Abstract
Background and Purpose
Warburg Micro syndrome (WARBM) is a rare autosomal recessive genetic disease characterized by ocular, neurologic, and endocrine anomalies. WARBM is a phenotypically and genetically heterogeneous syndrome caused by mutations in RAB3GAP1, RAB3GAP2, RAB18, and TBC1D20. Here we present the clinical and genetic characterization of a consanguineous Tunisian family with a WARBM phenotype presenting two pathogenic variations, one of which is on RAB3GAP1.
Methods
We applied whole-exome sequencing (WES) to two affected young males presenting a WARBM-compatible phenotype.
Results
We reveal a new variation in RAB3GAP1 (NM_012233.3: c.297del, p.Gln99fs) and another variation in ABCD1 (NM_000033: c.896A>G, p.His299Arg). Each of these mutations, which in silico predictions concluded as being pathogenic variations, affects a critical protein region. Both affected males presented a WARBM-compatible phenotype, with severe intellectual disability, severe developmental delay, postnatal growth delay, postnatal microcephaly, congenital bilateral cataracts, general hypotonia, and a thin corpus callosum without a splenium. However, intrafamilial clinical heterogeneity was present, since only the oldest child had large ears, microphthalmia, foot deformities, and a genital anomaly, and only the youngest child had microcornea. Despite the mutation identified in ABCD1, our patients did not have any X-linked symptoms of adrenoleukodystrophy disorder that are usually caused by ABCD1 mutations, which prompted our interest in clinical monitoring.
Conclusions
WES analysis of a consanguineous Tunisian family with WARBM revealed a novel variation in RAB3GAP1 (NM_012233.3: c.297del, p.Gln99fs) that is most likely pathogenic and allowed us to confirm the diagnosis of WARBM.
Keywords: Warburg Micro syndrome; RAB3GAP1 protein, human; ABCD1 protein, human; mutation; whole exome sequencing
INTRODUCTION
Warburg Micro syndrome (WARBM), also known as Micro syndrome, is a genetically heterogeneous, autosomal recessive disease. It is extremely rare, but its true incidence is unknown.1 WARBM was first described by Warburg et al.2 in 1993 in a consanguineous Pakistani family with two affected siblings and their cousin. The use of the “Micro” term by Warburg et al.2 was inspired by the morphologic traits of the affected patients: microphthalmia, microcornea, microcephaly, and micrognathia. In that report they distinguished it from similar syndromes such as cerebro-oculo-facio-skeletal syndrome (MIM #214150), Cockayne syndromes A (MIM #216400) and B (MIM #133540), and Martsolf syndrome (MIM #212720).2 All of these diseases are characterized by intellectual disability, postnatal growth deficiency, microcephaly, cataract, contractures, and hypothalamic hypogonadism. In WARBM, intellectual disability is more severe and cranial magnetic resonance imaging (MRI) usually shows cortical dysplasia, in particular hypoplasia or agenesis of the corpus callosum. The congenital bilateral cataracts are not isolated in all of these syndromes, and is associated with microphthalmia, optic atrophy, and microcornea in WARBM.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 WARBM is also characterized by facial dysmorphia, with a hairy forehead, large anteverted ear, broad nasal root, and micrognathia.2,3,4,10,11,13,17,19,20
WARBM is classified into four subtypes based on the mutated gene: WARBM1 (MIM #600118), WARBM2 (MIM #614225), WARBM3 (MIM #614222), and WARBM4 (MIM #615663). These four subtypes are respectively caused by biallelic mutations in RAB3GAP1 (RAB3 GTPase-Activating Protein Catalytic Subunit; MIM *602536; 2q21.3), RAB3GAP2 (RAB3 GTPase-Activating Protein Noncatalytic Subunit; MIM *609275; 1q41), RAB18 (Ras-Associated Protein RAB18; MIM *602207; 10p21.1) and TBC1D20 (TBC1 Domain Family Member 20; MIM *611663; 20p13).1,5,7,9,23,24,25,26 It has thought that WARBM can be caused directly by the loss of function of the RAB18 protein, or indirectly by the loss of function of the RAB3GAP complex, or TBC1D20 protein.14,27
RAB3GAP1 consists of 24 exons and encodes for the catalytic subunit (p130) of the heterodimeric RAB3GAP complex. The noncatalytic subunit (p150) of this complex is encoded by RAB3GAP2.5,28,29,30,31 The RAB3GAP complex is a GTPase-activating protein (GAP) that regulates the Ca2+-mediated exocytosis of neurotransmitters and hormones5 by switching Rat Sarcoma-Associated Binding-Related Protein 3 (RAB3) between the RAB3-GTP “active form” and the RAB3-GDP “inactive form”.28,29,32 Indeed, the RAB3 subfamily of RAB proteins, which are small G proteins belonging to the rat sarcoma superfamily,32 are required for normal eye and brain development. 5 The RAB3GAP complex also functions as a guanine nucleotide exchange factor for RAB18 protein,33 and TBC1D20 protein exhibits modest GAP activity toward RAB18 protein.34
Since there are no hotspot mutations causing WARBM, the genetic analysis of the entire RAB3GAP1 gene as well as all of the other genes is necessary to identify the genetic cause of the syndrome.35 RAB3GAP1 mutations were the first reported and are now the most frequently (40%) reported in WARBM patients.1,3,5,25,36 More than 70 different pathogenic variations, which are commonly homozygous, have been identified in this gene1,3,5,11,17,20,25,27,31,35,36,37,38,39,40,41,42,43,44,45,46,47 (Supplementary Fig. 1 in the online-only Data Supplement).
Here we present the clinical and genetic characterization of a consanguineous Tunisian family with a WARBM phenotype presenting two pathogenic variations, one of which is on RAB3GAP1.
