Abstract
Background and Purpose
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily attacks the respiratory system, but there are also several reports of the involvement of the central nervous system, with one of the manifestations being encephalopathy. The relatively new emergence of COVID-19 means that few studies have investigated the clinical profile of encephalopathy associated with this disease. This study aimed to determine the clinical profile, laboratory, and imaging results of encephalopathy associated with COVID-19.
Methods
Three databases, namely PubMed/MEDLINE, Embase, and Scopus, were systematically searched for case reports and case series related to COVID-19-associated encephalopathy published from January 1, 2019 to July 20, 2020.
Results
This review included 24 studies involving 33 cases. The most-reported neurological symptoms were disorientation/confusion (72.72%), decreased consciousness (54.54%), and seizures (27.27%). Laboratory examinations revealed increases in the C-reactive protein level (48.48%), the lactate dehydrogenase level (30.30%), and lymphopenia (27.27%). Brain imaging did not produce any pathological findings in 51.51% of the cases. Electroencephalography showed generalized slowing in 45.45% of the cases. Elevated protein (42.42%) and lymphocytosis (24.24%) were found in the cerebrospinal fluid. Fifteen patients were reportedly discharged from the hospital in a stable condition, while four cases of mortality were recorded.
Conclusions
The clinical, laboratory, and imaging findings in this review support the hypothesis that cerebral damage in COVID-19-associated encephalopathy is caused by cytokine-immune-mediated inflammation rather than by direct invasion.
Keywords: COVID-19, encephalopathy, encephalitis, neuro immune, nerve inflammation, SARS-CoV-2
INTRODUCTION
In January 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified as the cause of an outbreak of pneumonia that developed into respiratory failure in Wuhan, China.1 The spread of SARS-CoV-2 subsequently expanded and led to an increase in the number of fatalities, with it being declared a pandemic by the World Health Organization in March 2020.2 As of July 2020, there were 15 million confirmed cases and more than 600,000 deaths.3
SARS-CoV-2 commonly attacks the respiratory system, causing a type of pneumonia called coronavirus disease 2019 (COVID-19), which has the initial symptoms of upper respiratory tract infection that rapidly develops into respiratory failure.4,5 While respiratory disorder is the main clinical manifestation of COVID-19, some studies have found that most patients also experience neurological symptoms.6,7 Helms et al.7 found neurological symptoms in 84% of the patients who entered an intensive care unit (ICU) without sedation and neuromuscular blockers, with symptoms of central nervous system (CNS) dysfunction such as agitation (69%) and confusion (65%). A systematic review by Ghannam et al.8 found that 23% of patients also had complications of encephalopathy. The results of both of these previous studies indicated the possibility of CNS involvement in the course of COVID-19, with the presence of SARS-CoV-2 neurotropism.8
The relatively new emergence of COVID-19 means that few studies have investigated the clinical profile of encephalopathy associated with this disease. There have been some reported cases of encephalopathy related to COVID-19, with varying clinical appearance and laboratory and imaging findings. The present study performed a systematic review with the aim of identifying the clinical profile and findings from laboratory and imaging investigations on encephalopathy associated with COVID-19.
METHODS
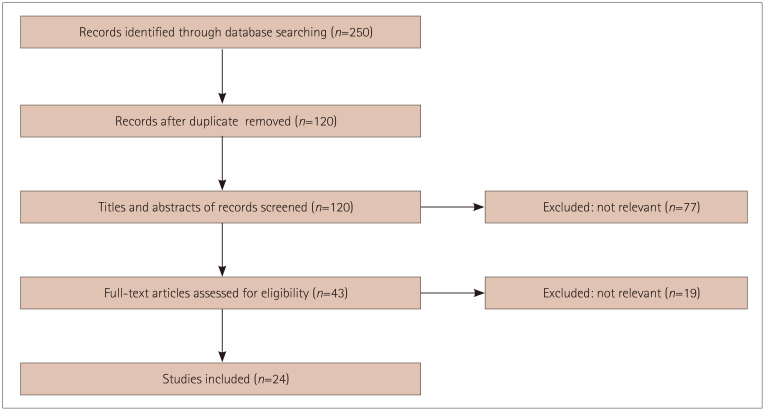
This review conformed to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement (Fig. 1).9
Fig. 1. Flow chart of study selection.
Search strategy
Two authors (V.P. and A.P.) searched three databases: PubMed/MEDLINE, Embase, and Scopus. Eligible titles and abstracts were marked for further review and screening. The articles included were only those published in English from January 1, 2019 to July 20, 2020. The search terms used were “COVID-19,” “SARS-CoV-2,” and “encephalopathy.” If a publication could not be accessed physically or digitally, the authors were contacted by email. The agreement between two of the present authors in selecting case reports was calculated using the Cohen’s kappa (κ) statistic, which was reported with the 95% CI. The risk of bias were calculated using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-1) assessment tool.10
Study selection
The studies included in this review were prospective or retrospective case reports or case series of encephalopathy patients related to COVID-19, while experimental studies were excluded. All potentially relevant articles were screened for eligibility. Publications that were not in English were excluded.
Data extraction
Data were extracted from all eligible reports by two authors (V.P. and A.P.). The extracted data included bibliographic information, demographic information, symptom onset, clinical symptoms (respiratory and neurological), investigation results (laboratory, imaging, and electroencephalography), therapy, and outcome. All disagreements were resolved by discussion between two authors [V.P. and A.P.].) with supervision by a third author (Y.M.T.S.). The collected data were entered into a Microsoft Excel worksheet. General data values were expressed as the percentage and number of patients.
