Abstract
Introduction
Patients with COVID‐19 may present different viral loads levels. However, the relationship between viral load and disease severity in COVID‐19 is still unknown. Therefore, this study aimed to systematically review the association between SARS‐CoV‐2 viral load and COVID‐19 severity.
Methods
The relevant studies using the keywords of “COVID‐19” and “viral load” were searched in the databases of PubMed, Scopus, Google Scholar, and Web of Science. A two‐step title/abstract screening process was carried out and the eligible studies were included in the study.
Results
Thirty‐four studies were included from the initial 1015 records. The vast majority of studies have utilized real‐time reverse transcription‐polymerase chain reaction of the nasopharyngeal/respiratory swabs to report viral load. Viral loads were commonly reported either as cycle threshold (C t) or log10 RNA copies/ml.
Conclusion
The results were inconclusive about the relationship between COVID‐19 severity and viral load, as a similar number of studies either approved or opposed this hypothesis. However, the studies denote the direct relationship between older age and higher SARS‐CoV‐2 viral load, which is a known risk factor for COVID‐19 mortality. The higher viral load in older patients may serve as a mechanism for any possible relationships between COVID‐19 viral load and disease severity. There was a positive correlation between SARS‐CoV‐2 viral load and its transmissibility. Nonetheless, further studies are recommended to precisely characterize this matter.
Keywords: COVID‐19, prognosis, SARS‐CoV‐2, severity, viral load
Graphical abstract
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an acute respiratory syndrome caused by coronavirus 2 (SARS‐CoV‐2) that causes inflammation and multiorgan involvement in the body. 1 , 2 , 3 The World Health Organization (WHO) declared this disease as a “public health emergency of international concern” on January 30, 2020. 4 , 5 As of September 3, 2021, there have been more than 218 million confirmed cases of COVID‐19 and 4,526,583 death have been reported around the world. 6 , 7
When SARS‐CoV‐2 enters lung cells, it attacks the lower respiratory tract and attaches strongly to its receptors in the lungs; namely, angiotensin‐converting enzyme receptors. 8 , 9 When an infection in the lower respiratory tract activates immune cells such as neutrophils and macrophages, it releases several chemokines and cytokines that activate the immune system like B and T cells, this irregular response eventually leads to elevated levels of cytokines, called cytokine storms or hypercytokinemia. 10 As a result, severe pneumonia involving various organs could develop that cause diverse symptoms and signs as well as consequent psychological harm. 1 The symptoms of COVID‐19 are fever, dry cough, dyspnea, headache, fatigue, loss of taste and/or smell, and gastrointestinal symptoms. 11 In laboratory results the liver enzymes are high, lymphocytes are low (lymphocytopenia), and C‐reactive protein levels are high. Eventually, the virus causes acute respiratory distress syndrome that may lead to death. 12 SARS‐CoV‐2 belongs to the Nidovirales order, Coronaviridae family, Coronavirinae subfamily, it is an enveloped virus with a positive‐sense, single‐stranded RNA genome of approximately 30 kb. 13
Since its emergence, the SARS‐CoV‐2 has undergone multiple mutations resulting in weaker or even or more dangerous variants of the virus. SARS‐CoV‐2 continuously evolves and potentially becomes more transmissible or fatal with each mutation. 2 Four variants of SARS‐CoV‐2 have been declared as the “variants of concern” by the WHO so far, which cause COVID‐19.
A. Alpha variant: Alpha variant, or the lineage B.1.1.7, is the first SARS‐CoV‐2 variant and can be substituted by 23 mutations. As a consequence of the mutation, the transmissibility of the virus increased by about 50% as compared to the wild strain, making it more infectious with more severe complications 14 ;
B. Beta variant: These mutations enhance the ability of the virus to attach to the human cells more easily in comparison with the previous variants 15 ;
C. Gamma variant: Gamma variant caused widespread infection in early 2021 and is currently considered as a “variant of concern” 16 ;
D. Delta variant: The Delta variant is more infectious and each infected person can transmit the virus to seven or more people. 17
For the clinical management of COVID‐19 disease, it is substantial to quantify the viral load of the blood. 18 Viral load indicates active viral proliferation and is used to identify the severe viral infections of the respiratory tract and monitor the disease progression and treatment. 19 The viral load can be obtained from the patient's viral RNA with a certain concentration (the value that exceeds the threshold) by testing the value of the C t cycle threshold of the reverse transcription‐polymerase chain reaction (RT‐PCR). The lower the C t values than a patient's sample, the higher the viral load. 20 The relationship between the viral load and severity of disease in COVID‐19 patients has not yet been fully understood. The investigation demonstrated that patients with COVID‐19 who have been treated in the intensive care unit with a severe illness have a relatively higher viral load. A study also suggested that in large hospital groups, a high viral load is associated with an increased risk of death. 21 Thus, the study of the correlation between COVID‐19 viral load and the progression of the disease and the treatment and prevention of COVID‐19 helps to science promotion significantly. 22
A Chinese study working on the association of viral load with the development of COVID‐19 found that patients with more viral load had fewer lymphocytes but more neutrophils. 23 In another study that examined the relationship between viral load and disease severity with COVID‐19 clinical results, viral load in severe disease was much higher than in mild or asymptomatic disease. 24 However, conflicts exist regarding the effects of SARS‐CoV‐2 viral load on disease severity. Therefore, the present study systematically reviewed the association between SARS‐CoV‐2 viral load and COVID‐19 severity.
2. METHODS
2.1. Data sources
Relevant articles were systematically searched from the keywords “COVID‐19” and “viral load” in the online databases of PubMed, Science Direct, Scopus, and Web of Science. All the relevant literature published from December 2019 to August 2021 was retrieved and further screened using EndNote.
2.2. Study objectives
The principal aim was to investigate the relationship between the COVID‐19 viral load and its severity. However, the relationship between viral load and COVID‐19 infectivity as well as the patients' age and viral load was also discussed.
2.3. Study selection and inclusion/exclusion criteria
We conducted a two‐phase screening process; first, the studies were evaluated based on their title and abstract, and then the eligible ones were screened based on their full texts. We included peer‐reviewed articles that studied the association between SARS‐CoV‐2 viral load and the COVID‐19 disease severity or mortality. The selected articles were cross‐examined by other researchers to avoid duplication.
The exclusion criteria were as follows:
-
‐
Literature with no available full‐texts including the conference papers and abstracts;
-
‐
Literature with the main focus of nonhuman experiments of any kind like in vitro studies, animal trials, or literature without justifying details;
-
‐
Reviews, systematic reviews, or meta‐analyses;
-
‐
Case reports.
2.4. Data extraction
Two independent investigators summarized and extracted the following information from the included publications: The first author's ID (Reference), year, and type of publication (e.g., cross‐sectional study), country of study, the sample size of the study, patient mean age and gender, sampling site, measured viral load, and disease outcome; the data were further gathered in a specifically designed sheet and organized into tables.
2.5. Quality/risk of bias assessment
We used the Newcastle–Ottawa Scale to assess the quality of the studies. 25 This scale yields a total score out of 9 to the studies based on their selection, comparability, and exposure/outcomes.
