Summary
Differentiation of specialized cell types requires precise cell-cycle control. Plant stomata are generated through asymmetric divisions of a stem-cell-like precursor followed by a single symmetric division that creates paired guard cells surrounding a pore. The stomatal-lineage-specific transcription factor MUTE terminates the asymmetric divisions and commits to differentiation. However, the role of cell-cycle machineries in this transition remains unknown. We discover that the symmetric division is slower than the asymmetric division in Arabidopsis. We identify a plant-specific cyclin-dependent kinase inhibitor, SIAMESE-RELATED4 (SMR4), as a MUTE-induced molecular brake that decelerates the cell cycle. SMR4 physically and functionally associates with CYCD3;1 and extends the G1 phase of asymmetric divisions. By contrast, SMR4 fails to interact with CYCD5;1, a MUTE-induced G1 cyclin, and permits the symmetric division. Our work unravels a molecular framework of the proliferation-to-differentiation switch within the stomatal lineage and suggests that a timely proliferative cell cycle is critical for stomatal-lineage identity.
Keywords: cell cycle, cyclin-dependent kinase inhibitor, cell fate, stomatal development, asymmetric cell division, symmetric cell division
Graphical abstract
Highlights
-
•
During stomatal differentiation, asymmetric divisions are faster than terminal divisions
-
•
Upon commitment to differentiation, MUTE induces the cell-cycle inhibitor SMR4
-
•
SMR4 decelerates the asymmetric cell division cycle via selective binding to cyclin D
-
•
Regulating duration of the G1 phase is critical for epidermal cell fate specification
Stomata, which are cellular valves in the plant epidermis, differentiate via fast asymmetric divisions of a precursor, followed by a single slower symmetric division. Han et al. identify a plant-specific, cyclin-dependent kinase inhibitor that regulates the length of cell cycles in the stomatal lineage to enable the transition from proliferation to differentiation.
Introduction
Growth and development of multicellular organisms rely on faithful cell-cycle progression, in which fundamental mechanism is highly conserved across the eukaryote kingdoms (Elledge, 1996; Harashima et al., 2013). Accumulating evidence in metazoans emphasizes that cell-cycle machinery is modulated during development, operating distinctly in proliferating stem cells versus differentiating cells (Budirahardja and Gönczy, 2009). For example, early embryogenesis of flies, fish, and frogs, as well as murine embryonic stem cells, execute rapid cell-cycle mode due to shortened gap phases. As they undergo fate specification or differentiation, the duration of cell-cycle increases (Coronado et al., 2013; Dalton, 2015; Liu et al., 2019).
A typical eukaryotic cell cycle is composed of four distinct phases, G1-S-G2-M. Cell-cycle progression is driven by the oscillation of cyclin-dependent kinase (CDK) activity triggered by phase-specific cyclins, which are tightly regulated by the level of synthesis and proteolysis (Harashima et al., 2013; Morgan, 2007). CDK activity is negatively regulated by cyclin-dependent kinase inhibitors (CKIs). The G1/S transition is initiated by D-type cyclin (CyclinD) and CDK complex, which relieve retinoblastoma (Rb)-mediated repression on S phase gene expression (Bertoli et al., 2013; Desvoyes and Gutierrez, 2020). Accumulating studies suggest that the G1 extension is indicative of differentiation (Coronado et al., 2013; Liu et al., 2019).
Plants possess a large number of genes encoding cyclins, CDKs, and CKIs (Inzé and De Veylder, 2006). Studies have shown how specific cell-cycle components are coupled to developmental patterning. For example, during Arabidopsis root development, transcription factors SHORTROOT and SCARECROW directly induce a CyclinD, CYCD6;1, that drives a formative cell division to create root endodermis and cortex cells (Sozzani et al., 2010). Another example is lateral root formation, in which auxin-induced formative division is modulated by CYCD2;1 and plant CKI, KIP-RELATED PROTEIN2 (KRP2, also known as ICK2) (Sanz et al., 2011). Some highly specialized plant cell types, such as epidermal pavement cells and trichomes, undergo endoreduplication at the onset of terminal differentiation (Inzé and De Veylder, 2006). Plant-specific CKIs, including SIAMESE (SIM) and SIAMESE-RELATED1 (SMR1, also known as LGO) regulate morphogenesis of trichomes and sepal giant cells, respectively, by promoting endoreduplication presumably via inhibiting CDK activity (Hamdoun et al., 2016; Roeder et al., 2010; Walker et al., 2000). It remains unclear if the modulation of cell cycle contributes to switching the cell division mode—from stem cell divisions to differentiating cell divisions—in plants.
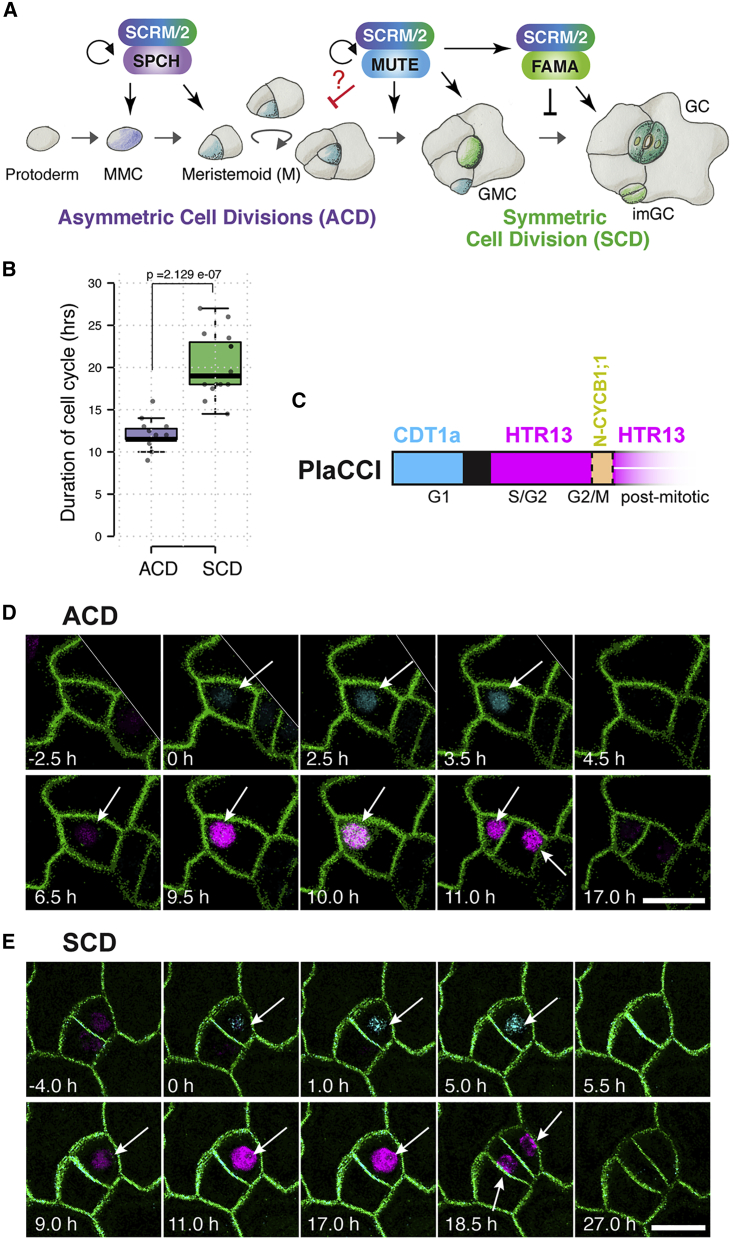
Development of stomata, valves on the plant aerial epidermis for gas exchange and water control, is an accessible model of de novo initiation and differentiation of lineage-specific stem cells. In Arabidopsis, birth of pores begins with the stomatal lineage fate specification of protodermal cells, which forms bipotent meristemoid mother cells (MMC) able to become either stomata or pavement cells (Han and Torii, 2016; Lau and Bergmann, 2012). A series of asymmetric cell division (ACD) follows to amplify the number of stomatal lineage precursor cells: meristemoids and stomatal lineage ground cells (SLGCs). The meristemoid renews itself after ACD, thus behaving as a transient stem cell. After a few rounds of ACDs, a single round of terminal symmetric cell division (SCD) of a guard mother cell (GMC) proceeds, completing a stoma composed of paired guard cells (Han and Torii, 2016; Lau and Bergmann, 2012) (Figure 1A).
Figure 1.
Cell-cycle duration between asymmetric cell division and symmetric cell division during stomatal development
(A) Cartoon of the heterodimeric transcription factors specifying stomatal development. A series of ACD is triggered by SPCH·SCRM/2 and a single symmetric cell division (SCD) is coordinated by MUTE·SCRM/2 and FAMA·SCRM/2. How the mode of cell cycle switches from ACD to SCD is not known (red line and question mark). MMC, meristemoid mother cell; M, meristemoid; GMC, guard mother cell; imGC and GC, immature and mature guard cell.
(B) Duration of the cell-cycle time of stomatal precursors undergoing ACD and SCD in wild type. n = 15 for each cell division mode. Two-tailed Student t test was performed. p = 2.129e-07.
(C) PlaCCI color code. Cyan: CDT1a-CFP signal, onset of G1 phase; black: short period with no fluorescence signal; magenta: HTR13-mCherry signal, S/G2 through late M; orange: CYCB1;1-YFP signal. Postmitotic referred to G1 or G0 (terminal state).
(D and E) Representative time-lapse confocal images of ACD (D) and SCD (E) in stomatal lineage cells from 1- to 3-day-old cotyledon of Col-0 expressing both PlaCCI and LTi6B (green). CDT1a-CFP signal (cyan) marks the starting point (0 h) at the onset of G1 phase for ACD and SCD. Note that the CYCB1;1-YFP (D, green nucleus/chromosomes, M phase) is not always visible due to time-lapse recordings obtained at 30 min time intervals. Arrows point to nuclei with a fluorescent signal in different cell stages. Scale bar, 10 μm. See also Figure S1 and Table S1.
Accumulating evidence supports that master-regulatory bHLH proteins SPEECHLESS (SPCH), MUTE, and FAMA govern cell-state transitions within the stomatal lineage, in part via directly regulating the expression of cell-cycle genes (Adrian et al., 2015; Hachez et al., 2011; Han et al., 2018; Lau et al., 2014) (Figure 1A). SPCH initiates and sustains the ACDs of a meristemoid in part via upregulating CyclinDs CYCD3;1 and 3;2 (MacAlister et al., 2007; Vatén et al., 2018). MUTE terminates proliferative cell state and drives final SCD by activating a large subset of cell-cycle regulators, including CYCD5;1 (Han et al., 2018; Pillitteri et al., 2007). FAMA and a Myb protein, FOUR LIPS, are directly induced by MUTE and inhibit SCD via direct suppression of the cell-cycle genes, thereby ensuring that the SCD occurs just once (Hachez et al., 2011; Han et al., 2018; Xie et al., 2010). However, it is not known how proliferative ACD switches to terminal SCD, and whether the core cell-cycle machinery contributes to this process.
Through time-lapse imaging of stomatal development using plant cell-cycle marker, Plant Cell-Cycle Indicator (PlaCCI) (Desvoyes et al., 2020), we discovered that the stomatal SCD cycle is slower than that of ACDs. Subsequent transcriptomic and ChIP-sequencing analyses identified that MUTE directly induces the expression of SMR4 during meristemoid-to-GMC transition. Through loss-of-function and stomatal-lineage-specific overexpression of SMR4 as well as its functional interaction studies with CyclinDs, we elucidate that SMR4 acts as a molecular brake to decelerate cell cycle in G1 phase to ensure termination of the ACD cycle and facilitate faithful progression to SCD. Slowing down the ACD cycle resulted in skewed stomata with pavement cell-like characters. Taken together, we reveal a molecular framework of the cell proliferation-to-differentiation switch within the stomatal lineage and suggest that a timely proliferative ACD cycle is critical for the generation of stomata with proper GC size, shape, and identity.
Results
The single symmetric division of stomatal precursor is slower than amplifying asymmetric division
The stomatal precursor cells execute a unique transition from amplifying ACD to a single SCD, a step coordinated by the bHLH protein MUTE (Figure 1A). To understand if a switch from the ACD-to-SCD division mode links to the cell-cycle dynamics, we first performed time-lapse imaging of developing cotyledon epidermis by using the multi-color PlaCCI (Desvoyes et al., 2020) (Figure 1C) and examined each phase of cell cycle during asymmetric and symmetric divisions (Figures 1B, 1D, and 1E; Video S1). The average cell-cycle time of ACD of meristemoid and SCD of GMC was 12 ± 1.64 and 20.27 ± 3.73 h, respectively (Figure 1B; Table S1), indicating that ACD is faster by ∼7.5 h than SCD that creates a pair of guard cells. Measuring cell division time using a plasma-membrane GFP marker LTI6b (Kurup et al., 2005) yielded essentially the same results (Figure S1). On the basis of these findings, we conclude that the switching from ACD to SCD involves cell-cycle slow down.
Shown are representative asymmetric cell division (ACD; left) and symmetric cell division (SCD; right) in wild-type plants expressing the membrane marker LTi6B-GFP (green) and the cell cycle marker PlaCCI (cyan = G1, magenta = S/G2/lateM, and green nucleus = M). Small maximum projections of time laps recordings obtained at a 30-min time interval of an abaxial cotyledon epidermis 24 h after preparation. Played back at 5 fps. Hours are indicated at the top in 30-min intervals. Scale bar, 5 μm.