RESULTS
Twenty-four studies involving 33 patients with encephalopathy and COVID-19 were collected from the 3 databases (PubMed/MEDLINE, Scopus, and Embase).11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34 The patients comprised 11 females and 22 males aged 47.90±16.65 years (mean±SD). The onset of respiratory or systemic symptoms occurred 6.0±4.3 days after respiratory/systemic symptoms, while CNS disorders appeared after 11.10±7.85 days; however, in two cases the neurological symptom appeared 1–3 days earlier than the respiratory symptoms. Demographic data are provided in Table 1. The agreement in selecting case reports between the two authors was excellent, as shown by a Cohen’s κ statistic of 0.95 (95% CI=0.93–0.98). Assessments performed using the ROBINS-I assessment tool showed a low risk of bias.
Table 1. Clinical profile and demographic data of the 24 studies including 33 cases.
| Author | Country | Age and sex | Respiratory/ systemic symptom onset | Symptoms | Onset | Method of COVID-19 diagnosis | CSF profile | Brain imaging (CT/MRI) | EEG | Cytokines profile |
|---|---|---|---|---|---|---|---|---|---|---|
| Duong et al.11 | USA | 41 years, female | NR | Seizure | NR | RT-PCR | Lymphocytic pleocytosis, increased RBC | Normal brain CT with contrast | Generalized slowing with no epileptic discharge | NR |
| Bernard-Valnet et al.12 | Switzerland | 64 years, female | 5 days | Tonic-clonic seizures, disorientation, psychosis | Acute (not specified) | RT-PCR | Lymphocytic pleocytosis, increased protein | Normal brain MRI | Nonconvulsive focal status epilepticus | NR |
| 67 years, female | 17 days | Headache, confused | Few hours before admission | RT-PCR | Lymphocytic pleocytosis, increased protein | Normal brain MRI | NR | NR | ||
| Ye et al.13 | China | Male (age NR) | 4 days | Altered consciousness, confusion | Acute (not specified) | RT-PCR | Normal | Normal brain CT | NR | NR |
| McAbee et al.14 | USA | 11 years, male | 2 days | Status epilepticus | Acute (not specified) | RT-PCR | Neutrophilic pleocytosis, increased protein and RBC | Normal brain CT | Frontal intermittent delta activity | NR |
| Andriuta et al.15 | France | Middle-aged female | 7 days | Gait disturbance, hypopallesthesia, bladder and bowel incontinence | Progressively developed on day 16 of admission | RT-PCR | NR | Brain MRI: medial mesencephalic hyperintensity | Normal | NR |
| Middle-aged male | NR | Altered consciousness, flaccid tetraparesis | NR | RT-PCR | NR | Brain MRI: bilateral diffuse white-matter hyperintensities | NR | NR | ||
| Chaumont et al.16 | France | 69 years, male | 7 days | Confusion, headache | 1 day before admission | RT-PCR/CT | Lymphocytic pleocytosis, increased protein | Normal brain MRI | Bilateral slowing | NR |
| Sohal and Mansur17 | USA | 72 years, male | NR | Weakness, lightheadedness, seizure | Day 3 of admission | RT-PCR | NR | Brain CT: chronic microvascular changes | Six left temporal seizures | NR |
| Pilotto et al.18 | Italy | 60 years, male | 2 days | Altered consciousness | 5 days before admission | RT-PCR | Lymphocytic pleocytosis, increased protein | Normal brain CT | Generalized slowing with reduced reactivity to acoustic stimuli | Increased IL-6, IL-8, TNF-α, and β2-microglobulin |
| Al-Olama et al.19 | UAE | 36 years, male | 2 days | Drowsy, headache | 4 days after respiratory symptom | RT-PCR | NR | Brain CT: right frontal intracerebral hematoma with subarachnoid hemorrhage in ipsilateral sylvian fissure and frontal and temporal lobes suggestive of viral encephalitis | NR | NR |
| Moriguchi et al.20 | Japan | 24 years, male | 1 day | Generalized seizure, unconsciousness | 9 days after respiratory symptom | CT/RT-PCR CSF | Increased opening pressure, mononuclear pleocytosis | Right lateral ventriculitis and encephalitis in right mesial lobe and hippocampus | NR | NR |
| Vandervorst et al.21 | Belgium | 29 years, male | 7 days | Generalized weakness, disorientation | 10 days after respiratory symptom | RT-PCR | Normal | Hyperintensity in the left medial temporal lobe with mild gyral expansion | General excess beta rhythm with left temporal delta activity | NR |
| Wong et al.22 | UK | 40 years, male | 10 days | Unsteady gait | Day 3 of admission | RT-PCR | Normal | Suggestive of inflammatory rhombencephalitis/ myelitis | NR | NR |
| Zandifar and Zandifar23 | Iran | 49 years, male | 2 days | Tonic–clonic seizure, altered consciousness | Acute (not specified) | Clinical, imaging, and exclusion of other possibilities | Pleocytosis, increased protein | Diffuse brain edema | NR | NR |
| 39 years, male | 5 days | Tonic seizure, altered consciousness, disorientation | Day 3 of admission | RT-PCR | NR | NR | NR | NR | ||