3. RESULTS
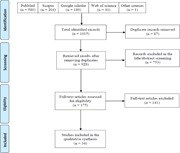
The search strategies resulted in 1015 records, being 928 remaining after removing the duplicates. Of which, 753 records were excluded in the title/abstract screening, and 175 full texts were reviewed. Finally, 34 studies met the eligibility criteria to be included after full‐text screening (Figure 1). Most of the studies were from China (n = 7); three studies per following countries: Japan, Spain, Turkey, and the USA; two studies per following countries: Italy, South Korea, and Switzerland; and one study for the following countries: Brazil, Czech Republic, England, France, Germany, India, Israel, and Singapore (Table 1). The studies had overall acceptable quality, all of them scoring 4 and above (Table 2).
Figure 1.
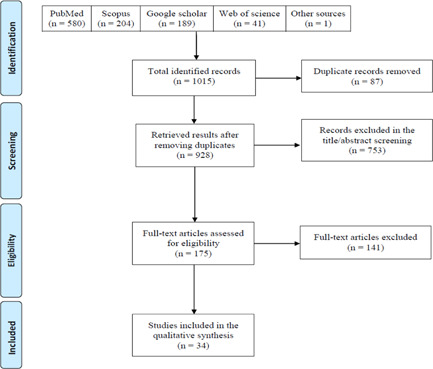
Prisma flow diagram of the study's selection process
Table 1.
Summary of the findings of the included studies
| ID | First author (reference) | Type of study | Publication year | country | Study population | age | Gender | Sampling method | Viral load and its association with disease severity | Sign/symptom | Comorbidities | Lab test | Clinical outcome | Transmission | Important finding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aoki et al. 26 | Cross‐sectional | 2021 | Japan | 24 | N/A | Female/male | RT‐PCR | There was a high correlation between viral load calculated using the RT‐PCR cycle threshold value and antigen concentration. The tendency to decrease antigen concentration over time after disease onset is associated with viral load. C t value: 25 | N/A | N/A | N/A | N/A | N/A | SQT is fully compatible with RT‐PCR and should be useful in diagnosing COVID‐19 in any clinical setting |
| Nasopharyngeal swab | |||||||||||||||
| 2 | Aydin et al. 27 | Case series | 2021 | Turkey | 125 | 62,1 | 48.8% male | RT‐PCR | The effect of SARS‐CoV‐2 viral load on saliva and other substances was not found in their prognosis. C t value: 22.28 | N/A | Hypertension, COPD, DM, malignancy, immune deficiency, cardiovascular disease, and asthma | N/A | N/A | N/A | The viral load of saliva in the early stages of COVID‐19 infection may have a high prognostic value in predicting disease progression in patients over 45 years of age. Saliva is a good substance in COVID‐19 screening |
| Oronasopharyngeal (ONP) samples and saliva samples | |||||||||||||||
| 3 | Berastegui‐Cabrera et al. 28 | Cross‐sectional | 2021 | Spain | 72 | 66 | 56% male | RT‐PCR | No association was found between viral load in NP samples and the presence of SARS‐CoV‐2 RNAemia. The absence of differences in NP viral load between patients with SARS‐CoV‐2 RNAemia and without it proves that the clinical development index of COVID‐19 patients is better than that of NP viral load. The median viral load in NP swabs = 6.98 log10 copies/ml (IQR, 5.15–8.20) | Arthromyalgias, coryza, cough, dyspnea, headache, odynophagia, diarrhea, anosmia, weakness, and dysgeusia | Chronic kidney disease, solid‐organ transplantation, connective tissue disease, and chronic liver disease | Leukocytes: 5.22, 7.00, Neutrophils: 3.49, 4.79, Lymphocytes: 0.58, 1.36, Platelets: 158, 248, Hemoglobin: 13, 13.8, AST: 37, 26, ALT: 33, 23, Bilirubin: 0.59, 0.46, Sodium: 2, 4, Potassium: 2,1, Creatinine: 4, 6, C‐reactive protein: 97.9, 44.9, Ferritin: 625.6, 442, D‐dimers: 1430, 620, LDH: 450, 251.5, | ARDS, multiple organ failure, IMV, ICU admission, mortality | N/A | The presence of RNAemia SARS‐CoV‐2, in the first emergency assessment, is more common in patients with severe chronic underlying disease, such as chronic liver disease and solid organ transplantation, with viral load in the upper respiratory tract and with adverse outcomes |
| Nasopharyngeal swabs | |||||||||||||||
| 4 | Buetti et al. 29 | Cross‐sectional | 2021 | Switzerland | 90 | 62.5 | 78.9% male | RT‐PCR Nasopharyngeal swab | Viral load (log10 copies/ml), median [IQR]: 3.3 [1.8; 5.2] That viral load in the LRT was associated with the 6‐week mortality | Cardiovascular, Immunosuppression, DM, Renal failure, Cancer, and Chronic respiratory failure | N/A | N/A | N/A | Delay in LRT virus averaged approximately 30 days in critically ill patients, and viral load in LRT was associated with 6‐week mortality | |
| 5 | Buder et al. 30 | Cohort | 2021 | Germany | 59 | Median: 48 years | 49% | Quantitative real‐time PCR of respiratory samples | Median viral load (IQR): 6.80 × 104 (4.75 × 103–1.81 × 106) RNA copies/ml | N/A | 10 patients had immunosuppression | N/A | 34 outpatient, 20 admitted to ICU | Higher viral load correlated with a higher chance of viral transmission | SARS‐CoV‐2 infectivity correlated with viral load, with the best predictor of infectivity being viral loads above 1.0 × 107 RNA copies/ml. The probability of virus isolation from respiratory samples also correlated positively with viral load. Seroconversion terminated SARS‐CoV‐2 infectivity |
| 6 | Cho et al. 31 | Pospective observational | 2020 | China | 75 | 36.4 ± 16.3 | 48% male | RT‐PCR Nasopharyngeal and deep throat swabs | There was no correlation between the recovery time of olfactory or gustatory disorders and the Ct value of PCR was sampled indirectly from nasopharyngeal swabs and deep throat reflected the viral load of SARS‐CoV‐2. C t value: 28.3 ± 6.7 | Rhinorrhea, Purulent nasal discharge, Taste change, Nasal blockage, Epistaxis, Cough, Fever, Dyspnea, and smell change. | N/A | N/A | N/A | N/A | There is no association between severity and improvement of olfactory and taste disorders with SARS‐CoV‐2 viral load |
| 7 | Chua et al. 32 | Cross‐sectional | 2021 | China | 91 | Asymptomatic Male:8.6 (4.3–11.0), Symptomatic Mean (IQR): 9.2 (4.0–15.0) | Asymptomatic 57.1% male, Symptomatic 44.4% male | RT‐PCR Nasopharyngeal swab (NPS), and saliva samples collected on admission | The onset days of symptoms for all patients were inversely related to the NPS and saliva viral loads. Viral load (log10 copies/ml): lymphopenia (NPS, Saliva): 6.7, 5.8 viral load (log10 copies/ml):: nonlymphopenia (NPS, Saliva): 6.2, 4.9 | N/A | Total white cell count (×109/L): 6, 5.8‐Hemoglobin (g/dl): 12.8, 13.2‐Platelets (×109/L): 258.4, 278.1‐Urea (mmol/L): 3.4, 3.9‐Creatinine (µmol/L): 41.6, 44.9‐Creatine Kinase (U/L):122.5, 99.7‐Troponin I (ng/l): 1.9, 11.3‐C Reactive Protein (mg/dl): 1.4, 1.7‐Erythrocyte Sedimentation Rate (mm/h):8.6, 12‐ | N/A | N/A | Salivary viral loads in hospitalized children with clinical and immune profiles are better than NPS | |
