Abstract
Alkaloids are a type of natural compound possessing different pharmacological activities. Natural products, including alkaloids, which originate from plants, have emerged as potential protective agents against neurodegenerative disorders (NDDs) and chronic inflammations. A wide array of prescription drugs are used against these conditions, however, not free of limitations of potency, side effects, and intolerability. In the context of personalized medicine, further research on alkaloids to unravel novel therapeutic approaches in reducing complications is critical. In this review, a systematic survey was executed to collect the literature on alkaloids and their health complications, from which we found that majority of alkaloids exhibit anti-inflammatory action via nuclear factor-κB and cyclooxygenase-2 (COX-2), and neuroprotective interaction through acetylcholinesterase (AChE), COX, and β-site amyloid precursor protein activity. In silico ADMET and ProTox-II-related descriptors were calculated to predict the pharmacological properties of 280 alkaloids isolated from traditional medicinal plants towards drug development. Out of which, eight alkaloids such as tetrahydropalmatine, berberine, tetrandrine, aloperine, sinomenine, oxymatrine, harmine, and galantamine are found to be optimal within the categorical range when compared to nicotine. These alkaloids could be exploited as starting materials for novel drug synthesis or, to a lesser extent, manage inflammation and neurodegenerative-related complications.
1. Introduction
Since ancient times, natural products have been utilized to treat a wide variety of health complications and have high therapeutic potential; varieties of plants containing bioactive metabolites are used to treat inflammation, neurodegenerative disorders, and correlated complications with good efficiency [1]. Among them, alkaloids are a part of chemical defense in plants, structurally varied category defend themselves chemically, structurally varied category of nitrogen-containing secondary metabolites with strong pharmacological effects, and account for 60% of plant-derived drugs [2]. Alkaloids are prevalent in several botanical families like Amaryllidaceae, Apocynaceae, Papaveraceae, Asteraceae, Solanaceae, Rutaceae, Fabaceae, and Rubiaceae [3].
In modern medicine, plant-derived alkaloids get much attention in a steady supply of medication to treat chronic diseases such as cancer, diabetes, and neurological disorders. They not only protect plants from herbivores but also curb fungal and bacterial infestation, which broadens their use in medicine and other fields [4]. Because of their actions, alkaloids have a wide variety of pharmacological appliances in the therapeutic area such as analgesic (e.g., morphine), antiasthmatic (e.g., ephedrine), anticancer (e.g., vincristine), antihypertensive (e.g., reserpine), antipyretic (e.g., quinine), and antihyperglycemic (e.g., piperine) effects [5].
Plant-derived alkaloids have been discovered to show anti-inflammatory activities by suppressing a range of pro-inflammatory protein complexes implicated in inflammatory signaling pathways. This complex includes nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-kB), extracellular signal-regulated protein kinase 1/2 (ERK1/2), Akt, and signal transducer and activator of transcription 1(STAT1) as well as inflammatory mediators, that is, prostaglandin E2 (PEG2), nitric oxide (NO), cytokines, and chemokines [6, 7]. Inflammatory condition is distinguished by immune cell infiltration, activation, and production of several inflammatory mediators and cytokines, in excessive amounts [8]. Overproduction of inflammatory mediators leads to various inflammatory disorders such as bowel disease, rheumatoid arthritis, and cardiovascular diseases, and associated diseases such as diabetes, cancer, chronic kidney disease, neurodegenerative disorders (NDDs), and aging [9]. Likewise, NDDs were the second leading cause of high mortality worldwide in 2016 [10]. Alzheimer's diseases, dementia, Parkinson's disease, and amyotrophic lateral sclerosis are frequently observed NDDs. By 2040, they are predicted to overtake cancer as the second greatest cause of death following cardiovascular disease, according to the World Health Organization [11]. Alkaloids have been shown to improve the pathophysiology of NDDS by acting as monoamine oxidase (MAO) inhibitors, acetylcholinesterase, and butyrylcholinesterase inhibitors, and N-methyl-D-aspartate (NMDA) antagonists as well as muscarinic and adenosine receptor agonists [12].
Many pieces of evidence show that traditional medicine formulations, mostly made from plant-based components, could help treat inflammation, allergic disorders, and NDDs with minimum systemic toxicity. Therefore, this review highlights the specific role of alkaloids to modulate inflammatory and neurological conditions and aid the constant search for alkaloid-based therapy of both inflammatory and neurodegenerative diseases.
2. Methodology
We have collected scientific information about inflammation, NDDs, and other health complications from peer-reviewed articles published in scientific journals. Mostly, medicinal plants and phytochemicals were considered during the study. To obtain the relevant data, Google Scholar, PubMed, Scopus, Science-Direct, conference papers, AMED, Cochrane Library, and other electronic literature databases were searched using the terms “alkaloids,” “medicinal uses,” “anti-inflammatory,” “analgesic,” “phytochemicals,” “medicinal plants,” “natural products,” “herbal,” “ethnopharmacology,” “neurodegenerative disorders,” etc. All the results were tallied via the utmost range of accessible literature. The potential pharmacokinetic properties such as absorption, distribution, metabolism, excretion, and toxicity (ADMET) of 280 alkaloids obtained during the literature survey were evaluated through the chemoinformatic tool pkCSM [13]. Furthermore, computational-based toxicity of the alkaloids was examined through the ProTox-II webserver, which gives information on various levels of indicators such as organ toxicity, oral toxicity, toxicological endpoints (carcinogenicity, cytotoxicity, hepatotoxicity, immunotoxicity, and mutagenicity), toxicological pathways, and active targets with a confidence score [14]. Figure 1 shows the schematic progress of the study.
Figure 1.
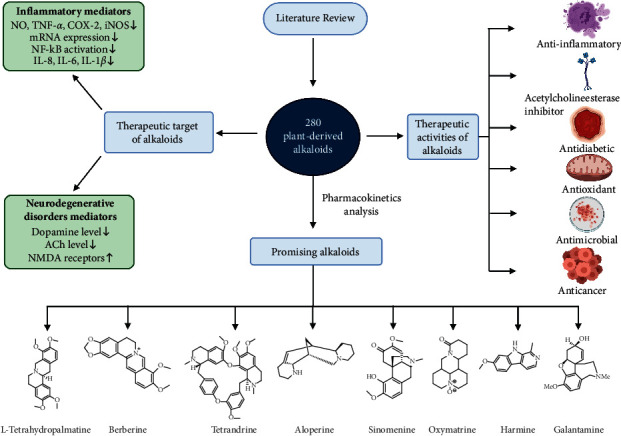
Schematic workflow of the study (note: TNF-α = tumor necrosis factor-alpha, IL-1β = interleukin-1 beta, IL-8 = interleukin-8, IL-6 = interleukin-6, COX-2 = cyclooxygenase-2, iNOS = inducible nitric oxide synthase, NO = nitric oxide, ACh = acetylcholine, NMDA = N-methyl-D-aspartate).
3. Biosynthesis and Physiological Role of Plant-Derived Alkaloids
The biosynthesis route of plant-derived alkaloids involves different synergistic steps, differentiated based on whether they are intramolecular or intermolecular. Its biosynthesis usually starts with amino acid precursors such as ornithine, arginine, lysine, phenylalanine, tyrosine, and tryptophan [15] or aldehyde precursors, followed by the generation of an iminium cation and then a Mannich-like reaction. This final step is sometimes referred to as the scaffolding step or the first dedicated step in a pathway as shown in Figure 2 [16]. The precursors from which alkaloids are biosynthesized are shown in Table S1.
Figure 2.
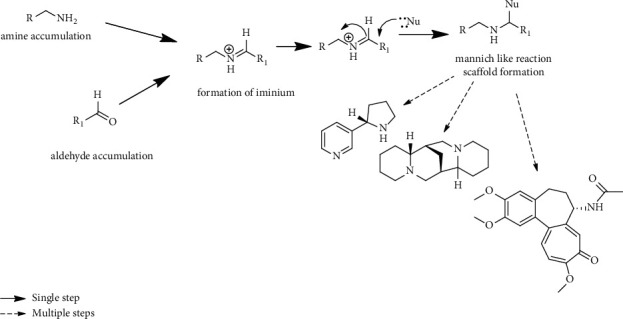
Basic biosynthetic route of plant-derived alkaloids.
Pyrrolizidine alkaloids, which are esters between necine bases derived from arginine or ornithine using putrescine intermediates and tiglic acid, and related C5 necine acids are derived from isoleucine in which homospermidine synthase catalyzes the initial step in biosynthesis. The majority of such alkaloids have been exclusively observed in Senecio species [17]. Biosynthesis of quinolizidine occurs within the genus Lupinus and some species of legumes, including Lupinus angustifolius, L. luteus, L. albus, and L. mutabilis [18]. Quinolizidine alkaloids are biosynthesized with the decarboxylation of lysine by lysine decarboxylase [19] to produce cadaverine amine, which on oxidation with amine oxidase generate spontaneous intramolecular Schiff base formation, resulting in a ring formation, and the addition of various functional groups yields the final product [18]. Furthermore, Lotus (Nelumbo nucifera) predominantly accumulates benzylisoquinoline [20], and biosynthesis of the majority of these alkaloids is biologically synthesized by the decarboxylation of tyrosine or L-dihydroxyphenylalanine (L-DOPA) to yield 4-hydroxyphenylacetaldehyde (4-HPAA) and L-dopamine, respectively, catalyzed by tyrosine/DOPA decarboxylase, followed by the condensation of L-dopamine and 4-HPAA to form (S)-norcoclaurine [21]. Similarly, the biosynthesis of ophiorrhines A and B, and monoterpenoid alkaloids was found via (4 + 2) Diels–Alder cycloaddition reaction forming two different intermediates. The first intermediate involves the oxidation of 5-oxodolichantoside, whereas the second intermediate, that is, 3-keto intermediate, is formed by the condensation of tryptamine and secologanin, and then, imine ion is formed from N-methylation and oxidation [22]. Pyruvic acid reductase plays an important role in tropane alkaloid synthesis. In this process, tropinone formed from putrescine is reduced to tropine, which on condensation with phenyl lactic acid gives littorine. Phenyl pyruvic acid reductase (PPAR) reduces phenyl pyruvic acid formed from littorine into phenyl lactic acid; thus, formed phenyl lactic acid is converted into hyoscyamine aldehyde, the precursor of anisodamine and scopolamine [23].
The initial step in the biosynthesis of benzylisoquinolines is the conversion of L-tyrosine to dopamine and 4-hydroxyphenylacetaldehyde, which is catalyzed by P450, and 6-0-methyltransferase, N-methyl transferase, and 4′-O-methyltransferase catalyze the methylation process to form the intermediate (S)-reticuline, it produces different benzylisoquinoline alkaloid (BIA) on multistep transformation [24]. The holy lotus genome has been shown to contain genes for BIA synthesis, norcoclaurine synthase (NCS), O- and N-methyltransferases, and cytochrome (CYP) monooxygenases CYP80A, CYP80G, and CYP719A [25], while the enzyme CYP80G associated with aporphine alkaloid biosynthesis, which catalyzes the conversion of (S)-reticuline to (S)-corytuberine through intramolecular C-C coupling [26]. Enzyme alcohol dehydrogenase (ADH) and cytochrome P450 (CYP450) catalyzed the conversion of strictosidine aglycone into the strychnos alkaloids akuammicine and acted as an important precursor for anticancer agent vinblastine and vincristine [27]. Similarly, the 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoic acid acts as an intermediary in tropinone formation, showing that the tropinone is synthesized by a polyketide synthase after two rounds of decarboxylation by malonyl-CoA [28]. Alkaloids have been proved to be the most effective against protective agents of metabolic operations and often act as neurotransmitters and signaling systems. They have been used to treat various disorders, including inflammation, allergies, cancer, diabetes, and many others [29]. Along with these, alkaloids play a crucial role in protecting from extreme temperature, salinity, water, radiation, heavy metals, and herbicidal injury [30].