| Farhadian et al.24 | USA | 78 years, female | 2 days | Uncontrolled limb movements | 3 days before admission | RT-PCR | Brain MRI: atrophy and patchy periventricular and subcortical white matter hyperintensities | Mild generalized slowing | Increased IL-6, IL-8, and IFN-γ-induced protein 10 | |
| Chalil et al.25 | Canada | 48 years, female | 14 days | Altered consciousness | Day 15 of admission | RT-PCR+CT | Neutrophilic pleocytosis | Brain CT: extensive bilateral parietal and occipital intraparenchymal hemorrhage and interventricualr extension with hydrocephalus | NR | NR |
| Afshar et al.26 | Iran | 39 years, female | 9 days | Altered consciousness, tonic–clonic seizure | 1 day after respiratory symptom | Clinical, imaging, and serology | Normal | Brain MRI: hyperintensities in bilateral thalami, medial temporal lobe, and pons | NR | NR |
| Bodro et al.27 | Spain | 25 years, male | 1 day | Confusion and agitation | 12 hours after respiratory symptom | RT-PCR | Lymphocytic pleocytosis, increased protein | Normal brain CT and MRI | NR | NR |
| 49 years, male | 7 days | Disorientation | Few hours after admission | RT-PCR | Lymphocytic pleocytosis, increased protein | Normal brain CT and MRI | NR | NR | ||
| Abdi et al.28 | Iran | 58 years, male | No complaint | Altered consciousness | 1 month, progressive over 2 days | RT-PCR | Increased glucose | Brain MRI: diffuse confluent white-matter hyperintensities | NR | NR |
| Delamarre et al.29 | France | 51 years, male | 10 days | Altered consciousness | 11 days after respiratory symptom | RT-PCR | Albumin-cytological dissociation | Brain MRI: bilateral hyperintensities in bilateral thalami | Low-voltage symmetrical delta activity | |
| Huang et al.30 | USA | 40 years, female | NR | Syncope and altered mental status | NR | RT-PCR | Normal | NR | NR | NR |
| Khoo et al.31 | UK | 65 years, female | Respiratory symptoms at 2 weeks before admission | Involuntary movements, diplopia, cognitive decline | 7 days before admission | RT-PCR | Normal | Normal brain MRI | Normal | NR |
| Zambreanu et al.32 | UK | 66 years, female | NR | Altered consciousness, confusion | Few hours before admission | RT-PCR | Increased protein | Hyperintensities in mesial temporal lobes and medial thalami | NR | NR |
| Panariello et al.33 | Italy | 23 years, male | NR | Psychosis | 3 days | RT-PCR | Normal | Normal | 6 Hz theta activity | Increased IL-6 |
| Dogan et al.34 | Turkey | 49 years, male | NR | Altered consciousness | NR | RT-PCR | Increased protein | Suggestive of encephalitis | NR | Increased IL-6 |
| 59 years, male | NR | Altered consciousness | NR | RT-PCR | Increased protein | Suggestive of encephalitis | NR | Increased IL-6 | ||
| 59 years, male | NR | Altered consciousness | NR | RT-PCR | Increased protein | Normal | NR | Normal | ||
| 51 years, female | NR | Altered consciousness | NR | RT-PCR | Increased protein | Normal | NR | Normal | ||
| 55 years, male | NR | Altered consciousness | NR | RT-PCR | Increased protein | Normal | NR | Normal | ||
| 22 years, male | NR | Altered consciousness | NR | RT-PCR | Increased protein | Suggestive of encephalitis | NR | Increased IL-6 |
COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; CT, computed tomography; EEG, electroencephalography; IFN-γ, interferon gamma; NR, not reported; RBC, red blood cells; RT-PCR, reverse-transcription PCR.
The most-common respiratory/systemic symptoms (Table 2) were fever (54.54%), fatigue/myalgia (48.48%), cough (42.42%), and shortness of breath (30.30%). The most-prominent symptoms of the CNS were disorientation/confusion (72.72%), loss of consciousness (54.54%), and seizures (27.27%). Physical examinations revealed a extensor plantar response, the meningeal irritation sign, and motor weakness in 18.18%, 12.12%, and 12.12% of the cases, respectively. In laboratory examinations, the most frequently recorded findings were increases in the C-reactive protein (CRP) level (48.48%), the lactate dehydrogenase (LDH) level (30.30%), and lymphopenia (27.27%).
Table 2. Summary of the case reports and case series findings.
| Variable | Cases (n=33) | ||
|---|---|---|---|
| Comorbidities | |||
| HT | 7 (21.21) | ||
| DM | 4 (12.12) | ||
| Obesity | 3 (9.09) | ||
| CAD | 1 (3.03) | ||
| ESKD | 1 (3.03) | ||
| Kidney transplant | 1 (3.03) | ||
| Dyslipidemia | 1 (3.03) | ||
| AD | 1 (3.03) | ||
| OA | 1 (3.03) | ||
| GERD | 1 (3.03) | ||
| Closed-angle glaucoma | 1 (3.03) | ||
| Autism | 1 (3.03) | ||
| Substance abuse | 1 (3.03) | ||
| COVID-19 systemic/respiratory symptoms | |||
| Fever | 18 (54.54) | ||
| Cough | 14 (42.42) | ||
| Nasal congestion | 3 (9.09) | ||
| Sore throat | 1 (3.03) | ||
| Dyspnea | 10 (30.30) | ||
| Fatigue/myalgia | 16 (48.48) | ||
| Headache | 10 (30.30) | ||
| Anosmia | 3 (3.03) | ||
| Ageusia | 2 (6.06) | ||
| Diarrhea | 4 (12.12) | ||
| Neurological symptoms | |||
| Loss of consciousness | 18 (54.54) | ||
