1399-3038.2009.00961.x [DOI] [PubMed] [Google Scholar]
- 57. Sorensen K, Cawood AL, Gibson GR, Cooke LH, Stratton RJ. Amino acid formula containing synbiotics in infants with cow's milk protein allergy: a systematic review and meta‐analysis. Nutrients. 2021;13(3):935. 10.3390/nu13030935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shu SA, Yuen AWT, Woo E, et al. Microbiota and food allergy. Clin Rev Allergy Immunol. 2019;57(1):83‐97. 10.1007/s12016-018-8723-y [DOI] [PubMed] [Google Scholar]
- 59. Cukrowska B, Bierła JB, Zakrzewska M, Klukowski M, Maciorkowska E. The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti‐allergic mechanisms in early life. Nutrients. 2020;12(4), 946. 10.3390/nu12040946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fox A, Bird JA, Fiocchi A, et al. The potential for pre‐, pro‐ and synbiotics in the management of infants at risk of cow's milk allergy or with cow's milk allergy: an exploration of the rationale, available evidence and remaining questions. World Allergy Organ J. 2019;12(5):100034. 10.1016/j.waojou.2019.100034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sorensen K, Cawood AL, Cooke LH, Acosta‐Mena D, Stratton RJ. The use of an amino acid formula containing synbiotics in infants with cow's milk protein allergy—effect on clinical outcomes. Nutrients. 2021;13(7):2205. 10.3390/nu13072205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. El‐Hodhod MA, Hamdy AM, El‐Deeb MT, Elmaraghy MO. Cow's milk allergy is a major contributor in recurrent perianal dermatitis of infants. ISRN Pediatr. 2012; 2012:408769. 10.5402/2012/408769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Uphoff EP, Bird PK, Antó JM, et al. Variations in the prevalence of childhood asthma and wheeze in MeDALL cohorts in Europe. ERJ Open Res. 2017;3(3), 00150‐2016. 10.1183/23120541.00150-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meyer R, Smith C, Sealy L, Mancell S, Marino L. The use of extensively hydrolysed and amino acid feeds beyond cow's milk allergy: a national survey. J Hum Nutr Diet. 2020;34:34‐23. 10.1111/jhn.12794 [DOI] [PubMed] [Google Scholar]
- 65. Health and Social Care Information Centre . Infant feeding survey. 2010. https://data.gov.uk/dataset/infantfeeding-survey-2010. Accessed 16 September 2021.
- 66. Oakley LL, Renfrew MJ, Kurinczuk JJ, Quigley MA. Factors associated with breastfeeding in England: an analysis by primary care trust. BMJ Open. 2013;3(6):e002765. 10.1136/bmjopen-2013-002765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kelly YJ, Watt RG, Nazroo JY. Racial/ethnic differences in breastfeeding initiation and continuation in the United Kingdom and comparison with findings in the United States. Pediatrics. 2006;118(5):e1428‐e1435. 10.1542/peds.2006-0714 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


