Abstract
Interstitial ciprofloxacin concentrations in soft tissues were measured by microdialysis following intravenous administration of 200 mg to each of eight healthy volunteers. Interstitial ciprofloxacin concentrations were significantly lower than corresponding total serum drug concentrations; the interstitium-to-serum concentration ratios ranged from 0.55 to 0.73. An in vitro simulation based on interstitial pharmacokinetics showed a substantially lower antimicrobial activity than did the simulation based on serum pharmacokinetics. Thus, ciprofloxacin concentrations at the site of effect may be subinhibitory although effective concentrations are attained in serum.
Antibiotic dosing is usually tailored to obtain serum drug concentrations that exceed the minimum concentration required to inhibit bacterial growth (i.e., MIC) for the infecting microorganism. However, as the majority of infections are located in peripheral tissues, it is considered more appropriate to study the process of drug penetration of the target site, rather than merely assessing serum pharmacokinetics (7).
One antibiotic frequently employed in peripheral compartment infections is ciprofloxacin. The rationale for administering ciprofloxacin to individuals with these conditions relies on biopsy studies describing a favorable tissue penetration of ciprofloxacin (1, 13). Measurement of total tissue drug concentrations, however, may be misleading since only the free, interstitial drug concentration exerts antibacterial activity in most cases (12, 14). Unbound-drug concentrations may be substantially lower than total tissue drug concentrations and may even be severalfold below corresponding concentrations in serum (15). In light of these considerations, subinhibitory concentrations of ciprofloxacin in the sites of effect may provide an explanation for those cases in which ciprofloxacin failed to eradicate the relevant pathogen despite documented in vitro susceptibility (9).
The present study therefore was undertaken to measure unbound ciprofloxacin concentrations in the interstitial space of skeletal muscle and subcutaneous adipose tissue by means of microdialysis in healthy volunteers (15).
The study was approved by the local ethics committee. All volunteers were given a detailed description of the study, and their written consent was obtained. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline of the European Commission. Eight healthy, drug-free, male volunteers of normal weight (mean ± standard error [SE], 76 ± 4 kg) and ranging from 28 to 37 years old were enrolled in the study.
For the measurement of free interstitial ciprofloxacin concentrations we employed in vivo microdialysis as described previously (15–17). Two microdialysis probes (both CMA 10; CMA, Stockholm, Sweden) were inserted, one into the musculus vastus medialis and one into the subcutaneous adipose layer of the thigh, and perfused with Ringer’s solution at a flow rate of 1.5 μl/min. After a 30-min baseline sampling period, in vivo probe calibration was performed as described previously by a retrodialysis procedure (21) with a perfusion medium containing 30 μg of ciprofloxacin/ml for 30 min (15, 16). Following a 30-min washout period, ciprofloxacin (Ciproxin; Bayer, Leverkusen, Germany) was administered as a single intravenous dose of 200 mg over 10 min.
The unbound ciprofloxacin fraction in serum was obtained by employing Ultrafree-MC Centrifugal Filter Units with a molecular mass cutoff of 10 kDa (Millipore Corporation). One hundred fifty microliters of serum was placed into the Ultrafree-MC Centrifugal Filter Units and centrifuged at 14,000 × g and 37°C for 20 min. Ciprofloxacin concentrations in microdialysates and serum were analyzed by a published high-performance liquid chromatography method (11). In order to generate a pharmacodynamic model which allows for the description of the antibacterial activity of ciprofloxacin in serum and the interstitial-space fluid, the in vivo time-concentration profile of ciprofloxacin was simulated in vitro. For that purpose, Mueller-Hinton broth which was kept in a water bath at 37°C was inoculated with select bacteria at an approximate concentration of 108 CFU/ml. Subsequently, the time-ciprofloxacin concentration profiles obtained in vivo were simulated in vitro by changing ciprofloxacin concentrations in broth as described previously (4, 18). After vortexing of the culture tube, 40-μl samples were withdrawn at select time points and serially diluted with 0.9% sodium chloride. Twenty-microliter samples obtained at each dilution step were then plated onto agar plates and incubated at 37°C for 18 to 26 h. Subsequently, the colonies were counted and back-extrapolated to the original volume.
Data are presented as means ± SEs. Interstitial concentrations were calculated from dialysate concentrations as described previously (15, 16). Individual data sets were fitted by a commercially available computer program (Topfit 2.0; Gustav Fischer, Stuttgart, Germany) according to a two-compartment model for serum values and according to a one-compartment model for tissues as described previously (16). The ratios for the areas under the concentration-time curves (AUCs) AUCmuscle/AUCfree serum and AUCsubcutaneous/AUCfree serum were calculated as a measure of drug penetration into the peripheral compartments.