| 8 | de la Calle et al. 33 | Cross‐sectional | 2021 | Spain | 455 | 64.9 ± 18.1 | 56% male | rRT‐PCR nasopharyngeal | Patients with respiratory failure had a higher viral load at admission than those who did not. Low viral load (C t > 30), Intermediate viral load (C t 25–30): 1.81, high viral load (C t < 25): 2.99 | Fever, Vomiting, Cough, Tachypnea, Diarrhea, SpO2 < 90% air room, Myalgia and Dyspnea | Cardiovascular disease, chronic renal disease, chronic lung disease, DM, immunosuppression, obesity, current or former smoker, and chronic liver disease | LDH (U/L): 326.6, GOT (U/L): 32, GPT (U/L): 25, CPK (U/L): 86, TnT (U/L): 10.5, C‐reactive protein (mg/dl): 7.7, Ferritin (mg/dl): 699, D‐dimers (ng/ml): 664 | Need for supplemental oxygen, ARDS, noninvasive mechanical ventilation, ICU admission, Septic shock, Prone position, MACE event, Acute kidney injury (AKI), Venous thrombosis, Hepatitis, Respiratory failure, Invasive mechanical ventilation, and mortality | N/A | The SARS‐CoV‐2 viral load, measured by Ct value of rRT‐PCR in pharyngeal swabs at admission, is a good indicator of the prognosis for respiratory failure |
| 9 | He et al. 34 | Cohort | 2020 | China | 23 | 41 | 43.5% males | qRT‐PCR Pharyngeal swab | Minimum viral load: 40 C t. Asymptomatic type patients had lower viral loads than common and severe types | Fever, cough, nasal congestion, dizziness, fatigue, arthralgia, | human endogenous retrovirus‐H (Hervs) and Human picobirnavirus (HPBV) | Patients with severe disease had more abnormal laboratory test results (including leukopenia and lymphocytopenia) | no significant correlation was observed between age and Ct value also no association between Ct value and severity of illness was observed. Significant positive relation has been detected between peak viral load and severity of illness. | Weaker transmission capacity of asymptomatic cases due to the lower viral load | Asymptomatic type patients had lower viral loads than common and severe types |
| 10 | Jacot et al. 35 | Cross‐sectional | 2020 | Switzerland | N/A | 0‐99 years | Female/male | RT‐PCR Nasopharyngeal swab | Range: 3–10 log copies/ml. Median: 6.78 log10 copies/ml In the first period of covid‐19 outbreak viral load was higher SARS‐CoV‐2 viral load seem to be a substandard predictor of disease outcome, COVID‐19 disease severity is not significantly related to viral replication in the upper and lower respiratory tracts | Fever cough | N/A | N/A | In the first period of covid‐19 outbreak viral load was higher | below 1000 copies/ml values can be considered at slight risk of transmission | Despite there are significant differences between viral loads of different viruses, SARS‐Cov‐2 had a alike viral load to Respiratory syncytial virus and influenza B than other coronaviruses |
| 11 | Jain et al. 36 | Comparative | 2021 | India | 200 | group A 35.23 ± 11.99, group B 35.32 ± 12.92 | 60% male | RT‐PCR Nasopharyngeal swab | Group A with olfactory and taste dysfunction: 24.43 C t. Group B without OTD: 27.39 C t. The patients with taste and olfactoryimpairment at diagnosis had more viral load than patients without taste and olfactoryimpairment | Loss of smell and taste malaise sore throat cough fever nasal discharge | N/A | RT‐PCR was utilized to test The COVID‐19, with 3 gene detection: RdRp (RNA‐dependent RNA polymerase), E (Envelope encoding) gene, and N (Nucleocapsid encoding) gene. For analysis cycle threshold was utilized. | N/A | N/A | The patients with OTD at diagnosis had more viral load than patients without OTD |
| 12 | Kam et al. 37 | Cohort | 2021 | Singapore | 17 | 7.7 | Female/male | RT‐PCR Nasopharyngeal swab | Symptomatic: 28.6 C t. Asymptomatic: 36.7 C t higher viral loads was seen in symptomatic children in comparison to asymptomatic children | Upper respiratory tract symptoms with mild sickness signs | N/A | N/A | patients with mild and severe chest CT involvement had significantly lower viral load in comparison to patients with no chest CT lesions. | symptomatic children in had high viral load in the first stage of sickness indicates the transmission potential of presymptomatic children. | Children with symptomshad higher viral loads than children without symptoms |
| 13 | Karahasan Yagci et al. 38 | Cohort | 2020 | turkey | 730 | 35 | 49.9% female | RT‐qPCR Nasopharyngeal swab | Without CT scan involvement: 24.9 mild CT involvement: 27.8 moderate CT involvement: 29.4 severe CT involvement: 27.9. The oppositecorrelation of chest CT Total severity score (TSS) and viral load was seen. Significantly higher viral loads was observed in patients with no chest CT lesions in comparison to patients with mild and severe chest CT involvement | Fever, cough and dyspnea | Hypertension, diabetes mellitus, cardiovascular disease, chronic obstructive pulmonary diseases (COPD), cancers, HIV, collagenosis, and chronic liver disease | N/A | 284 (39%) patients were admitted to hospital and 27 of patientsexpired during the hospitalization. | N/A | The oppositecorrelation of chest CT total severity score (TSS) and viral load |
| 14 | Kawasuji et al. 39 | Case‐control | 2020 | Japan | 28 | Median age: 45.5 years | 53.6% male | rRT‐PCR Nasopharyngeal swab | 33.6 ± 5.5 C t. A significant viral load and recovery time differencewas observed between patients with pulmonary involvement and patients without pulmonary involvement | N/A | N/A | N/A | Significantly higher viral load at the beginning of sampling in symptomatic patients than in asymptomatic patients was observed. Also, Children had significantly higher viral load than adults in the beginning of sampling. | A high nasopharyngeal viral load can be connected to the secondary transmission of COVID‐19 | Secondary transmission of COVID‐19 can be related to high nasopharyngeal viral load. Additionally, the viral load can help describe why transmission is observed in some patients, but not in others, particularly among patients who live in same house |
| 15 | Kim et al. 40 | Retrospective | 2021 | South Korea | 106 | Mean age: 28.0 ± 9.3 years | 43.4% male | RT‐PCR Nasopharyngeal/oropharyngeal swab | 33.6 ± 5.5 C t. Viral load and recovery time were significantly different between pulmonary involvement patients and patients without pulmonary involvement was observed | Cough, fever, headache, hyposmia, rhinorrhea, sputum, muscle pain, diarrhea, chest pain, ocular pain | Rhinitis, asthma, migraine, iron deficiency, anemia, hyperlipidemia, endometriosis, depression disorder, hair loss, atopic dermatitis | N/A | Recovery times were significantly slower in the patients with pulmonary involvement than patients without involvement. | N/A | Viral load and recovery time were significantly different between pulmonary involvement patients and patients without pulmonary involvement was observed. The cycle threshold cutoff value for the existence of pneumonia was 31.38 |
