Abstract
Background and Purpose. Diabetes mellitus (DM), hyperglycemia, and hypertension can result in diabetic retinopathy (DR), which is a major cause of blindness on a global scale. Development of DR is associated with decreased endothelial cells, increased basal membrane thickness, permeation of the retinal blood barrier, and neovascularization in patients. The purpose of the present review is to provide an overview of the findings regarding applications of phytochemicals for DR treatment and could be a beneficial resource for further clinical studies and also a basis for pharmaceutical purposes for drug design. Materials and Methods. A narrative literature review was performed from electronic databases including Web of Science, PubMed, and Scopus to analyze the effects of different phytochemicals to prevent or treat oxidation, angiogenesis, and inflammation in diabetic retinopathy. The inclusion criteria were original studies, which included the effects of different phytochemicals on diabetic retinopathy. The exclusion criteria included studies other than original articles, studies which assessed the effects of phytochemicals on nondiabetic retinopathy, and studies which used phytochemical-rich extracts. Results and Conclusions. Studies have shown that increased levels of inflammatory cytokines, angiogenic, and oxidative stress factors are involved in the progression and pathogenesis of DR. Therefore, phytochemicals with their anti-inflammatory, antiangiogenic, and antioxidant properties can prevent DR progression and retinal damage through various cellular mechanisms. It is also shown that some phytochemicals can simultaneously affect the inflammation, oxidation, and angiogenesis in DR.
1. Introduction
Diabetic retinopathy (DR) is one of the important microvascular complications of diabetes mellitus (DM), in which chronic hyperglycemia and hypertension affect the retinal microvasculature and cause critical damage leading to blindness worldwide [1–4]. It is estimated that, by 2030, the number of people with diabetes will exceed 500 million, making it a global threat, and one-third of people will have DR [5, 6]. Decreased endothelial cells, increased basal membrane thickness, permeation of the retinal blood barrier, and neovascularization are some of the impairments seen in patients with DR [7]. DR has two stages based on the severity of the symptoms: nonproliferative DR (NPDR) and proliferative DR (PDR). NPDR is associated with microaneurysm and “dot and blot” hemorrhages, whereas PDR is characterized by retinal neovascularization. PDR is also associated with retinal hemorrhage and detachment, resulting in partial or complete loss of vision. Loss of central vision occurs due to increased retinal vessel permeability and edema [8]. Retinal neovascularization plays an important role in the pathogenesis of DR [1]. Vascular endothelial growth factor (VEGF) is an angiogenic factor that is expressed in a large number of retinal cells exposed to hyperglycemia. This factor increases proliferation, migration, and tubal formation. Therefore, in patients with DR, VEGF levels in the retina significantly increase, which is directly related to the progression of the disease [1, 9]. VEGF antagonists can be used as a treatment to reduce angiogenesis in patients with DR. Inflammation and oxidative stress are important in the pathogenesis and development of DR [10, 11]. Chronic hyperglycemia in patients with DR causes high production of inflammatory cytokines, including tumor necrosis factor (TNF-α) and interleukin (IL-1β), and subsequently destroys retinal cells [12]. In the inflammatory process of patients with DR, hyperglycemia increases the expression of nuclear factor kappa B (NF-κB), which causes many disorders, including increased retinal vascular leakage, leukocytosis, increased expression of inflammatory cytokines, and intercellular adhesion molecule-1 (ICAM-1) [13, 14]. TNF-α stimulates the expression of genes TNF-α involved in apoptosis and migration [11]. Therefore, inhibition of inflammatory factors can prevent the progression of DR [14]. Increased amounts of free oxygen radicals (FORs) lead to the development of DR and increased retinal damage [15]. Hyperglycemia also increases the amount of plasma-free radical and malondialdehyde (MDA) and disrupts the antioxidant system [12, 15]. Phytochemicals are compounds found in certain plants [16, 17]. Studies have shown that phytochemicals can prevent DR progression through their antiangiogenic, antioxidant, and anti-inflammatory characteristics [7, 18, 19]. In this paper, we intend to summarize an overview of the antiangiogenesis, anti-inflammatory, and antioxidant effects of phytochemicals on DR.
2. Antiangiogenic Phytochemicals against DR
Angiogenesis remains normal in the physiological state, but in disease conditions, the expression of proangiogenic factors such as VEGF, fibroblast growth factor (FGF), and others increases. Angiogenesis is often associated with malignancy and causes its progression [20, 21]. The formation of new vessels by germination is a process that has several stages, including the destruction of the basement membrane by enzymatic mechanisms, the proliferation of epithelial cells, migration, germination, the formation of branches and tubes [22]. Aloe-emodin is a derivative of anthraquinone and is found in various plants, including Aloe vera. Recent studies suggest that aloe-emodin is effective in treating many diseases, including cancer, viral, inflammatory, bacterial, parasitic, neurological, and hepatic diseases [23]. Aloe-emodin significantly reduced VEGF secretion, hypoxia-inducible factor- (HIF-) 1α, and prolyl hydroxylase domain protein 2 (PHD-2) expression in cultured ARPE-19 cells under hypoxia condition, thus reducing retinal angiogenesis. In a rat model of oxygen-induced retinopathy (OIR), oral intake of aloe-emodin reduced retinal neovascularization [24].
Andrographolide, a diterpenoid lactone, was obtained from Andrographis paniculata. It is effective in treating many diseases, including cancer, rheumatoid arthritis (RA), and upper respiratory tract infections [25]. Administrating andrographolide to STZ-induced DR mice inhibited retinal angiogenesis and vessel leakage. Andrographolide could also decrease VEGF and tissue factor (TF) expression, which played an important role in the process of angiogenesis [26]. Biochanin is an isoflavonoid obtained from Trifolium pratense and has various effects such as cancer prevention, nervous system protection, estrogen-like activity, anti-inflammatory, and antidiabetic [27]. In STZ-induced DR rats, oral administration of biochanin suppressed retinal TNF-α and IL-1β levels. Also, in the rats treated with this compound, VEGF levels and angiogenesis rate reduced, which led to improvements in symptoms in the retina [2]. Vaccinium virgatum contains high levels of anthocyanins, and their use has many effects on human health, including anticancer, neuroprotective, antioxidant, and blood pressure-lowering [16, 28]. In high glucose-induced HRCECs, the use of blueberry anthocyanin extract (BAE) and malvidin-3-glucoside (Mv-3-glc) controlled angiogenesis by reducing the content of VEGF and protein kinase B-1 (Akt) [16]. Chebulagic acid (CA), chebulinic acid (CI), and gallic acid (GA) are the active compounds in the alcoholic extract of Triphala, which suppresses the transmission of epithelial cells to the mesenchymal cells in retinal pigment cells [29]. In RF/6A cells under TNF-α, induced conditions intake of these three phytochemicals reduced matrix metalloprotease-9 (MMP-9) expression and angiogenic activity. Also, CA and CI reduced the expression of proangiogenic growth factor (PDGF-BB). These phytochemicals exerted their antiangiogenic effects by inhibiting the phosphorylation of p38, ERK, and NF-κB [30]. Chlorogenic acid is a polyphenolic compound found in coffee, and its consumption is associated with a reduced risk of cardiovascular disease and type 2 diabetes. Also, it is used to treat obesity in the daily diet [31, 32]. The use of chlorogenic acid inhibited the proliferative capacity, cell migration, and tube formation induced by VEGF in HRECs and monkey choroid-retinal endothelial cell line (RF/6A). It also reduced the amount of phosphorylated VEGF receptor 2 (VEFGR2), mitogen-activated extracellular regulated kinase (MEK1/2), ERK1/2, p38, the activity of microglia cells, and the expression of VEGF in these cells. Similarly, in STZ-induced DR C57BL/6 mice, it was demonstrated that, by receiving chlorogenic acid orally, retinal neovascularization decreased [5]. Chrysin is a flavonoid found in many natural products, including honey and many plants [33]. So far, many benefits have been discovered for this phytochemical, including anticancer, antioxidant, antiapoptotic, and treatment of movement problems [34, 35]. In glucose-exposed RPE cells, treatment with chrysin reduced the production of VEGF and insulin-like growth factor-I (IGF-1). In diabetic mice, treatment with chrysin improved the reduced thickness of the retina and also increased the secretion of pigment epithelium-derived factor (PEDF), which is a neovascular suppression factor. The expressions of various enzymes involved in the visual cycle, such as RPE65, level of lecithin retinol acyltransferase (LRAT), and retinol dehydrogenase 5 (RDH5), were increased due to chrysin administration and led to improved vision in diabetic mice. Chrysin also reduced advanced glycation end (AGE) secretion, AGE receptor (RAGE) induction, and endoplasmic reticulum (ER) stress caused by increased glucose in human retinal pigment epithelial (HRPE) cells and improved the visual cycle [3]. In another model of DR in vitro, the use of chrysin in HRMVECs in hyperglycemic conditions inhibited the apoptosis of retinal endothelial cells. It also decreased the expression of HIF-1α and VEGF and the Ang-Tie-2 pathway. Oral administration of chrysin in db/db mice reduced the expression of VEGFR-2, HIF-1α, and VEGF and increased the expression of intercellular-binding proteins, including VE-cadherin and ZO-1 junction proteins and platelet endothelial cell adhesion molecule-1 (PECAM-1), and thus, it was effective in inhibiting vascular leakage. The amount of cellular adhesion proteins reduced the loss of pericytes and endothelial cells and ultimately inhibited the formation of acellular capillaries [36]. Curcumin is a polyphenolic compound obtained from Curcuma longa. This phytochemical has a wide range of effects, including anti-inflammatory, antimicrobial, and antioxidant used in the treatment of cancer [37]. Oral administration of curcumin reduced retinal capillary BM thickness and alleviated angiogenesis in the retina of diabetic rats by decreasing VEGF expression [7]. Decursin is a pyranocoumarin obtained from the roots of Angelica gigas [38]. Decursin was demonstrated to reduce the expression of vascular endothelial growth factor receptor (VEGFR-2) and tube formation in human retinal microvascular endothelial cells (HRMVECs) in vitro and Sprague–Dawley (SD) rats in vivo [39]. Formononetin is an isoflavones compound Astragalus membranaceus and is found in various plants. It is widely used in traditional medicine, has antioxidant, anticarcinogenic, and neuroprotective effects, and is used in the treatment of neurodegenerative diseases such as Alzheimer's [40, 41]. In cultured ARPE-19 cells under hypoxia conditions, the use of formononetin reduced the expression of HIF-1α and inhibited VEGF secretion. In addition, intraperitoneal injection of formononetin reduced neovascularization in OIR model rats [41]. Genistein is an isoflavone mainly derived from Glycine max. It has many biological benefits, including anticancer activity [42]. Genistein affects acute retinal pigment epithelial-19 cells (ARPE-19), cells treated with normal and high glucose were evaluated, and the results showed a significant reduction in VEGF levels and retinal angiogenesis [42]. Gentiopicroside (GP) is extracted from the root of Gentianaceae and has antioxidant and liver protection effects. The main ingredient in this substance is iridoid glycosides [43]. In diabetic rats, oral use of GP decreased VEGF levels and significantly improved PEDF expression [13]. Hesperidin and its aglycone hesperetin are two citrus flavonoids, which have biological effects against many diseases such as cardiovascular diseases and cancer [44]. Findings showed that hesperidin (100 and 200 mg/kg/day) could normalize VEGF level and retinal and plasma abnormalities in streptozotocin- (STZ-) induced DR rats through downregulation of retinal angiogenesis. Blood retinal barrier permeability and retinal thickness were also increased after hesperidin treatment [45]. Hesperetin administration to STZ-induced DR rats led to inhibition of retinal angiogenesis, dilation of the vessels, and thickening of the basement membrane (BM). VEGF and protein kinase C β (PKCβ) were also inhibited by hesperetin which are the basic mechanisms for angiogenesis [46]. Kaempferol is a flavonoid compound found in many plants, including cabbage, beans, cumin, and onions. Kaempferol has various pharmacological effects, including anticancer, antimicrobial, antioxidant, anti-inflammatory, and protection of vital organs, including skin, liver, colon, ovary, pancreas, stomach, and bladder [47, 48]. Therefore, in many cancers, it is recommended to eat foods rich in kaempferol [48]. Kaempferol treatment inhibited the ability of cellular reproduction and migration due to increased glucose in HREC cells. The findings also suggested that kaempferol inhibited angiogenesis in HREC cells under high glucose conditions by suppressing VEGF and placenta growth factor (PGF) expression. High glucose could increase the activation of extracellular regulated protein kinase (Erk1/2), Src, protein kinase B-1 (Akt1), and phosphoinositide 3-kinases (PI3K) expression, and kaempferol could reverse these effects [1]. Scutellarin is a flavonoid glucuronide that has several pharmacological activities, including antioxidant, anti-inflammation, vascular relaxation, and antiplatelet [49]. Scutellarin administration to human retinal endothelial cells (HRECs) downregulated VEGF and hypoxia-inducible factor-1α (HIF-1α). As a result, scutellarin showed an inhibitory effect on retinal angiogenesis, proliferation, and tube formation [50]. In HRC cells under hypoxia and hyperglycemia condition, treatment with scutellarin reduced cell proliferation and migration, tube formation, and VEGF expression, thereby exerting its antiangiogenic effect. This compound also inhibited phosphorylation of extracellular regulated protein kinase (ERK), p-Src, and focal adhesion kinase (FAK) in these cells [9].