SMR4 is expressed in stomatal lineage cells and directly induced by MUTE
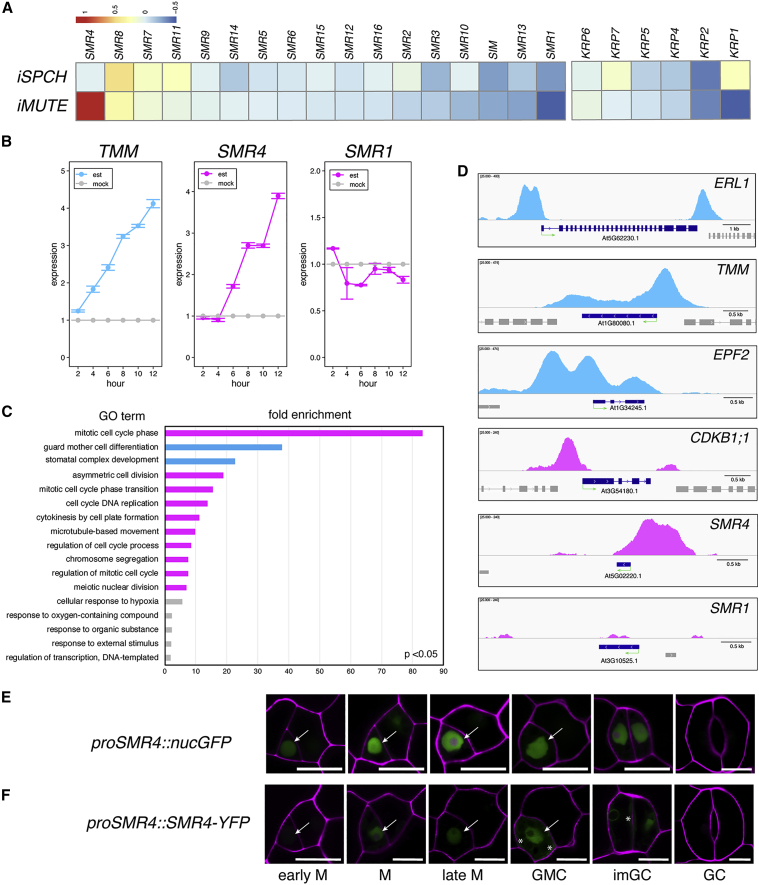
In eukaryotic cells, CKIs negatively regulate cell-cycle progression. To identify a factor that plays a role in the decelerating cell cycle during the ACD-to-SCD transition, we surveyed publicly available transcriptome data (Han et al., 2018; Lau et al., 2014) to search for CKIs that are induced by SPCH and MUTE. Among the 7 KRP and 17 SIM/SMR genes (Kumar and Larkin, 2017; Peres et al., 2007), only SMR4 exhibits marked increase by the induced MUTE overexpression (iMUTE) (Figure 2A). On the other hand, a majority of SMRs and KRPs is either downregulated or unchanged upon iMUTE or iSPCH (Figure 2A). Subsequently, we performed time course induction analysis. Consistent with the transcriptome data, SMR4 expression was increased by iMUTE with similar kinetics to a known direct MUTE target, TMM (Han et al., 2018) (Figure 2B). In addition, iMUTE (and in a lesser extent, iSPCH) weakly induced SMR8 (Figure S2).
Figure 2.
SMR4, one of the plant-specific CKIs expresses in stomatal lineage and is a direct target of MUTE
(A) Heatmap represents the changes in expression of 24 CKIs in Arabidopsis by SPCH or MUTE induction. RNA-seq data adapted from (Lau et al., 2014) (iSPCH) and (Han et al., 2018) (iMUTE). Heatmap denotes log2 ratio of changes in expression compared with non-induced control.
(B) Time course expression for 12 h with 2-h interval of SMR4 and SMR1 by iMUTE monitored by qRT-PCR. TMM was used as a positive control for a MUTE inducible gene. est: 10 μM estradiol treated, mock: non-treated control (DMSO only). Data are presented as mean ± SEM.
(C) GO categories of direct MUTE targets (MUTE bound, iMUTE up) ranked by fold enrichment compared with background genome. p < 0.05. Pink bars: “cell cycle,” “division,” “mitotic” categories; blue bars: “stomatal” categories; gray bars: others.
(D) IGV snapshots of ChIP-seq profile of MUTE binding to the promoters of SMR4, SMR1, and known MUTE targets (ERL1, TMM, EPF2, and CDKB1;1) (Han et al., 2018; Qi et al., 2017). No MUTE binding was detected to SMR1 loci. A green arrow under the gene annotation indicates gene orientation and transcriptional start sites.
(E and F) Expression patterns of SMR4 transcriptional and translational reporters. proSMR4::nucGFP (E) and proSMR4::SMR4-YFP (F) in stomatal lineage precursor cell specific on the epidermis. White arrows: nuclei with GFP or YFP signal. Asterisks: cytoplasmic YFP signal. Scale bar, 10 μm.
See also Figures S2 and S3 and Table S2.
Our previous transcriptome study (Han et al., 2018) found that MUTE induces a suite of cell-cycle- and mitotic-division-related genes driving the SCD of stomata. To test whether these genes are indeed direct MUTE targets, we performed genome-wide MUTE ChIP-sequencing (see STAR Methods) (Table S2). MUTE-bound genes as well as those MUTE-bound genes that are induced by MUTE are highly enriched in the Gene Ontology (GO) categories (Figure 2C; Table S2): “mitotic cell-cycle phase (83.22-fold enrichment, p = 2.15e-02),” “ACD (18.91-fold enrichment, p = 2.4e-02)” and other cell-cycle/mitosis-related categories (Figure 2C, pink bars), as well as the genes involved in stomatal development: “GMC differentiation (37.83-fold enrichment, p=8.34e-04)” and “stomatal complex development (22.7-fold enrichment, p = 1.75e-12)” (Figure 2C, cyan bars). Strong MUTE-bound peaks are detected at the 5′- and 3′- regions of known MUTE target loci, ERL1, TMM, EPF2, and CDKB1;1 (Figure 2D). Most importantly, MUTE robustly bound to the 5′ region of SMR4, indicating that SMR4 is a direct MUTE target. As expected, no MUTE binding peak was detected in SMR1 loci, which is not induced by iMUTE and thus not a direct MUTE target (Figures 2B and 2D).
We further characterized the SMR4 expression patterns using seedlings expressing nuclear-localized GFP driven by the SMR4 promoter (proSMR4::nucGFP; Figure 2E). A strong GFP signal was detected in stomatal lineage cells, with the highest expression in a late meristemoid to GMC and persisted in immature GCs (Figure 2E). Likewise, a translational reporter of SMR4-YFP fusion protein driven by the SMR4 promoter (proSMR4::SMR4-YFP) exhibited similar accumulation patterns predominantly in the nuclei (Figure 2F, arrows). A weak SMR4-YFP signal was also detected in the cytoplasm (Figure 2F, asterisks), which may imply the regulation of SMR4 proteins. These expression patterns mirror that of MUTE (Pillitteri et al., 2007). Finally, to address whether MUTE is required for the SMR4 expression during the meristemoid-to-GMC transition, we examined the SMR4 reporters in the MUTE-null mutant, mute-2 (Pillitteri et al., 2008) (Figure S3). SMR4-YFP was not detected in the arrested mute-2 meristemoids, and transcriptional reporter proSMR4::nucGFP signals were diminished (Figure S3). Combined, our results indicate that MUTE directly promotes the SMR4 expression in stomatal precursor cells before the onset of the SCD and that MUTE is both necessary and sufficient for this boosted expression. We also noted weak, background-level of nucGFP signals in few meristemoids (Figure S3B), implying a putative role for SMR4 in a MUTE independent process. The SMR4 expression suggests its distinct role from that of canonical CKIs in endorduplication.
SMR4 suppresses cell proliferation in part with SMR8
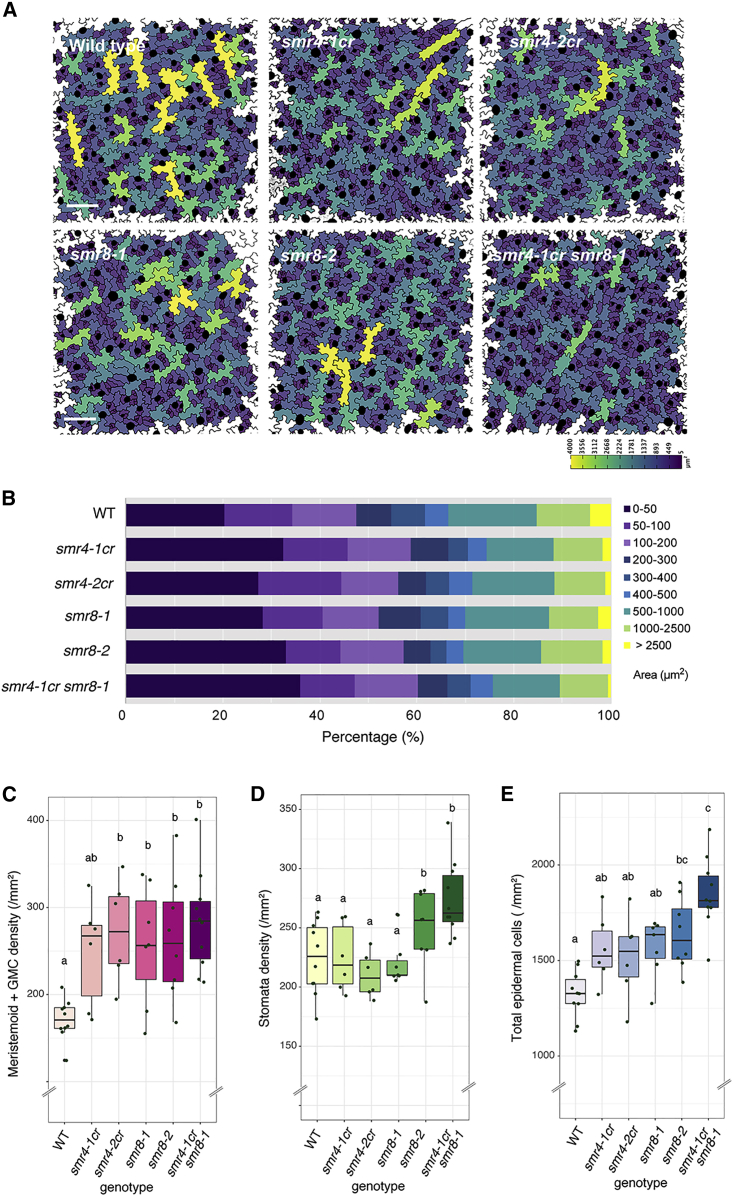
To understand the role of SMR4 in stomatal development, we next sought to characterize its loss-of-function phenotypes. Because no T-DNA insertion line is available for SMR4, presumably owing to its short coding sequence (219 bp), we employed CRISPR-Cas9 system (Tsutsui and Higashiyama, 2017) (see STAR Methods). A guide RNA targeting to SMR4 yielded either a base-pair deletion (smr4-1cr) or insertion (smr4-2cr) at 80 bp from the translation start site, which leads to a frameshift and premature stop codon (Figures S4A and S4B). A quantitative analysis of segmented epidermal cells (see STAR Methods) revealed that smr4cr epidermis is increased in small cells (<50 μm2) and concomitantly decreased in large pavement cells (>4,000 μm2) (Figures 3A, 3B, and 3E). Stomatal precursor cell (meristemoid and GMC) density is also increased in smr4cr alleles (Figure 3C). On the other hand, stomatal density was not significantly changed in smr4cr (Figure 3D), suggesting that SMR4 primarily restricts the divisions of early stomatal precursor cells. Introduction of functional SMR4 transgene (proSMR4::HA-SMR4) fully rescued the phenotypes of smr4-1cr (Figures S4D–S4G), indicating that increased numbers of stomatal precursor cells in smr4 mutant are due to the loss of function of SMR4.
Figure 3.
smr4 CRISPR knockout mutants produce smaller cells, the phenotype is enhanced by smr8
(A) Abaxial cotyledon epidermis from 4-day-old seedlings of wild type, smr4-1cr, smr4-2cr, smr8-1, smr8-2, and smr4-1cr smr8-1double mutant. Epidermal cells size is color coded as a color scale at bottom. GCs are marked in black. Scale bar, 100 μm.
(B) Bar graphs showing the percentage of each category of cell area (rightmost) from the images for the genotype presented in (A). GCs are not included in the category of cell area.
(C–E) Density of stomatal precursor cells (meristemoid+GMC) (C), stomata (D), and total epidermal cell (E) 1.0 mm−2 area for the genotypes shown in (A). One-way ANOVA followed by Tukey’s post hoc test was performed for comparing all genotypes. Different letter denotes significant difference. Double letter denotes insignificance. p < 0.05 or p < 0.01. The number of plants from each genotype, WT: n = 10, smr4-1cr: n = 6, smr4-2cr: n = 6, smr8-1: n = 7, smr8-2: n = 8, smr4-1cr smr8-1: n = 10.
See also Figure S4.
Because SMR8 expression was marginally increased by iMUTE (Figure S2), we further characterized the loss-of-function phenotypes of SMR8. Two T-DNA insertion lines, smr8-1 and smr8-2, accumulate a reduced-level of SMR8 transcripts (Figure S4C). Like smr4, smr8 mutants conferred an increase in small epidermal and stomatal precursor cells (Figures 3A–C, and 3E), and the total epidermal cell numbers become most exaggerated in the smr4 smr8 double mutant (Figure 3E). Therefore, SMR4 plays a role in repressing ACD in part redundantly with SMR8.