| 16 | Kociolek et al. 41 | Retrospective | 2020 | USA | 817 | 0‐17 years | 52.1% male | RT‐PCR Nasopharyngeal swab | Asymptomatic children: 2.0 × 103 copies/ml symptomatic children: 1.3 × 107 copies/ml. In children without symptoms lower viral load was found in their nasopharynx/oropharynx than children with symptoms | Cough, fever/chills, dyspnea, pharyngitis, loss of taste or smell, headache, abdominal pain, diarrhea, fatigue, myalgias, congestion/rhinorrhea, nausea/vomiting, rash, or conjunctivitis | Immunocompromised = 51. Diabetes = 19 | N/A | Ct values were significantly higher in children without symptoms than children with symptoms. Also, significantly lower viral loads was observed in asymptomatic than symptomatic children. | N/A | Asymptomatic children had low viral loads in their nasopharynx/oropharynx than children with symptoms |
| 17 | Kriegova et al. 42 | Prospective | 2021 | Czech Republic | 1038 | 50.0 ± 3.3 | Female/male | RT‐PCR Nasopharyngeal swab | Asymptomatic and mild group 23.65 (±7.62) C t. Moderate group 27.68 (±6.98) C t. Severe and critical group 26.52 (±4.82) C t. High levels of virus in the respiratory tract and excessive producing of chemokines and cytokines between first 2 weeks from the onset of symptoms were significantly related to severity of the COVID‐19 | N/A | N/A | N/A | self‐conductnasal‐swab in combination with direct RT‐qPCRare easy, low‐cost and quick CoV‐2 testing method which could significantly increase the extent of the teststrategies which are needed to control the expansion of COV‐19 during and post‐pandemic era | N/A | High levels of virus in the respiratory tract and too much productionof chemokines and cytokines and between the first two weeks from the onset of symptoms were significantly related to severity of the COVID‐19 |
| 18 | Kwon et al. 43 | Prospective | 2020 | South Korea | 31 | 32‐72 years | 58% female | Nasopharyngeal swab RT‐PCR | Initial viral load at five toten days from onset of symptoms in the asymptomatic and mild group, moderate group, and the severe and critical group was 32.65 (±7.62), 27.68 (±6.98), and 26.52 (±4.82) cycles | Fever, chill, cough, sputum, sore throat, dyspnea, rhinorrhea chest pain, headache, myalgia, nasal congestion, hyposmia, hypogeusia, pneumonia | Diabetes mellitus, hypertension, chronic lung disease, chronic liver disease, obesity (body mass index > 25), smoking | Old age, initial low WBC count, low platelet count, high CRP level, and fever were identified as factors associated with severity | Early increases in type I IFN response might be involved in the pathophysiology of severe COVID‐19 by eliciting subsequent excessive responses of multiple cytokines and chemokines | N/A | Higher viral load, stronger antibody response, and excessive inflammation at first two weeks from onset of symptoms are related to the COVID‐19 severity |
| 19 | Le Borgne et al. 44 | Retrospective | 2021 | France | 287 | 50.0 to 73.0, median age: 63.1 | 65.8% male | Pharyngeal swabs qRT‐PCR | 4.76 (3.29–6.06) log10 copies/reaction Nasopharyngeal viral load measured by RT‐PCR during beginning emergency department (ED) viral load is not predictor of severity and mortality in COVID‐19 patients | N/A | Hypertension, cardiovascular disease, diabetes mellitus, renal insufficiency, dialysis, COPD, malignancies, immunotherapy, corticosteroids | At emergency department admission, patients who didn't survive in comparison to survived patients. had significantly higher C‐reactive protein (122 vs. 74 mg/L, p = .007) and creatinine (p = .036). Nonsurvivors were also more likely to present with anemia (p = .003) and lymphopenia (p = .02) than survivors | Forty‐two patients (14.6%) died. | Nasopharyngeal viral load was measured by RT‐PCR at emergency department admission viral load isn't predictor of severity and mortality in COVID‐19 patients | |
| 20 | Piubelli et al. 45 | Cross‐sectional | 2021 | Italy | 273 | N/A | Female/male | RT‐PCR Nasal and Pharyngeal swabs | Viral load decreased during 2 months of quarantine (C t decreased from 24 to 34). Alongside, the number of patients who need intensive care significantly decreased because of the reduction of viral load | N/A | N/A | More probable in high‐transmission setting compared with low‐transmission setting | ICU admission (5.3%) | N/A | N/A |
| 21 | Rauch et al. 46 | Cohort | 2021 | USA | 1808 | 27.3 ± 11 | 53% male | RT‐qPCR and CRISPR‐based assay Nasopharyngeal swab | Viral load = 286–510,000 copies/μl. The shift of viral load is shown in those who stayed at home | Nasal congestion, sore throat, fatigue, anosmia | N/A | 8 positive participants by CRISPR‐based assay and 9 by RT‐qPCR were detected | All were alive at the end of the study | N/A | The prevalence of SARS‐CoV‐2 in cohort 2 was changed and it was because of decreased community restrictions and increased social interactions |
| 22 | Sarkar et al. 47 | Cross‐sectional | 2020 | India | 138 | N/A | Female/male | RT‐PCR Nasopharynx swab (NPS) and oropharynx swab (OPS) | In those with C t values between 17 and 23, patients had severe infections | N/A | N/A | N/A | N/A | In high viral load cases, the rate of transmission was 8‐times more than low viral load cases. Patients with Ct above 33‐34 were not contagious | In individuals with high viral load, the possibility of transmission was almost 8 times higher compared to low viral load individuals. Of those who were infected, 7% had a high viral load, 9% moderate viral load, and 84% low viral load based on Ct values. The probability of transmission in those with high viral load was 6.25 in comparison with law viral load with 0.8 |
| 23 | Shlomai et al. 48 | Cross‐sectional descriptive | 2020 | Israel | 170 | 62 | 58% Male | Nasopharyngeal samples RT‐PCR | Viral load was significantly higherin ventilated and nonsurvivors patients (eightfold more than other patients). Low viral load was associated with decreased risk of mortality and intensive care | Hypoxemia | N/A | N/A | 21 death | N/A | Viral load was directly linked to hypoxemia. Viral load was significantly related toblood oxygen saturation. The patient's age significantly correlated with viral load |
| 24 | Shrestha et al. 49 | Cohort | 2020 | USA | 230 health care personnel (HCP) | N/A | Male 36% | PCR Nasopharyngeal swab | Viral load in 2 or 3 days after onset of symptoms was the peak. Time since onset of symptoms was significantly related to viral load | N/A | Chronic lung disease, current smoker, chronic heart disease, hypertension, liver cirrhosis, immunocompromised, diabetes mellitus, chronic kidney disease | N/A | N/A | N/A | 86.5% of transmission potential was in the first 5 days since onset of symptoms |
| 25 | Singanayagam et al. 50 | Cross‐sectional | 2020 | England | 754 samples from 425 symptomatic cases | 0‐100 years old | Female/male | RT‐PCR Nose, throat, combined nose‐and‐throat and nasopharyngeal swabs | There was no difference in C t value between asymptomatic (C t = 31.23), mild to moderate (C t = 30.94), and severe cases (C t = 32.55). In the first week of onset of symptoms, viral load was higher than the second week | N/A | N/A | In 42% of cases, culture was positive. The culture positivity during the first week of infection was significantly higher than the second week | N/A | N/A | Cases in the 81–100 year age group were more asymptomatic than other groups |