4. Therapeutic Targets of Alkaloids
4.1. Inflammatory Mediators
Inflammation is a nonspecific and immediate response mechanism of the body's innate system against infectious (bacteria, viruses, fungi, and parasites) and noninfectious stimuli. Innate immune cells include white blood cells such as dendritic cells, neutrophils, natural killer cells, monocytes/macrophages, eosinophils, and basophils. Innate immune cells can produce and release pro-inflammatory mediators such as NO, cytokines, chemokines, hormones, growth factors, and adhesion molecules to sustain their communication and orchestrate immune responses [31].
Nuclear factors, such as NF-κB, a family of inducible transcription factors, play a significant role in the inflammatory process because they regulate genes involved in immunological and inflammatory responses [32]. NF-κB is responsible for the transcriptional induction of several cytokines, chemokines, growth factors, cell adhesion molecules, and some acute-phase proteins. The activation of NF-κB involves the phosphorylation and degradation of its inhibitory protein via proteasomal degradation. As a result, free NF-κB is released into the nucleus, where it binds to κB binding sites in the promoter site of target genes, causing pro-inflammatory cytokine transcription. The major cytokines it regulates include tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-8, and IL-12 Il-18. Furthermore, it also regulates the expression of chemokines, such as monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), C-X-C motif chemokine ligand (CXCL)-1, and CXCL-10 [33]. NF-κB also modulates the inflammasome, a multiprotein complex composed of pattern recognition receptors that act as an innate immune system sensor to infectious microorganisms and host inflammatory proteins [34].
Overall, positive feedback mechanism and inflammatory cytokines again activate NF-κB in innate immune cells, thereby inducing cytokines and chemokines in greater quantity leading to further infiltration of inflammatory cells to disseminate the inflammation [35]. This reaction further helps on-site differentiation and infiltration of adaptive immune cells to eliminate microbes and harmful antigens [36]. Usually, inflammation and release of inflammatory mediators are beneficial to the host as it helps resolve the diseases; however, the dysregulation of the inflammatory responses results in severe tissue damages and contributes to the development of acute or chronic inflammatory diseases. Therefore, in the later case, the inhibition of inflammatory mediators or their receptors remains beneficial to prevent further tissue damage.
Prostaglandins are also important in inducing an inflammatory response. They are the lipids biosynthesized in inflamed and damaged tissue and, therefore, associated with the development of acute inflammation symptoms, swelling, and redness. The generation of prostaglandins from arachidonic acid, an essential fatty acid, involved cyclooxygenase (COX) isoenzymes. The prostaglandin synthesis is blocked by nonsteroidal anti-inflammatory drugs (NSAIDs), inhibiting COX activities [37]. Nitric oxide (NO) activates and regulates COX enzyme activity during the inflammatory condition [38], besides targeting prostaglandins. Hence, NO and/or COX are also common possible therapeutic targets to suppress the inflammatory pathogenesis of diseases. Figure 3 illustrates the mechanism of alkaloids produced from plants in treating inflammation.
Figure 3.
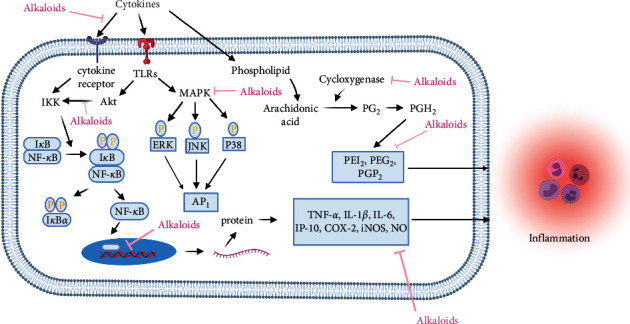
Mechanisms of plant-derived alkaloids in the treatment of inflammation (promotion ↑, inhibition ⊥). (Note: TLRs = toll-like receptors, IKK = IkB kinase, NF-kB = nuclear factor-kappa B MAPK = mitogen-activated protein kinase, ERK = extracellular signal-regulated kinase, JNK = c-Jun N-terminal kinase, AP-1 = activator protein-1, PGH2 = prostaglandin H2, PEG2 = prostaglandin E2, TNF-α: tumor necrosis factor-alpha, IL-1β = interleukin-1 beta, COX-2 = cyclooxygenase-2, iNOS = inducible nitric oxide synthase, NO = nitric oxide).
4.2. Mediators of Neurodegenerative Disorders (NDDs)
The continuous and irreparable damage of the structure or function of neurons coupled with pathologically changed proteins that accumulate in the human brain, leading to neuron degeneration, is known as neuron degeneration disease (NDD) [39]. The neuronal cell death may lead to different NDDs, such as Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, Huntington's disease, brain trauma, progressive supranuclear palsy, prion diseases, and spinocerebellar ataxias. The common mechanism underlying these NDDs includes proteasomal dysfunction, as a result of which misfolded protein cleared insufficiently and correctly, leading to its aggregation in the brain. Furthermore, oxidative stress and production of free radicals/reactive oxygen species (ROS), mitochondrial malfunction and DNA repairs, fragmentation of neuronal cellular complexity, and neuroinflammation are directly associated with NDD etiology [40].
The presence of acetylcholinesterase (AChE) is linked to a plaque of extracellular β-amyloid protein (Aβ) deposits and neurofibrillary tangles in Alzheimer's disease, the most common NDD. Aβ is a small polypeptide generated from the processing of a larger transmembrane β-amyloid precursor protein (APP) by β-site amyloid precursor protein cleaving enzyme (BACE-1) [41]. To treat Alzheimer's disease, administered drugs can only reduce the symptoms or delay disease progression by inhibiting AChE [42]. Alkaloids could function as a neuroprotective agent by inhibiting several cellular activities, such as inhibiting AChE enzyme activity, increasing the level of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter in the mammalian brain, by partially blocking NMDA receptors and enhancing cellular autophagy function and many more mechanisms [12]. Similarly, the etiology of Parkinson's disease, another common NDDs, involves genetic, nongenetic [43], and environmental factors [43]. The common characteristics are an accumulation of misfolded protein aggregates, proteasomal dysfunction, mitochondrial DNA damage, oxidative stress, neuroinflammation, and genetic mutations. The hallmark of Parkinson's disease is the dopaminergic neuronal loss in the substantia nigra pars compacta (SNpc) and reduced dopamine levels [44]. Several investigations have found that numerous misfolded protein aggregates, such as Aβ, p-tau, and α-synuclein, are frequently observed in human post-mortem brains of patients with mixed dementia with Lewy bodies and Parkinson's disorder with dementia [45]. Catalyzing dopamine by the enzyme monoamine oxidase-B (MAO-B), which is increased in the brain, is the cause of decreased dopamine levels, hence indicating MAO-B is a good target to maintain dopamine levels in Parkinson's diseases [46]. Figure 4 explains the mechanism of the plant-isolated alkaloids for medicating neurodegeneration.
Figure 4.
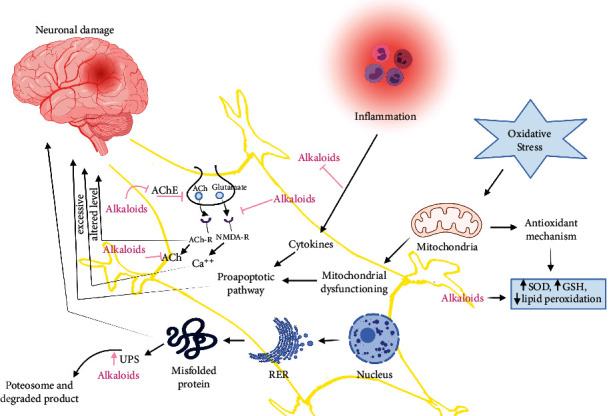
Mechanisms of plant-derived alkaloids in treating neurodegenerative disorders (increase ↑, inhibition ⊥). (Note: AChE = acetylcholinesterase, ACh = acetylcholine, ACh-R = acetylcholine receptor, NMDA-R= N-Methyl-D-Aspartate receptor, UPS = ubiquitin-proteasome system, SOD = superoxide dismutases, GSH = glutathione, RER = rough endoplasmic reticulum).
5. Ethnopharmacological Survey of Plants Producing Therapeutic Alkaloids
Many modern pharmaceutical treatments are derived from traditional and natural remedies. Humans have relied on herbs and plants as sources of effective medicine for treating illness for thousands of years. In conventional medicine, more than 53,000 plant species from worldwide have been used [47]. For instance, Berberis vulgaris, rich in berberine alkaloids, is traditionally used as a herb that is effective in preventing coronary artery disease and shows anti-inflammatory and immunomodulatory effects. The trigonelline alkaloid, isolated from Trigonella foenum-graecum in China, is effective for curing diabetes and central nervous system (CNS) diseases [48]. Due to synergistic actions of secondary ingredients, such as oxindole and L-stachydrine from Capparis tomentosa has been traditionally used for inflammation in Tanzania [49]. Similarly, the traditional Chinese herbal plant Lycoris radiata, natural isoquinoline alkaloid, has different biological utilities, including anti-inflammatory-related activity [50]. Similarly, evolitrine, a key component identified in the leaves of Acronychia pedunculata, a traditional medicinal plant in Sri Lanka, was discovered to have NO inhibitory, anti-nociceptive, antihistamine, and antioxidant properties [51]. Anti-inflammatory and antioxidant activities have been revealed in alkaloids found in Malaysian and Thai Erythroxylum cuneatum leaf extract [52]. Neolamarckia cadamba, a plant traditionally used in China to treat inflammation, fever, and itch, contained 3-dihydrocadambine, a key chemical with anti-inflammatory efficacy in vitro and in vivo studies [53]. The acridone alkaloids obtained from the barks of Citrus aurantium have been reportedly used in Nigerian traditional medicine to treat inflammatory diseases and for the management of cancer [54]. Similarly, mistletoe, i.e., Viscum album L., a culturally significant plant in Europe, has been used to treat neurological conditions such as epilepsy, hysteria, nervousness, and Alzheimer's diseases [55]. Likewise, the aerial part of the plant Sida acuta (Malvaceae family), used in traditional Ayurvedic Indian medicine, contained a good quantity of cryptolepine, which has anti-inflammatory properties [56]. In addition, Stephania rotunda (Menispermaceae) has been utilized as a folk medicine in several Asian nations, which is prevalent in hilly regions of Cambodia and found to contain several bisbenzylisoquinoline alkaloids like 2-norcepharanthine, cepharanoline, and fangchinoline that is used for the management of inflammatory diseases [57]. Around 392 species of African plants have been found to possess isoquinoline alkaloids (19%) and have been used for the exclusive treatment of cancer and inflammation-related diseases [58]. Sceletium contains alkaloids of the mesembrine type, including Δ7 mesembrenone, mesembranol, mesembrenone, mesembrine, and epimesembranol, which are used as a traditional medicine in South Africa to treat neurodegenerative disease [59]. In the Guangxi and Yunnan provinces of China, Stephania cepharantha has been widely used to treat stomach aches and snakebites, and the alkaloids present in it have been found to possess potential anti-neuroinflammatory agents [60]. We have also tried to report and shed light on the listed alkaloids in Table S2 comprehensively. Furthermore, the structures of the topmost 30 alkaloids based on ADMET and ProTox-II are shown in Figure 5. The structure of the rest of the alkaloids taken in this review is shown in Figure S1.