Stomatal-lineage-specific expression of CKIs reveals their unique functions
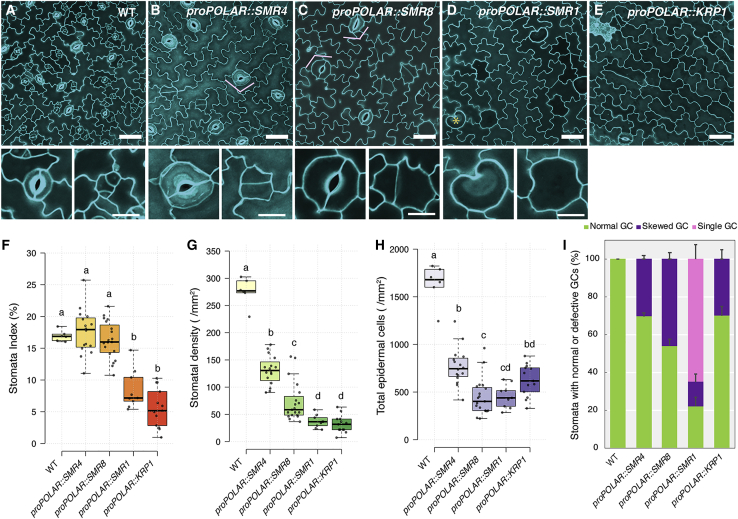
Our study revealed that SMR4 is a direct MUTE target and expresses during the transition from proliferating meristemoid state to differentiating GMC state. SMR proteins are known to promote endoreduplication in trichomes, pavement cells, and sepal giant cells (Hamdoun et al., 2016; Kumar and Larkin, 2017; Roeder et al., 2010; Walker et al., 2000). However, unlike trichomes and pavement cells, stomatal lineage cells do not undergo endoreduplication (Melaragno et al., 1993). We thus hypothesized that SMR4 may function differently from the other canonical SMRs. To address this, SMR4, SMR8, and SMR1 along with KRP1 are ectopically expressed in the stomatal lineage cells (MMC, meristemoids and SLGC) by using POLAR promoter (Pillitteri et al., 2011) (Figures 4 and S5). Unlike SMR1 or KRP1, POLAR-promoter-driven SMR4 and SMR8 did not significantly changed stomatal index ([number of stomata/number of stomata and non-stomatal epidermal cells] ×100) (Figure 4F), reflecting reduction in the number of both stomata and epidermal cells (Figures 4G and 4H). Whereas GMCs of POLAR-promoter-driven SMR4 executed SCD, they occasionally formed stomata composed of skewed guard cells (Figure 4B, pink bracket; Figures 4I and S5). This suggests that SMR4 does not inhibit the final SCD per se. Similar deformed stomata were also observed in proPOLAR::SMR8 (Figure 4C, pink bracket; Figures 4I and S5).
Figure 4.
Stomatal lineage overexpression phenotype of a suite of CKIs reveal their unique activities
(A–E) Epidermal phenotype of abaxial cotyledons from 4-day-old wild type (A), proPOLAR::SMR4 (B), proPOLAR::SMR8 (C), proPOLAR::SMR1 (D), and proPOLAR::KRP1 (E). Scale bars, 50 μm. Insets: enlarged mature guard cell and precursor cells from each genotype. Scale bars, 20 μm. Orange asterisks, undivided single-celled stomata. Pink brackets, skewed stomata.
(F–I) Quantification of epidermal cell number of abaxial cotyledon from 4-day-old wild-type and transgenic plants. Stomatal index (F), stomatal density (G), total epidermal cells (H), and fraction of normal (light green), skewed (purple), and single-celled stomata (pink) found on each genotype (I) in 1.0-mm−2 area. One-way ANOVA with Tukey’s post hoc test was performed to compare all genotypes. The number of plants from each genotype, WT: n = 6, proPOLAR::SMR4: n = 16, proPOLAR::SMR8: n = 19, proPOLAR::SMR1: n = 13, proPOLAR::KRP1: n = 14.
See also Figure S5.
Unlike SMR4, ectopic stomatal lineage expression of SMR1 and KRP1 displayed cell division defects with unique consequences. proPOLAR::SMR1 produced abnormally large undivided GMC-like cells (Figure 4D, asterisks; Figure S5), which constitute over 60% of the all stomata (Figure 4I). This result is consistent with the known role of SMR1 in suppressing the activity of CDKB1;1 thereby promoting endoreduplication (Kumar et al., 2015). Finally, proPOLAR::KRP1 severely inhibited the ACDs, resulting in epidermis vastly consisted of pavement cells with low stomatal index (Figures 4E and 4F), resembling spch mutant (MacAlister et al., 2007; Pillitteri et al., 2007). Among those proPOLAR::KRP1 stomata, approximately one-third were deformed (Figure 4I).
The phenotype of proPOLAR::SMR4 (and SMR8) is consistent with the diminished proliferative activity of meristemoids. To uncouple formative step from differentiation, we introduced proPOLAR::SMR4 into mute mutant, in which a number of amplifying ACDs are increased and meristemoids arrest (Pillitteri et al., 2007) (Figure S5). Indeed, proPOLAR::SMR4 significantly reduced the number of ACDs, resulted in low number of larger meristemoids (Figures S5J–S5N). Since MUTE is absolutely required for GMC identity (Pillitteri et al., 2007), these enlarged meristemoids never became stomata. Taken together, we conclude that SMR4, to some extent SMR8, possesses a unique feature different from canonical CKIs to specifically terminate (but not fully inhibit) ACDs of meristemoids but allow final SCD to proceed in GMC. Furthermore, the formation of skewed irregular-shaped stomata, some resembling pavement cells (Figure 4B), implies that excessive SMR4 activity disrupts guard cell morphogenesis.
SMR4 balances between cell proliferation and differentiation
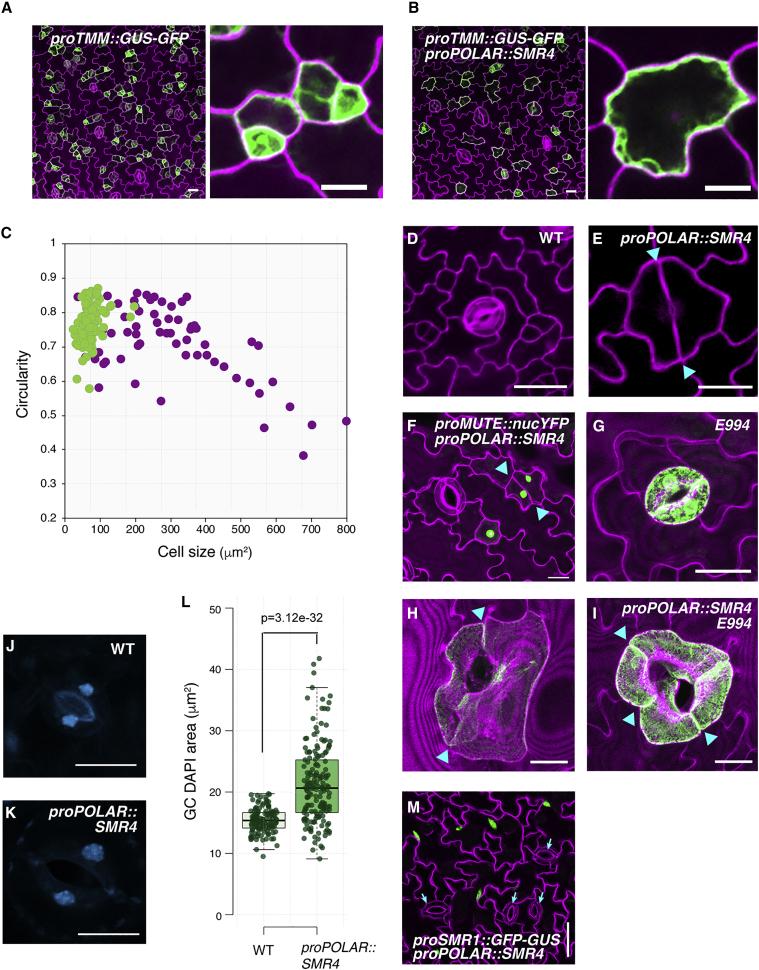
To further understand the identity of abnormally shaped stomata observed in proPOLAR::SMR4, stomatal lineage markers were introduced. In wild type, stomatal lineage precursor marker proTMM::GUS-GFP (Nadeau and Sack, 2002) was detected in stomatal lineage cells with the brightest signal in triangular shaped meristemoids (Figure 5A). Surprisingly, both stomatal lineage cells and enlarged pavement cell-like cells in proPOLAR::SMR4 plants expressed proTMM::GUS-GFP (Figures 5B and S6). Some of these GFP-expressing cells in proPOLAR::SMR4 plants divide symmetrically even without ACD or after a single round of ACD with significantly extended duration (Figures 5B, 5E, and 5F cyan arrows, Figure S6 pink arrows; Video S2). Further quantitative analysis shows that, compared with wild type, these proTMM::GUS-GFP-positive cells in proPOLAR::SMR4 are greater in size range (50∼800 μm2) and in addition have low circularity (Figure 5C). This might reflect mixed cell fate, pavement cell shape with stomatal identity. Some of these cells express stomatal fate commitment marker proMUTE::nucYFP (Figure 5F) and finally differentiate into mature guard cells (Figures 5G–5I) exhibiting large wavy and skewed shape (Figures 5H and 5I) but expressing a mature guard cell marker, E994. Thus, the large skewed GCs in proPOLAR::SMR4 originate from the enlarged stomatal lineage precursors caused by delayed and fewer ACD cycles (Figure S6; Video S2).
Figure 5.
Stomatal lineage overexpression of SMR4 reduces proliferative activity of meristemoids
(A and B) (A) proTMM::GUS-GFP abaxial cotyledon from 4-day post-germination stage seedling (4 dpg), (B) proTMM::GUS-GFP in proPOLAR::SMR4, 4dpg, Scale bar, 20 μm. Insets: zoomed stomatal lineage cells expressing GFP. Scale bar, 10 μm.
(C) Size distribution versus circularity of the stomatal lineage precursor cells expressing proTMM::GUS-GFP in wild-type (green dots) and proPOLAR::SMR4 (purple dots) plants.
(D–I) Confocal images of representative stomata: wild-type stoma (D), mixed fate stoma developed proPOLAR::SMR4 (E), proMUTE::nYFP in proPOLAR::SMR4 (F), mature GC marker E994 in wild type (G), and proPOLAR::SMR4 (H and I). Cyan arrowheads, division site of GCs. Scale bars, 20 μm.
(J and K) DAPI-stained nuclei in mature GCs from wild-type (J) and proPOLAR::SMR4 plants (K).
(L) Quantitative analysis of DAPI-stained nuclear area in wild-type and proPOLAR::SMR4 GCs. Two-tailed Student’s t test was performed. p = 3.12e-32.
(M) Endoreduplication marker proSMR1::GFP-GUS expression in proPOLAR::SMR4 plants. Cyan arrows indicate enlarged GCs with no GFP expression. Scale bar, 50 μm.
See also Figures S6 and S7.
Shown are representative cotyledon epidermis expressing proTMM::GUS-GFP (pTMM::GUS-GFP) in wild type (left) and proPOLAR::SMR4 (pPOLAR::SMR4) transgenic plants (right). A cell of interest is marked with white arrowhead at the beginning of the movie. A magenta arrowhead marks when the division plane is first visible during ACD. A yellow arrow marks SCD division plane. Hours are presented relative to the time when ACD plane is first visible (0 h) from the cell of interest and indicated at the top of the left corner of the movie. Scare bar, 20 μm.
To address whether these enlarged GCs undergo endoreduplication process when SMR4 is ectopically expressed, the DNA content was measured using DAPI fluorescence (Figures 5J–5L). Half of GC populations exhibited the fluorescence similar to the wild type (the median value is 15 μm2 in wild type and 20 μm2 in proPOLAR::SMR4 plants), whereas some showed fluorescence values nearly doubled in proPOLAR::SMR4 plants (Figures 5J–L). Likewise, the GC nuclei size measurements using H2B-GFP (Maruyama et al., 2013) are consistent with the DAPI measurements (Figures S7A–S7D). The difference in nuclei size was more pronounced in pavement cells. Furthermore, none of the GCs expresses the endoreduplication marker proSMR1::nlsGFP-GUS (Bhosale et al., 2018) regardless of the GC size in proPOLAR::SMR4 plants, whereas pavement cells, where endoreduplication normally occurs, express GFP signal (Figure 5M). Combined, these results suggest that stomatal lineage overexpression of SMR4 may confer doubled DNA content, probably due to cell-cycle arrest in G2 after the S phase. Unlike SMR1, however, SMR4 does not trigger the endoreduplication cycle in the stomatal lineage. This feature distinguishes SMR4 from the known SIM/SMR-family of CKIs. The unique, non-canonical activity of SMR4 is also supported by systematic stomatal lineage overexpression of selected CKIs, where only SMR1 generated huge undivided GMC cells (Figures 4D and S5). Indeed, quantitative analysis showed that the nuclear size of proPOLAR::SMR1 GCs is ∼10 times larger than that of the control wild-type plants (Figures S7E–S7H), consistent with the known role of SMR1 in triggering endoreduplication (Hamdoun et al., 2016; Kumar et al., 2015; Schwarz and Roeder, 2016).