METHODS
This project was approved by our local ethics committee (no. CEBM/45/2021). Informed consent was obtained from the parents for molecular genetic analysis and for publication of the clinical and molecular data.
DNA extraction
Standard protocols were used to extract genomic DNA from peripheral leukocytes of the two patients as well as their healthy brother and parents.
Whole-exome sequencing
Whole-exome sequencing (WES) was performed for the two affected brothers. Exonic DNA libraries were prepared using the Illumina DNA Prep with Enrichment kit (Illumina, San Diego, CA, USA). The prepared libraries were quantified using qPCR according to the Illumina qPCR Quantification Protocol Guide. A standard curve of fluorescence readings and library sample concentrations was generated using Roche’s Rapid library standard quantification solution and calculator (F. Hoffmann-La Roche AG, Basel, Switzerland). The prepared libraries were sequenced on an Illumina NovaSeq 6000 system with 150 paired-end reads.
Exome sequence analysis
The paired-end sequence reads were aligned to the human genome GRCh38.p13 using Bowtie2 algorithm.48 The aligned reads were processed using the Picard SortSam algorithm (http://broadinstitute.github.io/picard/) to create and sort an alignment binary file, the Picard AddOrReplaceReadGroups algorithm to insert size information, and the Picard Mark-Duplicates algorithm to remove PCR duplicates. After duplication removal, the depth of coverage of targeted exome regions was calculated using the Picard BuildBamIndex algorithm. The variations were called using the GATK HaplotypeCaller algorithm, and the variation calling file was filtered using the GATK VariationFiltration algorithm.49 The variations were then annotated using Annovar software.50
Suspected pathogenic variations were identified using VarAFT software.51 We first selected the autosomal recessive and X-linked inheritance modes, and then filtered out synonymous and noncoding variations before selecting variations with a minor allele frequency of ≤0.01 in the dbSNP150, Exome Aggregation Consortium, gnomAD, 1000 Genomes Project, KaViar (Known Variations Project), and HRC (Haplotype Reference Consortium) databases, as well as in our local database. The pathogenicity of variations was analyzed using the following in silico tools: Provean,52 PolyPhen-2,53 MutationTaster,54 UMD-Predictor,55 SNPs&GO,56 PredictSNP,57 CADD,58 DANN,59 FATHMM,60 and GWAVA61 for nonsynonymous amino acid changes; MutationTaster for stop codons and in-frame insertions/deletions in coding regions; and Human Splicing Finder62 and ESEfinder (version 3.0)63 for splice-site variations. Pathogenic variations were retained if at least one in silico tool predicted them as pathogenic.
We interpreted the filtered variations according to the American College of Medical Genetics and Genomics (ACMG) guidelines,64 which classify variations into the following five categories using a criteria-based scoring system: pathogenic, likely pathogenic, uncertain significance, likely benign, or benign.
Suspected mutations were visually inspected using the Integrative Genomics Viewer.65
Sanger sequencing
The sequences of exon 5 of RAB3GAP1 and exon 1 of ABCD1 (ATP-binding cassette D1 subtype) were analyzed using PCR with the primer pairs listed in Supplementary Table 1 (in the online-only Data Supplement). PCR amplicons were then analyzed by direct DNA sequencing. DNA sequencing reactions were performed using the BigDye Terminator cycle sequencing kit (version 3.1, Life Technologies, Thermo Fisher Scientific, Carlsbad, CA, USA) and capillary electrophoresis on the ABI 3500 sequencer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Sequence data were analyzed using Sequencing Analyzed Software (version 6.0; Thermo Fisher Scientific, Waltham, MA, USA) and Applied Biosystems SeqScape (version 3.0) software. The NCBI sequences NM_012233.3 and NM_000033 were used as reference sequences for RAB3GAP1 and ABCD1, respectively.
RESULTS
Clinical features
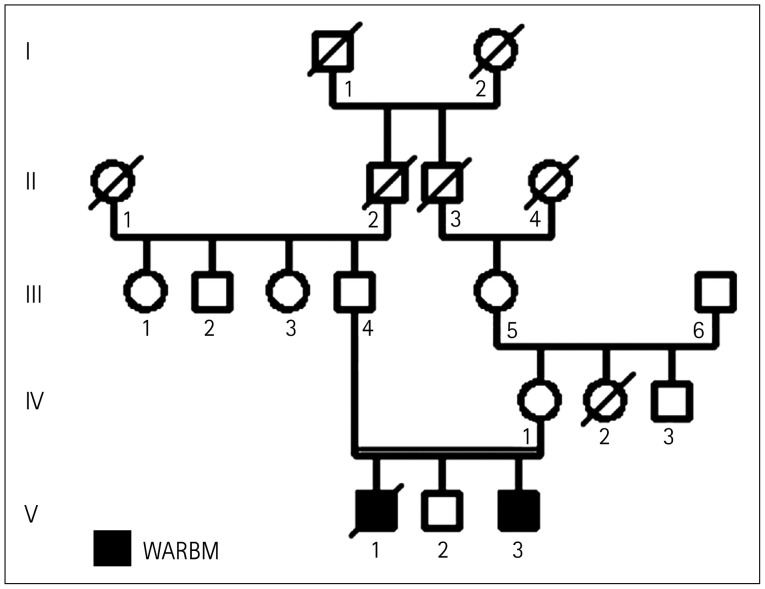
The analyzed family included three children: two affected males (the oldest child [V-1] and the youngest child [V-3]) and a healthy male child (V-2). The parents were second cousins, and both were healthy. There was no relevant abnormality in the family history (Fig. 1).
Fig. 1. Familial pedigree. Black and white symbols are affected and unaffected subjects, respectively. WARBM, Warburg Micro syndrome.