Figure 5.
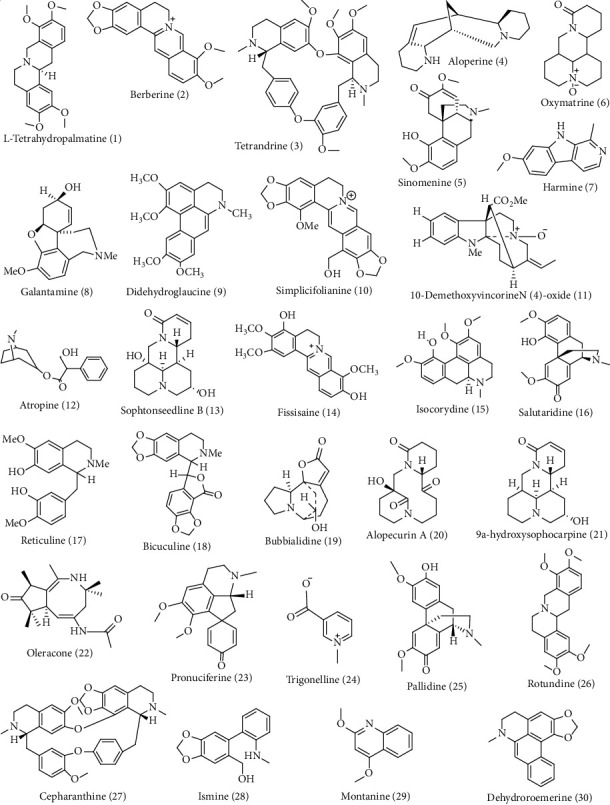
Selected plant-derived alkaloids isolated from various medicinal plants.
Overall, ethnopharmacological surveys have contributed to identifying potential metabolites in traditionally used medicinal plants. Many of these metabolites have been studied for their pharmacological applications, leading to the invention of drugs and the use of metabolites in modern medicine.
6. Therapeutic Activities of Plant-Derived Alkaloids
In both traditional and modern medical systems, the biological activities of alkaloids such as anticancer, antibacterial, anti-inflammatory, antimicrobial, antioxidant, AChE inhibitory activity, antimalarial, and antidiabetic activity have been examined as shown in Figure 6. Likewise, Figure 7 shows the diagrammatic graph of some plants and their pharmacological applications.
Figure 6.
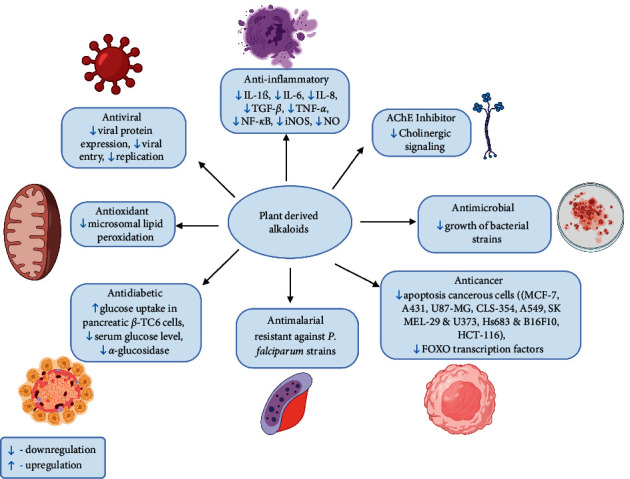
Biological applications of alkaloids (note: IL-1β = interleukin-1β, IL-6 = interleukin-6, IL-8 = interleukin-8, TGF-β = transforming growth factor-β, TNF-α = tumor necrosis factor α, NF-κB = nuclear factor-kappa B iNOS = inducible nitric oxide synthase, NO = nitric oxide, MCF-7 = Michigan cancer foundation-7, U87-MG = uppsala-87 malignant glioma).
Figure 7.
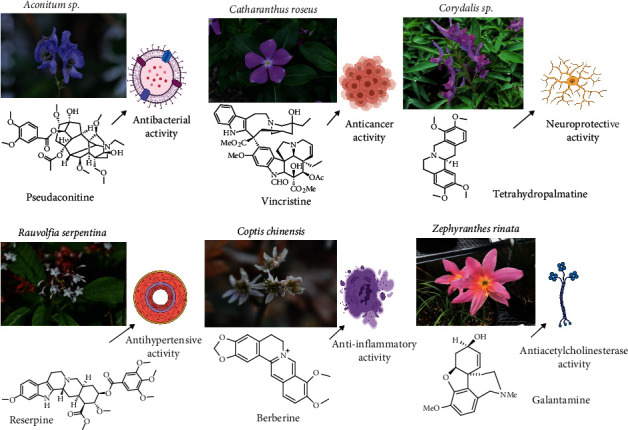
Diagrammatic graph shows some plants and their pharmacological applications.
Most plant-derived alkaloids have been demonstrated for antiproliferation, antiviral, antibacterial, insecticidal, and antimetastatic effects [61]. Due to the presence of protons receiving N-atoms and one or more protons donating amine H-atoms, alkaloids are also searched for their tendency to form hydrogen bonds with enzymes, receptors, and proteins in them [62]. Plant-derived alkaloids that show some biological activities are mentioned below.
6.1. Anti-Inflammatory Activity
Canthin-6-one alkaloids obtained from Ailanthus altissima stem bark have an anti-inflammatory effect by suppressing both NF-κB transcriptional activations and the Akt phosphorylation [63]. The plant-isolated alkaloids, berberine, showed promising results for treating Acne Vulgaris as it decreased pro-inflammatory cytokines, that is, IL-1 β, IL-6, IL-8, and TNF-α [64]. The anti-inflammatory response of the compound can be retrieved based on the NO inhibitory impacts on lipopolysaccharide (LPS) stimulated macrophages model [65]. Out of 23 compounds isolated from the roots of Isatis tinctoria, the NO production analysis revealed that tryptanthrin, 3-(2-carboxyphenyl)-4(3H)-quinazolinone, and 2-methyl-4(3H)-quinazolinone displayed inhibitory effects with the respective half-maximal inhibitory concentration (IC50) values of 1.2, 5.0, and 74.4 μM [66]. Likewise, ethanol extract of 1-carbomethoxy-β-carboline alkaloids from Portulaca oleracea showed the most effective anti-inflammatory activity [67] due to the stifling mitogen-activated protein kinase (MAPK) pathways and NF-κB, lowering the production of pro-inflammatory mediators such as inducible nitric oxide synthase (iNOS), TNF-α, IL-6, and IL-1β [67]. The alkaloids extracted from Chinese medical herbs are applicable in treating rheumatic immune diseases by invigorating the discharge of adrenal cortex hormones, obstructing the unleash of cytokines, and synchronizing the degree of NO [68, 69]. Oleracimine showed attenuating effect by limiting the production of NO and reducing the expression of IL-6, TNF-α, and PEG2 in both messenger ribonucleic acids (mRNAs) the protein level by inhibiting the activity of COX-2 and NO synthase enzyme [70]. Hence, the numerous plant-based alkaloids have mounting effects in the treatment of anti-inflammatory activity.
6.2. AChE Inhibitory Activity
AChE was taken as a valuable spot for controlling NDDs causing cholinergic signaling deficit [71]. The alkaloids were extracted from the root of Zanthoxylum rigidum; nitidine and avicine showed inhibitory action against AChE with IC50 of 0.65 ± 0.09 μM and 0.15 ± 0.01 μM, respectively [72]. Another study showed four pyrrolizidine alkaloids, namely, 7-O-angeloylechinatine-N-oxide, 3′-O-acetylheliospine-N-oxide, heliosupine N-oxide, and heliosupine, isolated from Solenanthus lanatus, which possess the AChE inhibitory activity with IC50 values ranging from 0.0001–0.60 mM [73]. Among monoterpene indole alkaloids extracted from the leaves of Rauvolfia vomitoria, rauvomitorine III alkaloids showed anti-AChE activity with an IC50 value of 16.39 ± 1.41 μM due to the existence of the N-methyl group in the vobasenal-type alkaloids as well as due to interactions with Trp133 and Trp86 moieties at hydrophobic appendages [74]. Similarly, mokluangin A-C and antidysentericin alkaloids extracted from the bark of Holarrhena pubescens showed strong AChE inhibitory activity with IC50 values ranging from 1.44 to 23.22 μM [75]. The alkaloids such as galantamine, caranine, N-demethylgalanthamine, and lycoramine obtained from leaves, roots, and bulbs of Amaryllidaceae species, Crinum, Habranthus, and Zephyranthes act as the most significant AChE inhibitors in correlation with chemical fingerprints [76]. Galantamine was found the most promising dual-site binding AChE inhibitor via the combinatorial library of Galantamine-curcumin hybrids [77]. These findings indicate that the plant-derived alkaloids have potent anti-AChE inhibitory activity.
6.3. Antioxidant Activity
Antioxidants are substances that fight against free radicals in the cells, which otherwise highly contribute to developing heart disease, cancer, and other diseases [78–80]. The phytochemical investigation of Nelumbo nucifera embryos revealed the antioxidant activity of its four main alkaloids, named neferine, isoliensinine, liensinine, and armepavine. Using the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 2,2,-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assays, the drug concentration eliciting 50% of the maximum simulation (SC50) values for these compounds was identified as 14.65, 12.07, 18.25, and 29.03 μM for ABTS and 33.37, 25.26, 44.21, and 79.34 μM for DPPH, respectively [81]. Similarly, among seven alkaloids isolated from Alphonsea cylindrical barks, iraqiine, muniranine, and kinabaline exhibit potent antioxidant activity against DPPH radical scavenging assay with half-maximal inhibitory concentration (IC50) values of 48.77 ± 1.01, 44.51 ± 1.12, and 64.28 ± 0.93 μg/ml, respectively [82]. Similarly, from the root of Stephania tetrandra, 15 different alkaloids have been isolated, among which three new aporphine alkaloids and two new phenanthrene alkaloids were reported. These alkaloids, (+)-dicentrine, (+)-neolitsine, (+)-glaucine, (-)-nuciferine, and stephenanthrine, also exhibit antioxidant activities, which were measured by determining malondialdehyde levels in rat liver microsomal lipid peroxidation induced by Fe2+/cysteine with the inhibitory values ranging from 62.50 ± 1.91 to 98.44 ± 0.34% at the concentration of 10 μM [83]. Likewise, alkaloids, mitraphylline, and isomitrphylline isolated from aqueous leaf extract of Uncaria tomentosa showed antioxidant activities, evaluated through DPPH, ABTS, & ferric ion reducing antioxidant power (FRAP) assay [84]. These overall findings indicate that plant-derived alkaloids possess antioxidant activity.