3. Antioxidant Phytochemicals against DR
The hyperglycemic conditions in diabetes lead to disruption of the electron transport chain and ultimately abnormal function of the retinal mitochondria, which in turn increases reactive oxygen species (ROS) production. Inflammatory pathways, oxidative stress, and ER stress regulate the level of autophagy and in severe stress conditions cause cell death and severe tissue damage [51]. Alpha-mangostin (α-MG) is a xanthoid that is isolated from Garcinia mangostana and has anti-inflammatory, antioxidant, and anticancer effects [52]. In STZ-induced DR rats, treatment with alpha-mangostin increased antioxidant capacity by reducing MDA level [53]. In a study, it was demonstrated that the main constituents of blueberry anthocyanins such as BAE, Mv, Mv-3-glc, and malvidin-3-galactoside (Mv-3-gal) significantly reduced ROS and by facilitating the expression of CAT and SOD antioxidant enzymes minimized the damaging effects of oxidative stress on HRCECs. Malvidin (Mv) decreased the production of superoxide products by reducing NADPH oxidase 4 (Nox4), but Mv-3-glc did not affect the Nox4 inhibiting. Mv, Mv-3-glc, and Mv-3-gal could also significantly alleviate nitric acid levels [16]. Curcumin use in STZ-induced DR rats increased antioxidant capacity and GSH levels in the retina. It also improved the elevated levels of nitrotyrosine and 8-OHdG [54, 55]. Curcumin significantly improved retinal dilatation and other vascular disorders caused by diabetes. Antioxidant enzymes that were reduced during diabetes were also compensated. Increased inflammatory factors such as TNF-α, VEGF, and BM thickness were also decreased after the use of curcumin. Curcumin administration also improved vascular endothelium and cytoplasmic damage in pericytes shown by retinal microscopic observations [54]. In STZ-induced PDR Wistar rats, oral administration also played a vital role in reducing oxidative stress by reducing MDA and increasing SOD, total antioxidant capacity (T-AOC) activity, and the ratio of B-cell lymphoma 2 (Bcl-2) to BCL-associated X (Bax) [7]. GSH, SOD, and CAT activation were also induced by curcumin [54]. In HG-induced ARPE-19 cells, curcumin treatment significantly reduced ROS production [12]. Consumption of curcumin in alloxan-induced DR rats increased the number of retinal ganglion cells under oxidative stress by increasing Brn3a expression [56]. Docosahexanoic acid (DHA) is a polyunsaturated fatty acid that is synthesized from the plant linolenic acid's fatty acid and is essential for the growth and development of the brain and retina that is also beneficial for pregnancy and lactation [57, 58]. β, ε-Carotene-3,3′-diol is a carotenoid with anti-inflammatory activity, with the ability to improve and prevent diseases that affect eye health and vision [59]. Studies on the effects of DHA and β, ε-carotene-3,3′-diol in STZ-induced male Wistar rats showed that these compounds exerted their antioxidant effects by weakly producing MDA and increasing GPx and GSH expression. In addition, β, ε-carotene-3,3′-diol could reduce the concentration of nitrotyrosine, while DHA had no such effect. They also improved the morphological changes caused by diabetes in the retina [60]. Eriodictyol is a flavonoid present in many citrus vegetables and medicinal plants and has anti-inflammatory, anticancer, antioxidant, and antiobesity effects [61]. The use of eriodictyol in rat retinal ganglion cells (RGCs) in hyperglycemic conditions increased the activity of antioxidant markers such as SOD, glutathione peroxidase (GPx), and CAT and ultimately reduced ROS production. Eriodictyol exerted its antioxidant activity by activating the Nrf2/HO-1 pathway [62]. Gastrodin is a phenolic glycoside, extracted from Gastrodia elata. The anti-inflammatory, antioxidant, and apoptotic effects of this compound have been identified. This phytochemical reduced apoptosis in HG-induced HRECs by regulating the sirtuin 1 (SIRT1)/Toll-like receptor 4 (TLR4)/NF-κBp65 pathway, increasing Bcl-2/Bax, and decreasing cytochrome C and cleaved caspase-3 expression. Administration of gastrodin also increased antioxidant capacity by reducing ROS production and decreased gene expression involved in oxidative stress such as HO-1, NQO1, and γ-glutamate-cysteine ligase modifier (GCLM) [63]. Genistein could inhibit the increased level of aldose reductase (ALR) caused by hyperglycemic conditions in ARPE-19 cells [42]. Genistein combined polysaccharide (GCP) is a drug rich in Glycine max isoflavones and has three main isoflavones, including genistein, daidzein, and glycetein. The use of this drug in the treatment of prostate cancer facilitates cell apoptosis and inhibits cell proliferation [64]. GCP caused an enhancement in antioxidant enzymes such as GSH-Px in postmenopausal women with DR [65]. The use of GP in STZ-induced DR rats was associated with low ROS production by regulating the levels of oxidative stress markers such as GSH, SOD, CAT, MDA, and carbonyl protein [13]. Administration of hesperidin in STZ-induced rats increased antioxidant capacity by decreasing aldose reductase (AR) activity and MDA as well as increasing superoxide dismutase (SOD) expression [45]. Hesperetin treatment in STZ-induced DR rats minimized cellular damage by affecting the activity of antioxidant enzymes such as total glutathione (tGSH), superoxide dismutase (SOD), and catalase (CAT) [66]. Lithospermic acid B (LSB) is a polyphenolic compound extracted from Salvia miltiorrhiza and was used in ancient China to treat heart diseases [67, 68]. Lithospermic acid administration in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, an animal model of type 2 diabetes, was associated with a decrease in 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative stress [69]. Naringin is a bioflavonoid used in the treatment of age-related diseases and has anticancer, anti-inflammatory, antiapoptotic, antiulcer, and antiosteoporotic effects [70, 71]. In STZ-induced rats, as well as in retinal Müller cells under hyperglycemic conditions, treatment with naringin activated antioxidant enzymes such as SOD and GSH [10]. Physcion 8-O-β-glucopyranoside (PG) is an anthraquinone, with a wide range of effects including anti-inflammatory, antioxidant, neuroprotective, and anticancer, causing liver and kidney toxicity and genetic damage [72]. Administration of PG on APRE-19 cells exposed to high glucose inhibited oxidative stress disorders in cells by reducing ROS [73]. Pterostilbene is a polyphenolic compound that, due to its two methoxyl groups, has broad effects in the prevention of type 1 and type 2 diabetes and hepatic steatosis and also reduces low-density lipoproteins (LDL) serum level [74]. In HRECs in hyperglycemic conditions, treatment with pterostilbene reduced ROS production by facilitating SOD activation [75]. Puerarin is an isoflavonoid derived from the root of Pueraria montana var. lobata and has various effects, such as antiaging, antidiabetic, and antihepatotoxic. This compound also inhibits cell proliferation by regulating mitogen-activated protein kinase (MAPK), mTOR/p70S6K, and Erk1/2 pathways and is used in cancer treatment [76]. Retinal pericyte cells exposure to AGE-BSA resulted in phosphorylation and activation of NADPH oxidase subunits, including p47phox and Rac1, so the production of ROS and the activity of enzymes involved in this pathway were stimulated. Administration of puerarin in these cells inhibited the activation of the NF-κB pathway, phosphorylation of p47phox, and Rac1 and ultimately reduced ROS production. In rats' eyes injected with AGE-RSA, puerarin also reduced the apoptosis of pericyte cells and decreased the expression of 8-OHdG [77]. Quercetin is a polyphenolic flavonoid with heart-protective, antioxidant, anti-inflammatory, antimicrobial, and antidiabetic effects [78–81]. The use of quercetin by regulating miR-29b, PTEN/AKT, and NF-κB pathways reduced the amount of ROS in ARPE-19 cells that were in hyperglycemic conditions [82]. Sauchinone is an anthocyanin extracted from Saururus chinensis and has been used in the past to treat fever, jaundice, and inflammatory diseases. This phytochemical is also useful for the treatment of hepatocellular carcinoma [83, 84]. Treatment of HG-induced ARPE-19 cells with sauchinone improved viability, reduced ROS production that facilitates the activity of antioxidant enzymes such as SOD, GPx, and CAT, and activated the Akt/Nrf2/HO-1 pathway. The rate of apoptosis in ARPE-19 cells is also alleviated by decreasing Bcl-2 and increasing Bax [85]. Scutellarin reduced the amount of ROS that induced the production of HIF-1α. It also inhibited oxidative stress-induced damage in HRECs under high glucose and hypoxic condition by reducing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity [50]. Sinomenine is an isoquinoline and one of the main components of Sinomenium acutum, widely used in the treatment of rheumatism. This substance also has anti-inflammatory, analgesic, antiangiogenic, and immunosuppressive effects [86, 87]. Treatment with sinomenine in retinal microglia cells in hyperglycemic conditions reduces the production of ROS and limits the damage caused by oxidative stress [88]. Sulforaphane is an isothiocyanate found in broccoli sprouts and is involved in preventing tumor growth, reducing inflammation, and oxidative stress [89, 90]. The use of this compound in STZ-induced DR SD rats and high glucose-induced retinal Müller cells enhanced antioxidant capacity by improving the binding of nuclear erythroid type 2 factor (Nrf2) to antioxidant response elements (AREs) and transcription of the heme-oxygenase-1 (HO-1) and NADPH quinone oxidoreductase 1 (NQO1) genes. Another effect of sulforaphane in diabetic rats was to facilitate the activation of antioxidant enzymes such as GSH, SOD, and CAT [91]. Taxifolin is a flavonoid compound present in olive oil and onion with a wide range of effects in improving inflammation, cancer, and diabetes [92]. In diabetic rats with alloxan, the use of taxifolin reduced MDA levels and increased tGSH level. Thus, oxidative stress was greatly alleviated [15].
4. Anti-Inflammatory Phytochemicals against DR
Activation of Müller glial cells, which produces inflammatory cytokines, triggers the inflammatory response in diabetic retinopathy. And the accumulation of these cytokines increases the apoptosis of neurons in the retina [93]. Increased levels of ICAM-1 produced by retinal endothelial cells and leukostasis in the retinal capillaries trigger inflammation [94]. Administration of α-MG via gavage in STZ-treated rats had anti-inflammatory and antiglycation effects, which were manifested by a decrease in AGE, RAGE, TNF-α, and VEGF [53]. Diabetic retinopathy increases nuclear factor κB (NF-κBp65), an inhibitor of kappa B (IκB), and IκB kinase (IκK) expression in mice. However, the use of andrographolide in these models negatively regulates the NF-κBp65, IκB, and IκK expression. The findings suggested that increased levels of IL-1β, IL-6, and TNF-α in mice with DR were significantly reduced by intraperitoneal injection of andrographolide, and therefore, this phytochemical reduced retinal inflammation. Early growth response-1 (Egr1) expression, which regulated the production of inflammatory cytokines, also decreased by andrographolide [26]. Baicalein is a flavone derived from the root of the plant Scutellaria baicalensis and has antiviral, antioxidant, and anticancer effects, which is also used in the treatment of cerebral ischemia due to its neuroprotective effect [95]. Treatment with baicalein in STZ-induced DR rats improved morphological changes, including decreasing the thickness of the inner and outer layers of the photoreceptors and the number of retinal ganglion cells and increasing the presence of pyknotic nuclei. This combination also improved vascular abnormalities. It was also involved in reducing the proliferation and activation of microglial cells. Baicalein downregulated glial fibrillary acidic protein (GFAP), VEGF, IL-18, TNF-α, and IL-1β, thereby reducing the inflammatory response [96]. In STZ-induced DR in rats, oral administration of biochanin suppressed retinal TNF-α and IL-1β levels, so this phytochemical improved the complications caused by inflammatory processes [2]. Mv and Mv-3-glc showed their anti-inflammatory effects by suppressing ICAM-1 expression and activating NF- κB in HRCECs treated with high glucose [16]. Treatment with chebulagic acid (CA), chebulinic acid (CI), and gallic acid (GA) in TNF-α-exposed RF/6A cells reduced the expression of inflammatory cytokines such as IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) by reducing phosphorylation of the p38, ERK, and NF-κB. In addition, CA and CI also reduced the number of inflammatory factors such as eotaxin, macrophage inflammatory protein-1 (MIP-1b), and RANTES and increased anti-inflammatory factors such as IL-10 and IL-13 [30]. Curcumin consumption in STZ-induced DR rats reduced vascular leakage. NF-κB is a factor that causes the secretion of proinflammatory cytokines, such as VEGF, inducible nitric oxide synthase (iNOS), and intercellular adhesion molecule-1(ICAM-1). Calcium/calmodulin-dependent protein kinase II (CaMKII) was also a factor that promoted retinal vascular damage. High levels of glucose increased the phosphorylation of these two factors. Treatment with curcumin in STZ-induced DR rats and Müller retinal cells in hyperglycemic conditions inhibited NF-κB phosphorylation and ultimately reduced inflammatory factors, such as VEGF, iNOS, and ICAM-1 [97]. The use of curcumin in alloxan-induced diabetic rats improved disorders of retinal morphology. It also reduced leakage in retinal vessels. Curcumin increased the number of ganglion cells under oxidative stress by increasing Brn3a expression. It exerted its anti-inflammatory effects by reducing VEGF, iNOS, and ICAM-1. It also elevated RecA expression and vascular density. The use of curcumin in cultured RGC cells derived from the retinas of diabetic rats increased neurite outgrowth in these cells [56]. ARPE-19 cell exposure to high glucose enhanced ROS/PI3K/AKT/mTOR signaling pathway and as a result increased TNF-α, IL-6, and IL-1β [12]. Treatment with curcumin in STZ-induced DR Lewis rats showed that it can inhibit the production of inflammatory cytokines, including IL-1β and NF-κB, and decrease VEGF expression [55]. Curcumolide, a sesquiterpene, extracted from Curcuma wenyujin has a wide range of effects, including anticancer, anti-inflammatory, and antioxidant [97]. The use of curcumolide in STZ-induced DR rats resulted in decreased expression of ICAM-1, leukocyte counts, and ultimately attenuated retinal vascular leakage [98]. In TNF-α-stimulated human umbilical vein endothelial cells (HUVECs), the use of this phytochemical inhibited the activity of p38 MAPK and NF-κB and proinflammatory factors [14]. As previously has been described, eriodictyol blocked apoptosis and has antioxidant properties. In line with this data, eriodictyol played an important role in the alleviation of the inflammatory response in HG-induced RGCs by reducing the production of inflammatory cytokines, including TNF-α and IL-8. The anti-inflammatory and antiapoptosis effects were facilitated by activation of the Nrf2/HO-1 pathway by this drug [62]. In STZ-induced DR rats treated with genistein, inhibition of TNF-α expression and release, as well as decreased retinal microglial cell activation, was observed. The use of genistein in cultured microglial cells treated with glycated albumin also reduced TNF-α release by inhibiting the phosphorylation of ERK and P38 MAPKs [99]. Gentiopicroside (GP) significantly limited inflammatory responses by attenuating the expression of NF-κB, TNF-α, IL-1β, and ICAM-1. In diabetic rats, oral use of GP decreased VEGF levels and significantly improved PEDF expression. The use of GP in the culture of retinal Müller cells under hyperglycemic conditions was associated with improved cell survival [13]. ICAM-1, TNF-α, and IL-1β were increased in the retina of diabetic rats. Administration of hesperetin reduced the levels of TNF-α and IL-1β and expression of caspase-3 in retinal Müller and astrocyte cells. This phytochemical also downregulated GFAP, AQP4, and capillary BM thickness and finally improved the morphological changes in the retinal layers [45, 66]. The use of lithospermic acid in OLETF rats caused morphological changes in the retina, including decreased vascular leakage, capillary atherosclerosis, and VEGF expression and increased thickness of the nerve layer, ganglion cells, and capillary BM layer. This phytochemical was derived from Salvia miltiorrhiza and reduced the serum levels of inflammatory markers, including urinary 8-OHdG, high-sensitivity C-reactive protein (hsCRP), MCP1, and TNF-α [69]. The use of β, ε-carotene-3, 3′-diol in STZ-induced DR rats reduced the amount of nitrotyrosine, which indicated the progression of inflammation [60]. Intraperitoneal injection of naringin in STZ rats increased ganglion cell count and ganglion cell layer (GCL) thickness and decreased GFAP levels. This compound inhibited the elevated level of retinal NF-κB p65 caused by diabetes. In rat Müller cell line (rMC1) exposed to diabetic conditions, the use of naringin significantly inhibited inflammation by reducing inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. It also reduced NF-κB p65 transmission from the cytoplasm to the nucleus. In STZ-induced DR rats, treatment with naringin decreased the number of inflammatory cytokines mentioned above [10]. Paeoniflorin is a monoterpene glycoside phytochemical that is isolated from Paeonia lactiflora and has anti-inflammatory effects. It is also helpful in diseases that affect the immune system, such as RA and systemic lupus erythematosus (SLE) [100, 101]. Paeoniflorin inhibited MMP-9 activation in BV2 cells and the transmission of NF-κB p65 to the nucleus and decreased p-p38 expression. It also increased the expression of suppressor of cytokine signaling 3 (SOCS3), an intracellular inhibitor of cytokines, by activating the TLR4 receptor. Other effects of paeoniflorin in STZ mice included reduced IL-1β and IBA-1 levels in inflammation and activation of microglial cells. This phytochemical also activated MMP-9 and increased SOCS3 expression in diabetic mice. Changes in the retina of diabetic mice, which included increased retinal thickness and retinal gliocyte proliferation, were also ameliorated by paeoniflorin [102]. The use of PG in APRE-19 cells treated with high glucose regulates the noncoding RNA activated by DNA damage (NORAD)/miR-125/STAT3 pathway. TNF-α and IL-1β levels and cellular apoptosis were also downregulated after treatment with PG [73]. The use of pterostilbene in HRECs under high glucose conditions reduced the proliferation of these cells, the production of TNF-α and IL-1β, and ultimately the inflammatory response. The reduction of NF-κB transcription was another benefit of this compound [75]. The effects of quercetin on miRNAs in different inflammatory conditions were previously studied [80]; miRNAs have vital regulatory roles in different diseases and conditions [103]. Treating APRE-19 cells in hyperglycemic conditions with quercetin led to the suppression of apoptosis and proinflammatory cytokines such as MCP-1 and IL-6, as well as inhibition of ROS production while upregulating miR-29b expression. Moreover, miR-29b overexpression resulted in the promotion of the PTEN/AKT pathway and inhibition of the NF-κB pathway in quercetin-treated cells. Taken together, quercetin exerted protective effects on DR via regulating the miR-29b, PTEN/AKT, and NF-κB pathways [82]. Resveratrol is a phytoestrogen that has anti-inflammatory, antioxidant, and neuroprotective effects and can regulate brain activity due to its ability to cross the blood-brain barrier [104]. Administration of resveratrol in STZ-induced DR rats had a wide range of effects, including decreased expression and activation of NF-κB, levels of inflammatory mediators such as TNF-α, IL-6, and prostaglandin-endoperoxide synthase 2 (COX-2), and mitigation of retinal cell apoptosis [19]. Sesamin, a lignan, is the main component of Sesamum indicum, has neuroprotective, antioxidant, and anti-inflammatory effects, and has been used in the treatment of patients with osteoarthritis in the past [105, 106]. Intraperitoneal injection of sesamin into STZ-induced DR mice reduced the activation of retinal microglia cells and proinflammatory factors such as TNF-α and ICAM-1. The antioxidant effect of this drug was manifested by decreasing the expression of inducible nitric oxide synthase (iNOS) and finally reducing the amount of ROS [107]. Shikonin, a naphthoquinone, is extracted from the root of Lithospermum erythrorhizon and has anti-inflammatory and anticancer effects. It has been used in the past to treat dermatitis, skin wounds, and burns [108]. Administration of shikonin in STZ mice could control the increased permeability of blood vessels in the retina. Diabetes reduced the thickness of the inner and middle layers of the retina, and the use of shikonin improved these effects. Oral use of shikonin reduced the expression of inflammatory factors such as COX-2, iNOS, and proapoptotic protein Bax, which were increased due to diabetes in the retina. It also reduced edema and damage to retinal cells. Shikonin treatment of RF cells in hypoxia and high glucose conditions reduced cell damage and also alleviated the expression of inflammatory factors such as COX-2, iNOS, and myeloperoxidase (MPO), and ZO-1, a protein for strong intercellular binding, is reduced in diabetic condition, while shikonin prevented changes in ZO-1 level [8]. Silybin is also known as silibinin, is a flavonolignan, and has been widely used to treat nonalcoholic fatty liver. Consumption of this phytochemical, obtained from Silybum marianum, also has anticancer and antidiabetic effects [109, 110]. The use of silybin in the treatment of STZ-induced DR rats reduced the number of obliterated retinal capillaries and improved leukostasis in retinal vessels. Administration of this compound also suppressed inflammation in the retina by reducing ICAM-1 expression [111]. Microglial cell culture in hyperglycemic conditions increased the expression and regeneration of inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Treatment with sinomenine reduced the levels of cytokines. Sinomenine also attenuated NF-κB p65 nuclear transport [88]. Administration of sulforaphane in STZ-induced DR SD rats significantly increased the number of ganglion cells. The anti-inflammatory effects of this compound could be concluded by observing the effects of sulforaphane on the reduction of TNF-α, IL-6, and IL-1β. In diabetic rats, the expression of four inflammatory factors, including pyrin domain-containing 3 (NLRP3), adaptor protein apoptosis-associated speck-like protein (ASC), cleaved caspase-1 p20 level, and IL-1β p17 increased. The use of this phytochemical in Müller cells in hyperglycemic conditions reduced the inflammatory response by lowering TNF-α and IL-6 levels [91]. Taxifolin improved inflammatory responses by significantly reducing IL-1β and TNF-α. Tissue damage to the retina also progressed to normalization with taxifolin. Congested and dilated arteries in the ganglion cell layer of the retina were also greatly improved after treatment with taxifolin in alloxan-induced DR rats [15]. Zerumbone, a monocyclic sesquiterpene, has been used in the past to treat diseases such as stomach pain, diabetes, and skin diseases. It has anti-inflammatory, antimicrobial, and antihypersensitivity effects [112]. Consumption of oral zerumbone extracted from Zingiber zerumbet in STZ-diabetic rats improved atrophy in the layers of nerve fibers and ganglion cells and increased retinal thickness. Decreased expressions of RAGE, TNF-α, IL-1β, IL-6, VEGF, ICAM-1, and vascular cell adhesion molecule-1 (VCAM-1) were other effects of this drug. Also, a decrease in NF-κB apoptotic activity after using this compound could be seen in diabetic rats [113].
5. Conclusion
Diabetic retinopathy is one of the most important complications in patients with diabetes and can greatly affect a person's quality of life. Current therapeutic techniques for diabetic retinopathy are anti-VEGF injection, photocoagulation, and administration of corticosteroids, which have been demonstrated to be effective; however, they possess significant limitations and serious side effects such as invasiveness, short-lasting mechanism, retinal detachment, blurred vision, fungal infection burning the retina, and loss of vision [114–116].
The present review provides an overview of the anti-inflammatory, antiangiogenesis, and antioxidant effects of phytochemicals (Table 1) in DR in vitro and in vivo. In Table 2, the effects of different phytochemicals on DR pathogenesis were summarized. Different types of phytochemicals including flavonoids, polyphenols, lignan, pyranocoumarin, iridoid glycosides, xanthoid, phytoestrogen, anthraquinone, naphthoquinone, sesquiterpene, monoterpene glycoside, isothiocyanate, anthocyanins, and isoquinoline inhibited angiogenic factors, such as VEGF, PKCβ, and HIF-1α, as well as oxidative stress by affecting the production of ROS and activity of antioxidant enzymes, including SOD, MDA, NADPH oxidase, and CAT. Furthermore, the inflammatory factors, including TNF-α, IL-6, and IL-1β, which could damage the retina, were also downregulated by phytochemicals. The molecular mechanisms of angiogenesis, inflammation, and oxidative stress in the pathophysiology of diabetic retinopathy are illustrated in Figure 1. Phytochemicals not only affect one of the pathogenic mechanisms of DR but also can affect several of them simultaneously (Figure 2).
Table 1.
Chemical structures of different phytochemicals used for diabetic retinopathy treatment.
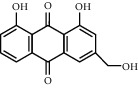
|
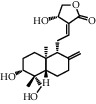
|
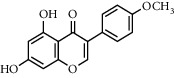
|
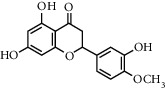
|
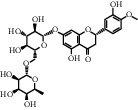
|
| Aloe-emodin | Andrographolide | Biochanin | Hesperetin | Hesperidin |
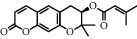
|
|
|
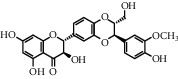
|