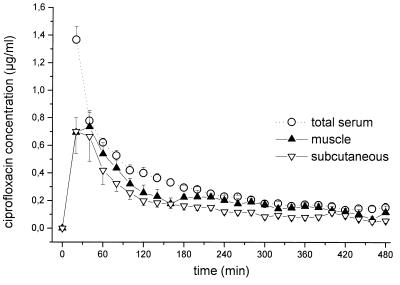
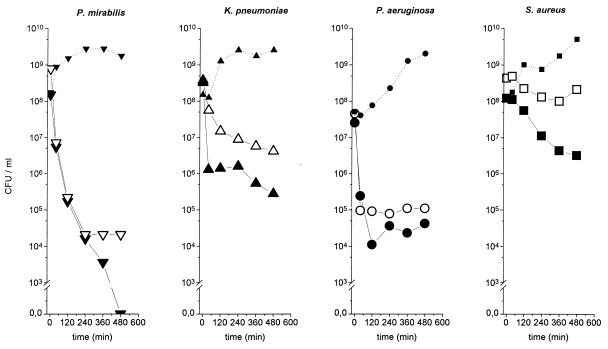
The time-concentration profiles for total ciprofloxacin concentrations in serum and in the interstitial-space fluid of skeletal muscle and subcutaneous adipose tissue are shown in Fig. 1. The maximum concentrations were 1.36 ± 0.09, 0.73 ± 0.80, and 0.86 ± 0.20 μg/ml, and the AUC values were 155.7 ± 9.9, 110.5 ± 16.7, and 85.1 ± 15.2 μg · min · ml−1, respectively. The half-life at β phase was 98 ± 1 min for ciprofloxacin in serum, and the times to maximum concentration of the drug in skeletal muscle and in subcutaneous adipose tissue were 19 ± 3 and 17 ± 5 min, respectively. The mean AUCmuscle/ AUCfree serum ratio was 1.23 ± 0.24, and the mean AUCsubcutaneous/AUCfree serum ratio was 0.89 ± 0.16. Time-kill curves for the in vitro simulation model are depicted in Fig. 2.
FIG. 1.
Time-concentration profiles for drug concentrations in total serum and interstitial space fluids of skeletal muscle and subcutaneous adipose tissue following administration of ciprofloxacin to healthy volunteers (single intravenous dose of 200 mg administered over 10 min; n = 8). Results are presented as means ± SEs. 0 to 10 min, time of administration.
FIG. 2.
Mean time-kill curves for the in vitro simulation model, in which the in vivo time course of ciprofloxacin concentration, obtained by measuring antibiotic concentrations in serum and the interstitial space fluid in the experiments shown in Fig. 1, was simulated in vitro for Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus. Solid symbols indicate time-kill data for serum drug concentrations; open symbols indicate time-kill data for interstitial subcutaneous concentrations; the dotted lines (small solid symbols) are the growth control curves.
The main finding of our study was that interstitial ciprofloxacin concentrations were significantly lower than corresponding total serum drug concentrations: the interstitial fluid/serum concentration ratios reached only about 0.7. Given that ciprofloxacin has a protein binding efficiency of approximately 30% (6), our results are in very good agreement with the notion that only the unbound fraction of the drug exerts antimicrobial activity and may penetrate into the interstitial space (12). This is further corroborated by our finding that the AUCtissue/AUCfree serum ratios were about 1 (range, 0.89 to 1.23). Our finding of substantially lower concentrations at the sites of effect than in serum, however, is in contrast to previous reports describing tissue/serum ciprofloxacin concentration ratios of up to 7 (5). How could this discrepancy be explained? The overestimation of concentrations measured from biopsy specimens is necessarily the result of an admixture of different compartmentalized drug fractions by tissue homogenization. For ciprofloxacin, a drug which is characterized by intracellular accumulation (2), concentrations in tissue homogenates reflect mean concentrations in the intra- and extracellular compartments but provide no information on those in the interstitium (20), since binding to tissue proteins is not accounted for. Total tissue measurements may thus overestimate the concentrations of antibiotics, like ciprofloxacin, that accumulate in the intracellular spaces at the site of effect and may underestimate concentrations at these sites of drugs, like beta-lactams, that equilibrate exclusively with the extracellular space (19). Our in vitro simulation experiments (Fig. 2) further showed that applying serum pharmacokinetics overestimated not only the antibiotic concentration but also the antibiotic activity at the target site. For instance, the simulation based on serum pharmacokinetics indicated a complete eradication for Proteus mirabilis, whereas that employing interstitial pharmacokinetic data indicated failure to eradicate the strain in vitro.
In conclusion, our study supports the concept that ciprofloxacin concentrations at the sites of effect may be subinhibitory although effective concentrations are attained in sera. These findings for healthy volunteers may warrant further studies on ciprofloxacin penetration in conditions like soft tissue infections, as the degrees of target site penetration could differ considerably in these situations.
Acknowledgments
This work was supported by a grant (Nr. P12659-MED) from the FWF, the Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung).