7. ADMET and ProTox-II Analysis
In silico ADMET studies are the most important considerations in drug discovery and development concerning pharmacokinetics properties [85]. To evaluate the alkaloids as promising therapeutic, pharmacokinetic properties ADMET (Table S3) and ProTox-II (Table S4) are analyzed. On predictive pkCSM, intestinal absorption values greater than 30% are considered to be better absorbed from the intestine after oral administration, which indicates that all alkaloids were able to be remarkably absorbed from the intestine of humans. Likewise, compounds with logPapp >0.90 are considered with high CaCO2 permeability. Compounds with log blood-brain barrier (logBBB) < −1 are weakly dispersed to the brain, whereas those with logBBB > 0.3 can pass the BBB. As indicated, compounds 2, 4, 7, 8, 12, 30, etc. were able to readily cross the BBB, whereas other compounds were unable to do so. Another important parameter, the volume of distributions (VD), is considered when logVDss < −0.15 and high when logVDss > 0.45 [13, 86]. Thus, the computational analysis showed relatively low water solubility, moderate CaCO2 permeability, and high intestinal absorption value for the following alkaloids: tetrahydropalmatine (1), berberine (2), tetrandrine (3), aloperine (4), sinomenine (5), oxymatrine (6), harmine (7), and galantamine (8). Furthermore, CYP (1A2, 2C9, 2C19, 2D6, and 3A4) parameters analyzed by ADMET are related to phase-1 drug bioinformatics in the metabolism of the drug [87]. The most important aspect of this study is CYP3A4, in which the alkaloids such as oxymatrine (6), bicucine (18), and pallidine (25) only inhibited it, whereas none of the other alkaloids were able to inhibit CYP3A4. This indicates that these mentioned alkaloids can be metabolized in the liver. It has an impact on total clearance and half-life. The total clearance describes the association between drug clearance rate and drug concentration in the body [88]. Compounds 1, 2, 10, 12, 14, 16-17, 19, 20, 25-26, etc. were found to show high clearance. Additionally, compounds with log PS > −2 would penetrate the central nervous system (CNS) and act as CNS-active drugs. Moreover, AMES toxicity is also an important parameter in selecting the drugs. Compounds 4, 5, 7, 27, 37, 38, 42, 45, 46, 49, 50, 66-70, 73, 75, 78-85, 87-89, 96-99, 104, 112, 113, 116, 118, 125, 128, 130-132, 134-136, 139, 141, 172, 174, 176, 179, 185-188, 195, 197, 204, 205, 212, 213, 218, 221, 223-229, 236, 240, 271, 273, 274, and 275 were found with AMES toxicity. The toxicity of secondary metabolites was evaluated using ProTox-II based on toxicity and lethal dose (LD50) values ranging from class 1 and 2 (fatal), class 3 (toxic), class 4 and 5 (harmful), and class 6 (non-toxic) [14]. The ADMET and ProTox-II properties of tetrahydropalmatine, berberine, tetrandrine, aloperine, sinomenine, oxymatrine, harmine, and galantamine were optimal within the categorical range in comparison with nicotine (Alzheimer's disease: NCT00018278 and Parkinson's disease: NCT01216904) [89].
8. Promising Plant-Derived Alkaloids
8.1. Tetrahydropalmatine
Tetrahydropalmatine (THP), an isoquinoline alkaloid, mainly extracted from Stephania and Corydalis genus, depicts anxiolytic, anti-inflammatory, analgesic, and cardioprotective activities [90, 91]. THP attenuated ketamine-induced surge in AChE activity, thus overruling the ketamine-induced decrease in ACh levels, demonstrating the protective action against nerve cell apoptosis in ketamine-induced mice [92]. Studies revealed that THP treatment rectified D-galactose-induced memory impairments associated with the decrease in malondialdehyde (MDA) and NO levels and increase in glutathione levels, and superoxide dismutase (SOD), catalase, and glutathione peroxidase activities [93]. Similarly, THP treatment showed a protective effect against ketamine-induced oxidative stress in mice, increasing glutathione peroxidase and SOD activities and decreasing MDA activities. Moreover, THP lowered TNF-α, IL-1β, and IL-6 expression-suppressed iNOS and NF-κB protein activities and induced glial cell-derived neurotrophic factor protein expression in ketamine-induced mice, demonstrating its anti-inflammatory actions. l-THP blocked TNF-α-induced adhesion of monocytes to human umbilical vein endothelial cells by inhibiting the production of both mRNA and protein levels of vascular cell adhesion molecule-1 (VCAM-1) along with the attenuation of TNF-α-stimulated NF-κB translocation in monocytes, highlighting its potential pharmacological action to intervene atherosclerosis [94]. Figure 8 shows the anti-inflammatory and neurodegenerative mechanism of l-THP. Thus, extensive pharmacological studies should be carried out further for rationalizing its anti-inflammatory and neuroprotective actions.
Figure 8.
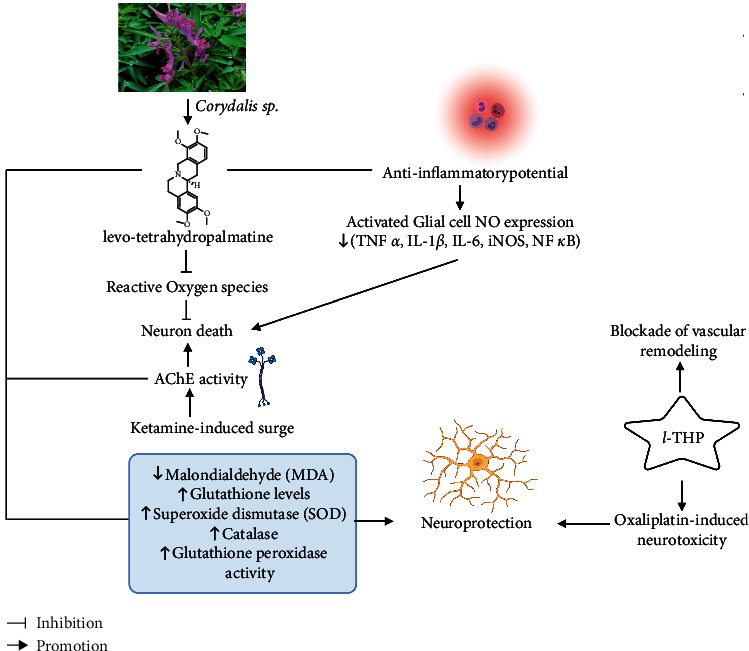
Anti-inflammatory and neurodegenerative mechanism involved in Levo-tetrahydropalmatine (l-THP). (Note: TNF-α = tumor necrosis factor α, IL-1β = interleukin-1β, IL-6 = interleukin-6, iNOS = inducible nitric oxide synthase, NF-κB = nuclear factor-kappa B).
8.2. Berberine
Berberine shows anti-inflammatory activity by reducing the pro-inflammatory response via the activation of AMP-activated protein kinase (AMPK) in macrophages and suppressing the expression of pro-inflammatory genes such as TNF-α, IL-Iβ, IL-6, monocyte chemoattractant protein-1 (MCP-1), COX-2, and iNOS [95]. Most importantly, berberine also inhibits the production of TNF-α and IL-6 in HepG2 cells, illustrating its anti-inflammatory activity in hepatocytes [96].
The use of berberine has been widely studied in the NDDs model. In a rat model of Alzheimer's disease, berberine chloride prevented neurodegeneration of the hippocampus and decreased the activity of BACE-1 [97]. In the transgenic mouse model of Alzheimer's disease, berberine significantly reduced Aβ plaque aggregation leading to the improvement of neuronal and mental disturbance by the inhibition of APP phosphorylation [98]. Moreover, oral administration of 50 mg/kg berberine for 5 weeks in Parkinson's disease mice managed memory loss symptoms by reducing apoptosis in the hippocampus and prohibiting dopaminergic neuronal loss in substantia nigra [99]. Currently, berberine is at different clinical trial phases to treat various diseases, including atherosclerosis (NCT03470376), hypercholesterolemia (NCT02078167), schizophrenia (NCT03470376), coronary artery disease (NCT03378934), and Alzheimer's disease (NCT03221894) [89]. Thus, berberine must be a significant drug for treating inflammatory and neurodegenerative diseases.
8.3. Tetrandrine
Tetrandrine is a bisbenzylisoquinoline alkaloid isolated from the roots of Stephania japonica, S. tetrandra, and S. Moore with a wide usage in treating inflammation [100]. Tetrandrine has been identified to inhibit the secretion of pro-inflammatory mediators, TNF-α, IL-6, and IL-1β expression by blocking the NF-kB signaling in LPS-induced macrophages [101]. Also, tetrandrine inhibits the expression of tissue metalloproteinase inhibitor-1, matrix metalloproteinase-3, and the production of PEG2 (prostaglandin E2) and NO (nitrite oxide) by inhibiting IkBα phosphorylation in ATDC5 cells and LPS-induced cells [102]. Similarly, intragastric administration of tetrandrine decreased the concentration of NO in serum and pancreatic tissue of the acute hemorrhagic necrotizing pancreatitis rat model, and it inhibited the activation of NF-κB by targeting the formation of IL-8, TNF-α, and IL-6 [103]. Likewise, the administration of tetrandrine proceeded intravenously in a rat model of Alzheimer's disease showed improvement in memorial and learning disability along with the decrease in the expression of TNF-α and IL-1β through the inhibition of the NF-κB pathway [104]. Interestingly, recently, a phase 4 clinical trial of tetrandrine tablets or tetrandrine has been used to treat COVID-19 patients (NCT04308317) as an anti-inflammatory drug [89]. Overall, the above evidence demonstrates that tetrandrine is a potent alkaloid in treating inflammation and neurodegenerative disease.
8.4. Aloperine
Aloperine, a kind of piperidine alkaloid, has a therapeutic effect on inflammation and neuropathic pain. It is isolated from Sophora alopecuroides, a plant used as a medicine widely distributed in Central and Western Asia [105, 106]. The ability of aloperine to inhibit the Toll-like receptor 4 (TLR4)-dependent inflammatory pathway in macrophages demonstrated its anti-inflammatory activity. It was thus shown to block the expression of TNF-α, IL-17A, and IL-6 and reduce the secretion of PEG2 via COX-2 and iNOS inhibition, consequently lowering NO production [107]. Similarly, aloperine treatment also reduced oxidized low-density lipoprotein, a marker of endothelial inflammation, and reduced MCP-1, VCAM-1, IL-6, and E-selection by reducing Kruppel-like factor 2 (KLF2) expression, suggesting the potential anti-atherosclerosis characteristics [105]. Further evidence shows that 80 mg/kg of aloperine was injected intraperitoneally that decreased neuropathic pain possesses by chronic constriction injury in the dorsal spinal cord by inhibiting the upregulation of NF-κB, IL-1β, and IL-6, which is related to the reduction of ROS through the suppression of NF-κB pathways [106]. Even though aloperine has a wide range of medical applications, additional research is necessary to develop aloperine as a drug for treating various diseases, including inflammation and neurodegenerative diseases.
8.5. Sinomenine
Sinomenine is a kind of benzyl alkaloid commonly found in Chinese herbal medicine that is mainly isolated from the root and stem of Sinomenium acutum [108]. It has a therapeutic application in treating chronic nephritis, rheumatoid arthritis, myocardial ischemia, ankylosing spondylitis, and other rapid arrhythmias and reduces associated foot swelling caused by formaldehyde, egg white, or carrageenan [109]. The anti-inflammatory activity of sinomenine is through the inhibition of c-Jun N-terminal kinases (JNKs) and NF-κB signaling pathways, thus suppressing the mRNA expression of cytokines, such as IL-1β and TNF- α [110]. In vivo study showed that 40 mg/kg of sinomenine administration on mice with experimentally induced rheumatoid arthritis remarkably reduced mechanical hypersensitivity during the peak of inflammation and the post-inflammatory phase [111].