| Disorientation/confusion | 24 (72.72) | ||
| Hallucination | 4 (12.12) | ||
| Psychotic | 2 (6.06) | ||
| Stiff neck | 1 (3.03) | ||
| Insomnia | 1 (3.03) | ||
| Aphasia | 2 (6.06) | ||
| Seizure | |||
| Tonic–clonic | 5 (15.15) | ||
| Tonic | 1 (3.03) | ||
| Myoclonic | 1 (3.03) | ||
| Unspecified | 2 (6.06) | ||
| Involuntary movements | 3 (9.09) | ||
| Unsteadiness | 3 (9.09) | ||
| Diplopia | 2 (6.06) | ||
| Dysphagia | 1 (3.03) | ||
| Incontinence bowel/bladder | 1 (3.03) | ||
| Neurological signs | |||
| Meningeal irritation sign | 4 (12.12) | ||
| Pupil anisocoria | 2 (6.06) | ||
| Akinetic mutism | 3 (9.09) | ||
| Motor weakness | |||
| Tetraplegia | 1 (3.03) | ||
| Paraplegia | 1 (3.03) | ||
| Hemiplegia | 2 (6.06) | ||
| Sensory deficit | 2 (6.06) | ||
| Extensor plantar response | 6 (18.18) | ||
| Photophobia | 1 (3.03) | ||
| Visual field defect | 1 (3.03) | ||
| Facial weakness | 1 (3.03) | ||
| Tongue weakness | 1 (3.03) | ||
| Nystagmus | 1 (3.03) | ||
| Loss of brainstem reflexes | 3 (9.09) | ||
| Sensory hemineglect | 1 (3.03) | ||
| Ataxia | 2 (3.03) | ||
| Laboratory findings | |||
| Lymphopenia | 9 (27.27) | ||
| Leukocytosis | 8 (24.24) | ||
| Leukopenia | 3 (9.09) | ||
| Thrombocytosis | 3 (9.09) | ||
| Thrombocytopenia | 3 (9.09) | ||
| Elevated CRP | 16 (48.48) | ||
| Elevated LDH | 10 (30.3) | ||
| Elevated procalcitonin | 3 (9.09) | ||
| Elevated ESR | 1 (3.03) | ||
| Elevated D-dimer | 6 (18.18) | ||
| Elevated CK | 3 (9.09) | ||
| Elevated AST/ALT | 2 (6.06) | ||
| Elevated LDH | 1 (3.03) | ||
| Chest imaging (n=22) | |||
| GGO+consolidation (CT) | 4 (18.18) | ||
| GGO without consolidation (CT) | 12 (54.54) | ||
| Infiltrate/consolidation (X-ray) | 3 (13.63) | ||
| Subpleural condensation (USG) | 1 (4.54) | ||
| Normal | 2 (9.09) | ||
| Brain imaging | |||
| Normal | 17 (51.51) | ||
| Diffuse edema | 1 (3.03) | ||
| Hyperintensities | |||
| Thalamus | 3 (9.09) | ||
| Cerebellar | 2 (6.06) | ||
| Periventricular | 2 (6.06) | ||
| Mesencephalic | 3 (9.09) | ||
| Pons | 3 (9.09) | ||
| White matter | 8 (24.24) | ||
| Temporal lobe | 3 (9.09) | ||
| Hemorrhagic | |||
| Pallidum | 1 (3.03) | ||
| Lobar | 2 (6.06) | ||
| Sulcus (SAH) | 4 (12.12) | ||
| Contrast enhancement | 4 (12.12) | ||
| CSF studies | |||
| Elevated WBC | 9 (27.27) | ||
| Elevated neutrophils | 1 (3.03) | ||
| Lymphocytosis | 8 (24.24) | ||
| Elevated RBC | 5 (15.15) | ||
| Elevated protein | 14 (42.42) | ||
| Decreased glucose | 1 (3.03) | ||
| Elevated pressure | 2 (6.06) | ||
| Antineuronal autoantibodies (n=8) | |||
| Positive | 1 (12.50) | ||
| Negative | 7 (87.50) | ||
| Inflammatory cytokines (n=7) | 7 (100) | ||
| CSF PCR (n=28) | |||
| Positive | 3 (10.71) | ||
| Negative | 25 (89.28) | ||
| EEG (n=11) | |||
| Background slowing | 7 (63.30) | ||
| Focal focus | |||
| Frontal | 1 (9.09) | ||
| Temporal | 2 (18.18) | ||
| Normal | 1 (9.09) | ||
| Outcomes (n=23) | |||
| Discharged | 15 (65.21) | ||
| Death | 4 (17.39) | ||
| Still in treatment | 4 (17.39) | ||
AD, Alzheimer’s disease; ALT, alanine transaminase; AST, aspartate transaminase; CAD, coronary artery disease; CRP, C-reactive protein; CSF, cerebrospinal fluid; CK, creatinine kinase; DM, diabetes mellitus; EEG, electroencephalography; ESKD, end-stage kidney disease; ESR, erythrocyte sedimentation rate; GERD, gastroesophageal reflux disease; GGO, ground-glass opacity; HT, hypertension; LDH, the lactate dehydrogenase; NR, not reported; OA, osteoarthritis; PCR, polymerase chain reaction; SAH, subarachnoid hemorrhage; USG, ultrasonography; WBC, white blood cells
Chest imaging was reported for 22 studies, with ground-glass opacities (72.72%) as the predominant finding, which is a typical finding in COVID-19 cases. Brain imaging did not produce pathological findings in 51.51% of the cases. Hyperintensity in the white matter was found in 24.24% of the cases. Electroencephalography (EEG) was carried out in 11 cases, with the most-common finding of generalized slowing (45.45%). The most-common findings in analyses of the cerebrospinal fluid (CSF) were elevated protein (42.42%) and lymphocytosis (24.24%). Significant increases in proinflammatory cytokines were found in all five studies (100%) that performed these analyses of the CSF: interleukin (IL)-6,18,24,27,33,34 IL-1β,27 IL-8,18,24 and TNF-α (tumor necrosis factor alpha).18 Antineuronal autoantibodies were only found in one (N-methyl-D-aspartate receptor [NMDA-R])33 out of the eight examined cases2,18,27,29,31,32,33 (12.5%). Polymerase chain reaction (PCR) examination of the CSF for SARS-CoV-2 was carried out in 28 cases, with positive results in 3 cases.19,20,30 PCR examinations of the CSF for other viruses such as herpes simplex virus (HSV), human herpes virus 6, cytomegalovirus, and varicella-zoster virus produced negative results.