|
| Decursin | Scutellarin | Sinomenine | Silybin | Genistein |

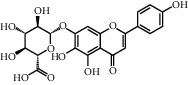
|

|

|

|
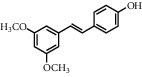
|
| Andrographolide | Lithospermic acid B | Alpha-mangostin | Formononetin | Pterostilbene |
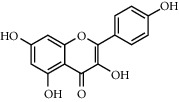
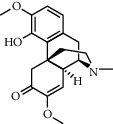
|
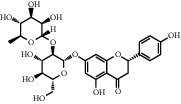
|
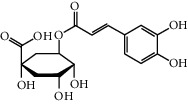
|
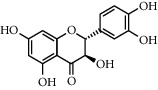
|

|
| Kaempferol | Naringin | Chlorogenic acid | Taxifolin | Chrysin |
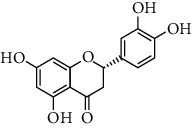
|

|
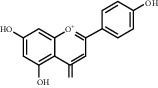
|
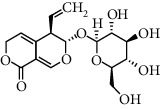
|
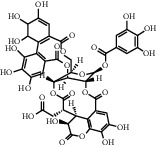
|
| Eriodictyol | Curcumin | Anthocyanin | Gentiopicroside | Chebulagic acid |

|

|

|
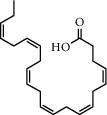
|
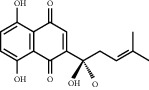
|
| Chebulinic acid | Gallic acid | β, ε-Carotene-3,3′-diol | Docosahexaenoic acid | Shikonin |

|
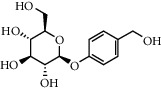
|
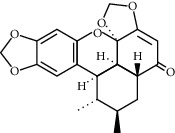
|
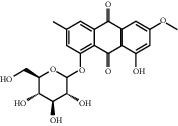
|

|
| Resveratrol | Gastrodin | Sauchinone | Physcion 8-O-β-glucopyranoside | Quercetin |
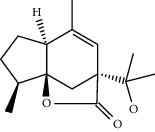
|