Similarly, sinomenine also stands to have a good therapeutic value against NDDs. It has been shown to inhibit ROS and NO generation in Aβ-treated human astrocytes, implying a favorable effect in Alzheimer's disease [112]. It also accounted for significant neuroprotective potential in the rat model induced with temporal lobe epilepsy in intrahippocampal kainate by decreasing the intensity of seizures, the incidence of status epilepticus, hippocampus abnormal mossy fiber sprouting (MFS), and deoxyribonucleic acid (DNA) fragmentation [113]. Clinical studies showed that 101 out of 120 patients improved rheumatoid arthritis by combining sinomenine with methotrexate as a therapeutic agent [114]. Therefore, sinomenine is an effective alkaloid for treating both inflammation and neurodegenerative disease by reducing the expression of pro-inflammatory cytokines.
8.6. Oxymatrine
Oxymatrine is a quinolizidine alkaloid extracted from the roots of Sophora flavescentis that has anti-inflammatory, antiallergic, antiviral, antifibrotic, anticancer, and cardiovascular protective properties [115]. Since oxymatrine reduced the production of cytokines, TNF-α, and IL-17A, as well as lowered the arthritic score and synovial inflammation, it has been shown to have a potent anti-inflammatory effect on collagen-induced arthritis (CIA) rats [116]. Oxymatrine carries remarkable protective effects on gastric ulcers via the suppression of gastric inflammatory reactions, oxidative stress, and pro-apoptotic actions. It was found to prevent several inflammatory mediators in ulcered tissue by blocking NF-κB translocation from the cytoplasm to the nucleus [117]. Oxymatrine is also reported for its anti-Alzheimer's disease effects by downregulating the densities of Aβ plaques and astrocyte clusters along with the improvement in the learning and cognitive abilities in the mice model [118]. Furthermore, by suppressing apoptosis and oxidative stress, it had effective neuroprotection against cerebral hypoxic-ischemic injury, which could be linked to the activation of protein kinase (Akt) and glycogen synthase kinase-3 (GSK3) and modification of the nuclear factor erythroid 2-related factor 2/heme oxygenase 1 (Nrf-2/HO-1) signaling pathway [119]. Through cathepsin-D-dependent regulation of the Toll-like receptor 4 (TLR4) signaling pathway, oxymatrine significantly attenuates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's diseases, and this provides dopamine-based neuroprotection and reduces microglia-mediated neuroinflammation [120]. It has been found as a potent anti-inflammatory and neurodegenerative drug, as 12 patients who have psoriasis (a type of skin inflammation) treated by the use of oxymatrine in the duration from 2012 to 2016 showed more significant improvement by inhibiting the excessive secretion of cell proliferation marker in skin surface [121]. With these findings of oxymatrine, further investigations with a wide range of therapeutical applications are in progress.
8.7. Harmine
Harmine, a β-carboline alkaloid, possesses considerable pharmacological importance such as anti-inflammatory, hallucinogenic, antioxidant, antitumor, antifungal, and antibacterial. The seeds of Peganum harmala were firstly used for its isolation [122]. The anti-inflammatory activity of harmine was demonstrated in LPS-injected mice. It was found to inhibit NF-κB activation, which lowered the serum level of IL-1β, TNF-α, and IL-6 [123]. Harmine-loaded ethosomes are beneficial for treating inflammation in a rat paw edema induced by carrageenan, potentially inhibiting the expression of inflammatory factors such as PEG-2, TNF-α, IL-1β, and NO [124]. Furthermore, it also downregulated TLR4 and nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR), and pyrin domain-containing protein 3 (NLRP3) expression myeloperoxidase activity and MDA production along with the enhancement in the activities of SOD [125]. TNF-α, NO generation, and myeloperoxidase activity were all reduced, as a result of which scopolamine-induced inflammation was lowered [126]. It inhibited NLRP3 inflammasome activation by reducing NLRP3, apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), cleaved caspase-1, IL-1β, and IL-18 levels, and thus, boosted the brain-derived neurotrophic factor/tropomyosin receptor kinase B (TrkB) signaling pathway, as a result of which cognitive impairment was attenuated in streptozotocin-induced diabetic rats [127]. Oral administration of 20 mg/kg harmine results in the improvement of memory impairment by increasing cholinergic function through the inhibition of AChE in scopolamine-induced mice, which shows that harmine may be potent for the treatment of neurodegenerative disease [128]. Drug harmine dimethyl-tryptamine (DMT) was used in clinical trials of 30 participants for the emotion mood cognitive function 1 (NCT04716335), and Social Empathy is in phase 4 clinical trial (NCT04716335) [89]. Therefore, harmine could stand as a potent anti-inflammatory and NDD drug.
8.8. Galantamine
Galantamine is a plant alkaloid, commonly isolated from Galanthus woronowii, Leucojum aestivum, and other members of the family Amaryllidaceae [129]. It is approved by the US Food and Drug Administration as a medication for treating Alzheimer's disease and is available under brand names Reminyl and Nivalin [130]. The relevant mechanism of galantamine in treating neurodegenerative diseases is depicted in Figure 9. Inhibition of the gliosis, cytokines (IL6, IL-1β, and TNF-α), and pro-inflammatory signaling molecules (NF-κB p65) and inflation of the synapse-associated proteins in the hippocampus of lipopolysaccharide-exposed mice mark galantamine as a promising treatment to ameliorate neuroinflammation and cognitive decline in neurodegenerative disorders [131]. Additionally, it displayed neuroprotective action through nicotinic receptors via the PI3K-Akt and Bcl signal transduction cascade [132]. Galantamine enhanced NMDA responses of rat cortical neurons, suggesting its importance in the improvement of learning/memory/cognition in Alzheimer's diseases patients [133]. It has been shown to induce hippocampal insulin-like growth factor 2 mRNA levels in mice on acute administration, suggesting its neurogenetic action [134]. Galantamine hydrobromide is in phase 3 clinical trials for Alzheimer, dementia, mental disorders, and brain diseases (NCT00216502) [89]. Thus, galantamine, which effectively inhibits the generation of cytokines and the expression of NF-κB, can be utilized to treat Alzheimer's disease.
Figure 9.
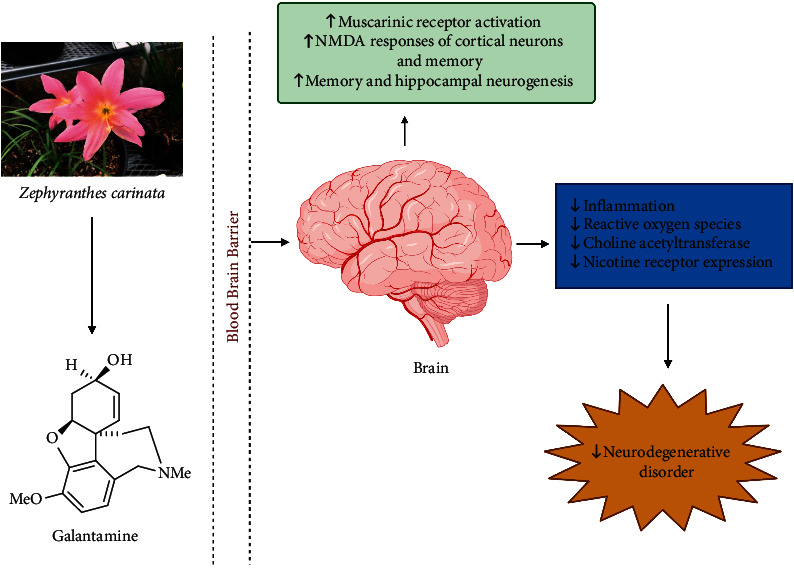
Involved mechanism of galantamine in treating the neurodegenerative disorder (promotion ↑, inhibition ⊥). (Note: NMDA = N-methyl-D-aspartate).
9. Conclusions
Alkaloids, one of the remarkable classes of natural compounds, exhibit extensive routes of structurally and/or functionally diverse molecules for the new potential preventative and/or therapeutical use in anti-inflammatory, AChE inhibition, and NDDs. Based on the literature survey and in silico ADMET analysis, alkaloids, namely, harmine, berberine, aloperine, oxymatrine, tetrandrine, sinomenine, tetrahydropalmatine, and galantamine, have the potential to serve as a lead compound against several anti-inflammation and NDDs. However, for further studies, it is imperative to explore extensively clinical trials, pharmacokinetic properties, health complications, and other important parameters before its medicinal applications. Many alkaloids are also toxic; thus, their safety profiles should be studied in detail. Collaborative research using alkaloids in combination with currently FDA-approved medicines could be investigated for improved and long-term anti-inflammatory and anti-AChE formulations. Hence, this review will be significant on medicinal chemistry, ethnopharmacological applications, and research on drug delivery regarding the alkaloids in the management of inflammation and NDDs.
Acknowledgments
The authors acknowledge Mr. Sagar Aryal for providing the figures through biorender.com.
Abbreviations
- AChE:
Acetylcholinesterase
- ADMET:
Absorption, distribution, metabolism, excretion, and toxicity
- Aβ:
β-amyloid
- Akt:
Protein kinase B
- ALS:
Amyotrophic lateral sclerosis
- AMPK:
5' adenosine monophosphate-activated protein kinase
- CIA:
Collagen-induced arthritis
- COX:
Cyclooxygenase
- COX-2:
Cyclooxygenase-2
- CYP:
O- and N-methyltransferases, and cytochrome
- IL-1β:
Interleukin-1β
- IL-6:
Interleukin-6
- IL-8:
Interleukin-8
- JNKs:
c-Jun N-terminal kinases
- MAPK:
Mitogen-activated protein kinase;
- MCP-1:
Monocyte chemoattractant protein-1
- MDA:
Malondialdehyde
- NCS:
Norcoclaurine synthase
- NDDs:
Neurodegenerative disorders
- NF-κB:
Nuclear factor-kappa B
- NMDA:
N-Methyl-D-aspartate
- PEG2:
Prostaglandin E2
- SOD:
Superoxide dismutase
- STAT-1:
Signal transducer and activator of transcription 1
- THP:
Tetrahydropalmatine
- TLR:
Toll-like receptor
- TNF-α:
Tumor necrosis factor α
- VCAM-1:
Vascular cell adhesion molecule-1.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
B. A., B. K. R., S. Bhattarai, S. Bhandari, P. T., K. G., K. S., D. R., R. T., D. A., A. O., H. P. D., and N. P. reviewed the literature on natural products and developed the manuscript. B. A., B. K. R., and D. A. performed ADMET and ProTox-II analysis of alkaloids. S. Bhattarai reviewed the inflammatory and neurodegenerative aspects. H. P. D. edited the manuscript in the area of natural products. N. P. conceptualized and supervised the project. All authors have read and agreed to the published version of the manuscript. Babita Aryal and Bimal Kumar Raut contributed equally to this work.
Supplementary Materials
Table S1: plant-derived alkaloids with their biosynthetic precursors and current research. Table S2: occurrence, pharmacology, and toxicity of alkaloids. Table S3: ADMET properties of promising plant alkaloids by pkCSM server. Table S4: prediction of toxicity of secondary metabolites inhibiting metabolic enzymes using ProTox-II. Figure S1: molecule structure of plant-derived alkaloids.