| 26 | Soria et al. 51 | Cohort | 2020 | Spain | 448 | 71.04 ± 18.29 | 45.7% male | RT‐PCR Nasopharyngeal swabs | Mean C t: mild (35.75 ± 0.45), moderate (32.69 ± 0.37), severe (29.58 ± 0.70). Viral load is a predictor of disease severity. High virus loading worsens the prognosis of the disease. C t value was significantly law in the severe group in comparison with the moderate and mild group | N/A | Hypertension, cardiovascular disease, diabetes. Obesity, asthma, COPD | N/A | Cases of the severe group include 23% of total cases and all of them were admitted. Also, 18.3% died during 90 days after diagnosis, 75 cases in the severe group, three cases in moderate, and four in the mild group | N/A | N/A |
| 27 | To et al. 52 | Cohort | 2020 | China | 23 | 62 | 56.5% male | RT‐qPCR Oropharyngeal saliva samples | The median viral load was 5 × 2 log10 copies/ml. The first week after the onset of symptoms, the viral load is high but decreases over time | Fever (96%), cough (22%), chills (17%), dyspnea (17%), runny and blocked nose, sore throat, chest discomfort, nausea, diarrhea, myalgia, malaise. In 15 (65%) CXR abnormalities were seen. In 17 (74%) multifocal ground‐glass lung opacities were seen | 48% had clinical medical illnesses including hypertension and diabetes | Those patients who had comorbidities had a lower anti‐RBD IgG OD compared to those without comorbidities | Five patients were admitted to ICU, two of them required intubation, and also two of them died | N/A | Older age was associated with a higher viral load. The antibody response occurred 10 days or later since the onset of symptoms |
| 28 | To et al. 53 | Cross‐sectional | 2020 | China | 12 | 62.5 | 58% male | RT‐qPCR Nasopharyngeal or sputum specimen | The median viral load was 3.3 × 106 copies/ml. On the first day of hospitalization viral load was slightly higher than other days. After day 11 viral load started to shed till being undetectable | N/A | N/A | According to viral culture, saliva contains live viruses and potentially can transmit the virus | At the end of the survey, all patients were alive | N/A | Saliva can be obtained from the patient without invasive procedure and it leads to reduce in nosocomial transmission of the virus |
| 29 | Trunfio et al. 54 | Retrospective cross‐sectional | 2021 | Italy | 200 | 56 | 58% male | RT‐PCR Nasopharyngeal swab | Viral load was associated with the severity of the disease | Gastrointestinal, neurological, respiratory, and systemic involvement, headache, olfactory and gustatory dysfunction, nausea and vomiting, diarrhea, fever, arthralgia, asthenia and malaise, cough, dyspnea, pharyngitis, and runny nose | Participants of group A (C t ≤ 20) had at least one comorbidity that was significantly different from the other two groups. Hypertension, COPD, asthma, obesity, active smoking, diabetes, cancer | N/A | 36.5% of cases were isolated at home and 63.5% were admitted to the hospital. Of those admitted, 16% died (including 20 cases in group A, 7 cases in group B, 5 cases in group c). 5% of all cases required intubation | N/A | Group A (C t ≤ 20) washospitalized more than group C (C t > 28). COVID‐19 severity and worse outcomes were significantly higher in group A compared with the other two groups (B: 20 < C t < 28). There was no association between viral load and prevalence of olfactory/taste disorder |
| 30 | Tsukagoshi et al. 55 | Cross‐sectional | 2021 | Japan | 286 | 39 ± 35 | 56.3% male | RT‐qPCR Nasopharyngeal swab | In fatal cases 3.57 × 109 ± 4.70 × 109 copies/ml; in survived cases 3.92 × 108 ± 1.60 × 109 copies/ml; in asymptomatic 4.92 × 107 ± 1.48 × 107 copies/ml. In fatal cases, viral load was significantly higher than symptomatic and asymptomatic cases. Poor prognosis in elderly patients was predicted in those with a high viral load | Fever, sore throat, cough | N/A | N/A | 5.2% of cases died | N/A | Pneumonia was more common in patients who died than in those who survived |
| 31 | Wang et al. 56 | Cross‐sectional | 2020 | China | 23 | 56 | 82.6% male | RT‐PCR Nasal swab, pharyngeal swab, sputum | In severe cases in comparison with mild cases, the viral load peak was significantly higher | N/A | N/A | N/A | 43.5% of cases admitted to ICU | N/A | N/A |
| 32 | Faíco‐Filho et al. 57 | Cohort | 2020 | Brazil | 875 | 48 | 49.1% male | RT‐PCR Nasal swab | Samples with C t values <40 were considered positive. Survivors presented a significantly higher initial C t value than that of nonsurvivors Mortality rates were 46% among patients with a high viral load (C t < 25) and 22% among patients with a low viral load | N/A | N/A | The higher the viral load, the worse the disease and the poorer the consequences | N/A | the Ct value could be used as a tool to help with the identification of patients at a higher risk for severe consequences | |
| 33 | Guo et al. 58 | Cohort | 2020 | china | 195 | 49.24 ± 15.99 | 48.2% males | RT‐PCR Nasopharyngeal swab | More severe patients seem to have a higher initial viral load. a significant increasing trend of initial viral load versus illness severity | Higher maximum body temperature within 24 h after hospitalization anddurationoffever (days) correlation with severe disease | Hypertension, Diabetes mellitus, Cardiovascular disease, Cerebrovascular disease, Chronic kidney disease | Higher plasma C‐reactive protein (CRP), D‐dimer, procalcitonin (PCT), and aspartate aminotransferase (AST); larger count of white blood cells (WBC) and neutrophil (NE), but relatively reduced lymphocyte count. A higher NE to lymphocyte ratio (NLR) was seen at a severe disease | N/A | N/A | Age, fever, peak body the temperature in 24 h after hospitalization, CRP, WBC, NE, NLR, AST, D‐Dimer, and PCT are positively correlated with severity, Patients with higher upper respiratory tract viral load at admission are more likely to develop severe symptoms and may need more aggressive treatment |
| 34 | Hasanoglu et al. 59 | Retrospective study | 2020 | Turkey | 60 | 33.9 | 48% males | RT‐PCR Saliva, urine, blood, and anal swab samples | The viral load of standards synthetic SARS‐CoV‐2 RdRp fragment/ml was between 2.5 × 102–5 copy/ml. No significant difference in the probability of PCR positivity across symptomatic and asymptomatic patients was found. PCR positivity does not always indicate infectivity | Cough and fatigue were the most observed symptoms on admission, 51.7%, and 30.5%, respectively | At least one comorbidity was present in 8 (13.3%) patients | N/A | Factors associated with poor prognosis are found to be significantly correlated with low viral load | N/A | A significant decrease in viral load of nasopharyngeal/oropharyngeal samples was observed with increasing disease severity |
Abbreviations: LRT, lower respiratory tract; NPS: nasopharyngeal swab.