|

|

|

|
| Curcumolide | Sulforaphane | Baicalein | Zerumbone | Sesamin |
Table 2.
Effects of different phytochemicals on diabetic retinopathy pathogenesis.
| Phytochemical | Plant | Model | Dose/concentration | Study type | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Aloe-emodin | — | ARPE-19 under hypoxia condition and OIR model rats | 0.2–5.0 µg/mL (in vitro) and 5.0 and 10.0 mg/kg/day (oral) | In vivo and in vitro | ↓ Retinal neovascularization and expressions of VEGFA, HIF-1α, and PHD-2 | Wu, 2016 |
| Alpha-mangostin | Garcinia mangostana | STZ-induced DR SD rat | 200 mg/kg/day | In vivo | ↓ MDA, AGE, RAGE, TNF-α, and VEGF | Jariyapongskul, 2015 |
| Andrographolide | Andrographis paniculata | STZ-induced NPDR and PDR C57BL/6 mice | 10 mg/kg/day (intraperitoneally) | In vivo | ↓ Retinal angiogenesis, inflammation, breakdown of BRB, VEGF, NF-κB, Egr-1 phospho-NF-κBp65, IKK, TNF-α, IL-6, IL-1β, serpine1, and TF | Yu, 2015 |
| Baicalein | — | STZ-induced DR SD female rats | 150 mg/kg/d orally | In vivo | ↓ Retinal inflammation, GFAP, VEGF, IL-18, TNF-a, IL-1, vascular abnormality, loss of ganglion cell layer (GCL), and microglial activation | Yang, 2009 |
| Biochanin | — | STZ-induced DR Wistar rats | 10 and 15 mg/kg/day (orally) | In vivo | ↓ Retinal angiogenesis, inflammation, VEGF, TNF-α, and IL-1β | Mehrabadi, 2018 |
| Blueberry anthocyanins | Vaccinium virgatum | High glucose-induced HRCECs | 10 μg/mL | In vitro | ↓ Retinal inflammation, oxidative stress, ROS, and VEGF production ↑ CAT and SOD activity BAE, Mv, and Mv-3-gal ↓ Nox4 protein expression BAE, Mv-3-glc, and Mv-3-gal ↓ Akt expression, Mv Mv-3-glc, and Mv-3-gal ↓ ICAM-1 levels and NF-κB | Huang, 2018 |
| Chebulagic acid (CA), chebulinic acid (CI), and gallic acid (GA) | — | RF/6A cells were stimulated with TNF-α CAM model | 1.0 to 100 µM; 25, 25, and 100 µM | In vivo and in vitro | ↓ CA, CI, and GA: retinal angiogenesis, inflammation, expression of MMP-9, IL-6, IL8, MCP-1, phosphorylation of p38, ERK and NF-κB CA, CI: eotaxin, MIP-1b, RANTES, PDGF-BB ↑ IL-10, and IL-13 | Shanmuga, 2018 |
| Chlorogenic acid | — | STZ-induced PDR C57BL/6 mice, HRECs, RF/6A cells, and microglia BV-2 cells | 1,10 mg/kg/day (oral); 0.3125–0.5 µM (in vitro) | In vivo and in vitro | ↓ Retinal angiogenesis, microglia cell activation, VEGF expression, phosphorylation of VEGFR-2, MEK1/2, ERK1/2, and p38 | Mei, 2018 |
| Chrysin | — | HRMVEC and adult male db/db mice C57BLKS | 1–20 µM (in vitro); 10 mg/kg (oral) | In vitro and in vivo | ↑ VE-cadherin, ZO-1 junction proteins, and PECAM-1 ↓ HIF-1α, VEGF, VEGF receptor-2, Ang-1, Ang-2, and Tie-2 proteins | Kang, 2016 |
| Chrysin | — | HRPE cell db/db mice | 1–20 µM; 10 mg/kg/day (oral) | In vivo and in vitro | ↓ Retinal neovascularization, VEGF, IGF-1, AGE secretion, RAGE, and ER stress ↑ Retinal thickness, PEDF, RPE65, LRAT, and RDH5 level | Kang, 2018 |
| Curcumin | — | STZ-induced DR Lewis rats | 0.5 g/kg, powdered diet | In vivo | ↓ Retinal inflammation, 8-OHdG, nitrotyrosine, IL-1β, VEGF, NF-κB, and ROS ↑ GSH activity | Kowluru, 2007 |
| Curcumin | — | STZ-induced DR male Wistar albino rats | 1g/kg body weight of rat orally | In vivo | ↓ Retinal inflammation, HbA1c level, vessel diameter, thickening of BM, TNF-a, and VEGF ↑ GSH, SOD, and CAT activities | Suresh,2011 |
| Curcumin | — | STZ-induced DR and rat retinal Müller cells | 100 mg/kg/day (oral); 5–15 μM | In vivo and in vitro | ↓ Retinal vascular leakage, inflammation, VEGF, iNOS, ICAM-1expession, phosphorylation of CaMKII, and NF-κB | Li, 2016 |
| Curcumin | Curcuma longa | Alloxan-induced DR in rats and RGC from diabetic and normal rats | 100 mg/kg/day (in vivo); 10–1000 nmol/mL (in vitro) | In vivo and in vitro | ↓ Retinal inflammation, oxidative stress, retinal capillary basement membrane thickness, phosphorylation p65subunit of NF-κB, CaMKII activity, VEGF, iNOS, and ICAM-1 expressions ↑ RecA and microvessel density, expression of Thy-1 and Brn3, and average neurite length | Pradhan, 2018 |
| Curcumin | — | STZ-induced PDR Wistar rat | 100 and 200 mg/kg/day (oral) | In vivo | ↓ Retinal angiogenesis, oxidative stress, apoptosis, retinal capillary basement membrane thickness, VEGF, and MDA ↑ Ratio of Bcl-2 to Bax, SOD, and T-AOC | Yang, 2018 |
| Curcumin | — | HG-induced ARPE-19 cells | 0–20 µmol/l | In vitro | ↓ TNF-α, IL-6, IL-1β, and ROS-AKT/mTOR | Ran, 2019 |
| Curcumolide | Curcuma wenyujin | STZ-induced DR, Wistar rats, and TNF-a-stimulated HUVECs | 2.5–20 μM intravitreal injection | In vivo and in vitro | ↓ Retinal inflammation, leukostasis, vascular permeability, TNF-a, ICAM-1, p38 MAPK, and NF-κB activation | Cai, 2017 |
| Decursin | Angelica gigas | HRMVEC cells and STZ-induced SD rats | 20 mg/kg/day (oral); 12.5–100 µM (in vitro) | In vivo and in vitro | ↓ Retinal proliferation and angiogenesis, VEGFR-2 expression, tube formation, and retinal neovascularization | Yang, 2013 |
| (1) Docosahexaenoic acid (2) β, ε-Carotene-3,3′-diol |
— | STZ-induced DR male Wistar rats | (1) CL and DL groups: 0.5 mg/kg (oral) (2) CDHA and DDHA groups: 13.3 mg/kg (oral) |
In vivo | (1) ↓ MDA (1,2) and nitrotyrosine level (2) ↑ GPX activity and GSH level (1, 2) |
Arnal, 2009 |
| Eriodictyol | — | HG-induced rat RGCs | 5–20 μM | In vitro | ↓ Retinal inflammation, production of ROS, TNF-α, IL-8, BAX, and cleaved caspase-3 ↑ SOD, GPX, CAT, Bcl-2, and activation of Nrf2/HO-1 | Lv, 2019 |
| Formononetin | Astragalus membranaceus | ARPE-19 cells under chemical hypoxia and OIR model rats | 5.0 and 10.0 mg/kg/day (intraperitoneal); 0.2–5 μg/mL (in vitro) | In vivo and in vitro | ↓ Retinal neovascularization, VEGF, HIF-1α, and PHD-2 | Wu, 2016 |
| Gastrodin | Gastrodia elata | HG-induced HRECs | 0.1–10 and 100 µM | In vitro | ↓ TLR4/NF-κB p65 signaling pathway, ROS, NADPH, NQO1, Nrf2, and GCLM ↑ SIRT1 | Zhang, 2018 |
| Genistein | — | ARPE-19 cells treated with normal and high glucose concentrations | 20 µM | In vitro | ↓ Retinal inflammation, angiogenesis, ALR, VEGF165, and VEGF secretion | Dongare, 2015 |
| Genistein combined polysaccharides | — | 12 postmenopausal Korean women | (Tablets) 2 g containing 120 mg of genistein and 57 mg of daidzein | In vivo | ↑ SHBG and GSH-Px activity | Oh, 2005 |
| Gentiopicroside | — | STZ-induced DR rats and rMC1 cells | 20–80 mg/kg/day; 10–100 µM (in vitro) | In vivo and in vitro | ↓ Retinal inflammation, oxidative stress, overexpression of HDAC, ROS expression, MDA, protein carbonyl expression, NF-κB, TNF-α, IL-1β, ICAM-1, GFAP, and VEGF expression ↑ HAT expression, GSH, SOD, CAT, and PEDF expression | Zhang, 2019 |
| Hesperetin | — | STZ-induced DR Wistar rats | 200 mg/kg/day (oral) | In vivo | ↓ Retinal angiogenesis, dilated vessels, VEGF, PKC-β, vascular permeability, and thickening of BM | Kumar, 2012 |
| Hesperetin | — | STZ-induced DR Wistar albino rats | 100 mg/kg body (oral) | In vivo | ↓ Retinal inflammation, ROS, TNF-α, IL-1β, caspase-3, GFAP, and AQP4 ↑ Retinal GSH SOD and CAT activity | Kumar, 2013 |
| Hesperidin | — | STZ-induced DR SD rats | 100 and 200 mg/kg/day (intragastrically) | In vivo | ↓ Retinal angiogenesis, oxidative stress, inflammation, permeability of the BRB, VEGF, ICAM-1, TNF-α, IL-1β, AGE, AR activity, and MDA ↑ SOD and retinal thickness | Shi, 2012 |
| Kaempferol | — | HRECs under high glucose condition | 5–25 µM | In vitro | ↓ Retinal angiogenesis, proliferative ability, migration, VEGF and PGF expression, expression of PI3K, activation of Erk1/2, Src, and Akt1 | Xu, 2017 |
| Lithospermic acid B | Salvia miltiorrhiza | OLETF rats | 10 or 20 mg/kg/day (oral) | In vivo | ↑ Thickness of the nerve layer, ganglion cells, and capillary BM layer ↓ VEGF, hsCRP, MCP1, TNF-α, and 8-OHdG | Jin, 2014 |
| Naringin | — | rMC1 STZ-induced DR rats | 20–80 mg/kg/day (intraperitoneally); 50 μM (in vitro) | In vivo and in vitro | ↓ Retinal inflammation, oxidative stress, TNF-α, IL-1β, IL-6, NF-κB level, and GFAP level ↑ SOD, GSH, GCL thickness, and ganglion cell number | Liu, 2017 |
| Paeoniflorin | — | Microglia BV-2 cells and STZ-induced DR CD-1 mice | 20 and 40 mg/kg/day (oral); 0.1–10 μM | In vivo and in vitro | ↓ Retinal inflammation, MMP-9 activation, expression of p-p38, NF-κB translocation, IBA-1, and IL-1β ↑SOCS3 expression and activating TLR4 | Zhu, 2017 |
| Physcion 8-O-Β-glucopyranoside | — | HG-disposed APRE-19 cell injury | 1.5 µM | In vitro | ↓ TNF-α, IL-1β, ROS generation, cell apoptosis, NORAD expression, and STAT3 ↑ miR-125 expression | Wan, 2020 |
| Pterostilbene | — | HRECs under high glucose environment | 1 mmol/l | In vitro | ↓ TNF-α, IL-1β, ROS production, and NF-κB mRNA and protein expression ↑ SOD activity | Shen, 2015 |
| Puerarin | — | AGE-RSA induced bovine retinal pericyte cells AGE-RSA injected to rat eyes | 1, 5, and 10 µM; 400 mM (intravitreally) | In vivo | ↓ NADPH oxidase activity, ROS, phosphorylation of p47 phox and Rac1, NF-κB, and 8-OHdG | Kim, 2012 |
| Quercetin | — | ARPE-19 cells were stimulated by high glucose | 30 μM | In vitro | ↓ Cell apoptosis, MCP-1, IL-6, ROS, PTEN, p-p65, and IκBα ↑ miR-29b expression and p-AKT | Wang, 2020 |
| Resveratrol (trans-3,5,40-trihydroxystilbene) | — | STZ-induced DR rats | 5 mg/kg per day orally | In vivo | ↓ Retinal inflammation, NF-κB, TNF-a, IL-6, and COX-2 | Ghadiri Soufi, 2015 |
| Sauchinone | — | Human RPE cell line/ARPE-19 | 5, 10, and 20 µM | In vitro | ↑ SOD, GPX, CAT, Bcl-2, P-Akt, nuclear nrf2, HO-1, and Akt/nrf2/HO-1 signaling pathway ↓ ROS and Bax | Shi, 2019 |
| Scutellarin | - | HRECs under high glucose and hypoxic condition | 0.1 nM; 1 µM | In vitro | ↓ Retinal angiogenesis and proliferation, tube formation, HIF-1α, VEGF, ROS, and NADPH oxidase activity | Wang, 2014 |
| Scutellarin | — | HRECs under high glucose and hypoxic condition and STZ-induced DR | 40 mg/kg/day (intragastrically); 10 μM (in vitro) | In vivo and in vitro | ↓ Retinal angiogenesis and proliferation, VEGF, phosphorylation of ERK, FAK, and Src | Long, 2019 |
| Sesamin | — | STZ-induced DR mice | 30 mg/kg BW (intraperitoneally), alternate day | In vivo | ↓ Retinal inflammation, TNF-α, ICAM, microglia activation, and iNOS | Ahmad, 2016 |
| Shikonin | — | STZ with whole-body hypoxia-induced DR in C57BL/6 mice and RPE cells | 0.5–50 mg/kg/day (oral); 0.1–10 μM (in vitro) | In vivo and in vitro | ↓ Retinal inflammation, vascular permeability, cell loss, COX-2, iNOS, Bax, MPO, and ZO-1 | Liao, 2017 |
| Silybin | — | STZ-induced DR SD rats | 15 and 30 mg/kg/day (orally) | In vivo | ↓ Retinal inflammation, obliterated retinal capillaries, retinal vascular leukostasis, and ICAM-1 | Zhang, 2014 |
| Sinomenine | Sinomenium acutum | Retinal microglia cells isolated from SD rats | 0.01–1 mM | In vitro | ↓ Retinal inflammation, microglial activation, IL-1β, TNF-α, IL-6, and ROS | Wang, 2007 |