SMR4 decelerates cell-cycle progression by G1 phase extension
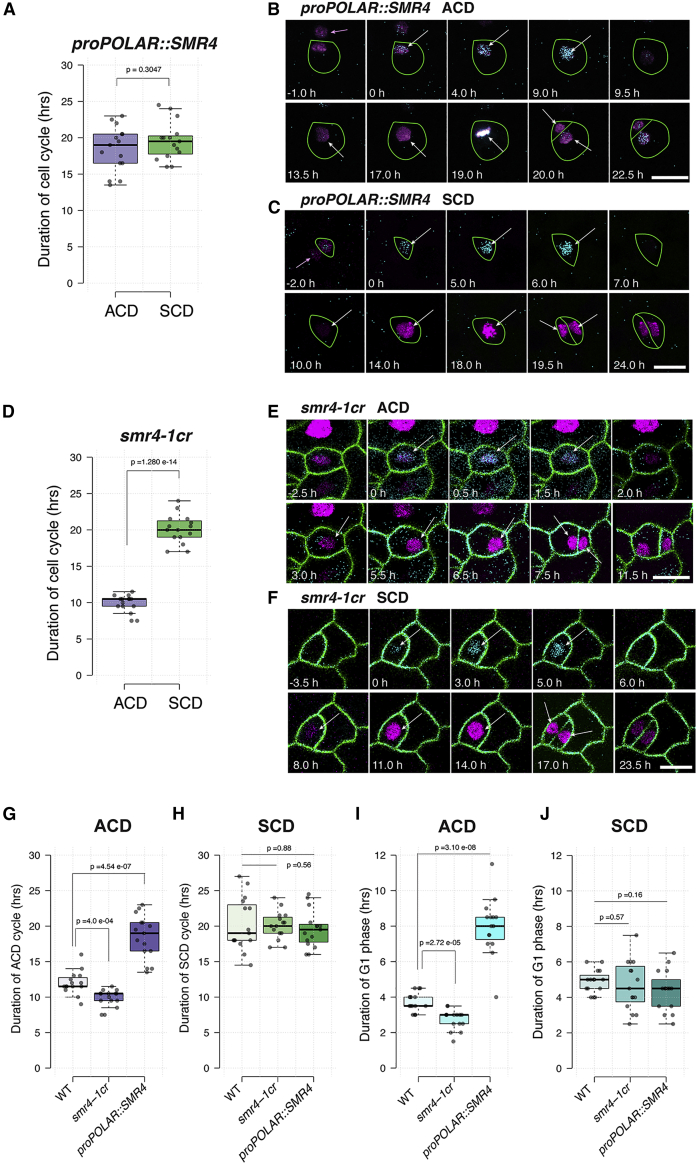
We elucidated that stomatal lineage ACDs are faster than the final SCD (Figures 1B and 1C). What is the ramification of stomatal lineage overexpression or loss-of-function mutation of SMR4 on cell-cycle duration of ACDs and SCD? To address this question, we introduced PlaCCI to proPOLAR::SMR4 and smr4-1cr mutant plants and performed time-lapse imaging (Figure 6; Videos S3 and S4). The stomatal precursor cells (meristemoids) in proPOLAR::SMR4 seedlings showed extended ACD cycle duration from 12.00 to 18.47 h (Figures 6A, 6B, and 6G; Table S1). This made the ACD cycle duration statistically non-significant from that of the SCD (Figure 6A). Further analysis of cell-cycle phase emphasized the striking extension of G1 phase, as determined by the time window from the onset of CDT1a-CFP expression to HTR13-mCherry expression (Desvoyes et al., 2020) (Figures 6B and 6C), from average of 3.73 to 7.97 h (Figure 6I; Video S3; Table S1). In contrast, the cell-cycle duration of the SCD was not significantly affected by proPOLAR::SMR4 (19.57 h, Figures 6A and 6C; Video S3; Table S1).
Figure 6.
SMR4 slows down the cell-cycle progression of ACD through G1 extension
(A and D) Cell-cycle duration of ACD and SCD measured by PlaCCI in proPOLAR::SMR4 (A) and smr4-1cr (D). n = 15 for each cell division mode.
(B, C, E, and F) Representative time-lapse confocal images of ACD and SCD in stomatal lineage cells from 1 to 3-day-old cotyledon of proPOLAR::SMR4 (B and C) and smr4-1cr (E and F) expressing PlaCCI. Cell outlines (green) for proPOLAR::SMR4 (B and C) were hand-drawn based on digital overexposure of confocal images. LTi6B (green) are introduced into smr4-1cr (E and F). For the color code and time setting, see Figure 1 legends. White arrows indicate the nuclei with fluorescent signal. Pink arrows indicate the nucleus of a sister cell from the prior round of ACD. Scale bar, 10 μm.
(G and H) Cell-cycle duration of ACD (G) and SCD (H) among WT, smr4-1cr and proPOLAR::SMR4.
(I and J) G1 phase duration of ACD (I) and SCD (J) among WT, smr4-1cr, and proPOLAR::SMR4.
(A, D, G, H, I, and J) Two-tailed Student’s t test was performed. p values were indicated on top of each boxplot.
See also Table S1, and Videos S3 and S4.
Shown are representative asymmetric cell division (ACD; left) and symmetric cell division (SCD; right) in plants expressing proPOLAR:SMR4 as well as the cell cycle marker PlaCCI (cyan = G1, magenta = S/G2/lateM, and green nucleus = M). Small maximum projections of time laps recordings obtained at a 30-min time interval of an abaxial cotyledon epidermis 24 h after preparation. Played back at 5 fps. Hours are indicated at the top in 30-min intervals. Scale bar, 5 μm.
Shown are representative asymmetric cell division (ACD; left) and symmetric cell division (SCD; right) in smr4-1cr single mutants expressing the membrane marker LTi6B-GFP (green) and the cell cycle marker PlaCCI (cyan = G1 and magenta = S/G2/lateM). Small maximum projections of time laps recordings obtained at a 30-min time interval of an abaxial cotyledon epidermis 24 h after preparation. Played back at 5 fps. Hours are indicated at the top in 30-min intervals. Scale bar, 5 μm.
During the ACD in smr4-1cr mutant, the G1 phase became shortened by 1 h compared with wild type (Figures 6E and 6I; Table S1; Video S4, WT: 3.73 h versus smr4-1cr: 2.73 h) while the S/G2 and M phases remained unchanged (Table S1), indicating that, in the absence of SMR4, the ACD cell cycle becomes accelerated. Again, the cell-cycle duration or the G1 phase duration of SCD was not significantly changed by the smr4-1cr mutation (Figures 6D, 6F, 6H, and 6J; Video S4; Table S1). Taken together, the results highlight that SMR4 is both necessary and sufficient to slow down cell-cycle progression by G1 phase extension, to prevent the further occurrence of ACDs once stomatal differentiation has been committed.
Physical and functional interactions of SMR4 with D-type cyclins underscore the switch from ACD to SCD
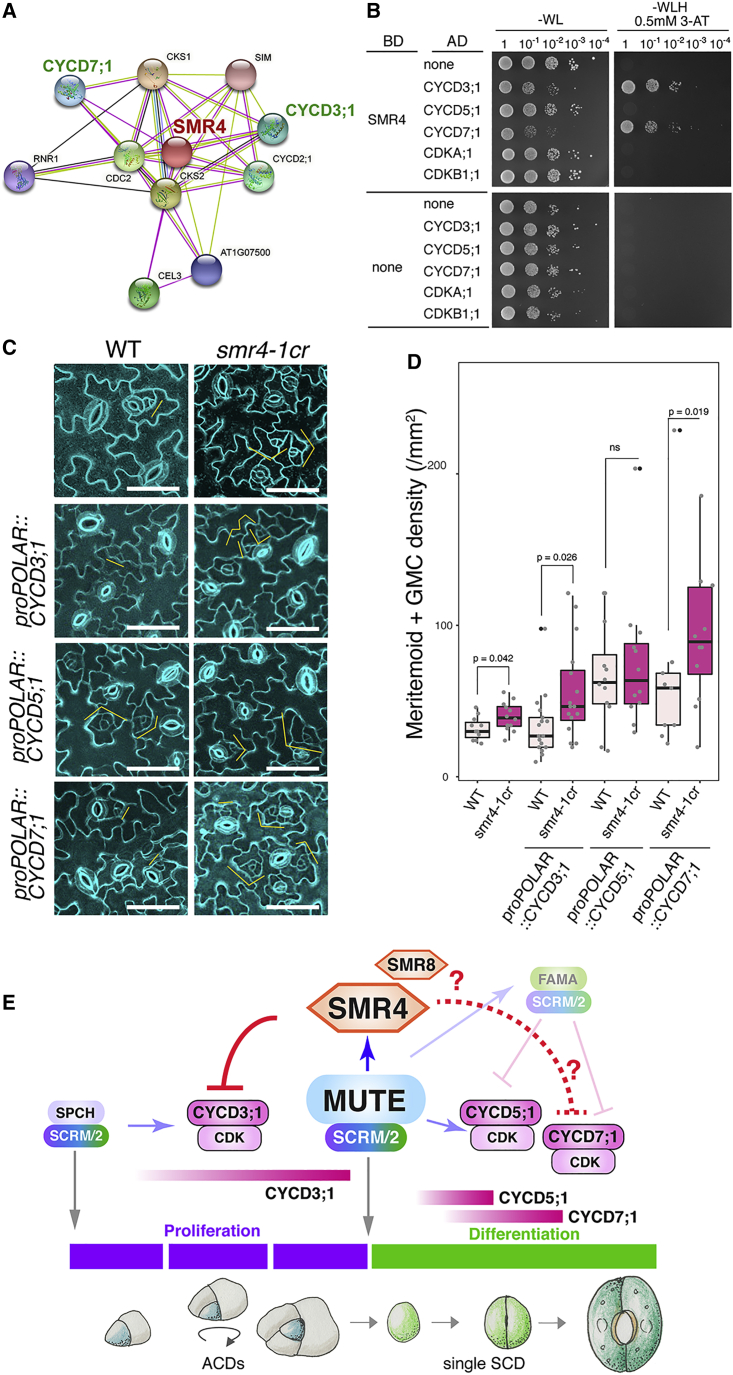
SIM is known to interact with CYCA2;3 to promote endoreduplication (Wang et al., 2020). Unlike SIM, stomatal lineage overexpression of SMR4 can extend G1 cycles of ACD but allow execution of the final SCD (Figures 4, 5, and 6). We thus predict that SMR4 regulates the G1-S transition via associating with CyclinDs. To understand the mode of action of SMR4, we first surveyed publicly available protein-protein interactome data (Szklarczyk et al., 2019), including a large-scale Arabidopsis in vivo mass-spectrometry-based interactome profiling of cell-cycle components through tandem-affinity purification-based technology (Van Leene et al., 2010). The known SMR4 interactors include major components of cell-cycle progression, CSK1, CKS2, CDKA;1 (CDC2), CYCD2;1, CYCD3;1 and CYCD7;1 (Van Leene et al., 2010) (Figure 7A). Among them, CYCD3;1 activity rises when cell re-enter the cell cycle (Riou-Khamlichi et al., 1999) and is highly induced by SPCH (Adrian et al., 2015), whereas CYCD7;1 is involved in the SCD of GMC (Weimer et al., 2018). Our yeast two-hybrid (Y2H) analysis shows that consistent with the interactome data (Figure 7A), SMR4 associates with CYCD3;1 and CYCD7;1 (Figure 7B). In contrast, no interaction was observed for SMR4 and CYCD5;1, a direct MUTE target initiating the single SCD (Han et al., 2018) (Figure 7B). We also did not observe the direct interaction of SMR4 with CDKA;1 or CDKB1;1 (Figure 7B).
Figure 7.
SMR4 decelerates the cell cycle via direct interactions with a selected set of D-type cyclins
(A) SMR4 interacting proteins from in vivo interactome (Van Leene et al., 2010) visualized by cytoscape.
(B) Yeast two-hybrid assays. Bait, the DNA-binding domain (BD) alone or fused to SMR4. Prey, the activation domain alone (AD) or fused to CYCD3;1, CYCD5;1, CYCD7;1, CDKA;1, and CDKB1;1. Transformed yeast were spotted in 10-fold serial dilutions on appropriate selection media.
(C) Transgenic plants harboring CYCD3;1, CYCD5;1, and CYCD7;1 driven by the POLAR promoter in wild type (WT) and smr4-1cr in comparison with wild type and smr4-1cr. Orange brackets: stomatal lineage precursors. Scale bars, 50 μm.
(D) Quantification of stomatal precursor cells in 1.0 mm−2 area from 7-day-old seedlings. Mann-Whitney test was performed. p values were marked on top of the boxplot. Independent T1 transgenic plants were analyzed. The number of plants used: WT: n = 11, smr4-1cr: n = 12, proPOLAR::CYCD3;1: n = 17, proPOLAR::CYCD3;1 smr4-1cr: n = 16, proPOLAR::CYCD5;1: n = 12, proPOLAR::CYCD5;1 smr4-1cr: n = 12. proPOLAR::CYCD7;1: n = 9, proPOLAR::CYCD7;1 smr4-1cr: n = 12.