The affected brothers (V-1 and V-3) were referred to the Department of Congenital and Hereditary Diseases at Charles Nicolle Hospital for multiple congenital anomalies. They had been born via spontaneous vaginal deliveries at term after uncomplicated pregnancies. The neonates had normal birth-weights, heights, and head circumferences.
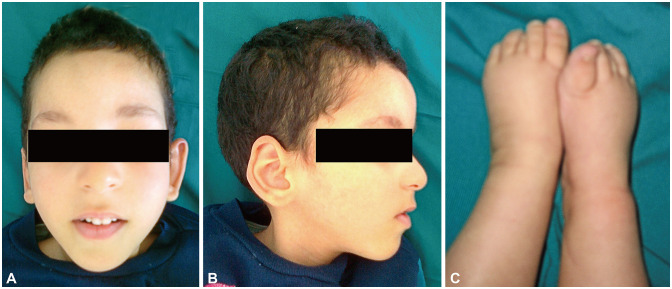
The oldest child (V-1) died at 10 years following a lung infection. He had a severe intellectual disability, and was unable to sit unaided, walk, or speak. His congenital bilateral cataracts were operated on at 4 months old. A physical examination performed at the age of 5 years revealed height, weight, and head circumference at -3.5 SDs, -5.1 SDs, and -5.4 SDs, respectively, as well as facial dysmorphia, with an elongated face, rarefied eyebrows, microphthalmia, beaked nose, small mouth, high arched palate, and protruding large ears. Moreover, he had hypoplastic scrotum, overlapping toes, and general hypotonia (Table 1 and Fig. 2).
Table 1. WARBM clinical features of our patients (V-1 and V-3) and literature data.
| V-1 | V-3 | WARBM1,2,3,4,5,10,11,13,17,19,20,25,27,35,36,38,40,41,42,44,46,47,69,70* | ||
|---|---|---|---|---|
| Age (yr) | Died at 10 years old following a lung infection | 8 | ||
| Growth | ||||
| Normal length and weight at birth | + | + | (15/19) | |
| Postnatal growth delay | + (height=-3.5 SDs/ weight=-5.1 SDs) |
+ (height=-4.7 SDs/ weight=-2.7 SDs) |
(35/55) | |
| Head and neck | ||||
| Postnatal microcephaly | + (head circumference=-5.4 SDs) | + (head circumference=-4 SDs) | (85/91) | |
| Congenital cataract | + | + | (89/93) | |
| Microphthalmia | + | - | (79/88) | |
| Microcornea | - | + | (50/80) | |
| Facial dysmorphism | Elongated face, beaked nose, rarefied eyebrows, protruding large ears, and high arched palate | Elongated face, curved and high forehead, strabismus, tented upper lip, and thin lower lip | Hairy forehead, large anteverted ear, broad nasal root, and micrognathia | |
| Skeletal | ||||
| Foot deformities | + (overlapping toes) | - | (9/38) | |
| Neurologic | ||||
| Intellectual disability | Severe | Severe | Severe to profound (93/93) | |
| Optic atrophy | - | - | (43/59) | |
| Hypotonia | + | + | (50/75) | |
| Spastic diplegia | - | - | (59/66) | |
| Seizure | - | - | (17/54) | |
| Sitting | - | - | (29/33) | |
| Walking | - | - | (50/53) | |
| Speech | - | - | (43/52) | |
| Cranial MRI | ||||
| Abnormal corpus callosum | Thin corpus callosum without a splenium | Thin corpus callosum without a splenium | Hypoplasia or agenesis (80/86) | |
| Cerebral atrophy | – | – | (26/69) | |
| Polymicrogyria | – | – | (33/71) | |
| Pachygyria | – | – | (18/62) | |
| Enlarged sylvian fissures | – | – | (6/65) | |
| Cerebellar hypoplasia | – | – | (14/73) | |
| Demyelination | – | – | (31/73) | |
| Others symptoms | ||||
| Genital abnormalities | + (hypoplastic scrotum) | – | (49/71) | |
*Pairs of values in round bracket indicate (a/b): a. number of cases with this clinical abnormality; b. total number of cases in which this feature was analyzed.
WARBM, Warburg Micro syndrome; +, present; -, absent; SD, standard deviation.
Fig. 2. Dysmorphic features noted in case of the oldest child (V-1). Facial dysmorphism of case V-1: frontal (A) and profile (B). These photographs show an elongated face, a beaked nose, rarefied eyebrows, and protruding large ears. (C) Foot deformities in case V-1. This photograph shows overlapping toes.
The youngest child (V-3) was an 8-year-old male. Like his older brother, he had severe intellectual disability and developmental delay. He had also received an operation for congenital bilateral cataracts, at 1 month old. A physical examination performed at the age of 5 years revealed growth delay (height=-4.7 SDs and weight=-2.7 SDs), microcephaly (head circumference=-4 SDs), general hypotonia, and dysmorphic features such as an elongated face, high forehead, strabismus, microcornea, tented upper lip, and thin lower lip (Table 1).
Cranial magnetic resonance imaging results
Cranial MRI of V-1 and V-3 at the ages of 8 and 7 months, respectively, revealed a thin corpus callosum without a splenium. No other cerebral anomalies were detected in either child.
Chromosome analysis
The two brothers had a normal male karyotype (46, XY).
Molecular findings
The analysis of the WES data of our two patients revealed the homozygous deletion c.297del (p.Gln99fs) in exon 5 of RAB3GAP1 (2q21.3) that has not been reported previously in the literature or in the public databases, and the hemizygous missense mutation c.896A>G (p.His299Arg) in exon 1 of ABCD1 (Xq28).
