Abstract
Previous studies have shown that the proton pump inhibitor omeprazole has antimalarial activity in vitro. The interactions of omeprazole with commonly used antimalarial drugs were assessed in vitro. Omeprazole and quinine combinations were synergistic; however, chloroquine and omeprazole combinations were antagonistic. Artemisinin drugs had additive antimalarial activities with omeprazole.
We recently identified the proton pump inhibitor omeprazole as a potential novel antimalarial agent (19). The precise mode of action of omeprazole against Plasmodium falciparum is unknown, but it may have mechanisms distinct from those of conventional drugs (19). Although omeprazole can be given parenterally (12), its terminal elimination half-life is short (40 to 60 min [10]), and relatively high concentrations in plasma may be required for clinically useful activity (19). Nevertheless, as in the case of the gastric parietal cell (22), the binding of omeprazole or its sulfenamide metabolite to a P. falciparum ATPase may prolong its antimalarial effect.
Although these considerations suggest that omeprazole may be unsuitable as antimalarial monotherapy, it could form part of combination treatment with conventional agents in order to improve cure rates and reduce the likelihood of parasite resistance (13). We have therefore examined the in vitro antimalarial activity of omeprazole in combination with chloroquine, quinine, and several commonly used artemisinin drugs.
Parasites were maintained in modified candle jars (21). Stock 1-mmol/liter solutions of artemisinin (Sigma, St. Louis, Mo.), dihydroartemisinin (Cotec Pharmaceutical Company, Guangxi, China), artemether (Brian Schuster, Walter Reed Army Institute of Research, Washington, D.C.), artesunic acid (Guilin Pharmaceuticals, Guangxi, China), omeprazole (Astra, Södertälje, Sweden), quinine (Sigma), and chloroquine (Sigma) were prepared in plasma-free RPMI and stored at −80°C. Dimethyl sulfoxide (1%, vol/vol) was used as the vehicle in all cases. Drug stocks were diluted in plasma-supplemented RPMI when required for assays.
Antimalarial drug combinations were assessed against clone 3D7 by isobolographic analysis (1). Assays were performed in 96-well microtiter plates containing 100 μl of 3D7 culture at 2% parasitemia and 2% hematocrit and 100 μl of the appropriate drug or drug combination. Parasite growth was determined by tritiated hypoxanthine incorporation (6). Media and vehicle controls were included on each plate. Experiments were repeated at least twice. Fifty percent effective concentrations (EC50) (Table 1) were determined by linear interpolation (11). Isobolograms were constructed from normalized fractional-inhibitory concentration (FIC) values in each experiment, and isoboles were fitted with a standard hyperbolic function defined by the parameter I (2, 3). The significance of the difference of I from zero was assessed with Student’s t test. Dose factor potentiations were also estimated (16).
TABLE 1.
EC50 of individual antimalarial drugs
| Drug | EC50 (mol/liter)a |
|---|---|
| Omeprazole | 1.4 × 10−5–3.6 × 10−5 |
| Quinine | 3.2 × 10−8–2.5 × 10−7 |
| Chloroquine | 8.0 × 10−9–2.0 × 10−8 |
| Artemisinin | 8.8 × 10−9–2.7 × 10−8 |
| Artesunate | 2.3 × 10−9–5.7 × 10−9 |
| Dihydroartemisinin | 1.6 × 10−9–3.4 × 10−9 |
| Artemether | 3.3 × 10−9–2.7 × 10−8 |
EC50 are presented as ranges determined from all isobologram experiments.
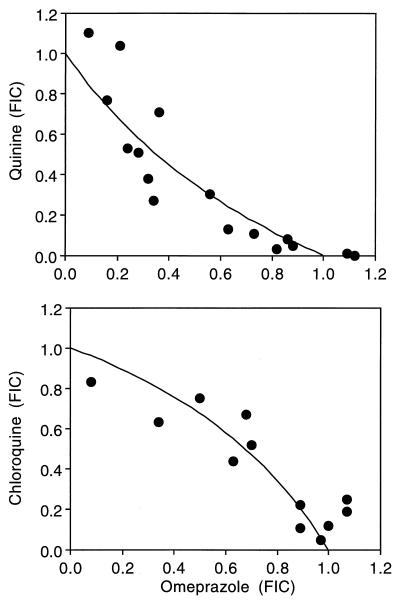
The interactions of omeprazole with quinine, chloroquine, and the artemisinin drugs ranged from significant synergism to mild antagonism. Combinations of omeprazole with quinine were significantly synergistic, but omeprazole and chloroquine were weakly antagonistic (Table 2; Fig. 1). Omeprazole and individual artemisinin drugs exhibited additivity in each case (Table 2). The combination of quinine and chloroquine was antagonistic (Table 2).