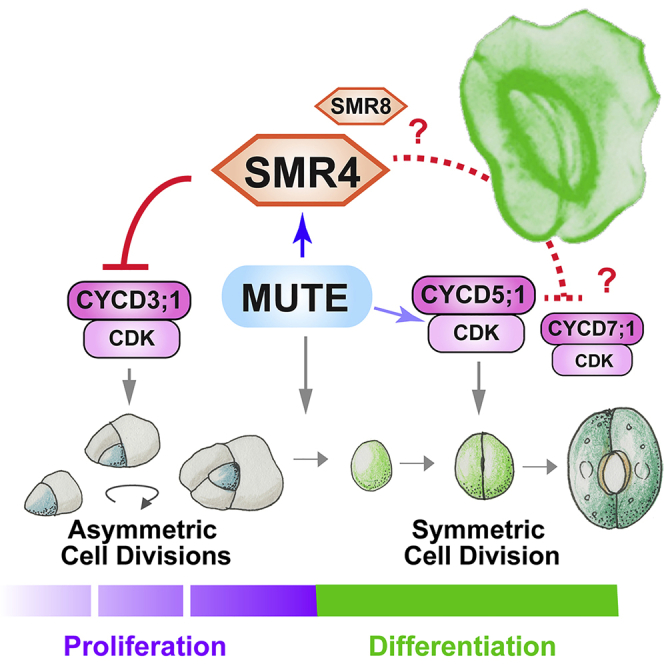
(E) Schematic model. SPCH·SCRM/2 initiate and sustain ACD and MUTE·SCRM/2 trigger SCD (gray arrows) by transcriptionally activating CYCD3;1 and CYCD5;1 (shaded blue arrows), respectively. MUTE directly up-regulates SMR4 transcription (Blue arrow). SMR4 (and SMR8 in part) suppress the activity of CYCD3;1 and possibly CYCD7;1 complexed with CDKs (red line), but not CYCD5;1, to terminate the ACD mode and ensure faithful progression of SCD. Question marks and dotted line indicate the possible roles of SMR8 in termination of ACD and SMR4 with CYCD7;1 in symmetric cell division, respectively.
Next, to address the biological significance of SMR4 interactions with CYCD3;1 and CYCD7;1 but not with CYC5;1, we examined the effects conferred by stomatal lineage overexpression of three CyclinDs in the presence or absence of functional SMR4. As shown in Figures 7C and 7D, in the absence of SMR4, POLAR-promoter-driven expression of CYCD3;1 and CYCD7;1 exaggerated the ACDs, resulting in significant increase in the density of stomatal precursor cells. Importantly, proPOLAR::CYCD3;1 did not influence the stomatal precursor cell density in wild type (which carries functional SMR4), whereas the ratio of the precursor cell density between wild type and smr4-1cr became greater in the presence of proPOLAR::CYCD3;1 (1.24 to 1.65). These results suggest that increase of stomatal precursors by CYCD3;1 requires the absence of SMR4 (Figure 7D). By contrast, proPOLAR::CYCD5;1 increased the stomatal precursor cells regardless of the presence or absence of SMR4, indicating that CYCD5;1 activity is SMR4-independent (Figures 7C and 7D). On the basis of these findings, we conclude that SMR4 can suppress the stomatal lineage divisions by direct association with CYCD3;1 and possibly with CYCD7;1, but not with CYCD5;1, and this differential interaction with CyclinDs underscores the transition from proliferative ACDs to final SCD (Figure 7E).
Discussion
In this study, we discovered that proliferative ACDs has faster cell-cycle duration than the single terminal SCD within the stomatal cell lineages. A subsequent genome-wide profiling of MUTE targets followed by phenotypic and functional characterizations identified SMR4 as a non-canonical CKI that sets a cell-cycle brake to facilitate transition from ACD to SCD. SMR4 is a direct MUTE target specifically induced by MUTE but not by SPCH (Figure 2), thus highlighting the orchestration of cell-state switch from proliferation (meristemoids) to differentiation (stomata) at the control of cell-cycle duration. This view is further supported by the findings that prolonged G1 phase specifically during the proliferative ACDs by stomatal lineage overexpression of SMR4 causes misspecification of guard cells (Figures 4 and 5).
In contrast of SIM and SMR1, known regulators of endoreduplication (Roeder et al., 2010; Wang et al., 2020), we found that SMR4 delays the G1/S transition during stomatal ACDs (Figure 6). It has been shown that SIM associates with CYCA2;3 but not with CYCD3;1 (Wang et al., 2020). Assuming that SMR1 functions similarly to SIM, the enlarged single-celled stomata conferred by the stomatal lineage overexpression of SMR1 (Figure 4) can be attributed to the direct inhibition of CYCA2;3-CDKB;1 complex by SMR1. Indeed, higher-order mutations in CYCA2s (cycA2;1, 2;2, 2,3 triple mutant) as well as the dominant-negative inhibition of CDKB1;1 exhibit the identical, single-celled stomata phenotypes (Boudolf et al., 2004; Vanneste et al., 2011). By contrast, we found that SMR4 functionally associates with CyclinDs (Figure 7). Thus, distinct functions among SIM/SMRs lie in their unique interaction potential with different cyclin-CDK complexes. During mammalian cell cycle, a series of CKIs exhibit inhibitory roles during G1/S transition via associating with CyclinD1/2/3-CDK4/6 and then with CyclinE/CDK2 complexes (Sherr and Roberts, 2004). Among them, p27KIP1 can bind with multiple cyclin-CDK complexes and exert different regulatory effects on each complex (Sherr and Roberts, 2004). Plants lack CyclinE, but the previous large-scale expression analysis of cell-cycle genes suggests that the plant CYCDs adopt both metazoan CyclinD and CyclinE functions (Menges et al., 2005). That SMR4 binds with different CyclinDs to negatively regulate G1/S phase therefore echoes its functional parallel to metazoan CKI, p27KIP1.
How could SMR4 decelerate cell cycle in proliferative ACDs but not in terminal SCD? Our results suggest that the specificity lies on preferential association of SMR4 with different CyclinDs, each with a unique expression pattern within the stomatal cell lineages. For example, CYCD3;1 and CYCD3;2 are induced by SPCH and promote ACDs (Adrian et al., 2015; Dewitte et al., 2007; Han et al., 2018; Lau et al., 2014). By contrast, CYCD5;1 is directly induced by MUTE to drive the terminal SCD (Han et al., 2018). CYCD7;1 is likely involved in the terminal SCD, however, its expression starts later and persists longer than CYCD5;1 (Han et al., 2018; Weimer et al., 2018). Based on the physical and functional associations of SMR4 with CYCD3;1 and CYCD7;1 but not with CYCD5;1, we propose the following model of regulatory circuit driving the asymmetric-to-symmetric division switch (Figure 7E): First, SPCH initiates and sustains the fast and reiterative ACDs of a meristemoid. During the meristemoid-to-GMC transition, MUTE directly induces SMR4, which directly associates with CYCD3;1 (and likely with CYCD3;2) and inhibit CYCD3;1-CKDA;1 complex to terminate amplifying ACDs. At the same time, MUTE directly induces CYCD5;1. Because CYCD5;1 is not directly inhibited by SMR4, the final SCD can start even in the presence of SMR4. SMR4 may fine-tune the SCD by being able to inhibit the later-expressed CYCD7;1, which is likely complexed with CDKA;1. The endogenous expression of SMR4 disappears immediately after the execution of SCD (Figure 2F); hence, the robust differentiation of stomata ensured. SMR8 has partially redundant role with SMR4 and is weakly induced by both SPCH and MUTE (Figures 2 and S2) as such, SMR8 is likely participating in fine-tuning of this transition. Unlike CycD3s and CycD7;1, CycD5;1 lacks part of the core domain (Strzalka et al., 2015), which may explain the differential SMR4 binding.
In addition to CKIs, Rb protein negatively regulates G1/S transition (Bertoli et al., 2013). The plant RETINOBLASTOMA RELATED (RBR) protein functions as key cell-cycle regulators during stomatal development, and its reduced expression confers excessive proliferative ACDs within the stomatal cell lineages, in part due to dysregulated SPCH expression (Borghi et al., 2010; Weimer et al., 2012). Whereas both CYCD3;1 and CYCD7;1 possess LxCxE RBR-binding motif, CYCD5;1 bears a variant motif, which may compromise the RBR association (Vandepoele et al., 2002). Thus, CYCD5;1's unique activity to execute the single SCD might involve the lack of negative regulation by RBR. Interestingly, RBR regulates the expression and activities of stomatal bHLH proteins, SPCH, and FAMA, respectively (Matos et al., 2014; Weimer et al., 2012), but not MUTE. Thus, the commitment to differentiation by MUTE-orchestrated network may be inherently resilient to inhibition at G1/S transition.
Our study showed that extended G1 phase by stomatal lineage overexpression of SMR4 conferred irregular-shaped meristemoids and eventual differentiation of stomata with skewed guard cells. Some guard cells exhibit a jigsaw-puzzled shape, which is indicative of pavement cell-like characteristics (Figures 4 and 5). Thus, without timely execution of an ACD, the stomatal precursor cell can adopt hybrid identity of a guard cell and pavement cell. An intrinsic polarity protein BASL ensures that only one of the two daughter cells, the meristemoid, maintains high SPCH levels, thereby able to reiterate proliferative ACDs (Dong et al., 2009). The remaining daughter cell readily loses SPCH protein and differentiate into a pavement cell. This process involves a dynamic subcellular re-localization of BASL protein between the nucleus and polarly localized cell cortex, the latter activates MAP kinase cascade that inhibits SPCH protein accumulation via phosphorylation (Zhang et al., 2015, 2016). It is not known whether the cell-cycle phase influences BASL behaviors, but our work implies that it could be the case.
The SMR4-mediated cell-cycle deceleration during the meristemoid-to-GMC transition mirrors the fundamental importance of G1-phase extension for cell fate decision and differentiation during development (Dalton, 2015; Liu et al., 2019). During mammalian adipogenesis, commitment of proliferating precursors to terminal differentiation is governed by the molecular competition of mitogens and differentiation stimuli at the G1-phase, in which timing determines the final numbers of adipocytes (Zhao et al., 2020). During plant stomatal differentiation, we found that the timing of G1-phase is not only critical for the commitment to differentiation but also maintaining the shape and size of stomatal lineage cells. In any event, the fine-tuning of the G1 phase duration may be the universal mechanism for proper cell-type differentiation in multicellular organisms. The direct role of MUTE to execute both termination of proliferative asymmetric divisions and orchestration of the single terminal symmetric division occurs through interwoven regulation of core cell-cycle drivers and a braker. Understanding how cell-cycle machineries in turn regulate the precise expression of MUTE, which likely involves epigenetic state changes, will provide a full picture of cell-cycle control of cell fate specification in plants.
Limitations of the study
We use CDT1a-CFP loading as a proxy for the G1 phase duration. We noticed that in stomatal lineage cells, CDF1a-CFP does not accumulate for the entire G1 phase. Nevertheless, it is clear that ACD is faster than SCD, based on both the quantitative analysis of the actual cell division time and the observed shorter duration of CDT1a-CFP signals in ACDs. In addition, the time-lapse imaging was performed using cotyledons grown under the microscope, and thus, it may not represent the exact cell-cycle time of stomatal precursors in vegetative leaves from field-grown plants.
