Summary
Background
Real-world data on the effectiveness of boosters against COVID-19, especially as new variants continue to emerge, is limited. Our objective was to assess demographic, clinical, and outcome variables of patients requiring hospitalization for severe SARS-CoV-2 infection comparing fully vaccinated and boosted (FV&B), fully vaccinated (FV), and unvaccinated (UV) patients.
Methods
This multicenter observational cohort analysis compared demographic, clinical, and outcome variables in FV&B, FV, and UV adults hospitalized for COVID-19. Partially vaccinated (PV) and individuals still hospitalized beyond the designated follow-up date of February 1, 2022 were excluded. The primary endpoint was in-hospital mortality. Secondary endpoints included characteristics and outcomes in subpopulations of intensive care and geriatric (age >65) patients.
Findings
Between August 12th, 2021 and January 20th, 2022, 8232 patient encounters had a primary diagnosis of COVID-19 and required inpatient treatment. Of the 8232 encounters requiring hospitalization, 448 (5.8%) were FV&B, 2257 (29.2%) were FV, and 5023 (65.0%) were UV; 357 PV and 147 still hospitalized were excluded. The median age of FV&B cohort was 73 (IQR 62, 82) compared to 70 (IQR 59, 80) for FV and 59 (IQR 45, 71) for UV (0.001). Most patients were female in both the FB&V and UV groups with 51.1% and 51.8%, respectively, while the FV group had a majority of males (51.3%). The median Elixhauser weighted score was 12 (IQR 3, 22) for FV&B, 10 (IQR 2, 20) for FV, and 9 (IQR 0, 17) for UV groups (p < 0.001). In-hospital mortality was 7.1% in the FV&B, 10.3% in the FV group, and 12.8% in the UV group (p < 0.001). The FV&B group had lower in-hospital mortality than both FV and UV groups (p = 0.045 and p = 0.001, respectively). The FV group had lower in-hospital mortality than the UV group (p = 0.004).
Interpretation
Fully vaccinated and boosted patients requiring hospital-level care for breakthrough COVID-19 have lower in-hospital mortality than fully vaccinated and unvaccinated patients despite being older and higher risk at baseline. Boosters offer added protection beyond full vaccination in preventing death. As COVID-19 continues to spread, larger expansive trials are needed to further identify risk factors for severe outcomes among the FV&B population.
Funding
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Keywords: COVID-19, SARS-CoV-2, Booster dose, Vaccination, Severe illness, Mortality, Hospitalization, Death
Research in context.
Evidence before this study
We searched PubMed on December 1, 2021 for evidence surrounding breakthrough COVID-19 in vaccinated and boosted patients leading to hospital or emergency care encounters using the search terms (booster OR booster shot OR third dose) AND (SARS-CoV-2 OR novel coronavirus OR COVID-19) AND (vaccination OR immunization) AND (hospitalization OR emergency visit) with no language or time restrictions. Vaccine efficacy has been established by pharmaceutical trials and has triggered the availability of vaccines across the globe. However, effectiveness of booster shots at preventing hospital-based treatment for COVID-19 in fully immunized patients in a real-world population is poorly understood with limited prior publications. Further, it is unclear if boosters offer an advantage over full vaccination for severe outcomes associated with COVID-19.
Added value of this study
This study is one of the first large, real world investigations in the United States addressing the impact of booster shots on in-hospital mortality. We found that fully vaccinated and boosted individuals were at baseline older and at higher risk of in-hospital mortality based on comorbidities compared to fully vaccinated and unvaccinated individuals. Despite the increased baseline risk, fully vaccinated and boosted individuals had a reduced in-hospital mortality compared to fully vaccinated and unvaccinated groups (p = 0.045 and p = 0.001, respectively).
Implications of all the available evidence
While vaccination provides a layer of protection against mortality compared to no vaccination, the booster dose enhances this defense and confers an additional mortality benefit.