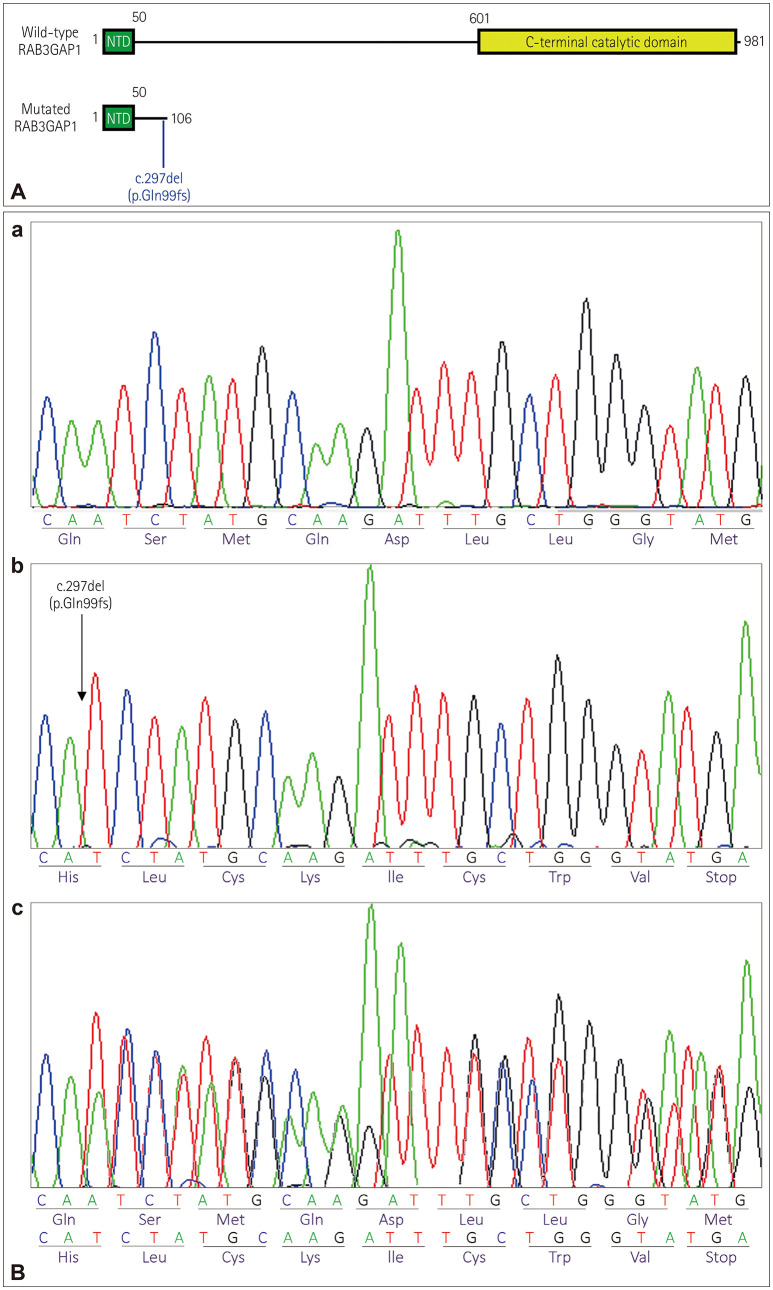
The c.297del (p.Gln99fs) variation resulted in a truncated protein without its C-terminal catalytic domain (Fig. 3A). In silico prediction using MutationTaster concluded that this was a disease-causing variation. Sanger sequencing of exon 5 of RAB3GAP1 confirmed the presence of this variation in a homozygous state in both affected brothers and in a heterozygous state in the parents and their healthy child (Fig. 3B). According to the ACMG guidelines,66 we classified the c.297del variation as pathogenic, since 1) it was predicted to be a null variation in RAB3GAP1, where a loss-of-function variation is a known mechanism of WARBM (PVS1), 2) it is absent from population databases (PM2), 3) it changes the RAB3GAP1 protein length (PM4), 4) it is predicted to be deleterious by in silico prediction tools (PP3), 5) it cosegregated with WARBM in two of the affected family members (PP1), and 6) the phenotype of the patients was highly specific for RAB3GAP1 (PP4). This variation has been submitted to the Clin-Var database (https://www.ncbi.nlm.nih.gov/clinvar/; c.297del variation ID: SCV001519035).
Fig. 3. Illustration of RAB3GAP1 protein (wild type and mutated) and Sanger sequencing validation of the RAB3GAP1 mutation. A: Wild-type and mutated RAB3GAP1 protein. B: Sanger sequencing validation of the RAB3GAP1 mutation. (a) Wild-type electropherogram. (b) Electropherogram of the two affected brothers. (c) Electropherogram of the parents and the unaffected son.
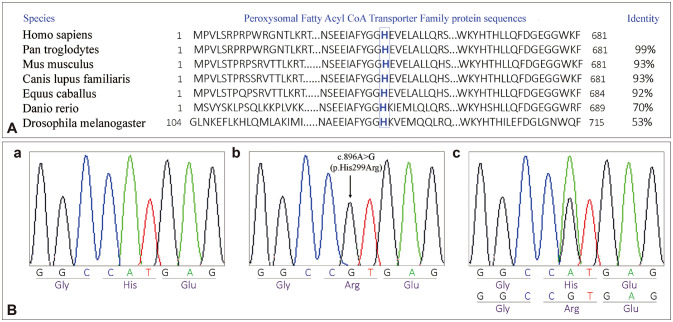
The c.896A>G (p.His299Arg) variation was predicted to be deleterious using in silico prediction tools (Supplementary Table 2 in the online-only Data Supplement). According to the NCBI SmartBLAST (https://blast.ncbi.nlm.nih.gov/smartblast/)67 and NCBI CD-search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)68 tools, the 299th histidine residue is localized in a highly conserved domain (Peroxysomal Fatty Acyl CoA Transporter Family protein 1-681aa) (Fig. 4A). Moreover, Human Splicing Finder and ESEfinder (version 3.0) showed that the c.896A>G variation created a new donor splicing site. The Sanger sequencing of exon 1 of ABCD1 confirmed the presence of this variation in a hemizygous state in the two children (V-1 and V-3) and in a heterozygous state in their mother (Fig. 4B).