The most commonly used pharmacological agents (Table 3) were hydroxychloroquine (48.48%), azithromycin (27.27%), and favipiravir (24.24%). Intravenous steroids were given in 8 cases (methylprednisolone in 5,18,26,29,31,32 dexamethasone in 2,28,33 and unspecified in 121), while immunotherapies such as plasmapheresis and intravenous immunoglobulin (IVIg) were given to 10 patients (plasmapheresis to 634 and IVIg to 418,26,29,32). Fifteen patients (65.21%) were reportedly discharged from the hospital in a stable condition, while four mortality cases (17.39%)17,23,28 were recorded.
Table 3. Pharmacological agents used in studies.
| Classification | Pharmacological agent | Cases (n=33) |
|---|---|---|
| Antiviral | Acyclovir | 9* (27.27) |
| Atazanavir | 1 (3.03) | |
| Arbidol | 1 (3.03) | |
| Oseltamivir | 1* (3.03) | |
| Darunavir/cobicistat | 1 (3.03) | |
| Lopinavir | 2 (6.06) | |
| Ritonavir | 2 (6.06) | |
| Favipiravir | 8 (24.24) | |
| Antibiotic | Ceftriaxone | 6 (4*; 18.18) |
| Vancomycin | 3 (1*; 9.09) | |
| Levofloxacin | 1 (3.03) | |
| Linezolid | 1 (3.03) | |
| Piperacillin-tazobactam | 1 (3.03) | |
| Amoxicillin | 2 (1*; 6.06) | |
| Ampicillin | 3* (9.09) | |
| Meropenem | 1 (3.03) | |
| Azithromycin | 9 (27.27) | |
| Antiepileptic | Levetiracetam | 6 (18.18) |
| Clonazepam | 2 (12.12) | |
| Valproate | 3 (9.09) | |
| Midazolam | 2 (6.06) | |
| Other | Hydroxychloroquine | 16 (48.48) |
| Mannitol | 1 (3.03) | |
| Norepinephrine | 1 (3.03) | |
| Methylprednisolone | 5 (15.15) | |
| Dexamethasone | 2 (6.06) | |
| Nebivolol | 1 (3.03) | |
| Amlodipine | 1 (3.03) | |
| Quetiapine | 2 (6.06) | |
| Aripiprazole | 1 (3.03) | |
| Haloperidol | 2 (6.06) | |
| Promazine | 1 (3.03) | |
| Paracetamol | 1 (3.03) | |
| Tocilizumab | 2 (6.06) | |
| Heparin, protamine sulfate | 1* (3.03) | |
| Vitamins B and C | 1 (3.03) | |
| Plasmapheresis | 6 (18.18) | |
| IVIg | 4 (12.12) |
Data are presented as n (%).
*Administration was stopped before full course completed.
DISCUSSION
Analysis of systematic reviews
The main target of organ damage by SARS-CoV-2 is the respiratory system, but there are several reports of affected patients also experiencing neurological problems ranging from mild manifestations (e.g., headaches and dizziness) to life-threatening complications (e.g., cerebrovascular disorders and encephalitis).6 Helms et al.7 found that agitation (69%) was a common complaint in COVID-19 patients receiving treatment in the ICU.
This review found that the common symptoms in encephalopathy patients were disorientation or confusion (72.72%), decreased consciousness (54.54%), and seizures (27.27%). These symptoms indicate damage in the CNS, especially the cerebral cortex, which is typically found in acute encephalopathy. This result was consistent with the clinical manifestations of encephalopathy, where disorientation was the most-common symptom reported in patients with positive HSV PCR (92%).35 Manifestations of seizures and headaches were found in 27.27% and 30.30% of the cases of COVID-19-associated encephalopathy, while HSV encephalitis seizures were found in 56% of the cases and headaches in 83% of the cases.35 Abnormal behavior that is characteristically found in HSV is rarely found in COVID-19 encephalopathy. The clinical appearance of COVID-19-associated encephalopathy was similar to that of encephalopathy associated with MERS-CoV-2 (Middle East respiratory syndrome coronavirus 2), with symptoms of upper respiratory tract infection (fever, cough, and fatigue), a decrease in mental status, and rapid respiratory failure.36 The meningeal irritation sign was only found in four cases (12.12%), which indicated that the pathological process was more dominant in the cortex than in the meninges. This also indicated that the involvement of the meninges in COVID-19-associated encephalopathy was less common than that of HSV, since the meningeal irritation sign appeared in 29% of HSV cases.35 However, these results can also be attributed to the loss of meningeal irritation in severe states of consciousness. CSF examinations predominantly showed elevated protein and pleocytosis, suggesting an inflammatory process in the brain that could be caused by an infection such as viral encephalitis, or an autoimmune condition such as limbic encephalitis or Guillain-Barre syndrome (GBS).