References
- 1.Yuan X., Tong B., Dou Y., Wu X., Wei Z., Dai Y. Tetrandrine ameliorates collagen-induced arthritis in mice by restoring the balance between Th17 and Treg cells via the aryl hydrocarbon receptor. Biochemical Pharmacology . 2016;101:87–99. doi: 10.1016/j.bcp.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Venkategowda A., Gowda L. Isolation and characterization of antimicrobial alkaloids from Plumeria alba flowers against Food borne pathogens. American Journal of Life Sciences . 2014;2:p. 1. [Google Scholar]
- 3.Yang L., Stöckigt J. Trends for diverse production strategies of plant medicinal alkaloids. Natural Product Reports . 2010;27(10):1469–1479. doi: 10.1039/c005378c. [DOI] [PubMed] [Google Scholar]
- 4.Adamski Z., Blythe L. L., Milella L., Bufo S. A. Biological activities of alkaloids: from toxicology to pharmacology. Toxins . 2020;12(4):p. 210. doi: 10.3390/toxins12040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng Y. P., Or T. C. T., Ip N. Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochemistry International . 2015;89:260–270. doi: 10.1016/j.neuint.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 6.IY M., Arumugam G., Archana S., et al. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders - a review. Recent Patents on Inflammation & Allergy Drug Discovery . 2018;12:39–58. doi: 10.2174/1872213X12666180115153635. [DOI] [PubMed] [Google Scholar]
- 7.Zhao L., Wang L., Di S.-N., et al. Steroidal alkaloid solanine A from Solanum nigrum Linn. exhibits anti-inflammatory activity in lipopolysaccharide/interferon γ-activated murine macrophages and animal models of inflammation. Biomedicine & Pharmacotherapy . 2018;105:606–615. doi: 10.1016/j.biopha.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Ngo Q. M. T., Tran P. T., Tran M. H., et al. Alkaloids from Piper nigrum exhibit antiinflammatory activity via activating the Nrf2/HO-1 pathway. Phytotherapy Research . 2017;31(4):663–670. doi: 10.1002/ptr.5780. [DOI] [PubMed] [Google Scholar]
- 9.Furman D., Campisi J., Verdin E., et al. Chronic inflammation in the etiology of disease across the life span. Nature Medicine . 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feigin V. L., Nichols E., Alam T., Bannick M. S., Beghi E., Blake N. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology . 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Alzheimer’s Disease, Wimo A., Ali G.-C., Guerchet M., Prince M., Prina M. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends . London, UK: International Alzheimer’s Disease; 2015. [Google Scholar]
- 12.Hussain G., Rasul A., Anwar H., et al. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. International Journal of Biological Sciences . 2018;14(3):341–357. doi: 10.7150/ijbs.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pires D. E. V., Blundell T. L., Ascher D. B. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. Journal of Medicinal Chemistry . 2015;58(9):4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee P., Eckert A. O., Schrey A. K., Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Research . 2018;46(W1):W257–W263. doi: 10.1093/nar/gky318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A., Aswal S., Semwal R. B., Chauhan A., Joshi S. K., Semwal D. K. Role of plant-derived alkaloids against diabetes and diabetes-related complications: a mechanism-based approach. Phytochemistry Reviews . 2019;18(5):1277–1298. doi: 10.1007/s11101-019-09648-6. [DOI] [Google Scholar]
- 16.Lichman B. R. The scaffold-forming steps of plant alkaloid biosynthesis. Natural Product Reports . 2021;38(1):103–129. doi: 10.1039/d0np00031k. [DOI] [PubMed] [Google Scholar]
- 17.Schramm S., Köhler N., Rozhon W. Pyrrolizidine alkaloids: biosynthesis, biological activities and occurrence in crop plants. Molecules . 2019;24(3):p. 498. doi: 10.3390/molecules24030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frick K. M., Kamphuis L. G., Siddique K. H., Singh K. B., Foley R. C. Quinolizidine alkaloid biosynthesis in lupins and prospects for grain quality improvement. Frontiers of Plant Science . 2017;8:p. 87. doi: 10.3389/fpls.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunsupa S., Katayama K., Ikeura E., et al. Lysine decarboxylase catalyzes the first step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid production in leguminosae. The Plant Cell Online . 2012;24(3):1202–1216. doi: 10.1105/tpc.112.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng X., Zhao L., Fang T., et al. Investigation of benzylisoquinoline alkaloid biosynthetic pathway and its transcriptional regulation in lotus. Horticulture Research . 2018;5:29–16. doi: 10.1038/s41438-018-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagel J. M., Facchini P. J. Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new World. Plant and Cell Physiology . 2013;54(5):647–672. doi: 10.1093/pcp/pct020. [DOI] [PubMed] [Google Scholar]
- 22.Feng T., Duan K.-T., He S.-J., et al. Ophiorrhines A and B, two immunosuppressive monoterpenoid indole alkaloids from Ophiorrhiza japonica. Organic Letters . 2018;20(24):7926–7928. doi: 10.1021/acs.orglett.8b03489. [DOI] [PubMed] [Google Scholar]
- 23.Qiu F., Yang C., Yuan L., et al. A phenylpyruvic acid reductase is required for biosynthesis of tropane alkaloids. Organic Letters . 2018;20(24):7807–7810. doi: 10.1021/acs.orglett.8b03236. [DOI] [PubMed] [Google Scholar]
- 24.He S.-M., Liang Y.-L., Cong K., et al. Identification and characterization of genes involved in benzylisoquinoline alkaloid biosynthesis in coptis species. Frontiers of Plant Science . 2018;9:p. 731. doi: 10.3389/fpls.2018.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menéndez-Perdomo I., Facchini P. Benzylisoquinoline alkaloids biosynthesis in sacred lotus. Molecules . 2018;23:p. 2899. doi: 10.3390/molecules23112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikezawa N., Iwasa K., Sato F. Molecular cloning and characterization of CYP80G2, a cytochrome P450 that catalyzes an intramolecular C-C phenol coupling of (S)-reticuline in magnoflorine biosynthesis, from cultured Coptis japonica cells. Journal of Biological Chemistry . 2008;283(14):8810–8821. doi: 10.1074/jbc.m705082200. [DOI] [PubMed] [Google Scholar]
- 27.Tatsis E. C., Carqueijeiro I., Dugé de Bernonville T., et al. A three enzyme system to generate the Strychnos alkaloid scaffold from a central biosynthetic intermediate. Nature Communications . 2017;8(1):p. 316. doi: 10.1038/s41467-017-00154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey A. J., O’Hagan D. Tropane alkaloid biosynthesis. A century old problem unresolved. Natural Product Reports . 2001;18(5):494–502. doi: 10.1039/b001713m. [DOI] [PubMed] [Google Scholar]
- 29.von Linné C. Alkaloids - Secrets of Life . Amsterdam, Netherlands: Elsevier; 2007. Biological significance of alkaloids. [Google Scholar]
- 30.Jan R., Asaf S., Numan M., Lubna K. K.-M., Kim K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy . 2021;11(5):p. 968. doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- 31.Marahatha R., Gyawali K., Sharma K., et al. Pharmacologic activities of phytosteroids in inflammatory diseases: mechanism of action and therapeutic potentials. Phytotherapy Research . 2021;35(9):5103–5124. doi: 10.1002/ptr.7138. [DOI] [PubMed] [Google Scholar]
- 32.Oeckinghaus A., Ghosh S. The NF- B family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology . 2009;1(4) doi: 10.1101/cshperspect.a000034.a000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J.-B., Han A.-R., Park E.-Y., et al. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-.KAPPA.B inactivation in RAW 264.7 macrophage cells. Biological and Pharmaceutical Bulletin . 2007;30(12):2345–2351. doi: 10.1248/bpb.30.2345. [DOI] [PubMed] [Google Scholar]
- 34.Guo H., Callaway J. B., Ting J. P.-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Medicine . 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmonds R. E., Foxwell B. M. Signalling, inflammation and arthritis: NF- B and its relevance to arthritis and inflammation. Rheumatology . 2008;47(5):584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 36.Porcelli S. A. Kelley and Firestein’s Textbook of Rheumatology . Amsterdam, Netherlands: Elsevier; 2017. Innate immunity; pp. 274–287. [DOI] [Google Scholar]
- 37.Gunaydin C., Bilge S. S. Effects of nonsteroidal anti-inflammatory drugs at the molecular level. The Eurasian journal of medicine . 2018;50:116–121. doi: 10.5152/eurasianjmed.2018.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clancy R., Varenika B., Huang W., et al. Nitric oxide synthase/COX cross-talk: nitric oxide activates COX-1 but inhibits COX-2-derived prostaglandin production. The Journal of Immunology . 2000;165(3):1582–1587. doi: 10.4049/jimmunol.165.3.1582. [DOI] [PubMed] [Google Scholar]
- 39.Poddar M. K., Chakraborty A., Banerjee S. Neurodegeneration: Diagnosis, Prevention, and Therapy . London, UK: IntechOpen; 2021. [Google Scholar]
- 40.Jellinger K. A. Basic mechanisms of neurodegeneration: a critical update. Journal of Cellular and Molecular Medicine . 2010;14(3):457–487. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Ayllón M.-S. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-amyloid. Frontiers in Molecular Neuroscience . 2011;4 doi: 10.3389/fnmol.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mirjana B. C., Danijela Z. K., Tamara D. L.-P., Aleksandra M. B., Vesna M. V. Acetylcholinesterase inhibitors: pharmacology and toxicology. Current Neuropharmacology . 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannon J. R., Greenamyre J. T. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicological Sciences . 2011;124(2):225–250. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dauer W., Przedborski S. Parkinson’s disease. Neuron . 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 45.Stefanis L. α-synuclein in Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine . 2012;2(2) doi: 10.1101/cshperspect.a009399.a009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishna R., Ali M., Moustafa A. A. Effects of combined MAO-B inhibitors and levodopa vs. monotherapy in Parkinson’s disease. Frontiers in Aging Neuroscience . 2014;6 doi: 10.3389/fnagi.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan S.-Y., Litscher G., Gao S.-H., Zhou S.-F., Yu Z.-L., Chen H.-Q. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evidence-based Complementary and Alternative Medicine . 2014;2014:20. doi: 10.1155/2014/525340.525340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J., Chan L., Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Current Medicinal Chemistry . 2012;19(21):3523–3531. doi: 10.2174/092986712801323171. [DOI] [PubMed] [Google Scholar]
- 49.Oguntibeju O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. Journal of Inflammation Research . 2018;11:307–317. doi: 10.2147/jir.s167789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Z., Yang P., Zhou Q. Multiple biological functions and pharmacological effects of lycorine. Science China Chemistry . 2013;56(10):1382–1391. doi: 10.1007/s11426-013-4967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ratnayake W. M. K. M., Suresh T. S., Abeysekera A. M., Salim N., Chandrika U. G. Acute anti-inflammatory and anti-nociceptive activities of crude extracts, alkaloid fraction and evolitrine from Acronychia pedunculata leaves. Journal of Ethnopharmacology . 2019;238 doi: 10.1016/j.jep.2019.111827.111827 [DOI] [PubMed] [Google Scholar]