Fig. 4. Protein alignment of ABCD1 orthologs and Sanger sequencing validation of the ABCD1 mutation. A: Protein alignment of seven ABCD1 orthologs showing conservation of histidine (in blue) from human to fruit fly. B: Sanger sequencing validation of the ABCD1 mutation. (a) Wild-type electropherogram. (b) Electropherogram of the two affected brothers. (c) Electropherogram of the mother.
DISCUSSION
WARBM is a rare autosomal recessive disease1 characterized by ocular, neurologic, and endocrine abnormalities1,2,3,4,5,10,11,13,17,19,20,25,27,35,36,38,40,41,42,44,46,47,69,70. It is a phenotypically and genetically heterogeneous syndrome caused by mutations in RAB3GAP1, RAB3GAP2, RAB18, and TBC1D201,5,7,9,23,24,25,26. In addition to WARBM, loss of function of the RAB3GAP complex is also associated with Martsolf syndrome9,25 that shares many of the characteristics of WARBM, although it is less frequently reported and less severe with only moderate intellectual disability and developmental delay, a long life expectancy, and pronounced cerebral anomalies in only rare cases.6,8,9,10,12,13,15,16,21,22,47 Amorphic mutations of RAB3GAP1 and RAB3GAP2 cause WARBM, while hypomorphic mutations could result in Martsolf syndrome.9 Only one homozygous RAB3GAP1 frameshift mutation was identified in two siblings with a moderate Martsolf phenotype. This mutation occurred so close to the transcriptional start site that translation of an alternative transcript might be initiated from the next in-frame ATG (start codon), resulting in a protein with reduced product level that lacks the first 50 amino acids of its N-terminal domain and has its entire C-terminal catalytic domain.25
Our two patients had a phenotype more compatible with WARBM than Martsolf syndrome, as they presented with severe intellectual disability, severe developmental delay, postnatal growth delay, postnatal microcephaly, congenital bilateral cataracts, general hypotonia, a thin corpus callosum without a splenium, and a loss-of-function frameshift mutation in RAB3GAP1, which results in a truncated protein without its C-terminal catalytic domain.
We noted an intrafamilial clinical heterogeneity, since only the oldest child (V-1) who died at 10 years had large ears, microphthalmia, foot deformities (overlapping toes), and a genital anomaly (hypoplastic scrotum), which are specific clinical features of WARBM. Moreover, microcornia, which is one of the characteristic symptoms of WARBM, was detected only in the youngest child (V-3). Certain WARBM anomalies were absent from our two patients, such as optic atrophy, micrognathia, and spastic diplegia1,2,3,4,5,10,11,13,17,19,20,25,27,35,36,39,40,41,42,44,46,47,69,70 (Table 1 and Fig. 2). In WARBM, the hypoplasia or agenesis of the corpus callosum is often associated with one or more of the following cerebral anomalies: cerebral atrophy, polymicrogyria, pachygyria, enlarged sylvian fissures, cerebellar hypoplasia, and abnormal myelination.1,25 In our patient, cranial MRI showed only a thin corpus callosum without a splenium.
Molecular analysis of the two affected children using WES revealed a new frameshift mutation in RAB3GAP1 (c.297del, p.Gln99fs), which created an early stop codon that resulted in a truncated protein without its C-terminal catalytic domain. Indeed, most RAB3GAP1 mutations are predicted to produce a truncated protein either before or within the C-terminal catalytic domain, resulting in a mutant protein without GAP activity.5,17,71 Inactivation of the RAB3GAP1 GTPase results in the accumulation of unlipidated LC3-I (microtubule-associated protein light chain 3–form I), impaired lipidation of Atg8 (autophagy-related protein 8) family members, and reduced SQSTM1 (sequestosome 1) protein levels, causing a disturbed autophagosome formation that influences the WARBM phenotype.26,72,73 Patients with truncated proteins before the catalytic domain show normal growth, postnatal microcephaly, severe intellectual deficiency and developmental delay, optic atrophy, spastic cerebral palsy, hypotonia, cerebral anomalies (hypogenesis of the corpus callosum with agenesis of the splenium, and decreased myelination of white matter), ocular anomalies (congenital cataracts, microphthalmia, and microcornia), and genital anomalies (micropenis, bifid scrotum, and cryptorchidism).5,17 These clinical features differ slightly from those found in our patients, confirming the clinical heterogeneity of WARBM.
The exome analysis also revealed another mutation (c.896A>G, p.His299Arg) in ABCD1 (MIM *300371), which was previously described in the ClinVar archive as a variation of uncertain significance (variation ID: VCV000834266) associated with X-linked adrenoleukodystrophy disorder (MIM #300100). This disease, which affects the nervous system and the adrenal glands, can appear at an advanced age.74,75 Since our patients were still young, clinical monitoring was necessary to diagnose this disease.
Footnotes
- Conceptualization: Nesrine Kerkeni, Maher Kharrat, Mediha Trabelsi.
- Formal analysis: Nesrine Kerkeni.
- Investigation: Nesrine Kerkeni, Mediha Trabelsi.
- Methodology: Nesrine Kerkeni, Maher Kharrat, Mediha Trabelsi.
- Project administration: Mediha Trabelsi.