The laboratory results showed that the inflammatory markers CRP and LDH as well as lymphopenia were increased in 48.48%, 30.30%, and 27.27% of the cases, respectively. These proportions are higher than those found in the general systematic review of COVID-19 studies conducted by Rodriguez-Morales et al.,37 where increases in CRP and LDH were found in 22.2% and 6.3% of the cases, respectively. These differences are attributable to frequent and severe inflammatory reactions in COVID-19-associated encephalopathy. Wang38 found that CRP levels were significantly higher in patients with severe symptoms than in those with moderate or mild symptoms (54.15 mg/dL vs. 16.76 mg/dL vs. 1.52 mg/dL, p<0.05). The presence of lymphopenia indicated the involvement of T cells, which caused the depletion of CD4 and CD8 cells.37,39 Lymphopenia can occur due to lymphocyte sequestration in specific target organs such as the lungs, gastrointestinal tract, and lymphoid tissue via the activation of angiotensin-converting enzyme 2 (ACE2) receptors by SARS-CoV-2. Other hypotheses are that the phenotype and mechanism of SARS-CoV-2 are similar to those of acute respiratory syndrome coronavirus (SARS-CoV), including the tendency for direct bone-marrow suppression, the immune-mediated destruction of lymphocytes,40,41,42,43 and lymphopenia manifestation.40
Brain imaging studies did not show significant pathological features in 51.51% of the cases. The most-common pathological finding was diffuse hyperintensity in T2-weighted/fluid-attenuated inversion recovery imaging, most frequently in the white matter (24.24%). These findings were consistent with those of the systematic review conducted by Katal et al.,44 where the proportion of normal magnetic resonance imaging (MRI) images was most frequently found (41%) in COVID-19 patients. Therefore, the neuroimaging results for encephalopathy associated with COVID-19 in the present review resembled those of encephalopathy/encephalitis found in the previous coronavirus outbreak. Two cases of SARS-CoV accompanied by severe neurological symptoms in the form of decreased consciousness and seizures had normal neuroimaging results.44,45 Arabi et al.36 reported on three Middle Eastern respiratory syndrome coronavirus (MERS-CoV) patients, whose MRI evaluations revealed that hyperintense lesions in T2-weighted images were spread widely and bilaterally in the white matter and subcortical areas, frontal lobes, temporal, parietal, and basal ganglia, as well as in the corpus callosum. These neuroimaging findings suggest similarities in the pathomechanism of CNS involvement in SARS-CoV-2, SARS-CoV,46 and MERS-CoV.
EEG examinations were performed in 11 cases, with the results showing nonspecific generalized slowing in 7 cases (63.30%) and epileptiform foci in 3 cases (27.27%): 2 in the temporal lobe and 1 in the frontal lobe. This was consistent with Canham et al.47 reporting that the predominant EEG feature in patients with severe COVID-19 was generalized slowing. These findings indicate that COVID-19 exerts diffuse and widespread effects in the CNS, in contrast to encephalitis caused by HSV, where atypical 2–3 Hz periodic lateralized epileptiform discharges originate from the temporal lobes.48 The CSF analysis showed increased levels of protein (42.42%), white blood cells (27.27%), and lymphocytes (24.24%). The negative results from the PCR analysis of the CSF for SARS-CoV-2 argue against direct invasion of the virus as an underlying mechanism of COVID-19 encephalopathy.
There is a large variety of pharmacological agents administered due to the dynamic changes in COVID-19 guidelines and protocols at each center. Acyclovir and ceftriaxone were generally given as empirical therapy, and stopped when there was no evidence of a bacterial infection or HSV. There was an increase in proinflammatory cytokines in the CSF,18,24,27,33,34 indicating a possible role of intravenous steroids and immunotherapy (IVIg and plasmapheresis) in the management of encephalopathy associated with COVID-19. Methylprednisolone and IVIg therapy given in the four cases of COVID-19-associated encephalopathy18,26,29,32 produced positive responses, with three patients discharged in a stable conditions18,29,32 and the fourth still receiving care but with significant improvement.26 Dogan et al.34 reported that plasmapheresis therapy produced dramatic improvements in both clinical and laboratory findings. This positive result supported the theory of a cytokine-mediated hyperinflammatory response as the basis for the pathomechanism of COVID-19-associated encephalopathy.18
Cytokine-immune-mediated inflammation as the underlying pathomechanism in SARS-CoV-2-associated inflammation