- 52.Li Z., Geng Y.-N., Jiang J.-D., Kong W.-J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evidence-based Complementary and Alternative Medicine . 2014;2014:12. doi: 10.1155/2014/289264.289264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan H.-L., Zhao Y.-L., Qin X.-J., et al. Anti-inflammatory and analgesic activities of Neolamarckia cadamba and its bioactive monoterpenoid indole alkaloids. Journal of Ethnopharmacology . 2020;260 doi: 10.1016/j.jep.2020.113103.113103 [DOI] [PubMed] [Google Scholar]
- 54.Segun P. A., Ismail F. M. D., Ogbole O. O., et al. Acridone alkaloids from the stem bark of Citrus aurantium display selective cytotoxicity against breast, liver, lung and prostate human carcinoma cells. Journal of Ethnopharmacology . 2018;227:131–138. doi: 10.1016/j.jep.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 55.Szurpnicka A., Kowalczuk A., Szterk A. Biological activity of mistletoe: in vitro and in vivo studies and mechanisms of action. Archives of Pharmacal Research . 2020;43(6):593–629. doi: 10.1007/s12272-020-01247-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abat J. K., Kumar S., Mohanty A. Ethnomedicinal, phytochemical and ethnopharmacological aspects of four medicinal plants of malvaceae used in Indian traditional medicines: a review. Medicines . 2017;4(4):p. 75. doi: 10.3390/medicines4040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baghdikian B., Mahiou-Leddet V., Bory S., et al. New antiplasmodial alkaloids from Stephania rotunda. Journal of Ethnopharmacology . 2013;145(1):381–385. doi: 10.1016/j.jep.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 58.Wansi J. D., Devkota K. P., Tshikalange E., Kuete V. Alkaloids from the medicinal plants of Africa. In: Kuete V., editor. Medicinal Plant Research in Africa . Oxford, UK: Elsevier; 2013. pp. 557–605. [DOI] [Google Scholar]
- 59.Patnala S., Kanfer I. HPLC analysis of mesembrine-type Alkaloids in sceletium plant material used as an African traditional medicine. Journal of Pharmacy & Pharmaceutical Sciences . 2010;13(4):558–570. doi: 10.18433/j3dk5f. [DOI] [PubMed] [Google Scholar]
- 60.Xiao J., Song J.-Y., Lin B., et al. Amide-iminoate isomerism in antineuroinflammatory isoquinoline alkaloids from Stephania cepharantha. Journal of Natural Products . 2020;83(4):864–872. doi: 10.1021/acs.jnatprod.9b00483. [DOI] [PubMed] [Google Scholar]
- 61.Qiu S., Sun H., Zhang A.-H., et al. Natural alkaloids: basic aspects, biological roles, and future perspectives. Chinese Journal of Natural Medicines . 2014;12(6):401–406. doi: 10.1016/s1875-5364(14)60063-7. [DOI] [PubMed] [Google Scholar]
- 62.Casciaro B., Mangiardi L., Cappiello F., et al. Naturally-occurring alkaloids of plant origin as potential antimicrobials against antibiotic-resistant infections. Molecules . 2020;25(16):p. 3619. doi: 10.3390/molecules25163619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho S.-K., Jeong M., Jang D., Choi J.-H. Anti-inflammatory effects of canthin-6-one alkaloids from Ailanthus altissima. Planta Medica . 2018;84(08):527–535. doi: 10.1055/s-0043-123349. [DOI] [PubMed] [Google Scholar]
- 64.Soleymani S., Farzaei M. H., Zargaran A., Niknam S., Rahimi R. Promising plant-derived secondary metabolites for treatment of acne vulgaris: a mechanistic review. Archives of Dermatological Research . 2020;312(1):5–23. doi: 10.1007/s00403-019-01968-z. [DOI] [PubMed] [Google Scholar]
- 65.Xu J., Zhao Y., Aisa H. A. Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. Pharmaceutical Biology . 2017;55(1):2095–2101. doi: 10.1080/13880209.2017.1357737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D., Shi Y., Li J., et al. Alkaloids with nitric oxide inhibitory activities from the roots of Isatis tinctoria. Molecules . 2019;24(22):p. 4033. doi: 10.3390/molecules24224033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim K.-H., Park E.-J., Jang H.-J., Lee S.-J., Park C. S., Yun B.-S. 1-carbomethoxy-β-Carboline, derived from Portulaca oleracea L., ameliorates LPS-mediated inflammatory response associated with MAPK signaling and nuclear translocation of NF-κB. Molecules . 2019;24(22):p. 4042. doi: 10.3390/molecules24224042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foyet H. S., Keugong Wado E., Ngatanko Abaissou H. H., Assongalem E. A., Eyong O. K. Anticholinesterase and antioxidant potential of hydromethanolic extract of Ziziphus mucronata (rhamnaceae) leaves on scopolamine-induced memory and cognitive dysfunctions in mice. Evidence-based Complementary and Alternative Medicine . 2019;2019:14. doi: 10.1155/2019/4568401.4568401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li S., Liu X., Chen X., Bi L. Research progress on anti-inflammatory effects and mechanisms of alkaloids from Chinese medical herbs. Evidence-based Complementary and Alternative Medicine . 2020;2020:10. doi: 10.1155/2020/1303524.1303524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li C.-Y., Meng Y.-H., Ying Z.-M., et al. Three novel alkaloids from Portulaca oleracea L. And their anti-inflammatory effects. Journal of Agricultural and Food Chemistry . 2016;64(29):5837–5844. doi: 10.1021/acs.jafc.6b02673. [DOI] [PubMed] [Google Scholar]
- 71.Moraga-Nicolás F., Jara C., Godoy R., et al. Rhodolirium andicola: a new renewable source of alkaloids with acetylcholinesterase inhibitory activity, a study from nature to molecular docking. Revista Brasileira de Farmacognosia . 2018;28:34–43. [Google Scholar]
- 72.Plazas E., Hagenow S., Avila Murillo M., Stark H., Cuca L. E. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ1-42 aggregation. Bioorganic Chemistry . 2020;98 doi: 10.1016/j.bioorg.2020.103722.103722 [DOI] [PubMed] [Google Scholar]
- 73.Benamar H., Tomassini L., Venditti A., Marouf A., Bennaceur M., Nicoletti M. Pyrrolizidine alkaloids from Solenanthus lanatus DC. with acetylcholinesterase inhibitory activity. Natural Product Research . 2016;30(22):2567–2574. doi: 10.1080/14786419.2015.1131984. [DOI] [PubMed] [Google Scholar]
- 74.Zhan G., Miao R., Zhang F., et al. Monoterpene indole alkaloids with acetylcholinesterase inhibitory activity from the leaves of Rauvolfia vomitoria. Bioorganic Chemistry . 2020;102 doi: 10.1016/j.bioorg.2020.104136.104136 [DOI] [PubMed] [Google Scholar]
- 75.Cheenpracha S., Jitonnom J., Komek M., Ritthiwigrom T., Laphookhieo S. Acetylcholinesterase inhibitory activity and molecular docking study of steroidal alkaloids from Holarrhena pubescens barks. Steroids . 2016;108:92–98. doi: 10.1016/j.steroids.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Shawky E., El Sohafy S. M., de Andrade J. P., de Souza Borges W. Profiling of acetylcholinesterase inhibitory alkaloids from some Crinum, Habranthus and Zephyranthes species by GC-MS combined with multivariate analyses and in silico studies. Natural Product Research . 2021;35(5):807–814. doi: 10.1080/14786419.2019.1598989. [DOI] [PubMed] [Google Scholar]
- 77.Stavrakov G., Philipova I., Lukarski A., et al. Galantamine-curcumin hybrids as dual-site binding acetylcholinesterase inhibitors. Molecules . 2020;25(15):p. 3341. doi: 10.3390/molecules25153341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aryal B., Adhikari B., Aryal N., Bhattarai B., Khadayat K., Parajuli N. LC-HRMS profiling and antidiabetic, antioxidant, and antibacterial activities of Acacia catechu (L.f.) willd. BioMed Research International . 2021;2021:16. doi: 10.1155/2021/7588711.7588711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aryal B., Niraula P., Khadayat K., et al. Antidiabetic, antimicrobial, and molecular profiling of selected medicinal plants. Evidence-based Complementary and Alternative Medicine . 2021;2021:16. doi: 10.1155/2021/5510099.7588711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacognosy Reviews . 2010;4(8):p. 118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang X.-L., Wang L., Wang E.-J., et al. Flavonoid glycosides and alkaloids from the embryos of Nelumbo nucifera seeds and their antioxidant activity. Fitoterapia . 2018;125:184–190. doi: 10.1016/j.fitote.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Obaid Aldulaimi A. K., Abdul Azziz S. S. S., Bakri Y. M., et al. Two new isoquinoline alkaloids from the bark of Alphonsea cylindrica king and their antioxidant activity. Phytochemistry Letters . 2019;29:110–114. doi: 10.1016/j.phytol.2018.11.022. [DOI] [Google Scholar]
- 83.Wang R., Zhou J., Shi G., Liu Y., Yu D. Aporphine and phenanthrene alkaloids with antioxidant activity from the roots of Stephania tetrandra. Fitoterapia . 2020;143 doi: 10.1016/j.fitote.2020.104551.104551 [DOI] [PubMed] [Google Scholar]
- 84.Azevedo B C., Roxo M., Borges C., et al. Antioxidant activity of an aqueous leaf extract from Uncaria tomentosa and its major alkaloids mitraphylline and isomitraphylline in Caenorhabditis elegans. Molecules . 2019;24:p. 3299. doi: 10.3390/molecules24183299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Durán-Iturbide N. A., Díaz-Eufracio B. I., Medina-Franco J. L. In-silico ADME/tox profiling of natural products: a focus on BIOFACQUIM. ACS Omega . 2020;5:16076–16084. doi: 10.1021/acsomega.0c01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clark D. E. In silico prediction of blood-brain barrier permeation. Drug Discovery Today . 2003;8(20):927–933. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 87.Šrejber M., Navrátilová V., Paloncýová M., et al. Membrane-attached mammalian cytochromes P450: an overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. Journal of Inorganic Biochemistry . 2018;183:117–136. doi: 10.1016/j.jinorgbio.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe R., Ohashi R., Esaki T., et al. Development of an in silico prediction system of human renal excretion and clearance from chemical structure information incorporating fraction unbound in plasma as a descriptor. Scientific Reports . 2019;9(1) doi: 10.1038/s41598-019-55325-1.18782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Clinical Trials. Gov. Home - ClinicalTrials.Gov, 2021, https://clinicaltrials.gov/
- 90.Chung Leung W., Zheng H., Huen M., Lun Law S., Xue H. Anxiolytic-like action of orally administered dl-tetrahydropalmatine in elevated plus-maze. Progress in Neuro-Psychopharmacology and Biological Psychiatry . 2003;27(5):775–779. doi: 10.1016/s0278-5846(03)00108-8. [DOI] [PubMed] [Google Scholar]
- 91.Wu L., Ling H., Li L., Jiang L., He M. Beneficial effects of the extract from Corydalis yanhusuo in rats with heart failure following myocardial infarction. Journal of Pharmacy and Pharmacology . 2007;59:695–701. doi: 10.1211/jpp.59.5.0010. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y., Sha R., Wang K., Li H., Yan B., Zhou N. Protective effects of tetrahydropalmatine against ketamine-induced learning and memory injury via antioxidative, anti-inflammatory and anti-apoptotic mechanisms in mice. Molecular Medicine Reports . 2018;17(5):6873–6880. doi: 10.3892/mmr.2018.8700. [DOI] [PubMed] [Google Scholar]
- 93.Qu Z., Zhang J., Yang H., et al. Protective effect of tetrahydropalmatine against d-galactose induced memory impairment in rat. Physiology & Behavior . 2016;154:114–125. doi: 10.1016/j.physbeh.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 94.Yang B.-R., Yu N., Deng Y.-H., et al. L-tetrahydropalamatine inhibits tumor necrosis factor-α-induced monocyte-endothelial cell adhesion through downregulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 involving suppression of nuclear factor-κ B signaling pathway. Chinese Journal of Integrative Medicine . 2015;21(5):361–368. doi: 10.1007/s11655-015-2165-7. [DOI] [PubMed] [Google Scholar]