| Sulforaphane | — | STZ-induced DR SD rats and rMC1 | 0.5 and 1 mg/kg/day 2.5 μM (in vitro) | In vivo and in vitro | ↓ Retinal inflammation, TNF-α, IL-6, IL-1β, NLRP3, ASC, and cleaved caspase-1 p20 level ↑Ganglion cells, antioxidant capacity, NRF2, transcriptions of HO-1, and NQO1 | Li, 2019 |
| Taxifolin | — | Alloxan-induced DR and albino Wistar male rats | 50 mg/kg/day (oral) | In vivo | ↓ Retinal inflammation, MDA, IL-1β, and TNF-α ↑ tGSH level | Ahiskali, 2019 |
| Zerumbone | Zingiber zerumbet | STZ-induced DR and male Wistar rats | 40 mg/kg once a day orally | In vivo | ↓ Retinal inflammation, NF-κB, apoptosis, thickness of retinal layers, AGEs, TNF-α, IL-1β, IL-6, VEGF, NF-κB, HbA1c, RAGE, ICAM-1, and VCAM-1 protein expression | Tzeng, 2016 |
∗ ↓: decrease; ↑: increase.
Figure 1.
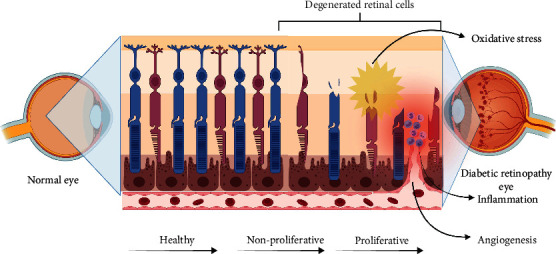
An insight into the molecular mechanism of angiogenesis, inflammation, and oxidative stress in the pathophysiology of diabetic retinopathy.
Figure 2.
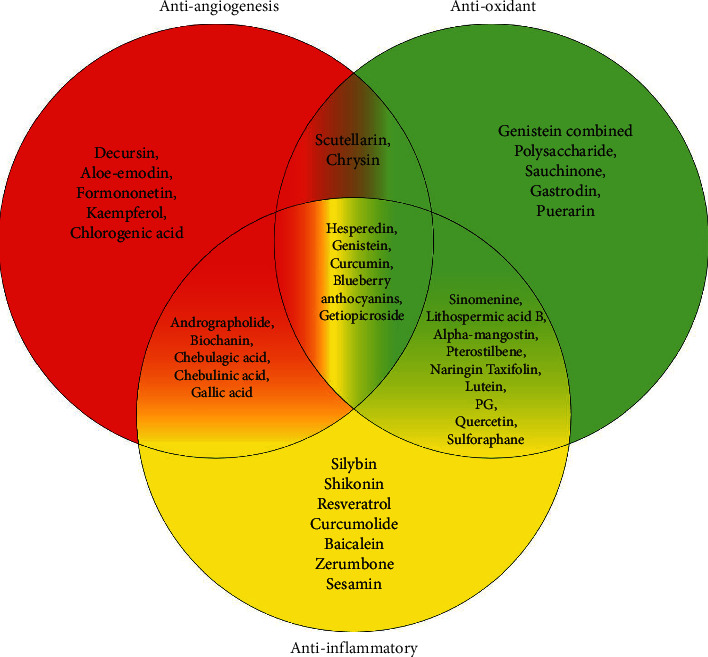
Simultaneous and individual effects of phytochemicals on angiogenesis, inflammation, and oxidative stress in diabetic retinopathy.
Based on the existing literature about the simultaneous effects of phytochemicals on the pathophysiology of DR, the present review provides some suggestions about using phytochemicals with multiple targets as a supplementary therapy for DR. It is also recommended to use different phytochemicals to enhance synergy and cover all aspects of DR by targeting multiple targets to prevent the disease or shorten the recovery time.
Although phytochemicals have various benefits, many factors should be noticed regarding their safety and production quality. Therefore, further pharmacokinetic and pharmacodynamic studies on the use of these products are necessary. Meanwhile, further studies are needed to investigate their cellular and molecular targets, alone and simultaneously.
Contributor Information
Ali Hafezi-Moghadam, Email: 126700200b@gmail.com.
Abouzar Bagheri, Email: a.bagherimg@gmail.com.
Data Availability
No data were used to support this study.
Disclosure
The authors understand that they will not benefit directly from participating in this research. The authors give their consent for the publication of identifiable details, which can include photograph(s) and/or videos and/or case history and/or details within the text (“material”) to be published in this journal and paper.
Conflicts of Interest
All the authors declare that they have no conflicts of interest.
Authors' Contributions
Mohammad Amin Khazeei Tabari and Razie Mirjalili contributed to conceptualization and drafting; Hooman Khoshhal, Elahe Shokouh, and Mohanna Khandan contributed to search strategy and drafting; Elham Hasheminasabgorji contributed to review and editing; Ali Hafezi-Moghadam and Abouzar Bagheri contributed to conceptualization, visualization, and supervision. Mohammad Amin Khazeei Tabari and Razie Mirjalili contributed equally to the preparation of the manuscript.
References
- 1.Xu X. H., Zhao C., Peng Q., Xie P., Liu Q. H. Kaempferol inhibited VEGF and PGF expression and in vitro angiogenesis of HRECs under diabetic-like environment. Brazilian Journal of Medical and Biological Research . 2017;50(3) doi: 10.1590/1414-431X20165396.e5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehrabadi M. E., Salemi Z., Babaie S., Panahi M. Effect of biochanin A on retina levels of vascular endothelial growth factor, tumor necrosis factor-alpha and interleukin-1beta in rats with streptozotocin-induced diabetes. Canadian Journal of Diabetes . 2018;42(6):639–644. doi: 10.1016/j.jcjd.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Kang M. K., Lee E. J., Kim Y. H., et al. Chrysin ameliorates malfunction of retinoid visual cycle through blocking activation of AGE-RAGE-ER stress in glucose-stimulated retinal pigment epithelial cells and diabetic eyes. Nutrients . 2018;10:8. doi: 10.3390/nu10081046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kordkheyli V. A., Mishan M. A., Tarsi A. K., et al. MicroRNAs may provide new strategies in the treatment and diagnosis of diabetic retinopathy: importance of VEGF. Iranian Journal of Basic Medical Sciences . 2021;24(3):267–279. doi: 10.22038/ijbms.2021.52164.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei X., Zhou L., Zhang T., Lu B., Sheng Y., Ji L. Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vascular Pharmacology . 2018;101:29–37. doi: 10.1016/j.vph.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Geneva, Switzerland: World Health Organization; 2021. Report of expert and stakeholder consultations on the WHO global diabetes compact. Report. [Google Scholar]
- 7.Yang F., Yu J., Ke F., et al. Curcumin alleviates diabetic retinopathy in experimental diabetic rats. Ophthalmic Research . 2018;60(1):43–54. doi: 10.1159/000486574. [DOI] [PubMed] [Google Scholar]
- 8.Liao P. L., Lin C. H., Li C. H., et al. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic. Scientific Reports . 2017;7 doi: 10.1038/srep44985.44985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long L., Li Y., Yu S., et al. Scutellarin prevents angiogenesis in diabetic retinopathy by downregulating VEGF/ERK/FAK/Src pathway signaling. Journal of Diabetes Research . 2019;2019:17. doi: 10.1155/2019/4875421.4875421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L. H., Zuo Z. F., Lu S. J., Liu A. H., Liu X. Z. Naringin attenuates diabetic retinopathy by inhibiting inflammation, oxidative stress and NF-kappa B activation in vivo and in vitro. Iranian Journal of Basic Medical Sciences . 2017;20(7):813–821. doi: 10.22038/IJBMS.2017.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platania C., Fidilio A., Lazzara F., et al. Retinal protection and distribution o curcumin in vitro and in vivo. Frontiers in Pharmacology . 2018;9 doi: 10.3389/fphar.2018.00670.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ran Z., Zhang Y., Wen X., Ma J. Curcumin inhibits high glucose-induced inflammatory injury in human retinal pigment epithelial cells through the ROS-PI3K/AKT/mTOR signaling pathway. Molecular Medicine Reports . 2019;19(2):1024–1031. doi: 10.3892/mmr.2018.9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Shi E., Yang L., Fu W. N., Hu F., Zhou X. S. Gentiopicroside attenuates diabetic retinopathy by inhibiting inflammation, oxidative stress, and NF-kappa B activation in rat model. European Journal of Inflammation . 2019;17 doi: 10.1177/2058739219847837. [DOI] [Google Scholar]
- 14.Cai Y., Li W., Tu H., et al. Curcumolide reduces diabetic retinal vascular leukostasis and leakage partly via inhibition of the p38MAPK/NF-κ B signaling. Bioorganic & Medicinal Chemistry Letters . 2017;27(8):1835–1839. doi: 10.1016/j.bmcl.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 15.Ahiskali I., Pinar C. L., Kiki M., et al. Effect of taxifolin on development of retinopathy in alloxan-induced diabetic rats. Cutaneous and Ocular Toxicology . 2019;38(3):227–232. doi: 10.1080/15569527.2019.1588289. [DOI] [PubMed] [Google Scholar]
- 16.Huang W., Yan Z., Li D., Ma Y., Zhou J., Sui Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxidative Medicine and Cellular Longevity . 2018;2018:10. doi: 10.1155/2018/1862462.1862462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishan M. A., Tabari K., Mahrooz A., Bagheri A. J. E. J. O. C. P. T. O. J. O. T. E. C. P. O. Role of microRNAs in the anticancer effects of the flavonoid luteolin: a systematic review. European Journal of Cancer Prevention . 2021;30 doi: 10.1097/cej.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 18.Lupo G., Cambria M. T., Olivieri M., et al. Anti-angiogenic effect of quercetin and its 8-methyl pentamethyl ether derivative in human microvascular endothelial cells. Journal of Cellular and Molecular Medicine . 2019;23(10):6565–6577. doi: 10.1111/jcmm.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghadiri Soufi F., Arbabi-Aval E., Rezaei Kanavi M., Ahmadieh H. Anti-inflammatory properties of resveratrol in the retinas of type 2 diabetic rats. Clinical and Experimental Pharmacology and Physiology . 2015;42(1):63–68. doi: 10.1111/1440-1681.12326. [DOI] [PubMed] [Google Scholar]
- 20.Okonkwo U., DiPietro L. Diabetes and wound angiogenesis. International Journal of Molecular Sciences . 2017;18(7):p. 1419. doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishan M. A., Khazeei Tabari M. A., Zargari M., Bagheri A. MicroRNAs in the anticancer effects of celecoxib: a systematic review. European Journal of Pharmacology . 2020;882 doi: 10.1016/j.ejphar.2020.173325.173325 [DOI] [PubMed] [Google Scholar]
- 22.Todorova D., Simoncini S., Lacroix R., Sabatier F., Dignat-George F. Extracellular vesicles in angiogenesis. Circulation Research . 2017;120(10):1658–1673. doi: 10.1161/circresaha.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X., Zeng Y., Liu Y., et al. Aloe-emodin: a review of its pharmacology, toxicity, and pharmacokinetics. Phytotherapy Research . 2020;34(2):270–281. doi: 10.1002/ptr.6532. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Ke X., Wang W., et al. Aloe-emodin suppresses hypoxia-induced retinal angiogenesis via inhibition of HIF-1α/VEGF pathway. International Journal of Biological Sciences . 2016;12(11):1363–1371. doi: 10.7150/ijbs.16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J., Ma Y., Wu J., et al. A review for the neuroprotective effects of andrographolide in the central nervous system. Biomedicine & Pharmacotherapy . 2019;117 doi: 10.1016/j.biopha.2019.109078.109078 [DOI] [PubMed] [Google Scholar]
- 26.Yu Z., Lu B., Sheng Y., Zhou L., Ji L., Wang Z. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochimica et Biophysica Acta (BBA)-General Subjects . 2015;1850(4):824–831. doi: 10.1016/j.bbagen.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Yu C., Zhang P., Lou L., Wang Y. Perspectives regarding the role of biochanin A in humans. Frontiers in Pharmacology . 2019;10:p. 793. doi: 10.3389/fphar.2019.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Liu H., Lv L., Yan H., Yuan Y. Antioxidant activity of blueberry anthocyanin extracts and their protective effects against acrylamide-induced toxicity in HepG2 cells. International Journal of Food Science & Technology . 2018;53(1):147–155. doi: 10.1111/ijfs.13568. [DOI] [Google Scholar]