Alt-text: Unlabelled box
Introduction
The Coronavirus Disease (COVID-19) pandemic landscape has changed dramatically since it began.1 In just one year, scientists, industry, and governments were able to develop and distribute safe and effective vaccinations that offer protection against the SARS-CoV-2 virus.2, 3, 4 However, despite these advances, the death toll has been substantial, with nearly 1 in 100 Americans over the age of 65 succumbing to this deadly virus.5 As SARS-CoV-2 infection continues to spread around the globe, one critical challenge is the continued viral mutation leading to new and potentially dangerous viral variants that may decrease the efficacy of current vaccines. Fortunately, studies on the B.1.351, B.1.1.7 (alpha), and B.1.617.2 (delta) variants revealed no major decrease in effectiveness with the Pfizer-BioNTech vaccine.6,7 However, as new variants continue to emerge, breakthrough SARS-CoV-2 infections in vaccinated individuals remain a significant concern. Smaller studies on fully vaccinated healthcare workers who subsequently developed SARS-CoV-2 infections demonstrated rates of breakthrough infection between 0.05% and 11.6%.8, 9, 10, 11
Early data from Israel and the United Kingdom also demonstrated that the effectiveness of the vaccine for preventing breakthrough infection and severe disease waned as time from vaccination increased.12,13 In order to combat this potential for waning immunity, the United States Food and Drug Administration authorized booster vaccinations in all individuals who completed the primary vaccination series at least six months after receiving the Moderna or Pfizer-BioNTech SARS-CoV-2 vaccine or at least two months after being vaccinated with the Janssen SARS-CoV-2 vaccine.14,15 To date, there is minimal data regarding the effectiveness of a booster dose in preventing breakthrough COVID-19 and progression to severe disease and death, especially in areas with a high concentration of viral variants. It is our objective to assess shared characteristics and outcomes of patients requiring hospitalization for severe SARS-CoV-2 infection comparing fully vaccinated and boosted (FV&B), fully vaccinated (FV) and unvaccinated (UV) patients.
Materials and methods
Study design, setting, and participants
We conducted a multicenter observational cohort analysis evaluating adult patients requiring hospital admission for COVID-19. We compared demographic, clinical, and outcomes data among patients who had completed a SARS-CoV-2 primary vaccination series and received a booster dose (FV&B), completed primary vaccination series (FV), and unvaccinated (UV) patients who required hospitalization for COVID-19 during the same time period. Patients were identified through analysis of electronic health records (EHR; Epic Systems, Verona, WI, USA). This study was conducted at Beaumont Health, an eight-hospital acute care hospital system in southeastern Michigan.
Consecutive adult patients greater than or equal to 18 years of age presenting to the ED and requiring hospital admission with a discharge diagnosis indicating active COVID-19 disease were eligible for inclusion. Patients who had laboratory-confirmed SARS-CoV-2 infection during a previous hospitalization, pediatrics, partially vaccinated, and those still admitted at the designated follow-up date of February 1, 2022 were excluded. The study was approved by the home organization's Institutional Review Board. The written informed consent requirement was waived due to the retrospective nature of the study.
Study definitions
Patients were categorized as FV&B, FV, or UV. Current Centers for Disease Control and Prevention (CDC) guidelines were used for classification.16 FV&B individuals had received either three doses of an mRNA vaccine (Pfizer, Moderna), one dose of a viral vector vaccine (Janssen) and one dose of the mRNA, one dose of a viral vector vaccine and two doses of the mRNA, or two doses of a viral vector vaccine. FV individuals had received either two doses of an mRNA vaccine (Pfizer, Moderna) or one dose of viral vector (Janssen).
UV individuals had no record of immunization against SARS-CoV-2 available in our EHR or within the Michigan Care Improvement Registry (MCIR), which tracks all vaccinations from any source across the state of Michigan.17 All individuals who received the correct number and combination of doses were included in the analysis irrespective of the timeline of dose administration. In-hospital mortality included death during the hospital stay as well as death within ten days of being discharged to hospice.
Data sources/measurement
All data were extracted from the EHR. This data included demographic, clinical, laboratory, and outcomes variables. Additionally, data included comorbidities, number of ED visits in the past six months, and body mass index (BMI) was extracted from the EHR after our cohort was identified. Comorbidities were assessed using the Agency for Healthcare Research and Quality (AHRQ) Elixhauser Comorbidity Index18 (Appendix A). Specific to the FV&B group, we extracted initial ED vital signs and laboratory values. A manual chart review was performed to identify the time from symptom onset to ED presentation for FV&B individuals that received ICU level of care or expired. For all cohorts, we evaluated hospital treatment and outcomes data. These variables included: need for supplemental oxygen stratified by level of support including mechanical ventilation, highest level of care, need for renal replacement therapy, vasopressor use, extracorporeal membrane oxygenation (ECMO) use, hospital length of stay, and hospital disposition.
Data from the MCIR included dates of vaccine administration as well as vaccine type and brand.
Immunocompromised individuals were identified using the AHRQ list of ICD-10 diagnoses and procedure codes that they have listed as defining an immunocompromised state (IMMUNID).19 All available EHR data were used to screen for these conditions and procedures and codes that were deemed active during the current hospitalization were included.