Table 2.
Quality assessment for the included studies using the Newcastle–Ottawa Scale
| The first author (reference) | Selection (out of 4) | Comparability (out of 2) | Exposure/outcome (out of 3) | Total score (out of 9) |
|---|---|---|---|---|
| Aoki et al. 26 | *** | – | ** | 5 |
| Aydin et al. 27 | *** | – | ** | 5 |
| Berastegui‐Cabrera et al. 28 | **** | ** | * | 7 |
| Buetti et al. 29 | *** | ** | *** | 8 |
| Buder et al. 30 | *** | ** | ** | 7 |
| Cho et al. 31 | ** | – | ** | 4 |
| Chua et al. 32 | ** | – | *** | 5 |
| de la Calle et al. 33 | *** | ** | *** | 8 |
| He et al. 34 | ** | ** | ** | 6 |
| Jacot et al. 35 | *** | – | ** | 5 |
| Jain et al. 36 | **** | ** | ** | 8 |
| Kam et al. 37 | *** | – | ** | 5 |
| Karahasan Yagci et al. 38 | *** | ** | *** | 8 |
| Kawasuji et al. 39 | ** | – | ** | 4 |
| Kim et al. 40 | *** | – | ** | 5 |
| Kociolek et al. 41 | **** | ** | ** | 8 |
| Kriegova et al. 42 | **** | – | ** | 6 |
| Kwon et al. 43 | *** | ** | *** | 8 |
| Le Borgne et al. 44 | **** | ** | ** | 8 |
| Piubelli et al. 45 | *** | ** | ** | 7 |
| Rauch et al. 46 | **** | ** | *** | 9 |
| Sarkar et al. 47 | ** | ** | ** | 6 |
| Shlomai et al. 48 | ** | – | ** | 4 |
| Shrestha et al. 49 | ** | ** | ** | 6 |
| Singanayagam et al. 50 | **** | ** | *** | 9 |
| Soria et al. 51 | *** | – | *** | 6 |
| To et al. 52 | *** | ** | ** | 7 |
| To et al. 53 | *** | ** | ** | 7 |
| Trunfio et al. 54 | *** | ** | *** | 8 |
| Tsukagoshi et al. 55 | ** | – | ** | |
| Wang et al. 56 | ** | – | ** | 4 |
| Faíco‐Filho et al. 57 | **** | ** | *** | 9 |
| Guo et al. 58 | *** | ** | *** | 8 |
| Hasanoglu et al. 59 | ** | ** | ** | 6 |
Most of the studies included adults and had a similar share of men and women. The vast majority of the studies have utilized real‐time RT‐PCR of the nasopharyngeal/respiratory swabs to report viral load. Viral load was usually reported in two categories; cycle threshold (C t) and log10 RNA copies/ml. Studies have reported viral load in several groups: mild, moderate, and severe patients, symptomatic versus asymptomatic patients, and groups sorted by age. The results were inconsistent; while some studies found a significant relationship between SARS‐CoV‐2 viral load and severity of illness, other studies were against it (Table 1).
4. DISCUSSION
SARS‐CoV‐2, the new coronavirus accountable for COVID‐19, was first detected in China in late 2019 and then spread out globally. The WHO declared this disease a public health emergency of international concern on January 30, 2020. Although having the potential of causing severe pneumonia, SARS‐CoV‐2 can also involve various organs and cause physical symptoms such as fever, cough, and dyspnea, as well as psychological and gastrointestinal symptoms. Several interventions and measures have been implemented to restrict the spread of the virus and control the situation, such as community education, border controls, lockdown, social distancing, wearing masks in public, hand hygiene, and schools shut down. These public health efforts not only slowed down SARS‐CoV‐2 transmission but also caused a decrease of mortality rate. 1 , 12
In the present study, the main hypothesis along with two minor ones was discussed against the similar available studies. The main hypothesis recommended a potential relationship between the viral load and the severity of the disease. The minor hypotheses, which were also frequently reported in the included studies, are the relation between the age and the viral load as well as the relation between viral load and virus transmissibility. Symptoms included in the table were aimed to represent the severity of the diseases and the included comorbidities were to avoid the bias of imposture relation between severity and the viral load.
For both hypotheses, the key method of measuring the viral loads was the RT‐PCR. Viral nucleic acid detection by RT‐PCR assays is the gold standard for the diagnosis of COVID‐19. Using this technique, we can gain an indirect viral load value (C t) easily and immediately after diagnosis. 33 The main hypothesis could be explained by the association between viral load and inflammatory factors that are also clearly connected with the disease severity. It is well‐known that excessive release of proinflammatory cytokines and chemokines contributes to the severity of clinical outcomes in various infections. Therefore, our findings that the plasma concentrations of IFN‐α, IFN‐γ, IP‐10, MIG, and IL‐6 were elevated in the severe and critical cases at 5–10 days from symptom onset suggest that the higher plasma concentrations of proinflammatory cytokines after approximately a week from symptom beginning may have a role in the enhancement of severity. Intriguingly, a recent longitudinal study showed that plasma IFN‐α continued to be high in patients with severe COVID‐19, whereas it dropped in those with moderate COVID‐19 during their clinical course. 43
Similar to our findings, He et al., 34 have identified that higher viral load was positively associated with COVID‐19 severity. This finding highlights the importance of monitoring the viral kinetics to identify patients at greater risk of progressing to severe pneumonia. Similarly, Guo et al., 58 have found that the upper respiratory tract viral RNA load of SARS‐CoV‐2 at the time of hospital admission is an independent predictive factor of COVID‐19. However, there were some studies with inconsistent results. The study performed by Hasanoglu et al., 59 is an example of this controversy. They demonstrated that asymptomatic patients have higher SARS‐CoV‐2 viral loads than symptomatic patients and unlike in the few study in the literature, a major decrease in viral load of nasopharyngeal/oropharyngeal samples was observed with increasing disease severity. Similarly, Cho et al. 31 have found that both severity and recovery from these symptoms have no associations with the viral load of SARS‐CoV‐2. Le Borgne et al., 44 have also found that respiratory viral load measurement on the first nasopharyngeal swab (by RT‐PCR) during initial ED management is neither a predictor of severity nor a predictor of mortality in SARS‐CoV‐2 infection.
To support our minor hypotheses suggesting the association between viral load and patient's age, the findings from the study by To et al., 52 suggested no relationships between severity of disease and viral load; their study only showed that the median viral load was 1 log10 higher in severe cases than in mild cases, but on the other hand, they found a direct connection between age and viral load. Similarly, Shlomai et al. 48 have found that low viral load was independently associated with reduced risk for mechanical ventilation and mortality; and interestingly, patients' age also correlated positively with the viral load. Aydin et al. 27 found that viral load detected in saliva in the early symptomatic period of infection may have a prognostic value in showing the course of the disease in patients over 45‐year‐old. Overall, the studies found a positive correlation between patients' age and viral load. This finding might be a rationale for any possible relationship between viral load and increased disease severity, as older age is related to worse COVID‐19 outcomes. 11 It also raises the alarm that older patients may be more likely to transmit the virus.