- 29.Shanmuganathan S., Angayarkanni N. Chebulagic acid and chebulinic acid inhibit TGF-β1 induced fibrotic changes in the chorio-retinal endothelial cells by inhibiting ERK phosphorylation. Microvascular Research . 2019;121:14–23. doi: 10.1016/j.mvr.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Shanmuganathan S., Angayarkanni N. Chebulagic acid chebulinic acid and gallic acid, the active principles of triphala, inhibit TNFα induced pro-angiogenic and pro-inflammatory activities in retinal capillary endothelial cells by inhibiting p38, ERK and NFkB phosphorylation. Vascular Pharmacology . 2018;108:23–35. doi: 10.1016/j.vph.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Bhandarkar N. S., Brown L., Panchal S. K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet–induced cardiovascular, liver, and metabolic changes in rats. Nutrition Research . 2019;62:78–88. doi: 10.1016/j.nutres.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z., Lam K. L., Hu J., et al. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Science & Nutrition . 2019;7(2):579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naz S., Imran M., Rauf A., et al. Chrysin: pharmacological and therapeutic properties. Life Sciences . 2019;235 doi: 10.1016/j.lfs.2019.116797.116797 [DOI] [PubMed] [Google Scholar]
- 34.Farkhondeh T., Samarghandian S., Roshanravan B. Impact of chrysin on the molecular mechanisms underlying diabetic complications. Journal of Cellular Physiology . 2019;234(10):17144–17158. doi: 10.1002/jcp.28488. [DOI] [PubMed] [Google Scholar]
- 35.El-Marasy S. A., El Awdan S. A., Abd-Elsalam R. M. Protective role of chrysin on thioacetamide-induced hepatic encephalopathy in rats. Chemico-Biological Interactions . 2019;299:111–119. doi: 10.1016/j.cbi.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Kang M. K., Park S. H., Kim Y. H., et al. Dietary compound chrysin inhibits retinal neovascularization with abnormal capillaries in db/db mice. Nutrients . 2016;8:12. doi: 10.3390/nu8120782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giordano A., Tommonaro G. Curcumin and cancer. Nutrients . 2019;11(10):p. 2376. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shehzad A., Parveen S., Qureshi M., Subhan F., Lee Y. S. Decursin and decursinol angelate: molecular mechanism and therapeutic potential in inflammatory diseases. Inflammation Research . 2018;67(3):209–218. doi: 10.1007/s00011-017-1114-7. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y., Yang K., Li Y., et al. Decursin inhibited proliferation and angiogenesis of endothelial cells to suppress diabetic retinopathy via VEGFR2. Molecular and Cellular Endocrinology . 2013;378(1-2):46–52. doi: 10.1016/j.mce.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Ong S., Shanmugam M., Fan L., et al. Focus on formononetin: anticancer potential and molecular targets. Cancers . 2019;11(5):p. 611. doi: 10.3390/cancers11050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J., Ke X., Ma N., et al. Formononetin, an active compound of Astragalus membranaceus (Fisch) Bunge, inhibits hypoxia-induced retinal neovascularization via the HIF-1α/VEGF signaling pathway. Drug Design, Development and Therapy . 2016;10:3071–3081. doi: 10.2147/dddt.s114022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dongare S. H., Rajendran S. H., Senthilkumari S., et al. Genistein alleviates high glucose induced toxicity and angiogenesis in cultured human RPE cells. International Journal of Pharmacy and Pharmaceutical Sciences . 2015;7(8) [Google Scholar]
- 43.Jin M., Feng H., Wang Y., et al. Gentiopicroside ameliorates oxidative stress and lipid accumulation through nuclear factor erythroid 2-related factor 2 activation. Oxidative Medicine and Cellular Longevity . 2020;2020:13. doi: 10.1155/2020/2940746.2940746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roohbakhsh A., Parhiz H., Soltani F., Rezaee R., Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sciences . 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 45.Shi X., Liao S., Mi H., et al. Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Molecules . 2012;17(11):12868–12881. doi: 10.3390/molecules171112868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar B., Gupta S. K., Srinivasan B. P., Nag T. C., Srivastava S., Saxena R. Hesperetin ameliorates hyperglycemia induced retinal vasculopathy via anti-angiogenic effects in experimental diabetic rats. Vascular Pharmacology . 2012;57(5-6):201–207. doi: 10.1016/j.vph.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Khazeei Tabari M. A., Iranpanah A., Bahramsoltani R., Rahimi R. Flavonoids as promising antiviral agents against SARS-CoV-2 infection: a mechanistic review. Molecules . 2021;26:13. doi: 10.3390/molecules26133900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imran M., Salehi B., Sharifi-Rad J., et al. Kaempferol: a key emphasis to its anticancer potential. Molecules . 2019;24(12):p. 2277. doi: 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L., Ma Q. Clinical benefits and pharmacology of scutellarin: a comprehensive review. Pharmacology & Therapeutics . 2018;190:105–127. doi: 10.1016/j.pharmthera.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Wang D., Wang L., Gu J., et al. Scutellarin inhibits high glucose-induced and hypoxia-mimetic agent-induced angiogenic effects in human retinal endothelial cells through reactive oxygen species/hypoxia-inducible factor-1α/vascular endothelial growth factor pathway. Journal of Cardiovascular Pharmacology . 2014;64(3):218–227. doi: 10.1097/fjc.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 51.Dehdashtian E., Mehrzadi S., Yousefi B., et al. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sciences . 2018;193:20–33. doi: 10.1016/j.lfs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Lee C. H., Ying T. H., Chiou H. L., et al. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget . 2017;8(29):47425–47439. doi: 10.18632/oncotarget.17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jariyapongskul A., Areebambud C., Suksamrarn S., Mekseepralard C. Alpha-mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. BioMed Research International . 2015;2015:10. doi: 10.1155/2015/785826.785826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta S. K., Kumar B., Nag T. C., et al. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. Journal of Ocular Pharmacology and Therapeutics . 2011;27(2):123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 55.Kowluru R. A., Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutrition & Metabolism . 2007;4(1):p. 8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pradhan D., Dasmohapatra T., Tripathy G. Pharmacognostic evaluation of curcumin on diabetic retinopathy in alloxan-induced diabetes through NF-KB and Brn3a related mechanism. Pharmacognosy Journal . 2018;10(2):324–332. doi: 10.5530/pj.2018.2.56. [DOI] [Google Scholar]
- 57.Calder P. C. Docosahexaenoic acid. Annals of Nutrition and Metabolism . 2016;69(1):8–21. doi: 10.1159/000448262. [DOI] [PubMed] [Google Scholar]
- 58.Echeverría F., Valenzuela R., Catalina Hernandez-Rodas M., Valenzuela A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: new dietary sources. Prostaglandins, Leukotrienes and Essential Fatty Acids . 2017;124:1–10. doi: 10.1016/j.plefa.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Buscemi S., Corleo D., Di Pace F., Petroni M., Satriano A., Marchesini G. The effect of lutein on eye and extra-eye health. Nutrients . 2018;10(9):p. 1321. doi: 10.3390/nu10091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnal E., Miranda M., Johnsen-Soriano S., et al. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Current Eye Research . 2009;34(11):928–938. doi: 10.3109/02713680903205238. [DOI] [PubMed] [Google Scholar]
- 61.Islam A., Islam M. S., Rahman M. K., Uddin M. N., Akanda M. R. The pharmacological and biological roles of eriodictyol. Archives of Pharmacal Research . 2020;43:582–592. doi: 10.1007/s12272-020-01243-0. [DOI] [PubMed] [Google Scholar]
- 62.Lv P., Yu J., Xu X., Lu T., Xu F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. Journal of Cellular Biochemistry . 2019;120(4):5644–5651. doi: 10.1002/jcb.27848. [DOI] [PubMed] [Google Scholar]
- 63.Zhang T. H., Huang C. M., Gao X., Wang J. W., Hao L. L., Ji Q. Gastrodin inhibits high glucose-induced human retinal endothelial cell apoptosis by regulating the SIRT1/TLR4/NF-Bp65 signaling pathway. Molecular Medicine Reports . 2018;17(6):7774–7780. doi: 10.3892/mmr.2018.8841. [DOI] [PubMed] [Google Scholar]
- 64.Batra N., Sam A., Woldemariam T., et al. Genistein combined polysaccharide (GCP) can inhibit intracrine androgen synthesis in prostate cancer cells. Biomedicines . 2020;8(8):p. 282. doi: 10.3390/biomedicines8080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh H. Y., Kim S. S., Chung H.-Y., Yoon S. Isoflavone supplements exert hormonal and antioxidant effects in postmenopausal Korean women with diabetic retinopathy. Journal of Medicinal Food . 2005;8(1):1–7. doi: 10.1089/jmf.2005.8.1. [DOI] [PubMed] [Google Scholar]
- 66.Kumar B., Gupta S. K., Srinivasan B. P., et al. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvascular Research . 2013;87:65–74. doi: 10.1016/j.mvr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Shih Y.-E., Chen C.-H., Lin N.-H., Tzen J. T. C. Development of indirect competitive ELISA for lithospermic acid B of salvia miltiorrhiza with its specific antibodies generated via artificial oil bodies. Molecules . 2019;24(10):p. 1952. doi: 10.3390/molecules24101952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pang B., Bian X., Xing J., Liu S., Liu Z., Song F. Effects of lithospermic acid on hIAPP aggregation and amyloid-induced cytotoxicity by multiple analytical methods. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics . 2020;1868(1) doi: 10.1016/j.bbapap.2019.140283.140283 [DOI] [PubMed] [Google Scholar]
- 69.Jin C. J., Yu S. H., Wang X.-M., et al. The effect of lithospermic acid, an antioxidant, on development of diabetic retinopathy in spontaneously obese diabetic rats. PLoS One . 2014;9(6) doi: 10.1371/journal.pone.0098232.e98232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen R., Qi Q.-L., Wang M.-T., Li Q.-Y. Therapeutic potential of naringin: an overview. Pharmaceutical Biology . 2016;54(12):3203–3210. doi: 10.1080/13880209.2016.1216131. [DOI] [PubMed] [Google Scholar]
- 71.Zeng X., Su W., Zheng Y., et al. Pharmacokinetics, tissue distribution, metabolism, and excretion of naringin in aged rats. Frontiers in Pharmacology . 2019;10:p. 34. doi: 10.3389/fphar.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y., Chu S., Yang S., et al. Physcion and physcion 8-O-β-glucopyranoside: a review of their pharmacology, toxicities and pharmacokinetics. Chemico-Biological Interactions . 2019;310 doi: 10.1016/j.cbi.2019.06.035.108722 [DOI] [PubMed] [Google Scholar]