While the mechanism of encephalopathy in COVID-19 remains unclear, previous studies have indicated the presence of neurotropism of SARS-CoV-2 that allows it to invade the CNS. There are two pathways that allow this invasion: 1) through the systemic circulation and 2) through the cribriform plate of ethmoid bone.49 SARS-CoV-2 binds with the ACE2 receptor via spike protein S1, allowing the attachment of virions to cell membranes.49,50 The systemic dissemination results from the attachment of SARS-CoV-2 to the ACE2 receptor in the capillary endothelium.50 ACE2 expression in glia cells and neurons is the pathway mechanism for cerebral damage.49,50 The occurrence of hyposmia or anosmia due to the spread of the virus in the olfactory bulb via the cribriform plate is an alternative pathway for invading the CNS.49
The findings of the present study do not support the pathomechanism of CNS damage in COVID-19-associated encephalopathy involving direct invasion by the virus. EEG findings indicate diffuse cerebral abnormalities, possibly resulting from severe and extensive inflammation. CSF analyses have shown an inflammatory process (denoting elevated protein) mediated by cytokines, supported by the results of increased proinflammatory cytokines in the CSF. The predominantly negative CSF PCR (89%) against SARS-CoV-2 does not support the hypothesis that direct viral invasion of the brain is the cause of encephalopathy. However, it should be noted that the negative PCR result for SARS-CoV-2 in the CSF does not imply the absence of the virus, because PCR in the CSF has a rather low sensitivity.37 The possibility of an autoimmune mechanism was considered based on several previous studies linking COVID-19 with GBS.51 There were two reported cases with autoimmune features: 1) NMDA-R33,52 and 2) limbic encephalitis, with T2-weighted hyperintense signal abnormalities in the limbic lobes, bilateral medial thalamus, and frontal white matter.32 The positive response exhibited by COVID-19 patients to intravenous steroid therapy and immunotherapy (IVIg and plasmapheresis) commonly used in autoimmune conditions such as myasthenia gravis and GBS suggest that an immune process plays a role in the occurrence of encephalitis.53
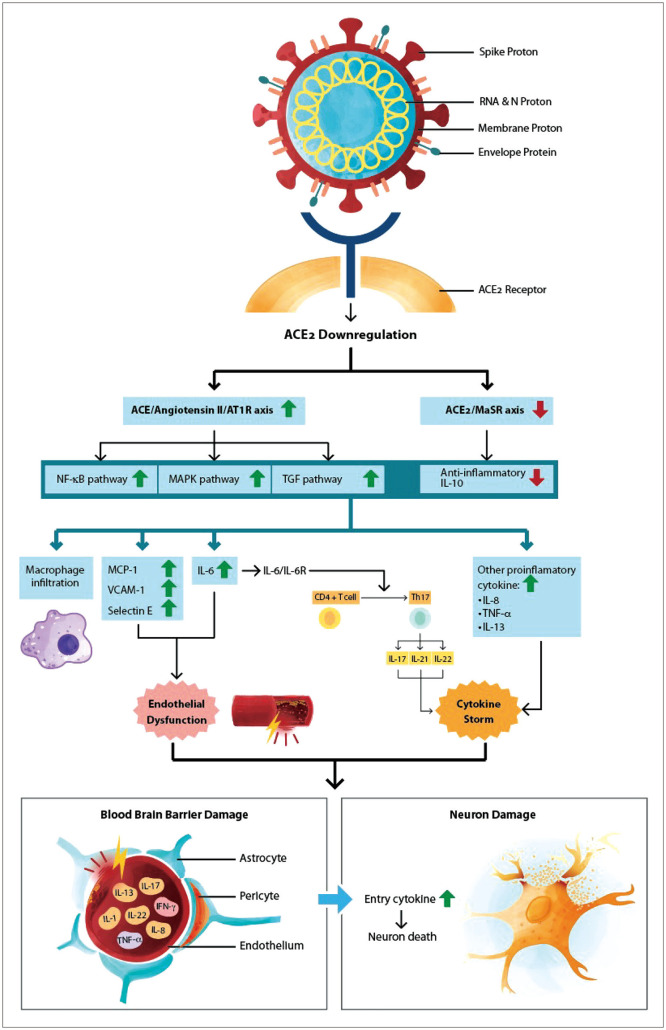
Fig. 2 illustrates the pathomechanism of immune-mediated cerebral damage in COVID-19-associated encephalopathy based on the theories from previous studies.54,55,56 The binding of SARS-CoV-2 to ACE2 receptor via spike protein causes ACE2 downregulation,50,54 followed by an increase in the ACE/angiotensin II/AT1R axis and a decrease in the MaSR (ACE2/Mas receptor) axis.54 These processes consequently result in the activation of the NF-κB (nuclear factor kappa B) and MAPK (mitogen-activated protein kinase) pathways, upregulation of TGF-β (tumor growth factor beta), and downregulation of the anti-inflammatory cytokine IL-10.54,55 These excitations of proinflammatory pathways increase the levels of MCP-1 (monocyte chemotactic protein 1), VCAM-1 (vascular cell adhesion molecule 1), selectin-E, and IL-6.55 IL-6 was found to be the core part of the cytokine storm.54,55,56 IL-6 activates CD4 and T cells into Th17 (T helper 17) cells, which aggravate the proinflammatory cytokines IL-17, IL-21, and IL-22.57 The cytokine storm and the endothelial dysfunction causing damage to the blood–brain barrier make it easier for proinflammatory cytokines to enter the brain parenchyma and also cause further neural damage.56 The pathomechanism underlying COVID-19 encephalopathy has been suggested to be a cytokine-immune-inflammatory process,18,34,56 but further research is needed to explain and confirm this hypothesis.
Fig. 2. Cytokine-immune-mediated inflammation and pathomechanism in COVID-19-associated encephalitis. ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein 1; NF-κB, nuclear factor kappa B; TGF, tumor growth factor; VCAM-1, vascular cell adhesion molecule 1; Th17, T helper 17.
The main limitation in this study was the large variety of case reports and the findings of certain techniques such as EEG and PCR not being reported for all studies. Inflammatory markers and antineuronal autoantibodies were only assessed in a few studies, and so whether the cytokine-immune-mediated inflammatory process is the cause of COVID-19 encephalopathy remains inconclusive.
In conclusion, The clinical, laboratory, and imaging findings in this review support the hypothesis that cerebral damage in COVID-19-associated encephalopathy is caused by cytokine-immune-mediated inflammation rather than by direct invasion. There have been several reports on the benefits of intravenous steroid therapy and immunotherapy in COVID-19-associated encephalopathy. Cytokine-immune-mediated inflammation may cause encephalopathy, but this remains inconclusive due to the inadequate data. Therefore, further examinations and research are needed to confirm this hypothesis.
Footnotes
- Conceptualization: Yusak Mangara Tua Siahaan.
- Data curation: all authors.
- Formal analysis: all authors.
- Funding acquistion: Yusak Mangara Tua Siahaan.
- Investigation: all authors.
- Methodology: all authors.
- Project administration: Vivien Puspitasari, Aristo Pangestu.
- Resources: all authors.