STAR★Methods
Key resources table
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Anti-GFP antibody - ChIP Grade | Abcam | Abcam Cat. ab290, Lot. GR318425-1 RRID: AB_303395 |
| Bacterial and virus strains | ||
| Argrobacterium GV3101::pMP90 | (Koncz et al., 1992) | n/a |
| Chemicals, peptides, and recombinant proteins | ||
| Propidium iodide | Sigma-Aldrich | P4170 |
| FM4-64 | Invitrogen | T13320 |
| DAPI | Sigma-Aldrich | D9542 |
| cOmplete™, Mini Protease Inhibitor Cocktail | Roche | 11836153001 |
| β-Estradiol | Sigma-Aldrich | E2758 |
| 3-amino-1,2,4-triazole | Sigma-Aldrich | A8056 |
| Deposited data | ||
| Raw and processed MUTE ChIP-seq data | This study | GEO: GSE173338 |
| iMUTE RNA-seq data | (Han et al., 2018) | GEO: GSE107018 |
| iSPCH RNA-seq data | (Lau et al., 2014) | GEO: GSE57953 |
| TAIR10 Arabidopsis annotation | TAIR | ftp://ftp.arabidopsis.org/home/tair/Genes/ TAIR10_genome_release/ |
| Experimental models: Organisms/strains | ||
| Arabidopsis thaliana, Wild type (Col-0) | ABRC | CS1093 |
| Arabidopsis thaliana, mute | (Pillitteri et al., 2007) | n/a |
| Arabidopsis thaliana, mute-2 | (Pillitteri et al., 2008) | n/a |
| Arabidopsis thaliana, iMUTE | (Han et al., 2018) | n/a |
| Arabidopsis thaliana, iSPCH | (Han et al., 2018) | n/a |
| Arabidopsis thaliana, proMUTE::MUTE-GFP scrm-D | (Qi et al., 2017; Han et al., 2018) | n/a |
| Arabidopsis thaliana, proSMR4::nucGFP (nls-3xGFP) | This study | n/a |
| Arabidopsis thaliana, proSMR4::nucGFP mute-2 | This study | n/a |
| Arabidopsis thaliana, proSMR4::SMR4-YFP | This study | n/a |
| Arabidopsis thaliana, proSMR4::SMR4-YFP mute-2 | This study | n/a |
| Arabidopsis thaliana, proSMR4::HA-SMR4 smr4-1cr | This study | n/a |
| Arabidopsis thaliana, smr4-1cr | This study | n/a |
| Arabidopsis thaliana, smr4-2cr | This study | n/a |
| Arabidopsis thaliana, smr8-1 | ABRC | SALK_126253 |
| Arabidopsis thaliana, smr8-2 | ABRC | SALK_074523 |
| Arabidopsis thaliana, smr4-1cr smr8-2 | This study | n/a |
| Arabidopsis thaliana, proPOLAR::SMR4 | This study | n/a |
| Arabidopsis thaliana, proPOLAR::SMR8 | This study | n/a |
| Arabidopsis thaliana, proPOLAR::SMR1 | This study | n/a |
| Arabidopsis thaliana, proPOLAR::KRP1 | This study | n/a |
| Arabidopsis thaliana, proTMM::GUS-GFP | ABRC (Nadeau and Sack, 2002) | CS65759 |
| Arabidopsis thaliana, proPOLAR::SMR4 proTMM::GUS-GFP | This study | n/a |
| Arabidopsis thaliana, proMUTE::nucYFP | (Qi et al., 2017) | n/a |
| Arabidopsis thaliana, proPOLAR::SMR4 pMUTE::nucYFP | This study | n/a |
| Arabidopsis thaliana, E994 | ABRC (Pillitteri et al., 2007) | CS70070 |
| Arabidopsis thaliana, proPOLAR::SMR4 E994 | This study | n/a |
| Arabidopsis thaliana, proSMR1::nlsGFP-GUS | (Bhosale et al., 2018) | n/a |
| Arabidopsis thaliana, proPOLAR::SMR4 proSMR1::nlsGFP-GUS | This study | n/a |
| Arabidopsis thaliana, PlaCCI | (Desvoyes et al., 2020) | n/a |
| Arabidopsis thaliana, LTI6b-GFP | (Kurup et al., 2005) ABRC | CS84726 |
| Arabidopsis thaliana, PlaCCI Lti6b-GFP | This study | n/a |
|
Arabidopsis thaliana, PlaCCI Lti6b-GFP proPOLAR::SMR4 |
This study | n/a |
| Arabidopsis thaliana, proPOLAR::SMR4 smr4-1cr | This study | n/a |
| Arabidopsis thaliana, proPOLAR::CYCD3;1 | This study | n/a |
| Arabidopsis thaliana, proPOLAR::CYCD3;1 smr4-1cr | This study | n/a |
| Arabidopsis thaliana, proPOLAR::CYCD5;1 | This study | n/a |
| Arabidopsis thaliana, proPOLAR::CYCD5;1 smr4-1cr | This study | n/a |
| Arabidopsis thaliana, proPOLAR::CYCD7;1 | This study | n/a |
| Arabidopsis thaliana, proPOLAR::CYCD7;1 smr4-1cr | This study | n/a |
| Arabidopsis thaliana, proSMR4::HA-SMR4 smr4-1cr | This study | n/a |
| Arabidopsis thaliana, proPOLAR::SMR4 mute | This study | n/a |
| Arabidopsis thaliana, proRPS5a::H2B-GFP | (Maruyama et al., 2013) | n/a |
| Arabidopsis thaliana, proPOLAR::SMR4 proRPS5a::H2B-GFP | This study | n/a |
| Saccharomyces cerevisiae (AH109 strain): | Clontech, (James et al., 1996) | n/a |
| AH109, pGBKT7::SMR4, pGADT7 | This study | n/a |
| AH109, pGBKT7::SMR4, pGADT7::CYCD3;1 | This study | n/a |
| AH109, pGBKT7::SMR4, pGADT7::CYCD5;1 | This study | n/a |
| AH109, pGBKT7::SMR4, pGADT7::CYCD7;1 | This study | n/a |
| AH109, pGBKT7::SMR4, pGADT7::CDKA;1 | This study | n/a |
| AH109, pGBKT7::SMR4, pGADT7::CDKB1;1 | This study | n/a |
| AH109, pGBKT7, pGADT7::CYCD3;1 | This study | n/a |
| AH109, pGBKT7, pGADT7::CYCD5;1 | This study | n/a |
| AH109, pGBKT7, pGADT7::CYCD7;1 | This study | n/a |
| AH109, pGBKT7, pGADT7::CDKA;1 | This study | n/a |
| AH109, pGBKT7, pGADT7::CDKB1;1 | This study | n/a |
| Oligonucleotides | ||
| Plasmid construction | Table S3, this paper | n/a |
| Single guide RNA for smr4 CRISPRing | Table S3, this paper | n/a |
| qRT-PCR primer/ genotyping | Table S3, this paper | n/a |
| Recombinant DNA | ||
| pKI1.1R | (Tsutsui and Higashiyama, 2017) | Addgene #85808 |
| pGWB440 | (Nakagawa et al., 2007) | Addgene #74826 |
| R4pGWB501 | (Nakagawa et al., 2008) | n/a |
| Other recombinant DNAs generated in this study | Table S3, this paper | n/a |
| Software and algorithms | ||
| Heatmapper | (Babicki et al., 2016) | http://www.heatmapper.ca/ |
| CCTop - CRISPR/Cas9 target online predictor | (Stemmer et al., 2015) | https://cctop.cos.uni-heidelberg.de:8043/ |
| R ver. 4.0.2 | R Development Core Team, 2008 | https://www.r-project.org/ |
| R ggplot2 package | (Wickham, 2016) | n/a |
| BoxPlotR | (Spitzer et al., 2014) | http://shiny.chemgrid.org/boxplotr/ |
| COLORBREWER 2.0 | Penn State Univ. | http://www.ColorBrewer.org |
| FIJI-ImageJ | (Schindelin et al., 2012) | https://imagej.net/Fiji |
| Bowtie2 | (Langmead and Salzberg, 2012) | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Samtools | (Li et al., 2009) | http://samtools.sourceforge.net/ |
| MACS (version 2.1.0.20140616) | (Feng et al., 2012) | http://liulab.dfci.harvard.edu/MACS |
| PANTHER | (Mi et al., 2019) | http://geneontology.org/ |
| iGV | (Robinson et al., 2011) | https://software.broadinstitute.org/software/igv/ |
| Other | ||
| RNeasy Plant mini kit | Qiagen | 74904 |
| ACCEL-NGS® 2S PLUS DNA LIBRARY KIT with 2S Set A MID Indexing Kit | Swift bioscience | 21024, 26148 |
| ChIP DNA Clean & Concentrator | Zymo Research | D5205 |
| ReverTra Ace™ qPCR RT Master Mix with gDNA Remover | TOYOBO | FSQ-301 |
| KAPA SYBR® FAST for LightCycle® 480 | KAPA Biosystems | KK4611 |
| Dynabeads™ Protein G | invitrogen | 1004D |
| SPRIselect | BECKMAN COULTER | B23317 |
| NEBuilder® HiFi DNA Assembly Master Mix | NEB | E2621 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact, Keiko U. Torii (ktorii@utexas.edu).
Materials availability
Plasmids and transgenic plants generated in this study will be available from the lead contact upon request.
Experimental model and subject details
The Arabidopsis Columbia (Col) accession was used for wild type. The loss-of function mutants, complementation and reporter transgenic lines were reported listed in the key resources table. The T-DNA insertion mutants were obtained from the Arabidopsis Biological Resource Center (ABRC) at Ohio State Univ. CRISPR gene editing was performed to obtain SMR4 knock-out mutants. Transgenic lines were introduced into respective mutant backgrounds by genetic crosses or Agrobacterium (GV3101 pMP90 strain)-mediated transformation (see method details and key resources table for the lines generated) and genotypes were confirmed by PCR or Sanger sequencing. All recombinant DNAs for transgenes introduced to Arabidopsis are listed in Table S3. For sequence of genotyping primers, sequencing, and cloning see Table S3. Seedlings and plants were grown in a long-day or constant light condition at 22°C. For yeast two hybrid analysis, AH109 strain was used and resulting transformants were grown at 30°C.
Method details
Plasmid construction and generation of CRISPR-based mutant alleles
For a detailed information of constructs generated in this study, see Table S3. Primers used for plasmid constructs were listed in Table S3. For generation of transgenic Arabidopsis, plasmid constructs were electroporated into Agrobacterium (GV3101/pMP90) and subsequently transformed by floral dipping. Loss-of-function mutant of SMR4 was generated by CRISPR by using pKAMA-ITACHI Vector (Addgene: 85808) as described previously (Tsutsui and Higashiyama, 2017). Briefly, primers for sgRNA were designed by the CCTop - CRISPR/Cas9 target online predictor (Stemmer et al., 2015). Primers were annealed and inserted into pKI1.1R vector cut with AarI. Resulting construct was introduced into wild-type Col-0 plants. T1 plants were screened by Hygromycin resistance. Six T1 lines were selected and sequenced to check whether mutations were introduced. One of the two sgRNAs was successful for generating mutations. In T2 generation, seeds that do not show OLE1-RFP signal were selected to exclude plants harboring transgene in the genome. We established two independent homozygous lines that contain 1 bp deletion (smr4-1cr) or 1 bp insertion (smr4-2cr) at SMR4 gene (Figure S4). Primers used single guide RNA (sgRNA) for SMR4 were listed in Table S3.
Plant growth condition and estradiol treatment
Arabidopsis accession Columbia (Col-0) was used as wild-type. The following mutants/transgenic lines are reported elsewhere: mute-2 (Pillitteri et al., 2008); Lti6b (Kurup et al., 2005); PlaCCI (Desvoyes et al., 2020). T-DNA insertion mutants of SMR8, smr8-1 (SALK_126253), smr8-2 (SALK_074523) were obtained from ABRC. Their genotype and transcript reduction were confirmed. The following higher-order mutants/marker lines were generated by genetic crosses: smr4-1cr smr8-1, proPOLAR::SMR4 mute-2, proSMR4::SMR4-YFP mute-2, proSMR4::nucGFP mute-2, PlaCCI Lti6b, proPOLAR::SMR4 PlaCCI, and smr4-1cr PlaCCI Lti6b. The presence of transgenes/mutant alleles were confirmed by genotyping. All plant materials used in this study were listed in key resources table. Sterilized seeds were grown on half strength of Murashige and Skoog (MS) media with 1% sucrose at 22°C under continuous light and 10∼14-day-old seedlings grown on MS media were transplanted to soil to harvest seeds. For phenotyping of smr mutants, cotyledons at 4-day post germination stage were imaged. For phenotyping of transgenic plants of proPOLAR::CKIs, T2 plants were grown on ½ MS agar media containing hygromycin (15 μg/ml), and imaged at day 4 and day 8. For proPOLAR::CYCD transgenic plants, multiple independent T1 plants were selected from ½ MS agar media containing hygromycin (15 μg/ml) and imaged at day 7. For the complementation test of SMR4, T3 homozygous plants of proSMR4::HA-SMR4 were germinated on ½ MS agar media were imaged at 5-day post germination (dpg).
Confocal microscopy
For confocal microscopy, cell peripheries of seedlings were visualized with either propidium iodide (Sigma, P4170) or FM4-64 (Invitrogen, T13320). Images were acquired using LSM800 (Zeiss) or SP5-WLL (Leica) using a 63x water lens. The Zeiss LSM800 was used to image the GFP and RFP reporter with excitation at 488 nm and an emission filter of 490 to 546 nm, and with excitation at 555 nm and 583-617 nm emission range, respectively. PlaCCI lines (Desvoyes et al., 2020) were imaged using SP5-WLL with the following conditions: CFP, excitation at 458 nm and emission from 468 to 600 nm; GFP, excitation at 488 nm and emission from 490 to 546 nm; YFP, excitation at 514 nm and emission from 524 to 650 nm; mCherry, excitation at 560 nm and emission from 590 to 650 nm. Signals were visualized sequentially using separate HyD detectors. DIC images were taken to delineate the cell outlines (shown in magenta). Raw data were collected with 1024 x 1024 pixel image and imported into Fiji-ImageJ to generate CYMK images using the channel merge function. The time-lapse were collected at 30-min intervals using a 20x lens (Peterson and Torii, 2012; Qi et al., 2017). Raw images were imported into Fiji-ImageJ to generate time projections using the Stacks function. For higher quality time-lapse imaging of PlaCCI x LTi6b lines in different genetic backgrounds, we used Leica Stellaris 8 FALCON with the following conditions: CFP, excitation at 458 nm and emission from 464 to 510 nm; YFP excitation at 514 nm and emission from 520 to 560 nm; mCherry, excitation at 561 nm and emission from 570 to 620 nm. Signals were visualized sequentially using separate HyD detectors (HyDX/HyDS) in TauSeparation mode. The time-lapses were collected at 30-min intervals using a 63x oil-lens, zoom factor 1.5. Raw data were collected with 512 x 512 pixel image and imported into Fiji-ImageJ v1.8.0_66 to generate RGB images/z-stacks using the channel merge function. To correct for drift of multichannel z-stacks the “StackReg” plugin was applied.