TABLE 2.
In vitro efficacies of antimalarial drug combinations against P. falciparum clone 3D7
| Drug combination | Interaction factor
|
Dose factor potentiation | ||
|---|---|---|---|---|
| I | SD | P | ||
| Omeprazole-quinine | 0.622 | 0.234 | <0.001 | 2.0 |
| Omeprazole-chloroquine | −0.730 | 0.205 | <0.001 | 0.6 |
| Omeprazole-dihydroartemisinin | 0.026 | 0.359 | 0.802 | 1.0 |
| Omeprazole-artemisinin | −0.095 | 0.184 | 0.088 | 1.0 |
| Omeprazole-artesunate | 0.021 | 0.216 | 0.739 | 1.0 |
| Omeprazole-artemether | 0.006 | 0.332 | 0.978 | 1.0 |
| Quinine-chloroquine | −2.836 | 2.826 | 0.005 | 0.3 |
FIG. 1.
Isobolograms illustrating interactions of omeprazole with quinine (synergism) and chloroquine (antagonism). Axes are normalized FIC values. Each duplicate experiment was repeated three times with omeprazole and quinine and twice with omeprazole and chloroquine.
Quinine remains a first-line treatment for severe falciparum malaria, but there are concerns regarding declining efficacy in some countries (15, 17). Our in vitro data demonstrate that omeprazole given to a severely ill patient may increase the effects of quinine and improve clinical outcome. The results of recent research suggest that omeprazole inhibits CYP3A-catalyzed 3-hydroxylation of quinine by human liver microsomes (23), an effect that may increase serum quinine concentrations in vivo but at the risk of increased toxicity. Since omeprazole is currently used for gastric acid suppression and as a part of the therapy for Helicobacter pylori infection, field studies of the quinine-omeprazole combination could be carried out to examine these issues.
The in vitro additive antimalarial activity of omeprazole plus an artemisinin derivative may be influenced by pharmacokinetic factors in vivo. Nevertheless, parasite clearance in vivo is already relatively rapid with the artemisinin derivatives, and it seems unlikely that the additive activity demonstrated in our in vitro study would be beneficial in the acute phase of infection. Nevertheless, the different actions of these drugs may both reduce recrudescence and prevent the development of parasite resistance.
In addition to possible clinical implications, our data may shed light on the mechanisms of action of some conventional antimalarial drugs. Our data confirm that chloroquine and quinine are antagonistic (20). Consistent with the observation that these drugs have similar stage-specific activities against pigment-producing parasites (18), both interfere with hemozoin formation (7). However, this probably occurs indirectly, before any ferriprotoporphyrin binding, with chloroquine increasing the amount of a negative regulator of hemozoin formation (8). Although the final target of quinine is unknown, it may reverse chloroquine-induced inhibition of ferriprotoporphyrin sequestration (8). The contrasting interactions of omeprazole with chloroquine and quinine in the present study provide additional indirect evidence that these quinoline drugs have different actions against the parasite.
Factors relating to the cellular effects of omeprazole might explain why omeprazole and quinine behave synergistically while chloroquine-omeprazole combinations are antagonistic. Omeprazole may inhibit growth of P. falciparum by inhibiting parasite ATPase activity, which, in turn, increases the pH of the acidic food vacuole. Since both omeprazole and chloroquine are weak bases which accumulate in acidic environments (14), a higher pH and/or competition with omeprazole would also inhibit accumulation of chloroquine at its site of action. In addition, chloroquine accumulation may be driven by a P-type ATPase (4), which may also be affected by omeprazole. Unlike chloroquine, quinine is more lipophilic and is not concentrated exclusively in food vacuoles (9). Indeed, there is evidence that quinine inhibits membrane ATPases in the same way as omeprazole (5).
Our data provide important preliminary evidence of interactions between the novel antimalarial agent omeprazole and conventional drugs that may have clinical implications. At present, parasite resistance limits therapeutic options for malaria, and the artemisinin derivatives are not yet available in many Western countries. Omeprazole may prove to be a valuable adjunctive antimalarial agent in this context.
Acknowledgments
We thank A. Cowman and G. Brown for supplying 3D7. We are also grateful to Shane Powell for help with laboratory procedures and to Hugh Barrett for assistance with data analysis.