- 73.Wan W., Wan W., Long Y., et al. RETRACTED ATICLE: physcion 8-O-β-glucopyranoside exerts protective roles in high glucose-induced diabetic retinopathy via regulating lncRNA NORAD/miR-125/STAT3 signalling. Artificial Cells, Nanomedicine, and Biotechnology . 2020;48(1):463–472. doi: 10.1080/21691401.2019.1709861. [DOI] [PubMed] [Google Scholar]
- 74.Gómez-Zorita S., Milton-Laskíbar I., Aguirre L., Fernández-Quintela A., Xiao J., Portillo M. P. Effects of pterostilbene on diabetes, liver steatosis and serum lipids. Current Medicinal Chemistry . 2021;28 doi: 10.2174/0929867326666191029112626. [DOI] [PubMed] [Google Scholar]
- 75.Shen H., Rong H. Pterostilbene impact on retinal endothelial cells under high glucose environment. International Journal of Clinical and Experimental Pathology . 2015;8(10):12589–12594. [PMC free article] [PubMed] [Google Scholar]
- 76.Li J., Guo C., Lu X., Tan W. Anti-colorectal cancer biotargets and biological mechanisms of puerarin: study of molecular networks. European Journal of Pharmacology . 2019;858 doi: 10.1016/j.ejphar.2019.172483.172483 [DOI] [PubMed] [Google Scholar]
- 77.Kim J., Kim K. M., Kim C.-S., et al. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free Radical Biology and Medicine . 2012;53(2):357–365. doi: 10.1016/j.freeradbiomed.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 78.Jaisinghani R. N. Antibacterial properties of quercetin. Microbiology Research . 2017;8:1. doi: 10.4081/mr.2017.6877. [DOI] [Google Scholar]
- 79.Patel R. V., Mistry B. M., Shinde S. K., Syed R., Singh V., Shin H.-S. Therapeutic potential of quercetin as a cardiovascular agent. European Journal of Medicinal Chemistry . 2018;155:889–904. doi: 10.1016/j.ejmech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 80.Akbari Kordkheyli V., Khonakdar Tarsi A., Mishan M. A., et al. Effects of quercetin on microRNAs: a mechanistic review. Journal of Cellular Biochemistry . 2019;120(8):12141–12155. doi: 10.1002/jcb.28663. [DOI] [PubMed] [Google Scholar]
- 81.Khazeei Tabari M. A., Khoshhal H., Tafazoli A., Khandan M., Bagheri A. Applying computer simulations in battling with COVID-19, using pre-analyzed molecular and chemical data to face the pandemic. Informatics in Medicine Unlocked . 2020;21 doi: 10.1016/j.imu.2020.100458.100458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X., Li H., Wang H., Shi J. Quercetin attenuates high glucose induced injury in human retinal pigment epithelial cell line ARPE-19 by up-regulation of miR-29b. Journal of Biochemistry . 2020;167 doi: 10.1093/jb/mvaa001. [DOI] [PubMed] [Google Scholar]
- 83.Kim Y. W., Jang E. J., Kim C.-H., Lee J.-H. Sauchinone exerts anticancer effects by targeting AMPK signaling in hepatocellular carcinoma cells. Chemico-Biological Interactions . 2017;261:108–117. doi: 10.1016/j.cbi.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 84.Xiao J., Wang J., Chen Y., Zhou Z., Gao C., Guo Z. Sauchinone ameliorates intestinal inflammation and promotes Th17 cell production of IL-10 via Blimp-1. Biochemical and Biophysical Research Communications . 2020;522(2):435–441. doi: 10.1016/j.bbrc.2019.11.122. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y., Zhang Y., Li Y., Tong C. Sauchinone inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells. RSC Advances . 2021;9(30):17065–17071. doi: 10.1039/c9ra02817j. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Feng Z.-T., Yang T., Hou X.-Q., et al. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomedicine & Pharmacotherapy . 2019;113 doi: 10.1016/j.biopha.2019.108759.108759 [DOI] [PubMed] [Google Scholar]
- 87.Deng F., Ma Y.-X., Liang L., Zhang P., Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomedicine & Pharmacotherapy . 2018;97:1269–1274. doi: 10.1016/j.biopha.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 88.Wang A. L., Li Z., Yuan M., Yu A. C. H., Zhu X. A., Tso M. O. M. Sinomenine inhibits activation of rat retinal microglia induced by advanced glycation end products. International Immunopharmacology . 2007;7(12):1552–1558. doi: 10.1016/j.intimp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 89.Jiang X., Liu Y., Ma L., et al. Chemopreventive activity of sulforaphane. Drug Design, Development and Therapy . 2018;12:2905–2913. doi: 10.2147/dddt.s100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santín-Márquez R., Alarcón-Aguilar A., López-Diazguerrero N. E., Chondrogianni N., Königsberg M. Sulforaphane-role in aging and neurodegeneration. GeroScience . 2019;41(5):655–670. doi: 10.1007/s11357-019-00061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S., Yang H., Chen X. Protective effects of sulforaphane on diabetic retinopathy: activation of the Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Experimental Animals . 2019;68(2):221–231. doi: 10.1538/expanim.18-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie X., Feng J., Kang Z., et al. Taxifolin protects RPE cells against oxidative stress-induced apoptosis. Molecular Vision . 2017;23:520–528. [PMC free article] [PubMed] [Google Scholar]
- 93.Rübsam A., Parikh S., Fort P. E. Role of inflammation in diabetic retinopathy. International Journal of Molecular Sciences . 2018;19(4):p. 942. doi: 10.3390/ijms19040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Semeraro F., Morescalchi F., Cancarini A., Russo A., Rezzola S., Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: therapeutic implications. Diabetes & Metabolism . 2019;45(6):517–527. doi: 10.1016/j.diabet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Liang W., Huang X., Chen W. The effects of baicalin and baicalein on cerebral ischemia: a review. Aging and Disease . 2017;8(6):p. 850. doi: 10.14336/ad.2017.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang L.-P., Sun H.-L., Wu L.-M., et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investigative Opthalmology & Visual Science . 2009;50(5):2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 97.Li J., Wang P., Ying J., Chen Z., Yu S. Curcumin attenuates retinal vascular leakage by inhibiting calcium/calmodulin-dependent protein kinase II activity in streptozotocin-induced diabetes. Cellular Physiology and Biochemistry . 2016;39(3):1196–1208. doi: 10.1159/000447826. [DOI] [PubMed] [Google Scholar]
- 98.Lin W., Tu H., Zhu Y., et al. Curcumolide, a unique sesquiterpenoid from Curcuma wenyujin displays anti-angiogenic activity and attenuates ischemia-induced retinal neovascularization. Phytomedicine . 2019;64 doi: 10.1016/j.phymed.2019.152923.152923 [DOI] [PubMed] [Google Scholar]
- 99.Ibrahim A. S., El-Shishtawy M. M., Peña A., Liou G. I. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Molecular Vision . 2010;16(215-19):2033–2042. [PMC free article] [PubMed] [Google Scholar]
- 100.Tu J., Guo Y., Hong W., et al. The regulatory effects of paeoniflorin and its derivative paeoniflorin-6′-o-benzene sulfonate CP-25 on inflammation and immune diseases. Frontiers in Pharmacology . 2019;10:p. 57. doi: 10.3389/fphar.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li H., Jiao Y., Xie M. Paeoniflorin ameliorates atherosclerosis by suppressing TLR4-mediated NF-κB activation. Inflammation . 2017;40(6):2042–2051. doi: 10.1007/s10753-017-0644-z. [DOI] [PubMed] [Google Scholar]
- 102.Zhu S.-H., Liu B.-Q., Hao M.-J., et al. Paeoniflorin suppressed high glucose-induced retinal microglia MMP-9 expression and inflammatory response via inhibition of TLR4/NF-κB pathway through upregulation of SOCS3 in diabetic retinopathy. Inflammation . 2017;40(5):1475–1486. doi: 10.1007/s10753-017-0571-z. [DOI] [PubMed] [Google Scholar]
- 103.Khazeei Tabari M. A., Mishan M. A., Moradi M., et al. Noncoding RNA roles in pharmacogenomic responses to aspirin: new molecular mechanisms for an old drug. BioMed Research International . 2021;2021:14. doi: 10.1155/2021/6830560.6830560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lange K. W., Li S. Resveratrol, pterostilbene, and dementia. BioFactors . 2018;44(1):83–90. doi: 10.1002/biof.1396. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y.-L., Xu Z.-M., Yang G.-Y., et al. Sesamin alleviates blood-brain barrier disruption in mice with experimental traumatic brain injury. Acta Pharmacologica Sinica . 2017;38(11):1445–1455. doi: 10.1038/aps.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kong P., Chen G., Jiang A., et al. Sesamin inhibits IL-1β-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget . 2016;7(50):83720–83726. doi: 10.18632/oncotarget.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmad S., ElSherbiny N. M., Jamal M. S., et al. Anti-inflammatory role of sesamin in STZ-induced mice model of diabetic retinopathy. Journal of Neuroimmunology . 2016;295-296:47–53. doi: 10.1016/j.jneuroim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Boulos J. C., Rahama M., Hegazy M.-E. F., Efferth T. Shikonin derivatives for cancer prevention and therapy. Cancer Letters . 2019;459:248–267. doi: 10.1016/j.canlet.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 109.Zhang B., Xu D., She L., et al. Silybin inhibits NLRP3 inflammasome assembly through the NAD+/SIRT2 pathway in mice with nonalcoholic fatty liver disease. The FASEB Journal . 2018;32(2):757–767. doi: 10.1096/fj.201700602r. [DOI] [PubMed] [Google Scholar]
- 110.Bijak M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—chemistry, bioavailability, and metabolism. Molecules . 2017;22(11):p. 1942. doi: 10.3390/molecules22111942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H.-T., Shi K., Baskota A., Zhou F.-L., Chen Y.-X., Tian H.-M. Silybin reduces obliterated retinal capillaries in experimental diabetic retinopathy in rats. European Journal of Pharmacology . 2014;740:233–239. doi: 10.1016/j.ejphar.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 112.Singh Y. P., Girisa S., Banik K., et al. Potential application of zerumbone in the prevention and therapy of chronic human diseases. Journal of Functional Foods . 2019;53:248–258. doi: 10.1016/j.jff.2018.12.020. [DOI] [Google Scholar]
- 113.Tzeng T. F., Liou S. S., Tzeng Y. C., Liu I. M. Zerumbone, a phytochemical of subtropical ginger, protects against hyperglycemia-induced retinal damage in experimental diabetic rats. Nutrients . 2016;8:8. doi: 10.3390/nu8080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stewart M. W. Treatment of diabetic retinopathy: recent advances and unresolved challenges. World Journal of Diabetes . 2016;7(16):p. 333. doi: 10.4239/wjd.v7.i16.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duh E. J., Sun J. K., Stitt A. W. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight . 2017;2:14. doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Osaadon P., Fagan X. J., Lifshitz T., Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye . 2014;28(5):510–520. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