Quantitative analysis of epidermal phenotype
For quantitative analysis of abaxial leaf epidermis of smr mutants and transgenic plants, confocal images were taken at the days after germination as indicated in the Figure legends. Preparation of images was done as described previously (Houbaert et al., 2018). For counting epidermal cell types, stomatal density, stomatal precursor cells (meristemoids and GMC), total epidermal cells (stomata, meristemoids, GMC and pavement cells) and stomata index (number of stomata / number of total epidermal cells x100) were calculated by counting cell types in an area of 0.45 mm2 (0.67 mm x 0.67 mm) at the developmental stage indicated with the cell counter plug-in in Fiji, and plotted as per mm2. The epidermal cell areas of smr mutants were color-coded-coded depending on the area calculated using ROI_Color_Coder with a range of min-max (0-4000) in Fiji. The epidermal cells were subdivided into 9 groups according to their size. One representative image from each genotype was analyzed and the cell size distribution was then calculated from 499 cells for Col-0 plants, 659 cells for smr4-1, 662 cells for smr4-2, 601 cells form smr8-1, 611 cells form smr8-1 and 755 cells for smr4-1cr smr8-1 double mutant. Guard cells were excluded for the cell size measurement.
For cell size and circularity measurement of stomatal lineage precursors, images of proTMM::GUS-GFP were set to grayscale and GFP expressing cells were colored in black while other cell area in white by photoshop, then the images were imported to Fiji. Imported images were subjected to Images > Threshold; Analyze > analyze particle. Shape descriptors box has to be checked in “Set measurement” under the Analyze tab to get circularity values from the selected cell area. For the meristemoid size in mute mutant background was measured by the same methods.
cDNA preparation and qRT-PCR
For chemical treatment, plants were grown on media containing either 10uM β-estradiol (Sigma, E8875) or DMSO. For time-course induction, estradiol-inducible MUTE or SPCH seeds were sown on 1/2 MS media, and subjected to stratification at 4°C for two to three days then grown for the four to five days under continuous light. Subsequent steps were performed as described Han et al. (2018) (Han et al., 2018). RNA was isolated using RNeasy Plant Mini Kit (Qiagen, 74904). 0.5 μg of RNA was converted to cDNA using ReverTra Ace™ qPCR RT Master Mix with gDNA Remover (TOYOBO, FSQ-301) according to the instructions of the manufacturer. qRT-PCR was performed as described in Han et al. (2018) (Han et al., 2018) using KAPA SYBR® FAST qPCR Kit Master Mix on LightCycler® 96 instrument (Roche). Relative expression was calculated by dividing ACTIN2 gene expression over the specific-gene expression and the fold change was calculated by dividing estradiol expression over DMSO (mock) expression at each time point indicated. See Table S3 for primer sequences used for qRT-PCR.
Chromatin immunoprecipitation sequencing (ChIP-seq)
For MUTE ChIP-seq experiments, transgenic plants proMUTE::MUTE-GFP scrm-D were prepared as described previously (Han et al., 2018) with following modifications. To shear the DNA, Bioruptor (Diagnode) was used, 30 sec on and 30 sec off cycle 15∼18 times. Immunoprecipitation against GFP was performed using anti-GFP antibody (Abcam, ab290, Lot. GR318425-1). DNAs from the immuno-complex was purified by kit (Zymo Research, D5205). The half of the purified DNA was subjected to library preparation using ACCEL-NGS® 2S PLUS DNA LIBRARY KIT with 2S Set A MID Indexing Kit (Swift bioscience, 21024, 26148) for next generation sequencing. Quantitative PCR (qPCR) was carried out using gene specific primers (Table S3) to confirm the library construction. The qPCR was run using KAPA SYBR® FAST qPCR Kit Master Mix on LightCycler® 96 instrument (Roche) as previously described (Han et al., 2018). Three biological replicates were used for MUTE ChIP-seq experiments. Size distribution of the libraries was validated by 2100 Bioanalyzer (Agilent). The prepared libraries (Col input and IP, MUTE-GFP input and IP with three replicates) were sequenced 35 bp paired-end in length with 30million coverage per sample on Illumina NextSeq 500 system. ChIP mapping and peak calling were performed as described in Feng et al. (2012) (Feng et al., 2012). Output reads were mapped to the TAIR10 genome assembly using bowtie2 and resulting bam files were sorted and indexed via samtool. The sorted bam files were subjected for MACS peak calling (version 2.1.0.20140616) (Table S2). Bedgraph file was generated and visualized in igv browser. Gene Ontology enrichment analysis was performed using GENE ONTOLOGY (http://geneontology.org/) combined with manual curation to remove redundant terms. Genes increased by MUTE more than Log2 FC (Fold Change) 0.4 and targeted by MUTE were tested (Table S2). Following multiple hypothesis testing correction (Bonferroni-correction), GO term with FDR <0.05 were called significantly enriched (Table S2). The ChIP-seq data generated in this study are deposited to the NCBI with an accession number GEO: GSE173338.
Measurement of DNA content and nuclei size
1st true leaves were harvested from 16-day old plants and fixed in a solution of 9:1 (v/v) ethanol: acetic acid for overnight. For DAPI staining, tissues were rehydrated with ethanol series. DAPI (4’6-diamidino-2’-phenylindole) staining was done in 5μg/ml final concentration for 15 minutes in dark. Nuclear DAPI fluorescence was excited at 405 nm captured with 410 -470 nm emission range. DAPI stained nuclei area from the guard cells was selected and measured by using FIJI software. Wild-type and two independent T2 proPOLAR::SMR4 transgenic lines were used. The number of guard cells measured is 129 (WT) and 234 (proPOLAR::SMR4). 10 or 11-day-old cotyledons from proRPS5A::H2B-GFP (Maruyama et al., 2013) transgenic plants were also imaged to measure the nuclei size of guard- and pavement cells. The area of nuclei (GFP) was selected and measured from the z-stack projection images using FIJI software. Number of guard cells and pavement cells measured; 155 and 108 (wild type), 191 and 103 (proPOLAR::SMR4 proRPS5A::H2B-GFP). DAPI-stained nuclear area of single-celled GCs in proPOLAR::SMR1 (n = 24) and normal GCs in wild type (n = 102) in 12-day old true leaves was measured.
Yeast two hybrid assay
Y2H assays were performed using the Matchmaker™ Two-Hybrid System (Clontech). Bait (pGBKT7) and prey (pGADT7) constructs were co-transformed into the yeast strain AH109 according to manufactural instruction (Clontech). The resulting transformants were spotted on SD (−Leu, −Trp) and SD (−Trp, −Leu, −His) selection media containing different concentration of 3-amino-1,2,4-triazole (Sigma, A8056) as previously reported (Putarjunan et al., 2019). All constructs and primer information are listed in the Table S3.
Quantification and statistical analysis
A series of Z-stack confocal images were taken to obtain images covering the epidermis and capturing GFP signal from the reporter lines. The area and the number of epidermal cells were quantified by using FIJI-ImageJ. Statistical analyses were performed using R ver. 4.0.2. For the multiple sample comparison, one-way ANOVA with post-hoc Tukey HSD test was performed. For the two-sample comparison, either student t-test or Mann-Whitney U test were performed. Graphs were generated using R ggplot2 package, BoxPlotR or Microsoft Excel (Listed in key resources table). The color of Boxplot graph was based on ColorBrewer.org. The value of n, the number of each experiment or samples, means of error bars, and how statistical significance was defined are indicated in a relevant figure legend.
Acknowledgments
We thank Lieven De Veylder for proSMR1::nls-GFP-GUS, Daisuke Kurihara for proRPS5A::H2B-GFP, ABRC for GFP-Lti6B and SMR8 T-DNA lines, and James Green, Machiko Arakawa and Ayami Nakagawa for assistance in plant care. This work is supported by MEXT KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas (17H06476), WPI-ITbM, and the start-up funds from the UT Austin to K.U.T.; grant RTI2018-094793-B-I00 from Spanish Ministry of Science and Innovation and grant 2018-AdG_833617 from European Research Council to C.G. K.U.T. acknowledges the support from Howard Hughes Medical Institute and Johnson & Johnson Centennial Chair in Plant Cell Biology at the UT Austin. S.-K.H. was supported by the Young Leader Cultivation Program from Nagoya University. A.H. is supported by the Walter Benjamin Program, Deutsche Forschungsgemeinschaft (447617898).
Author contributions
Conceptualization, S.-K.H. and K.U.T.; experimental design, S.-K.H., E.-D.K., and K.U.T.; performance of experiments, S.-K.H., A.H., J.Y., R.I., and T.S.; bioinformatics analysis, S.-K.H., S.K., E.-D.K., and K.U.T.; visualization, S.-K.H., A.H., J.Y., and K.U.T.; essential materials and tools, B.D. and C.G.; Writing – original draft, S.-K.H. and K.U.T.; Writing – review & editing, S.-K.H., A.H., J.Y., B.D., C.G., E.-D.K., and K.U.T.; project administration, K.U.T.; funding acquisition, C.G. and K.U.T.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community.
Published: February 10, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.devcel.2022.01.014.
Supplemental information
Data and code availability
-
•
The ChIP-seq data generated in this study have been deposited at the NCBI Gene Expression Omnibus (GEO: GSE173338) and are publicly available. All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Adrian J., Chang J., Ballenger C.E., Bargmann B.O., Alassimone J., Davies K.A., Lau O.S., Matos J.L., Hachez C., Lanctot A., et al. Transcriptome dynamics of the stomatal lineage: birth, amplification, and termination of a self-renewing population. Dev. Cell. 2015;33:107–118. doi: 10.1016/j.devcel.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babicki S., Arndt D., Marcu A., Liang Y., Grant J.R., Maciejewski A., Wishart D.S. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli C., Skotheim J.M., de Bruin R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013;14:518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale R., Boudolf V., Cuevas F., Lu R., Eekhout T., Hu Z., Van Isterdael G., Lambert G.M., Xu F., Nowack M.K., et al. A spatiotemporal DNA endoploidy map of the Arabidopsis Root reveals roles for the endocycle in root development and stress adaptation. Plant Cell. 2018;30:2330–2351. doi: 10.1105/tpc.17.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi L., Gutzat R., Fütterer J., Laizet Y., Hennig L., Gruissem W. Arabidopsis RETINOBLASTOMA-RELATED is required for stem cell maintenance, cell differentiation, and lateral organ production. Plant Cell. 2010;22:1792–1811. doi: 10.1105/tpc.110.074591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V., Barrôco R., de Almeida Engler J., Verkest A., Beeckman T., Naudts M., Inzé D., De Veylder L. B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana. Plant Cell. 2004;16:945–955. doi: 10.1105/tpc.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budirahardja Y., Gönczy P. Coupling the cell cycle to development. Development. 2009;136:2861–2872. doi: 10.1242/dev.021931. [DOI] [PubMed] [Google Scholar]
- Coronado D., Godet M., Bourillot P.-Y., Tapponnier Y., Bernat A., Petit M., Afanassieff M., Markossian S., Malashicheva A., Iacone R., et al. A short G1 phase is an intrinsic determinant of naive embryonic stem cell pluripotency. Stem Cell Res. 2013;10:118–131. doi: 10.1016/j.scr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Dalton S. Linking the cell cycle to cell fate decisions. Trends Cell Biol. 2015;25:592–600. doi: 10.1016/j.tcb.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvoyes B., Arana-Echarri A., Barea M.D., Gutierrez C. A comprehensive fluorescent sensor for spatiotemporal cell cycle analysis in Arabidopsis. Nat. Plants. 2020;6:1330–1334. doi: 10.1038/s41477-020-00770-4. [DOI] [PubMed] [Google Scholar]
- Desvoyes B., Gutierrez C. Roles of plant retinoblastoma protein: cell cycle and beyond. EMBO J. 2020;39:e105802. doi: 10.15252/embj.2020105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W., Scofield S., Alcasabas A.A., Maughan S.C., Menges M., Braun N., Collins C., Nieuwland J., Prinsen E., Sundaresan V., Murray J.A.H. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. USA. 2007;104:14537–14542. doi: 10.1073/pnas.0704166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., MacAlister C.A., Bergmann D.C. BASL controls asymmetric cell division in Arabidopsis. Cell. 2009;137:1320–1330. doi: 10.1016/j.cell.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S.J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Feng J., Liu T., Qin B., Zhang Y., Liu X.S. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 2012;7:1728–1740. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C., Ohashi-Ito K., Dong J., Bergmann D.C. Differentiation of Arabidopsis guard cells: analysis of the networks incorporating the basic helix-loop-helix transcription factor, FAMA. Plant Physiol. 2011;155:1458–1472. doi: 10.1104/pp.110.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdoun S., Zhang C., Gill M., Kumar N., Churchman M., Larkin J.C., Kwon A., Lu H. Differential roles of two homologous cyclin-dependent kinase inhibitor genes in regulating cell cycle and innate immunity in Arabidopsis. Plant Physiol. 2016;170:515–527. doi: 10.1104/pp.15.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.-K., Qi X., Sugihara K., Dang J.H., Endo T.A., Miller K.L., Kim E.-D., Miura T., Torii K.U. MUTE directly orchestrates cell-state switch and the single symmetric division to create stomata. Dev. Cell. 2018;45:303–315.e5. doi: 10.1016/j.devcel.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Han S.-K., Torii K.U. Lineage-specific stem cells, signals and asymmetries during stomatal development. Development. 2016;143:1259–1270. doi: 10.1242/dev.127712. [DOI] [PubMed] [Google Scholar]