This study was supported by the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Berenbaum M C. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 2.Brueckner R P, Milhous W K, Canfield C J. Program and abstracts of the 40th Annual Meeting of the American Society of Tropical Medicine and Hygiene. Am. J. Trop. Med Hyg. 45:1–313. 1991. Quantitative isobolic analysis of antimalarial drug interactions; p. 190. [Google Scholar]
- 3.Canfield C J, Pudney M, Gutteridge W E. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol. 1995;80:373–381. doi: 10.1006/expr.1995.1049. [DOI] [PubMed] [Google Scholar]
- 4.Chandra S, Adhikary G, Sikdar R, Sen P C. The in vivo inhibition of transport enzyme activities by chloroquine in different organs of the rat is reversible. Mol Cell Biochem. 1992;118:15–21. doi: 10.1007/BF00249690. [DOI] [PubMed] [Google Scholar]
- 5.Choi I, Mego J L. Purification of Plasmodium falciparum digestive vacuoles and partial characterization of the vacuolar membrane ATPase. Mol Biochem Parasitol. 1988;31:71–78. doi: 10.1016/0166-6851(88)90146-6. [DOI] [PubMed] [Google Scholar]
- 6.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan T J, Ross D C, Adams P A. Quinoline anti-malarial drugs inhibit spontaneous formation of beta-haematin (malaria pigment) FEBS Lett. 1994;351:54–57. doi: 10.1016/0014-5793(94)00921-x. [DOI] [PubMed] [Google Scholar]
- 8.Fitch C D. Involvement of heme in the antimalarial action of chloroquine. Trans Am Clin Climatol Assoc. 1998;109:97–105. [PMC free article] [PubMed] [Google Scholar]
- 9.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance. J Parasitol. 1997;27:231–240. doi: 10.1016/s0020-7519(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 10.Hochwald C, Farrington E. Omeprazole. Pediatr Nurs. 1996;5:453–454. [PubMed] [Google Scholar]
- 11.Huber W, Koella J C. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993;55:257–261. doi: 10.1016/0001-706x(93)90083-n. [DOI] [PubMed] [Google Scholar]
- 12.Jacqz-Aigrain E, Bellaich M, Faure C, Andre J, Rohrlich P, Baudouin V, Navarro J. Pharmacokinetics of intravenous omeprazole in children. Eur J Clin Pharmacol. 1994;47:181–185. doi: 10.1007/BF00194970. [DOI] [PubMed] [Google Scholar]
- 13.Landau I, Chabaud A, Cambie G, Ginsburg H. Chronotherapy of malaria: an approach to malaria chemotherapy. Parasitol Today. 1991;7:350–352. doi: 10.1016/0169-4758(91)90218-d. [DOI] [PubMed] [Google Scholar]
- 14.Lapenna D, Gioia D D, Coifani G, Festi D, Cuccurullo F. Antioxidant properties of omeprazole. FEBS Lett. 1996;382:189–192. doi: 10.1016/0014-5793(96)00155-x. [DOI] [PubMed] [Google Scholar]
- 15.Looareesuwan S, Charoenpan P, Ho M, White N J, Karbwang J, Bunnag D, Harinasuta T. Fatal Plasmodium falciparum malaria after an inadequate response to quinine treatment. J Infect Dis. 1990;161:577–580. doi: 10.1093/infdis/161.3.577. [DOI] [PubMed] [Google Scholar]
- 16.Poch G. Dose factor of potentiation derived from isoboles. Arzneim-Forsch. 1980;30:2195–2196. [PubMed] [Google Scholar]
- 17.Pukrittayakamee S, Supanaranound W, Looareesuwan S, Vanijanonta S, White N J. Quinine in severe falciparum malaria: evidence of declining efficacy in Thailand. Trans R Soc Trop Med Hyg. 1994;88:324–327. doi: 10.1016/0035-9203(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 18.Skinner T S, Manning L S, Johnston W A, Davis T M E. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and the artemisinin drugs. J Parasitol. 1996;26:519–525. doi: 10.1016/0020-7519(96)89380-5. [DOI] [PubMed] [Google Scholar]
- 19.Skinner-Adams T S, Davis T M E, Manning L S, Johnston W A. The efficacy of benzimidazole drugs against Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1997;91:580–584. doi: 10.1016/s0035-9203(97)90035-3. [DOI] [PubMed] [Google Scholar]
- 20.Stahel E, Druilhe P, Gentilini M. Antagonism of chloroquine and other antimalarials. Trans R Soc Trop Med Hyg. 1988;82:222. doi: 10.1016/0035-9203(88)90417-8. [DOI] [PubMed] [Google Scholar]
- 21.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;89:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Wilde M I, McTavish D. Omeprazole. An update of its pharmacology and therapeutic use in acid-related disorders. Drug Eval. 1994;48:91–132. doi: 10.2165/00003495-199448010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X J, Ishizaki T. Metabolic interactions of selected antimalarial and non-antimalarial drugs with the major pathway (3-hydroxylation) of quinine in human liver microsomes. Br J Clin Pharmacol. 1997;44:505–511. doi: 10.1046/j.1365-2125.1997.t01-1-00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]