REFERENCES
- 1.Arcieri G, August R, Becker N, Doyle C, Griffith E, Gruenwaldt G, Heyd A, O’Brien B. Clinical experience with ciprofloxacin in the USA. Eur J Clin Microbiol. 1986;5:220–225. doi: 10.1007/BF02013994. [DOI] [PubMed] [Google Scholar]
- 2.Capecchi P L, Blardi P, De Lalla A, Ceccatelli L, Volpi L, Pasini F L, Di Perri T. Pharmacokinetics and pharmacodynamics of neutrophil-associated ciprofloxacin in humans. Clin Pharmacol Ther. 1995;57:446–454. doi: 10.1016/0009-9236(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 3.Cavet M E, West M, Simmons N L. Fluoroquinolone (ciprofloxacin) secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1997;121:1567–1578. doi: 10.1038/sj.bjp.0701302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalla Costa T, Nolting A, Rand K, Derendorf H. Pharmacokinetic-pharmacodynamic modelling of the in vitro antiinfective effect of piperacillin-tazobactam combinations. Int J Clin Pharmacol Ther. 1997;35:426–433. [PubMed] [Google Scholar]
- 5.Daschner F D, Westenfelder M, Dalhoff A. Penetration of ciprofloxacin into kidney, fat, muscle and skin tissue. Eur J Clin Microbiol. 1986;5:212–213. doi: 10.1007/BF02013992. [DOI] [PubMed] [Google Scholar]
- 6.Davis R, Markham A, Balfour J A. Ciprofloxacin. An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs. 1996;51:1019–1074. doi: 10.2165/00003495-199651060-00010. [DOI] [PubMed] [Google Scholar]
- 7.Eichler H G, Müller M. Drug distribution. The forgotten relative in clinical pharmacokinetics. Clin Pharmacokinet. 1998;34:95–99. doi: 10.2165/00003088-199834020-00001. [DOI] [PubMed] [Google Scholar]
- 8.Fang G, Brennen C, Wagener M, Swanson D, Hilf M, Zadecky L, DeVine J, Yu V L. Use of ciprofloxacin versus use of aminoglycosides for therapy of complicated urinary tract infection: prospective, randomized clinical and pharmacokinetic study. Antimicrob Agents Chemother. 1991;35:1849–1855. doi: 10.1128/aac.35.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fass R J. Treatment of skin and soft tissue infections with oral ciprofloxacin. J Antimicrob Chemother. 1986;18(Suppl. D):153–157. doi: 10.1093/jac/18.supplement_d.153. [DOI] [PubMed] [Google Scholar]
- 10.Fink M P, Snydman D R, Niederman M S, Leeper K V, Jr, Johnson R H, Heard S O, Wunderink R G, Caldwell J W, Schentag J J, Siami G A, et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother. 1994;38:547–557. doi: 10.1128/aac.38.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krol G J, Beck G W, Bentham T. HPLC analysis of ciprofloxacin and ciprofloxacin metabolites in body fluids. J Pharm Biomed Anal. 1995;14:181–190. doi: 10.1016/0731-7085(95)01611-2. [DOI] [PubMed] [Google Scholar]
- 12.Kunin C M, Craig W A, Kornguth M, Monson R. Influence of binding on the pharmacologic activity of antibiotics. Ann N Y Acad Sci. 1973;26:214–224. doi: 10.1111/j.1749-6632.1973.tb20483.x. [DOI] [PubMed] [Google Scholar]
- 13.Licitra C M, Brooks R G, Sieger B E. Clinical efficacy and levels of ciprofloxacin in tissue in patients with soft tissue infection. Antimicrob Agents Chemother. 1987;31:805–807. doi: 10.1128/aac.31.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrikin D J, Briant J, Rolinson G N. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother. 1983;11:233–238. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- 15.Müller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Agneter E, Eichler H G, Pehamberger H. Characterization of peripheral compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller M, Mader R M, Steiner B, Steger G G, Jansen B, Gnant M, Helbich T, Jakesz R, Eichler H G, Blöchl-Daum B. 5-Fluorouracil kinetics in the interstitial tumor space: clinical response in breast cancer patients. Cancer Res. 1997;57:2598–2601. [PubMed] [Google Scholar]
- 17.Müller M, Schmid R, Georgopoulos A, Buxbaum A, Wasicek C, Eichler H G. Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther. 1995;57:371–380. doi: 10.1016/0009-9236(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 18.Nolting A, Dalla Costa T, Rand K H, Derendorf H. Pharmacokinetic-pharmacodynamic modeling of the antibiotic effect of piperacillin in vitro. Pharmacol Res. 1996;13:91–96. doi: 10.1023/a:1016085402278. [DOI] [PubMed] [Google Scholar]
- 19.Ryan D M, Cars O. A problem in the interpretation of beta-lactam antibiotic levels in tissues. J Antimicrob Chemother. 1983;12:281–284. doi: 10.1093/jac/12.3.281. [DOI] [PubMed] [Google Scholar]
- 20.Schentag J J, Gengo F M. Principles of antibiotic tissue penetration and guidelines for pharmacokinetic analysis. Med Clin N Am. 1982;66:39–49. doi: 10.1016/s0025-7125(16)31440-7. [DOI] [PubMed] [Google Scholar]
- 21.Stahle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis. III. Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49:1853–1858. doi: 10.1016/0024-3205(91)90488-w. [DOI] [PubMed] [Google Scholar]