- Resources: Faouzi Maazoul, Hela Boudabous, Ridha M’rad, Mediha Trabelsi.
- Software: Nesrine Kerkeni.
- Supervision: Mediha Trabelsi.
- Validation: Maher Kharrat, Ridha M’rad, Mediha Trabelsi.
- Writing—original draft: Nesrine Kerkeni.
- Writing—review&editing: Mediha Trabelsi.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2022.18.2.214.
Reported RAB3GAP1 mutations in patients with Warburg Micro syndrome.
Primer sequences
Results from in silico prediction tools for the ABCD1 mutation
References
- 1.Morris-Rosendahl DJ, Segel R, Born AP, Conrad C, Loeys B, Brooks SS, et al. New RAB3GAP1 mutations in patients with Warburg micro Syndrome from different ethnic backgrounds and a possible founder effect in the Danish. Eur J Hum Genet. 2010;18:1100–1106. doi: 10.1038/ejhg.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg M, Sjö O, Fledelius HC, Pedersen SA. Autosomal recessive microcephaly, microcornea, congenital cataract, mental retardation, optic atrophy, and hypogenitalism. Micro syndrome. Am J Dis Child. 1993;147:1309–1312. doi: 10.1001/archpedi.1993.02160360051017. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Salam GM, Hassan NA, Kayed HF, Aligianis IA. Phenotypic variability in micro syndrome: report of new cases. Genet Couns. 2007;18:423–435. [PubMed] [Google Scholar]
- 4.Ainsworth JR, Morton JE, Good P, Woods CG, George ND, Shield JP, et al. Micro syndrome in Muslim Pakistan children. Ophthalmology. 2001;108:491–497. doi: 10.1016/s0161-6420(00)00540-6. [DOI] [PubMed] [Google Scholar]
- 5.Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, et al. Mutations of the catalytic subunit of RAB3GAP cause Warburg micro syndrome. Nat Genet. 2005;37:221–223. doi: 10.1038/ng1517. [DOI] [PubMed] [Google Scholar]
- 6.Aligianis IA, Morgan NV, Mione M, Johnson CA, Rosser E, Hennekam RC, et al. Mutation in Rab3 GTPase-activating protein (RAB3GAP) noncatalytic subunit in a kindred with Martsolf syndrome. Am J Hum Genet. 2006;78:702–707. doi: 10.1086/502681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bem D, Yoshimura S, Nunes-Bastos R, Bond FC, Kurian MA, Rahman F, et al. Loss-of-function mutations in RAB18 cause Warburg micro syndrome. Am J Hum Genet. 2011;88:499–507. doi: 10.1016/j.ajhg.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bora E, Cankaya T, Alpman A, Karaca E, Cogulu O, Tekgul H, et al. A new case of Martsolf syndrome. Genet Couns. 2007;18:71–75. [PubMed] [Google Scholar]
- 9.Borck G, Wunram H, Steiert A, Volk AE, Körber F, Roters S, et al. A homozygous RAB3GAP2 mutation causes Warburg micro syndrome. Hum Genet. 2011;129:45–50. doi: 10.1007/s00439-010-0896-2. [DOI] [PubMed] [Google Scholar]
- 10.Derbent M, Agras PI, Gedik S, Oto S, Alehan F, Saatçi U. Congenital cataract, microphthalmia, hypoplasia of corpus callosum and hypogenitalism: report and review of micro syndrome. Am J Med Genet A. 2004;128A:232–234. doi: 10.1002/ajmg.a.30109. [DOI] [PubMed] [Google Scholar]
- 11.Dursun F, Güven A, Morris-Rosendahl D. Warburg micro syndrome. J Pediatr Endocrinol Metab. 2012;25:379–382. doi: 10.1515/jpem-2011-0459. [DOI] [PubMed] [Google Scholar]
- 12.Ehara H, Utsunomiya Y, Ieshima A, Maegaki Y, Nishimura G, Takeshita K, et al. Martsolf syndrome in Japanese siblings. Am J Med Genet A. 2007;143A:973–978. doi: 10.1002/ajmg.a.31626. [DOI] [PubMed] [Google Scholar]
- 13.Graham JM, Jr, Hennekam R, Dobyns WB, Roeder E, Busch D. MICRO syndrome: an entity distinct from COFS syndrome. Am J Med Genet A. 2004;128A:235–245. doi: 10.1002/ajmg.a.30060. [DOI] [PubMed] [Google Scholar]
- 14.Handley M, Sheridan E. In: GeneReviews®. Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. Seattle: University of Washington; 2018. RAB18 deficiency. [PubMed] [Google Scholar]
- 15.Harbord MG, Baraitser M, Wilson J. Microcephaly, mental retardation, cataracts, and hypogonadism in sibs: Martsolf’s syndrome. J Med Genet. 1989;26:397–400. doi: 10.1136/jmg.26.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennekam RC, van de Meeberg AG, van Doorne JM, Dijkstra PF, Bijlsma JB. Martsolf syndrome in a brother and sister: clinical features and pattern of inheritance. Eur J Pediatr. 1988;147:539–543. doi: 10.1007/BF00441986. [DOI] [PubMed] [Google Scholar]
- 17.Koparir A, Karatas OF, Yilmaz SS, Suer I, Ozer B, Yuceturk B, et al. Revealing the functions of novel mutations in RAB3GAP1 in Martsolf and Warburg micro syndromes. Am J Med Genet A. 2019;179:579–587. doi: 10.1002/ajmg.a.61065. [DOI] [PubMed] [Google Scholar]
- 18.Martsolf JT, Hunter AG, Haworth JC. Severe mental retardation, cataracts, short stature, and primary hypogonadism in two brothers. Am J Med Genet. 1978;1:291–299. doi: 10.1002/ajmg.1320010305. [DOI] [PubMed] [Google Scholar]
- 19.Mégarbané A, Choueiri R, Bleik J, Mezzina M, Caillaud C. Microcephaly, microphthalmia, congenital cataract, optic atrophy, short stature, hypotonia, severe psychomotor retardation, and cerebral malformations: a second family with micro syndrome or a new syndrome? J Med Genet. 1999;36:637–640. [PMC free article] [PubMed] [Google Scholar]