- 95.Jeong H. W., Hsu K. C., Lee J.-W., et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. American Journal of Physiology-Endocrinology and Metabolism . 2009;296(4):E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 96.Lou T., Zhang Z., Xi Z., et al. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation . 2011;34(6):659–667. doi: 10.1007/s10753-010-9276-2. [DOI] [PubMed] [Google Scholar]
- 97.Panahi N., Mahmoudian M., Mortazavi P., Hashjin G. S. Experimental research Effects of berberine on β-secretase activity in a rabbit model of Alzheimer’s disease. Archives of Medical Science . 2013;1:146–150. doi: 10.5114/aoms.2013.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Durairajan S. S. K., Liu L.-F., Lu J.-H., et al. Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiology of Aging . 2012;33(12):2903–2919. doi: 10.1016/j.neurobiolaging.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 99.Kim M., Cho K.-H., Shin M.-S., et al. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. International Journal of Molecular Medicine . 2014;33(4):870–878. doi: 10.3892/ijmm.2014.1656. [DOI] [PubMed] [Google Scholar]
- 100.Liu T., Liu X., Li W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget . 2016;7(26):40800–40815. doi: 10.18632/oncotarget.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xue Y., Wang Y., Feng D.-c., Xiao B.-g., Xu L.-y. Tetrandrine suppresses lipopolysaccharide-induced microglial activation by inhibiting NF-κB pathway. Acta Pharmacologica Sinica . 2008;29(2):245–251. doi: 10.1111/j.1745-7254.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 102.Gao L.-N., Feng Q.-S., Zhang X.-F., Wang Q.-S., Cui Y.-L. Tetrandrine suppresses articular inflammatory response by inhibiting pro-inflammatory factors via NF-κB inactivation. Journal of Orthopaedic Research . 2016;34(9):1557–1568. doi: 10.1002/jor.23155. [DOI] [PubMed] [Google Scholar]
- 103.Bao G., Li C., Qi L., Wang N., He B. Tetrandrine protects against oxygen-glucose-serum deprivation/reoxygenation-induced injury via PI3K/AKT/NF-κB signaling pathway in rat spinal cord astrocytes. Biomedicine & Pharmacotherapy . 2016;84:925–930. doi: 10.1016/j.biopha.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 104.He F.-Q., Qiu B.-Y., Zhang X.-H., et al. Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-κB activation in a rat model of Alzheimer’s disease induced by amyloid-β (1-42) Brain Research . 2011;1384:89–96. doi: 10.1016/j.brainres.2011.01.103. [DOI] [PubMed] [Google Scholar]
- 105.Li W., Li Y., Zhao Y., Ren L. The protective effects of aloperine against ox-LDL-induced endothelial dysfunction and inflammation in HUVECs. Artificial Cells, Nanomedicine, and Biotechnology . 2020;48(1):107–115. doi: 10.1080/21691401.2019.1699816. [DOI] [PubMed] [Google Scholar]
- 106.Xu Y.-Q., Jin S.-J., Liu N., et al. Aloperine attenuated neuropathic pain induced by chronic constriction injury via anti-oxidation activity and suppression of the nuclear factor kappa B pathway. Biochemical and Biophysical Research Communications . 2014;451(4):568–573. doi: 10.1016/j.bbrc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 107.Ye Y., Wang Y., Yang Y., Tao L. Aloperine suppresses LPS-induced macrophage activation through inhibiting the TLR4/NF-κB pathway. Inflammation Research . 2020;69(4):375–383. doi: 10.1007/s00011-019-01313-0. [DOI] [PubMed] [Google Scholar]
- 108.Yang H., Wang J., Chen X., et al. Effects of sinomenine in LPS‐associated diseases are related to inhibition of LBP, Mac‐1, and L‐selectin levels. Journal of Veterinary Pharmacology and Therapeutics . 2019;42(6):732–737. doi: 10.1111/jvp.12807. [DOI] [PubMed] [Google Scholar]
- 109.Zhang L., Zhang W., Zheng B., Tian N. Sinomenine attenuates traumatic spinal cord injury by suppressing oxidative stress and inflammation via Nrf2 pathway. Neurochemical Research . 2019;44(4):763–775. doi: 10.1007/s11064-018-02706-z. [DOI] [PubMed] [Google Scholar]
- 110.Feng Z.-T., Yang T., Hou X.-Q., et al. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomedicine & Pharmacotherapy . 2019;113 doi: 10.1016/j.biopha.2019.108759.108759 [DOI] [PubMed] [Google Scholar]
- 111.Gao T., Shi T., Wiesenfeld-Hallin Z., Svensson C. I., Xu X.-J. Sinomenine alleviates mechanical hypersensitivity in mice with experimentally induced rheumatoid arthritis. Scandinavian Journal of Pain . 2015;7(1):9–14. doi: 10.1016/j.sjpain.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Singh D., Agrawal A., Singal C. M. S., Pandey H. S., Seth P., Sharma S. K. Sinomenine inhibits amyloid beta-induced astrocyte activation and protects neurons against indirect toxicity. Molecular Brain . 2020;13(1):p. 30. doi: 10.1186/s13041-020-00569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramazi S., Fahanik-Babaei J., Mohamadi-Zarch S.-M., et al. Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: involvement of oxidative stress, inflammation and pyroptosis. Journal of Chemical Neuroanatomy . 2020;108 doi: 10.1016/j.jchemneu.2020.101800.101800 [DOI] [PubMed] [Google Scholar]
- 114.Huang R.-Y., Pan H.-D., Wu J.-Q., et al. Comparison of combination therapy with methotrexate and sinomenine or leflunomide for active rheumatoid arthritis: a randomized controlled clinical trial. Phytomedicine . 2019;57:403–410. doi: 10.1016/j.phymed.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Xu Y., Ji W., et al. Anti-tumor activities of matrine and oxymatrine: literature review. Tumor Biology . 2014;35(6):5111–5119. doi: 10.1007/s13277-014-1680-z. [DOI] [PubMed] [Google Scholar]
- 116.Ma A., Yang Y., Wang Q., Wang Y., Wen J., Zhang Y. Anti-inflammatory effects of oxymatrine on rheumatoid arthritis in rats via regulating the imbalance between Treg and Th17 cells. Molecular Medicine Reports . 2017;15(6):3615–3622. doi: 10.3892/mmr.2017.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fu Y., Wu H.-Q., Cui H.-l., Li Y.-Y., Li C.-Z. Gastroprotective and anti-ulcer effects of oxymatrine against several gastric ulcer models in rats: possible roles of antioxidant, antiinflammatory, and prosurvival mechanisms. Phytotherapy Research . 2018;32(10):2047–2058. doi: 10.1002/ptr.6148. [DOI] [PubMed] [Google Scholar]
- 118.Chen Y., Qi Z., Qiao B., Lv Z., Hao Y., Li H. Oxymatrine can attenuate pathological deficits of Alzheimer’s disease mice through regulation of neuroinflammation. Journal of Neuroimmunology . 2019;334 doi: 10.1016/j.jneuroim.2019.576978. [DOI] [PubMed] [Google Scholar]
- 119.Ge X.-H., Shao L., Zhu G.-J. Oxymatrine attenuates brain hypoxic-ischemic injury from apoptosis and oxidative stress: role of p-Akt/GSK3β/HO-1/Nrf-2 signaling pathway. Metabolic Brain Disease . 2018;33(6):1869–1875. doi: 10.1007/s11011-018-0293-4. [DOI] [PubMed] [Google Scholar]
- 120.Gan P., Ding L., Hang G., Xia Q., Huang Z., Ye X. Oxymatrine attenuates dopaminergic neuronal damage and microglia-mediated neuroinflammation through cathepsin D-dependent HMGB1/TLR4/NF-κB pathway in Parkinson’s disease. Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shi H. J., Zhou H., Ma A. L., et al. Oxymatrine therapy inhibited epidermal cell proliferation and apoptosis in severe plaque psoriasis. British Journal of Dermatology . 2019;181(5):1028–1037. doi: 10.1111/bjd.17852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patel K., Gadewar M., Tripathi R., Prasad S., Patel D. K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pacific Journal of Tropical Biomedicine . 2012;2(8):660–664. doi: 10.1016/s2221-1691(12)60116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu X., Li M., Tan S., Wang C., Fan S., Huang C. Harmine is an inflammatory inhibitor through the suppression of NF-κB signaling. Biochemical and Biophysical Research Communications . 2017;489(3):332–338. doi: 10.1016/j.bbrc.2017.05.126. [DOI] [PubMed] [Google Scholar]
- 124.Jiang J., Ma T., Zhang L., Cheng X., Wang C. The transdermal performance, pharmacokinetics, and anti-inflammatory pharmacodynamics evaluation of harmine-loaded ethosomes. Drug Development and Industrial Pharmacy . 2020;46(1):101–108. doi: 10.1080/03639045.2019.1706549. [DOI] [PubMed] [Google Scholar]
- 125.Niu X., Yao Q., Li W., et al. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3 inflammasome signalling pathway in mice. European Journal of Pharmacology . 2019;849:160–169. doi: 10.1016/j.ejphar.2019.01.062. [DOI] [PubMed] [Google Scholar]
- 126.Li S. P., Wang Y. W., Qi S. L., et al. Analogous β-carboline alkaloids harmaline and harmine ameliorate scopolamine-induced cognition dysfunction by attenuating acetylcholinesterase activity, oxidative stress, and inflammation in mice. Frontiers in Pharmacology . 2018;9:p. 346. doi: 10.3389/fphar.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu P., Li H., Wang Y., et al. Harmine ameliorates cognitive impairment by inhibiting NLRP3 inflammasome activation and enhancing the BDNF/TrkB signaling pathway in STZ-induced diabetic rats. Frontiers in Pharmacology . 2020;11:p. 535. doi: 10.3389/fphar.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He D., Wu H., Wei Y., et al. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. European Journal of Pharmacology . 2015;768:96–107. doi: 10.1016/j.ejphar.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 129.Heinrich M., Lee Teoh H. Galanthamine from snowdrop-the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. Journal of Ethnopharmacology . 2004;92(2-3):147–162. doi: 10.1016/j.jep.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 130.Cronin J. R. The plant alkaloid galantamine: approved as a drug; sold as a supplement. Alternative & Complementary Therapies . 2001;7(6):380–383. doi: 10.1089/10762800152709741. [DOI] [Google Scholar]
- 131.Liu Y., Zhang Y., Zheng X., et al. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. Journal of Neuroinflammation . 2018;15(1):p. 112. doi: 10.1186/s12974-018-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matharu B., Gibson G., Parsons R., et al. Galantamine inhibits β-amyloid aggregation and cytotoxicity. Journal of the Neurological Sciences . 2009;280(1-2):49–58. doi: 10.1016/j.jns.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 133.Moriguchi S., Marszalec W., Zhao X., Yeh J. Z., Narahashi T. Mechanism of action of galantamine onN-Methyl-d-Aspartate receptors in rat cortical neurons. Journal of Pharmacology and Experimental Therapeutics . 2004;310(3):933–942. doi: 10.1124/jpet.104.067603. [DOI] [PubMed] [Google Scholar]
- 134.Kita Y., Ago Y., Takano E., Fukada A., Takuma K., Matsuda T. Galantamine increases hippocampal insulin-like growth factor 2 expression via α7 nicotinic acetylcholine receptors in mice. Psychopharmacology . 2013;225(3):543–551. doi: 10.1007/s00213-012-2841-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: plant-derived alkaloids with their biosynthetic precursors and current research. Table S2: occurrence, pharmacology, and toxicity of alkaloids. Table S3: ADMET properties of promising plant alkaloids by pkCSM server. Table S4: prediction of toxicity of secondary metabolites inhibiting metabolic enzymes using ProTox-II. Figure S1: molecule structure of plant-derived alkaloids.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