- Harashima H., Dissmeyer N., Schnittger A. Cell cycle control across the eukaryotic kingdom. Trends Cell Biol. 2013;23:345–356. doi: 10.1016/j.tcb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Houbaert A., Zhang C., Tiwari M., Wang K., de Marcos Serrano A., Savatin D.V., Urs M.J., Zhiponova M.K., Gudesblat G.E., Vanhoutte I., et al. POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature. 2018;563:574–578. doi: 10.1038/s41586-018-0714-x. [DOI] [PubMed] [Google Scholar]
- Inzé D., De Veylder L. Cell cycle regulation in plant development. Annu. Rev. Genet. 2006;40:77–105. doi: 10.1146/annurev.genet.40.110405.090431. [DOI] [PubMed] [Google Scholar]
- James P., Halladay J., Craig E.A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Németh K., Rédei G.P., Schell J. T-DNA insertional mutagenesis in Arabidopsis. Plant Mol. Biol. 1992;20:963–976. doi: 10.1007/BF00027166. [DOI] [PubMed] [Google Scholar]
- Kumar N., Harashima H., Kalve S., Bramsiepe J., Wang K., Sizani B.L., Bertrand L.L., Johnson M.C., Faulk C., Dale R., et al. Functional conservation in the SIAMESE-RELATED family of cyclin-dependent kinase inhibitors in land plants. Plant Cell. 2015;27:3065–3080. doi: 10.1105/tpc.15.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Larkin J.C. Why do plants need so many cyclin-dependent kinase inhibitors? Plant Signal. Behav. 2017;12:e1282021. doi: 10.1080/15592324.2017.1282021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup S., Runions J., Köhler U., Laplaze L., Hodge S., Haseloff J. Marking cell lineages in living tissues. Plant J. 2005;42:444–453. doi: 10.1111/j.1365-313X.2005.02386.x. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O.S., Bergmann D.C. Stomatal development: a plant's perspective on cell polarity, cell fate transitions and intercellular communication. Development. 2012;139:3683–3692. doi: 10.1242/dev.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O.S., Davies K.A., Chang J., Adrian J., Rowe M.H., Ballenger C.E., Bergmann D.C. Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science. 2014;345:1605–1609. doi: 10.1126/science.1256888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Michowski W., Kolodziejczyk A., Sicinski P. The cell cycle in stem cell proliferation, pluripotency and differentiation. Nat. Cell Biol. 2019;21:1060–1067. doi: 10.1038/s41556-019-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister C.A., Ohashi-Ito K., Bergmann D.C. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. [DOI] [PubMed] [Google Scholar]
- Maruyama D., Hamamura Y., Takeuchi H., Susaki D., Nishimaki M., Kurihara D., Kasahara R.D., Higashiyama T. Independent control by each female gamete prevents the attraction of multiple pollen tubes. Dev. Cell. 2013;25:317–323. doi: 10.1016/j.devcel.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Matos J.L., Lau O.S., Hachez C., Cruz-Ramírez A., Scheres B., Bergmann D.C. Irreversible fate commitment in the Arabidopsis stomatal lineage requires a FAMA and RETINOBLASTOMA-RELATED module. Elife. 2014;3:e03271. doi: 10.7554/eLife.03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaragno J.E., Mehrotra B., Coleman A.W. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell. 1993;5:1661–1668. doi: 10.1105/tpc.5.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M., de Jager S.M., Gruissem W., Murray J.A. Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J. 2005;41:546–566. doi: 10.1111/j.1365-313X.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Ebert D., Huang X., Thomas P.D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47:D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.O. New Science Press; 2007. The Cell Cycle : Principles of Control. [Google Scholar]
- Nadeau J.A., Sack F.D. Control of stomatal distribution on the Arabidopsis leaf surface. Science. 2002;296:1697–1700. doi: 10.1126/science.1069596. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Nakamura S., Tanaka K., Kawamukai M., Suzuki T., Nakamura K., Kimura T., Ishiguro S. Development of R4 gateway binary vectors (R4pGWB) enabling high-throughput promoter swapping for plant research. Biosci. Biotechnol. Biochem. 2008;72:624–629. doi: 10.1271/bbb.70678. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Suzuki T., Murata S., Nakamura S., Hino T., Maeo K., Tabata R., Kawai T., Tanaka K., Niwa Y., et al. Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 2007;71:2095–2100. doi: 10.1271/bbb.70216. [DOI] [PubMed] [Google Scholar]
- Peres A., Churchman M.L., Hariharan S., Himanen K., Verkest A., Vandepoele K., Magyar Z., Hatzfeld Y., Van Der Schueren E., Beemster G.T., et al. Novel plant-specific cyclin-dependent kinase inhibitors induced by biotic and abiotic stresses. J. Biol. Chem. 2007;282:25588–25596. doi: 10.1074/jbc.M703326200. [DOI] [PubMed] [Google Scholar]
- Peterson K.M., Torii K.U. Long-term, high-resolution confocal time lapse imaging of Arabidopsis cotyledon epidermis during germination. J. Vis. Exp. 2012;70:4426. doi: 10.3791/4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri L.J., Bogenschutz N.L., Torii K.U. The bHLH protein, MUTE, controls differentiation of stomata and the hydathode pore in Arabidopsis. Plant Cell Physiol. 2008;49:934–943. doi: 10.1093/pcp/pcn067. [DOI] [PubMed] [Google Scholar]
- Pillitteri L.J., Peterson K.M., Horst R.J., Torii K.U. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell. 2011;23:3260–3275. doi: 10.1105/tpc.111.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri L.J., Sloan D.B., Bogenschutz N.L., Torii K.U. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- Putarjunan A., Ruble J., Srivastava A., Zhao C., Rychel A.L., Hofstetter A.K., Tang X., Zhu J.-K., Tama F., Zheng N., Torii K.U. Bipartite anchoring of SCREAM enforces stomatal initiation by coupling MAP kinases to SPEECHLESS. Nat. Plants. 2019;5:742–754. doi: 10.1038/s41477-019-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Han S.-K., Dang J.H., Garrick J.M., Ito M., Hofstetter A.K., Torii K.U. Autocrine regulation of stomatal differentiation potential by EPF1 and ERECTA-LIKE1 ligand-receptor signaling. Elife. 2017;6:e24102. doi: 10.7554/eLife.24102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C., Huntley R., Jacqmard A., Murray J.A. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder A.H., Chickarmane V., Cunha A., Obara B., Manjunath B.S., Meyerowitz E.M. Variability in the control of cell division underlies sepal epidermal patterning in Arabidopsis thaliana. PLoS Biol. 2010;8:e1000367. doi: 10.1371/journal.pbio.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L., Dewitte W., Forzani C., Patell F., Nieuwland J., Wen B., Quelhas P., De Jager S., Titmus C., Campilho A., et al. The Arabidopsis D-type cyclin CYCD2;1 and the inhibitor ICK2/KRP2 modulate auxin-induced lateral root formation. Plant Cell. 2011;23:641–660. doi: 10.1105/tpc.110.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E.M., Roeder A.H. Transcriptomic effects of the cell cycle regulator LGO in Arabidopsis Sepals. Front. Plant Sci. 2016;7:1744. doi: 10.3389/fpls.2016.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C.J., Roberts J.M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Sozzani R., Cui H., Moreno-Risueno M.A., Busch W., Van Norman J.M., Vernoux T., Brady S.M., Dewitte W., Murray J.A., Benfey P.N. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466:128–132. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M., Wildenhain J., Rappsilber J., Tyers M. BoxPlotR: a web tool for generation of box plots. Nat. Methods. 2014;11:121–122. doi: 10.1038/nmeth.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer M., Thumberger T., Del Sol Keyer M., Wittbrodt J., Mateo J.L. CCTop: an intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS One. 2015;10:e0124633. doi: 10.1371/journal.pone.0124633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalka W.K., Aggarwal C., Krzeszowiec W., Jakubowska A., Sztatelman O., Banas A.K. Arabidopsis PCNAs form complexes with selected D-type cyclins. Front. Plant Sci. 2015;6:516. doi: 10.3389/fpls.2015.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H., Higashiyama T. pKAMA-ITACHI vectors for highly efficient CRISPR/Cas9-mediated gene knockout in Arabidopsis thaliana. Plant Cell Physiol. 2017;58:46–56. doi: 10.1093/pcp/pcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leene J., Hollunder J., Eeckhout D., Persiau G., Van De Slijke E., Stals H., Van Isterdael G., Verkest A., Neirynck S., Buffel Y., et al. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol. Syst. Biol. 2010;6:397. doi: 10.1038/msb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K., Raes J., De Veylder L., Rouzé P., Rombauts S., Inzé D. Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell. 2002;14:903–916. doi: 10.1105/tpc.010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., Coppens F., Lee E., Donner T.J., Xie Z., Van Isterdael G., Dhondt S., De Winter F., De Rybel B., Vuylsteke M., et al. Developmental regulation of CYCA2s contributes to tissue-specific proliferation in Arabidopsis. EMBO J. 2011;30:3430–3441. doi: 10.1038/emboj.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatén A., Soyars C.L., Tarr P.T., Nimchuk Z.L., Bergmann D.C. Modulation of asymmetric division diversity through cytokinin and SPEECHLESS regulatory interactions in the Arabidopsis stomatal lineage. Dev. Cell. 2018;47:53–66.e5. doi: 10.1016/j.devcel.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.D., Oppenheimer D.G., Concienne J., Larkin J.C. SIAMESE, a gene controlling the endoreduplication cell cycle in Arabidopsis thaliana trichomes. Development. 2000;127:3931–3940. doi: 10.1242/dev.127.18.3931. [DOI] [PubMed] [Google Scholar]
- Wang K., Ndathe R.W., Kumar N., Zeringue E.A., Kato N., Larkin J.C. The CDK inhibitor SIAMESE targets both CDKA;1 and CDKB1 complexes to establish endoreplication in trichomes. Plant Physiol. 2020;184:165–175. doi: 10.1104/pp.20.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer A.K., Matos J.L., Sharma N., Patell F., Murray J.A.H., Dewitte W., Bergmann D.C. Lineage- and stage-specific expressed CYCD7;1 coordinates the single symmetric division that creates stomatal guard cells. Development. 2018;145:dev160671. doi: 10.1242/dev.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer A.K., Nowack M.K., Bouyer D., Zhao X., Harashima H., Naseer S., De Winter F., Dissmeyer N., Geldner N., Schnittger A. Retinoblastoma related1 regulates asymmetric cell divisions in Arabidopsis. Plant Cell. 2012;24:4083–4095. doi: 10.1105/tpc.112.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Use R! Springer; 2016. ggplot2 : elegant graphics for data analysis; p. 1. online resource (XVI, 260 pages 232 illustrations, 140 illustrations in color. [Google Scholar]
- Xie Z., Lee E., Lucas J.R., Morohashi K., Li D., Murray J.A., Sack F.D., Grotewold E. Regulation of cell proliferation in the stomatal lineage by the Arabidopsis MYB four LIPS via direct targeting of core cell cycle genes. Plant Cell. 2010;22:2306–2321. doi: 10.1105/tpc.110.074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guo X., Dong J. Phosphorylation of the polarity protein BASL differentiates asymmetric cell fate through MAPKs and SPCH. Curr. Biol. 2016;26:2957–2965. doi: 10.1016/j.cub.2016.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang P., Shao W., Zhu J.-K., Dong J. The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev. Cell. 2015;33:136–149. doi: 10.1016/j.devcel.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.L., Rabiee A., Kovary K.M., Bahrami-Nejad Z., Taylor B., Teruel M.N. Molecular competition in G1 controls when cells simultaneously commit to terminally differentiate and exit the cell cycle. Cell Rep. 2020;31:107769. doi: 10.1016/j.celrep.2020.107769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shown are representative asymmetric cell division (ACD; left) and symmetric cell division (SCD; right) in wild-type plants expressing the membrane marker LTi6B-GFP (green) and the cell cycle marker PlaCCI (cyan = G1, magenta = S/G2/lateM, and green nucleus = M). Small maximum projections of time laps recordings obtained at a 30-min time interval of an abaxial cotyledon epidermis 24 h after preparation. Played back at 5 fps. Hours are indicated at the top in 30-min intervals. Scale bar, 5 μm.
Shown are representative cotyledon epidermis expressing proTMM::GUS-GFP (pTMM::GUS-GFP) in wild type (left) and proPOLAR::SMR4 (pPOLAR::SMR4) transgenic plants (right). A cell of interest is marked with white arrowhead at the beginning of the movie. A magenta arrowhead marks when the division plane is first visible during ACD. A yellow arrow marks SCD division plane. Hours are presented relative to the time when ACD plane is first visible (0 h) from the cell of interest and indicated at the top of the left corner of the movie. Scare bar, 20 μm.
Shown are representative asymmetric cell division (ACD; left) and symmetric cell division (SCD; right) in plants expressing proPOLAR:SMR4 as well as the cell cycle marker PlaCCI (cyan = G1, magenta = S/G2/lateM, and green nucleus = M). Small maximum projections of time laps recordings obtained at a 30-min time interval of an abaxial cotyledon epidermis 24 h after preparation. Played back at 5 fps. Hours are indicated at the top in 30-min intervals. Scale bar, 5 μm.
Shown are representative asymmetric cell division (ACD; left) and symmetric cell division (SCD; right) in smr4-1cr single mutants expressing the membrane marker LTi6B-GFP (green) and the cell cycle marker PlaCCI (cyan = G1 and magenta = S/G2/lateM). Small maximum projections of time laps recordings obtained at a 30-min time interval of an abaxial cotyledon epidermis 24 h after preparation. Played back at 5 fps. Hours are indicated at the top in 30-min intervals. Scale bar, 5 μm.
Data Availability Statement
-
•
The ChIP-seq data generated in this study have been deposited at the NCBI Gene Expression Omnibus (GEO: GSE173338) and are publicly available. All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.