- 20.Picker-Minh S, Busche A, Hartmann B, Spors B, Klopocki E, Hübner C, et al. Large homozygous RAB3GAP1 gene microdeletion causes Warburg micro syndrome 1. Orphanet J Rare Dis. 2014;9:113. doi: 10.1186/s13023-014-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez JM, Barreiro C, Freilij H. Two brothers with Martsolf’s syndrome. J Med Genet. 1985;22:308–310. doi: 10.1136/jmg.22.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strisciuglio P, Costabile M, Esposito M, Di Maio S. Martsolf’s syndrome in a non-Jewish boy. J Med Genet. 1988;25:267–269. doi: 10.1136/jmg.25.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpanini SM, McKie L, Thomson D, Wright AK, Gordon SL, Roche SL, et al. A novel mouse model of Warburg micro syndrome reveals roles for RAB18 in eye development and organisation of the neuronal cytoskeleton. Dis Model Mech. 2014;7:711–722. doi: 10.1242/dmm.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbeel L, Freson K. Rab proteins and Rab-associated proteins: major actors in the mechanism of protein-trafficking disorders. Eur J Pediatr. 2008;167:723–729. doi: 10.1007/s00431-008-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handley MT, Morris-Rosendahl DJ, Brown S, Macdonald F, Hardy C, Bem D, et al. Mutation spectrum in RAB3GAP1, RAB3GAP2, and RAB18 and genotype-phenotype correlations in Warburg micro syndrome and Martsolf syndrome. Hum Mutat. 2013;34:686–696. doi: 10.1002/humu.22296. [DOI] [PubMed] [Google Scholar]
- 26.Liegel RP, Handley MT, Ronchetti A, Brown S, Langemeyer L, Linford A, et al. Loss-of-function mutations in TBC1D20 cause cataracts and male infertility in blind sterile mice and Warburg micro syndrome in humans. Am J Hum Genet. 2013;93:1001–1014. doi: 10.1016/j.ajhg.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loiudice P, Napoli D, Ragone MC, Nardi M, Casini G. Novel RAB3GAP1 mutations causing Warburg micro syndrome in two Italian sisters. J Pediatr Neurosci. 2017;12:360–362. doi: 10.4103/jpn.JPN_45_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukui K, Sasaki T, Imazumi K, Matsuura Y, Nakanishi H, Takai Y. Isolation and characterization of a GTPase activating protein specific for the Rab3 subfamily of small G proteins. J Biol Chem. 1997;272:4655–4658. doi: 10.1074/jbc.272.8.4655. [DOI] [PubMed] [Google Scholar]
- 29.Nagano F, Sasaki T, Fukui K, Asakura T, Imazumi K, Takai Y. Molecular cloning and characterization of the noncatalytic subunit of the Rab3 subfamily-specific GTPase-activating protein. J Biol Chem. 1998;273:24781–24785. doi: 10.1074/jbc.273.38.24781. [DOI] [PubMed] [Google Scholar]
- 30.Sakane A, Manabe S, Ishizaki H, Tanaka-Okamoto M, Kiyokage E, Toida K, et al. Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3. Proc Natl Acad Sci U S A. 2006;103:10029–10034. doi: 10.1073/pnas.0600304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trkova M, Hynek M, Dudakova L, Becvarova V, Hlozanek M, Raskova D, et al. Early detection of bilateral cataracts in utero may represent a manifestation of severe congenital disease. Am J Med Genet A. 2016;170:1843–1848. doi: 10.1002/ajmg.a.37685. [DOI] [PubMed] [Google Scholar]
- 32.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerondopoulos A, Bastos RN, Yoshimura S, Anderson R, Carpanini S, Aligianis I, et al. Rab18 and a Rab18 GEF complex are required for normal ER structure. J Cell Biol. 2014;205:707–720. doi: 10.1083/jcb.201403026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handley MT, Carpanini SM, Mali GR, Sidjanin DJ, Aligianis IA, Jackson IJ, et al. Warburg micro syndrome is caused by RAB18 deficiency or dysregulation. Open Biol. 2015;5:150047. doi: 10.1098/rsob.150047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asahina M, Endoh Y, Matsubayashi T, Fukuda T, Ogata T. Novel RAB3GAP1 compound heterozygous mutations in Japanese siblings with Warburg micro syndrome. Brain Dev. 2016;38:337–340. doi: 10.1016/j.braindev.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Hamid MS, Abdel-Ghafar SF, Ismail SR, Desouky LM, Issa MY, Effat LK, et al. Micro and Martsolf syndromes in 34 new patients: refining the phenotypic spectrum and further molecular insights. Clin Genet. 2020;98:445–456. doi: 10.1111/cge.13825. [DOI] [PubMed] [Google Scholar]
- 37.Gillespie RL, O'Sullivan J, Ashworth J, Bhaskar S, Williams S, Biswas S, et al. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology. 2014;121:2124–2137.E2. doi: 10.1016/j.ophtha.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Gupta MD, Girish MP, Shah D, Rain M, Mehta V, Tyagi S, et al. Biochemical and genetic role of apelin in essential hypertension and acute coronary syndrome. Int J Cardiol. 2016;223:374–378. doi: 10.1016/j.ijcard.2016.07.242. [DOI] [PubMed] [Google Scholar]
- 39.Gupta N, Thakur S, Handley MT, Bokaria R, Saxena R, Kohli S. A genetic syndrome that mimics congenital TORCH infection. Genetic Clinics. 2016;9:2–8. [Google Scholar]
- 40.Imagawa E, Fukai R, Behnam M, Goyal M, Nouri N, Nakashima M, et al. Two novel homozygous RAB3GAP1 mutations cause Warburg micro syndrome. Hum Genome Var. 2015;2:15034. doi: 10.1038/hgv.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabzińska D, Mierzewska H, Senderek J, Kochański A. Warburg micro syndrome type 1 associated with peripheral neuropathy and cardiomyopathy. Folia Neuropathol. 2016;54:273–281. doi: 10.5114/fn.2016.62537. [DOI] [PubMed] [Google Scholar]