In the present study, the final hypothesis suggesting the association between viral load and the COVID‐19 infectivity could be supported by the findings of Kawasuji et al.'s study, which suggested that a high nasopharyngeal viral load may contribute to the secondary transmission of COVID‐19. 39 Similarly, Sarkar et al. found that 84% of cases had low viral load and practically will spread the virus even to very few their contacts, demonstrating the connection between viral load and transmission. 47 Buder et al. have also reported similar results that merely having no symptoms is not enough for recognizing whether the patients have the ability of transmission or not. They found that SARS‐CoV‐2 positively correlated with the infectivity of the patients, regardless of whether they are symptomatic or not. 30 Therefore, viral load is probably one of the factors influencing SARS‐CoV‐2 transmission.
There are some limitations in the present study. First and most important, a meta‐analysis was not conducted due to the significant heterogeneity that existed between the included studies. Furthermore, there were few studies on some of the discussed matters and this may decrease the validity and reliability of reported outcomes. However, this study may provide relevant insights for future research to conduct original studies and/or meta‐analyses to precisely determine the relationship between viral load and disease severity, and, in addition, to explore further discussed topics in this review, such as the correlation between age and SARS‐CoV‐2 viral load.
5. CONCLUSION
We have discussed three different hypotheses related to the viral load of COVID‐19. The results were inconclusive about the relationship between COVID‐19 severity and viral load, as a similar number of studies either approved or opposed this hypothesis. However, the included studies found a positive association between age and viral load. The higher viral load also appeared to be associated with the higher transmissibility of the disease. Nevertheless, such findings require careful meta‐analyses to be confirmed.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception and design of the study: Esmaeil Mehraeen, SeyedAhmad SeyedAlinaghi. Acquisition of data: Amirali Karimi, Nazanin Janfaza, Soheil Dehghani, and Fatemeh Afroughi. Analysis and interpretation of data: Pegah Mirzapour and Alireza Barzegary. Drafting the article: Amir Masoud Afsahi, Zahra Pashaei, Hengameh Mojdeganlou, Amirali Karimi, Pedram Habibi, Alireza Barzegary, Amirata Fakhfouri, Pegah Mirzapour, Nazanin Janfaza, Soheil Dehghani, Fatemeh Afroughi, Mohsen Dashti, Sepideh Khodaei, and Omid Dadras. Revising it critically for important intellectual content: SeyedAhmad SeyedAlinaghi, Esmaeil Mehraeen, and Omid Dadras. Final approval of the version to be submitted: Esmaeil Mehraeen, Omid Dadras, SeyedAhmad SeyedAlinaghi, Fabricio Voltarelli, and Jean‐Marc Sabatier.
ACKNOWLEDGMENTS
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Iranian Institute for Reduction of High‐Risk Behaviors, Tehran University of Medical Sciences, and Université Aix‐Marseille.
Dadras O, Afsahi AM, Pashaei Z, et al. The relationship between COVID‐19 viral load and disease severity: A systematic review. Immun Inflamm Dis. 2022;10:e580. 10.1002/iid3.580
DATA AVAILABILITY STATEMENT
The authors stated that all information provided in this article could be share.
REFERENCES
- 1. Dadras O, Alinaghi SAS, Karimi A, et al. Effects of COVID‐19 prevention procedures on other common infections: a systematic review. Eur J Med Res. 2021;26(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. SeyedAlinaghi S, Mirzapour P, Dadras O, et al. Characterization of SARS‐CoV‐2 different variants and related morbidity and mortality: a systematic review. Eur J Med Res. 2021;26(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. SeyedAlinaghi S, Afsahi AM, MohsseniPour M, et al. Late complications of COVID‐19; a systematic review of current evidence. Arch Acade Emerg Med. 2021;9(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehraeen E, Seyed Alinaghi SA, Nowroozi A, et al. A systematic review of ECG findings in patients with COVID‐19. Indian Heart J. 2020;72(6):500‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. SeyedAlinaghi S, Mehrtak M, MohsseniPour M, et al. Genetic susceptibility of COVID‐19: a systematic review of current evidence. Eur J Med Res. 2021;26(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdulhamid SM, Abd Latiff MS, Abdul‐Salaam G, et al. Secure scientific applications scheduling technique for cloud computing environment using Global League Championship Algorithm. PLoS One. 2016;11(7):e0158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mirzaei A, Rezakhani Moghaddam H, Habibi, et al. Identifying the predictors of turnover intention based on psychosocial factors of nurses during the COVID‐19 outbreak. Nurs Open. 2021;8:3469‐3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rabaan AA, Al‐Ahmed SH, Garout MA, et al. Diverse immunological factors influencing pathogenesis in patients with COVID‐19: a review on viral dissemination, immunotherapeutic options to counter cytokine storm and inflammatory responses. Pathogens. 2021;10(5):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabaan AA, Tirupathi R, Sule AA, et al. Viral dynamics and real‐time RT‐PCR Ct values correlation with disease severity in COVID‐19. Diagnostics. 2021;11(6):1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabaan AA, Al‐Ahmed SH, Muhammad J, et al. Role of inflammatory cytokines in COVID‐19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9(5):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehraeen E, Karimi A, Barzegary A, et al. Predictors of mortality in patients with COVID‐19‐a systematic review. Eur J Integr Med. 2020;40:101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. SeyedAlinaghi S, Abbasian L, Solduzian M, et al. Predictors of the prolonged recovery period in COVID-19 patients: a cross-sectional study. Eur J Med Res. 2021;26(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim D, Lee JY, Yang JS, et al. The architecture of SARS‐CoV‐2 transcriptome. Cell. 2020;181(4):914‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meng B, Kemp SA, Papa G, et al. Recurrent emergence of SARS‐CoV‐2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep. 2021;35(13):109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yadav PD, Sapkal GN, Ella R, et al. Neutralization of Beta and Delta variant with sera of COVID‐19 recovered cases and vaccinees of inactivated COVID‐19 vaccine BBV152/Covaxin. J Travel Med. 2021;28:taab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ranzani OT, Hitchings M, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID‐19 in Brazil: test negative case‐control study. BMJ. 2021;374:n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dove ES, Joly Y, Tassé AM, et al. Genomic cloud computing: legal and ethical points to consider. Eur J Hum Genet. 2015;23(10):1271‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen L, Wang G, Long X, et al. Dynamics of blood viral load is strongly associated with clinical outcomes in coronavirus disease 2019 (COVID‐19) patients: a prospective cohort study. J Mol Diag. 2021;23(1):10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January‐March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdulrahman A, Mallah SI, Alqahtani M. COVID‐19 viral load not associated with disease severity: findings from a retrospective cohort study. BMC Infect Dis. 2021;21(1):688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El Zein S, Chehab O, Kanj A, et al. SARS‐CoV‐2 infection: initial viral load (iVL) predicts severity of illness/outcome, and declining trend of iVL in hospitalized patients corresponds with slowing of the pandemic. PLoS One. 2021;16(9):e0255981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen W, Xiao Q, Fang Z, et al. Correlation analysis between the viral load and the progression of COVID‐19. Comput Math Methods Med. 2021;2021:9926249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsukagoshi H, Shinoda D, Saito M, et al. Relationships between viral load and the clinical course of COVID‐19. Viruses. 