- Software: Aristo Pangestu.
- Supervision: Vivien Puspitasari.
- Validation: Yusak Mangara Tua Siahaan.
- Visualization: all authors.
- Writing—original draft: all authors.
- Writing—review&editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
All data generated and analyzed during this study are included in this published article.
References
- 1.World Health Organization. Novel coronavirus (2019-nCoV) situation report-1 [Internet] Geneva: WHO; 2020. [cited 2020 Jul 22]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4. [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins University and Medicine. Coronavirus resource center [Internet] Baltimore: Johns Hopkins University; 2020. [cited 2020 Jul 22]. Available from: https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 4.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288:192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267:3135–3153. doi: 10.1007/s00415-020-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 10.Jeyaraman MM, Rabbani R, Al-Yousif N, Robson RC, Copstein L, Xia J, et al. Inter-rater reliability and concurrent validity of ROBINS-I: protocol for a cross-sectional study. Syst Rev. 2020;9:12. doi: 10.1186/s13643-020-1271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard-Valnet R, Pizzarotti B, Anichini A, Demars Y, Russo E, Schmidhauser M, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;27:e43–e44. doi: 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriuta D, Roger PA, Thibault W, Toublanc B, Sauzay C, Castelain S, et al. COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF. J Neurol. 2020;267:2810–2811. doi: 10.1007/s00415-020-09975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaumont H, Etienne P, Roze E, Couratier C, Roger PM, Lannuzel A. Acute meningoencephalitis in a patient with COVID-19. Rev Neurol (Paris) 2020;176:519–521. doi: 10.1016/j.neurol.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohal S, Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20:e00782. doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88:423–427. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Olama M, Rashid A, Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir (Wien) 2020;162:1495–1499. doi: 10.1007/s00701-020-04402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandervorst F, Guldolf K, Peeters I, Vanderhasselt T, Michiels K, Berends KJ, et al. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip Neurosurg. 2020;22:100821. doi: 10.1016/j.inat.2020.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong PF, Craik S, Newman P, Makan A, Srinivasan K, Crawford E, et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med (Lond) 2020;20:293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zandifar S, Zandifar Z. Acute viral encephalitis associated with SARS-CoV-2. Ann Clin Case Rep. 2020;5:1845 [Google Scholar]
- 24.Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20:248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalil A, Baker CS, Johnston RB, Just C, Debicki DB, Mayich MS, et al. Acute hemorrhagic encephalitis related to COVID-19. Neurol Clin Pract. 2020;11:e147–e151. doi: 10.1212/CPJ.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afshar H, Yassin Z, Kalantari S, Aloosh O, Lotfi T, Moghaddasi M, et al. Evolution and resolution of brain involvement associated with SARS-CoV2 infection: a close clinical-paraclinical follow up study of a case. Mult Scler Relat Disord. 2020;43:102216. doi: 10.1016/j.msard.2020.102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodro M, Compta Y, Llansó L, Esteller D, Doncel-Moriano A, Mesa A, et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7:e821. doi: 10.1212/NXI.0000000000000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdi S, Ghorbani A, Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416:117001. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delamarre L, Gollion C, Grouteau G, Rousset D, Jimena G, Roustan J, et al. COVID-19-associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J Neurol Neurosurg Psychiatry. 2020;91:1004–1006. doi: 10.1136/jnnp-2020-323678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoo A, McLoughlin B, Cheema S, Weil RS, Lambert C, Manji H, et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J Neurol Neurosurg Psychiatry. 2020;91:1013–1014. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- 32.Zambreanu L, Lightbody S, Bhandari M, Hoskote C, Kandil H, Houlihan CF, et al. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J Neurol Neurosurg Psychiatry. 2020;91:1229–1230. doi: 10.1136/jnnp-2020-323839. [DOI] [PubMed] [Google Scholar]
- 33.Panariello A, Bassetti R, Radice A, Rossotti R, Puoti M, Corradin M, et al. Anti-NMDA receptor encephalitis in a psychiatric covid-19 patient: a case report. Brain Behav Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dogan L, Kaya D, Sarikaya T, Zengin R, Dincer A, Akinci IO, et al. Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: case series. Brain Behav Immun. 2020;87:155–158. doi: 10.1016/j.bbi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sili U, Kaya A, Mert A HSV Encephalitis Study Group. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–118. doi: 10.1016/j.jcv.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li T, Qiu Z, Zhang L, Han Y, He W, Liu Z, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004;189:648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Z, Zhao C, Dong Q, Zhuang H, Song S, Peng G, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: a systematic review in 116 patients. J Neuroradiol. 2020;48:43–50. doi: 10.1016/j.neurad.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of A case. Acta Neurol Taiwan. 2006;15:26–28. [PubMed] [Google Scholar]
- 47.Canham LJW, Staniaszek LE, Mortimer AM, Nouri LF, Kane NM. Electroencephalographic (EEG) features of encephalopathy in the setting of Covid-19: a case series. Clin Neurophysiol Pract. 2020;5:199–205. doi: 10.1016/j.cnp.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhuri A, Kennedy PG. Diagnosis and treatment of viral encephalitis. Postgrad Med J. 2002;78:575–583. doi: 10.1136/pmj.78.924.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 50.Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. doi: 10.1016/j.wneu.2020.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27:361–368. doi: 10.1097/WCO.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ropper AH, Samuels MA, Klein JP. Adams and Victor’s principles of neurology. 10th ed. New York: McGraw-Hill; 2014. [Google Scholar]
- 54.Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fotuhi M, Mian A, Meysami S, Raji CA. Neurobiology of COVID-19. J Alzheimers Dis. 2020;76:3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this published article.