- 42.Rump P, Jazayeri O, van Dijk-Bos KK, Johansson LF, van Essen AJ, Verheij JB, et al. Whole-exome sequencing is a powerful approach for establishing the etiological diagnosis in patients with intellectual disability and microcephaly. BMC Med Genomics. 2016;9:7. doi: 10.1186/s12920-016-0167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawyer SL, Schwartzentruber J, Beaulieu CL, Dyment D, Smith A, Warman Chardon J, et al. Exome sequencing as a diagnostic tool for pediatric-onset ataxia. Hum Mutat. 2014;35:45–49. doi: 10.1002/humu.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivastava P, Saxena D, Joshi S, Phadke SR. Consanguinity as an adjunct diagnostic tool. Indian J Pediatr. 2016;83:258–260. doi: 10.1007/s12098-015-1764-9. [DOI] [PubMed] [Google Scholar]
- 45.Tasdemir S, Sahin I, Morris-Rosendahl DJ, Marzioglu E, Cayir A, Yuce I, et al. Recurrent RAB3GAP1 mutations in the Turkish population. Genet Couns. 2015;26:415–423. [PubMed] [Google Scholar]
- 46.Tenawi S, Al Khudari R, Alasmar D. Novel mutation in the RAB3GAP1 gene, the first diagnosed Warburg micro syndrome case in Syria. Oxf Med Case Reports. 2020;2020:omaa031. doi: 10.1093/omcr/omaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yüksel A, Yesil G, Aras C, Seven M. Warburg micro syndrome in a Turkish boy. Clin Dysmorphol. 2007;16:89–93. doi: 10.1097/MCD.0b013e328054c404. [DOI] [PubMed] [Google Scholar]
- 48.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desvignes JP, Bartoli M, Delague V, Krahn M, Miltgen M, Béroud C, et al. VarAFT: a variant annotation and filtration system for human next generation sequencing data. Nucleic Acids Res. 2018;46:W545–W553. doi: 10.1093/nar/gky471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 55.Salgado D, Desvignes JP, Rai G, Blanchard A, Miltgen M, Pinard A, et al. UMD-predictor: a high-throughput sequencing compliant system for pathogenicity prediction of any human cDNA substitution. Hum Mutat. 2016;37:439–446. doi: 10.1002/humu.22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabrese R, Capriotti E, Fariselli P, Martelli PL, Casadio R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum Mutat. 2009;30:1237–1244. doi: 10.1002/humu.21047. [DOI] [PubMed] [Google Scholar]
- 57.Bendl J, Stourac J, Salanda O, Pavelka A, Wieben ED, Zendulka J, et al. PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput Biol. 2014;10:e1003440. doi: 10.1371/journal.pcbi.1003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quang D, Chen Y, Xie X. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31:761–763. doi: 10.1093/bioinformatics/btu703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ, et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat. 2013;34:57–65. doi: 10.1002/humu.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shihab HA, Rogers MF, Gough J, Mort M, Cooper DN, Day IN, et al. An integrative approach to predicting the functional effects of non-coding and coding sequence variation. Bioinformatics. 2015;31:1536–1543. doi: 10.1093/bioinformatics/btv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, et al. Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018;39:1517–1524. doi: 10.1002/humu.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44:D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, et al. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodríguez Criado G, Rufo M, Gómez de Terreros I. A second family with micro syndrome. Clin Dysmorphol. 1999;8:241–245. [PubMed] [Google Scholar]
- 70.Yildirim MS, Zamani AG, Bozkurt B. Warburg micro syndrome in two children from a highly inbred Turkish family. Genet Couns. 2012;23:169–174. [PubMed] [Google Scholar]
- 71.Clabecq A, Henry JP, Darchen F. Biochemical characterization of Rab3-GTPase-activating protein reveals a mechanism similar to that of Ras-GAP. J Biol Chem. 2000;275:31786–31791. doi: 10.1074/jbc.M003705200. [DOI] [PubMed] [Google Scholar]
- 72.Sou YS, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T, et al. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell. 2008;19:4762–4775. doi: 10.1091/mbc.E08-03-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spang N, Feldmann A, Huesmann H, Bekbulat F, Schmitt V, Hiebel C, et al. RAB3GAP1 and RAB3GAP2 modulate basal and rapamycin-induced autophagy. Autophagy. 2014;10:2297–2309. doi: 10.4161/15548627.2014.994359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engelen M, Kemp S, de Visser M, van Geel BM, Wanders RJ, Aubourg P, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis. 2012;7:51. doi: 10.1186/1750-1172-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turk BR, Theda C, Fatemi A, Moser AB. X-linked adrenoleukodystrophy: pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int J Dev Neurosci. 2020;80:52–72. doi: 10.1002/jdn.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reported RAB3GAP1 mutations in patients with Warburg Micro syndrome.
Primer sequences
Results from in silico prediction tools for the ABCD1 mutation
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.