2021;13(2):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molina‐Molina A, Ruiz‐Malagón EJ, Carrillo‐Pérez F, et al. Validation of mDurance, a wearable surface electromyography system for muscle activity assessment. Front Physiol. 2020;11:606287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aoki K, Nagasawa T, Ishii Y, et al. Clinical validation of quantitative SARS‐CoV‐2 antigen assays to estimate SARS‐CoV‐2 viral loads in nasopharyngeal swabs. J Infect Chemother. 2021;27(4):613‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aydin S, Benk IG, Geckil AA. May viral load detected in saliva in the early stages of infection be a prognostic indicator in COVID‐19 patients? J Virol Methods. 2021;294:114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berastegui‐Cabrera J, Salto‐Alejandre S, Valerio M, et al. SARS‐CoV‐2 RNAemia is associated with severe chronic underlying diseases but not with nasopharyngeal viral load. J Infect. 2021;82(3):e38‐e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buetti N, Wicky PH, Le Hingrat Q, et al. SARS‐CoV‐2 detection in the lower respiratory tract of invasively ventilated ARDS patients. Crit Care. 2020;24(1):610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buder F, Bauswein M, Magnus CL, et al. SARS‐CoV‐2 infectivity correlates with high viral loads and detection of viral antigen and is terminated by seroconversion. J Infect Dis. 2021.jiab415. 10.1093/infdis/jiab415 [DOI] [Google Scholar]
- 31. Cho R, To Z, Yeung Z, et al. COVID‐19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope. 2020;130(11):2680‐2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chua GT, Wong JSC, To KKW, et al. Saliva viral load better correlates with clinical and immunological profiles in children with coronavirus disease 2019. Emerg Microbes Infect. 2021;10(1):235‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de la Calle C, Lalueza A, Mancheño‐Losa M, et al. Impact of viral load at admission on the development of respiratory failure in hospitalized patients with SARS‐CoV‐2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(6):1209‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He Y, Xu X, Lu Q, et al. High viral load suggests increased COVID‐19 severity in a longitudinal cohort. 2020.
- 35. Jacot D, Greub G, Jaton K, et al. Viral load of SARS‐CoV‐2 across patients and compared to other respiratory viruses. Microbes Infect. 2020;22(10):617‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jain A, Pandey AK, Kaur J, et al. Is there a correlation between viral load and olfactory & taste dysfunction in COVID‐19 patients? Am J Otolaryngol. 2021;42(3):102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kam KQ, Yung CF, Maiwald M, et al. Clinical utility of buccal swabs for severe acute respiratory syndrome coronavirus 2 detection in coronavirus disease 2019‐infected children. J Pediatric Infect Dis Soc. 2020;9(3):370‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karahasan Yagci A, Sarinoglu RC, Bilgin H, et al. Relationship of the cycle threshold values of SARS‐CoV‐2 polymerase chain reaction and total severity score of computerized tomography in patients with COVID 19. Int J Infect Dis. 2020;101:160‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawasuji H, Takegoshi Y, Kaneda M, et al. Transmissibility of COVID‐19 depends on the viral load around onset in adult and symptomatic patients. PLoS One. 2020;15(12):e0243597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim C, Kim W, Jeon JH, et al. COVID‐19 infection with asymptomatic or mild disease severity in young patients: clinical course and association between prevalence of pneumonia and viral load. PLoS One. 2021;16(4):e0250358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kociolek LK, Muller WJ, Yee R, et al. Comparison of upper respiratory viral load distributions in asymptomatic and symptomatic children diagnosed with SARS‐CoV‐2 infection in pediatric hospital testing programs. J Clin Microbiol. 2020;59(1):e02593‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kriegova E, Fillerova R, Raska M, et al. Excellent option for mass testing during the SARS‐CoV‐2 pandemic: painless self‐collection and direct RT‐qPCR. Virol J. 2021;18(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kwon J‐S, Kim JY, Kim M‐C, et al. Factors of severity in patients with COVID‐19: cytokine/chemokine concentrations, viral load, and antibody responses. Am J Trop Med Hyg. 2020;103(6):2412‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Le Borgne P, Solis M, Severac F, et al. SARS‐CoV‐2 viral load in nasopharyngeal swabs in the emergency department does not predict COVID‐19 severity and mortality. Acad Emerg Med. 2021;28(3):306‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piubelli C, Deiana M, Pomari E, et al. Overall decrease in SARS‐CoV‐2 viral load and reduction in clinical burden: the experience of a hospital in northern Italy. Clin Microbiol Infect. 2021;27(1):131.e1‐131.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rauch JN, Valois E, Ponce‐Rojas JC, et al. Comparison of severe acute respiratory syndrome coronavirus 2 screening using reverse transcriptase‐quantitative polymerase chain reaction or CRISPR‐based assays in asymptomatic college students. JAMA Netw Open. 2021;4(2):e2037129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarkar B, Sinha RN, Sarkar K. Initial viral load of a COVID‐19‐infected case indicated by its cycle threshold value of polymerase chain reaction could be used as a predictor of its transmissibility—an experience from Gujarat, India. Indian J Community Med. 2020;45(3):278‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shlomai A, Ben‐Zvi H, Bendersky AG, et al. Nasopharyngeal viral load predicts hypoxemia and disease outcome in admitted COVID‐19 patients. Crit Care. 2020;24(1):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shrestha NK, Marco Canosa F, Nowacki AS, et al. Distribution of transmission potential during nonsevere COVID‐19 illness. Clin Infect Dis. 2020;71(11):2927‐2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT‐PCR cycle threshold values in cases of COVID‐19, England, January to May 2020. Euro Surveill. 2020;25(32):2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Soria ME, Cortón M, Martínez‐González B, et al. High SARS‐CoV‐2 viral load is associated with a worse clinical outcome of COVID‐19 disease. Access Microbiol. 2021;3(9):000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. To KK, Tsang OT, Yip CC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trunfio M, Venuti F, Alladio F, et al. Diagnostic SARS‐CoV‐2 cycle threshold value predicts disease severity, survival, and six‐month sequelae in COVID‐19 symptomatic patients. Viruses. 2021;13:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsukagoshi H, Shinoda D, Saito M, et al. Relationships between viral load and the clinical course of COVID‐19. Viruses. 2021;13(2):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID‐19 severity. J Clin Invest. 2020;130(10):5235‐5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Faíco‐Filho KS, Passarelli VC, Bellei N. Is higher viral load in SARS‐CoV‐2 associated with death? Am J Trop Med Hyg. 2020;103(5):2019‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo X, Jie Y, Ye Y, et al. Upper respiratory tract viral rna load at hospital admission is associated with COVID‐19 disease severity. Open Forum Infect Dis. 2020;7(7):ofaa282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hasanoglu I, Korukluoglu G, Asilturk D, et al. Higher viral loads in asymptomatic COVID‐19 patients might be the invisible part of the iceberg. Infection. 2021;49(1):117‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors stated that all information provided in this article could be share.