Outcome measures
The primary outcome of this study was in-hospital mortality. Secondary endpoints included characteristics and outcomes in ICU and elderly (age >65) subpopulations including mechanical ventilation, vasopressors, renal replacement therapy, ECMO, supplemental oxygen (none, low flow therapy, high flow therapy), and hospital length of stay.
Statistical analysis
All data were summarized through descriptive statistics. Numerical results are reported as means and standard deviation or medians and interquartile ranges. Categorical variables are reported as counts and percentages. The Chi-square or Fisher's exact test was used for categorical variables and the Kruskal-Wallis (exact) test was used for numerical variables. When comparing FVB, FV, and UV, the multiple comparison method was applied; the post hoc Holm-Bonferonni procedure was used for categorical variables and the Tukey-Kramer method was used for numerical variables.20 All tests performed in this analysis were two-sided tests, with p < 0.05 or a confidence interval of 95% indicating statistical significance. Analysis was performed using R software, version 4.1.2 (R Foundation for Statistical Computing) and Excel (Microsoft).
Ethics committee approval
This study was approved by the Beaumont Health Institutional Review Board.
Role of the funding source
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Results
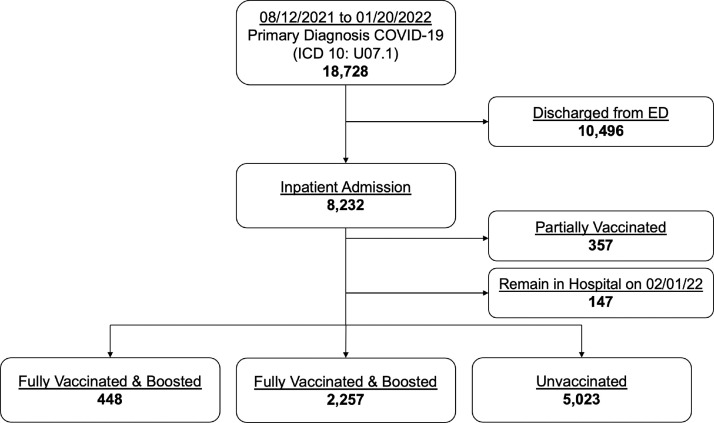
Between August 12th, 2021 and January 20th, 2022, there were 18,728 ED encounters with a primary diagnosis of COVID-19 (ICD 10: U07.1). After excluding 10,496 encounters that were discharged from the ED, 147 encounters that remained hospitalized at our designated follow-up date, and 357 partially vaccinated encounters, a total of 7728 encounters remained in the final analysis. Of these encounters, 448 (5.8%) were FV&B, 2257 (29.2%) were FV, and 5023 (65.0%) were UV (Figure 1).
Figure 1.
Enrollment profile of COVID-19 emergency and inpatient encounters.
The median age of FV&B patients was 73 (Interquartile range (IQR) 62, 82) compared to 70 (IQR 59, 80) for FV and 59 (IQR 45, 71) for UV (p < 0.001). The majority of patients were female in both the FB&V and UV groups with 51.1% and 51.8%, respectively, and male in the FV group (51.3%) (p = 0.049). FV&B had a lower median BMI of 28.3 (IQR 24.2, 34.0), compared to FV (29.2 (IQR 24.9, 34.7)), and UV (30.0 (IQR 25.7, 35.9)) (p < 0.001). The proportion of immunocompromised and pre-existing end-stage renal disease (ESRD) in the FV&B cohort were 29.5% and 13.6%, respectively, compared to 17.9% and 6.2% in the FV cohort, respectively, and 11.3% and 2.3% in the UV cohort, respectively (p < 0.001 for both). The median Elixhauser weighted score was 12 (IQR 3, 22) for FV&B, 10 (IQR 2, 20) for FV, and 9 (IQR 0, 17) for UV groups (p < 0.001). 4.9% of FV&B individuals required mechanical ventilation compared to 6.5% of FV, both of which were lower than the UV group (10.2%) (p < 0.001). In-hospital mortality was 7.1% in the FV&B, which was significantly lower than the FV group (10.3%; p = 0.045) and UV group (12.8%; p = 0.001) (Table 1 and Supplementary Table 1).
Table 1.
Demographics, pre-existing comorbidities, in-hospital therapies, and outcomes among FV&B, FV, and UV individuals.
| Vaccination Status |
|||||
|---|---|---|---|---|---|
| Variables‡ | All | FV&B | FV | UV | p value |
| N | 7728 | 448 (5.80%) | 2257 (29.21%) | 5023 (65.00%) | |
| Demographics | |||||
| Age, years | |||||
| Mean (SD) | 61.87 (17.64) | 70.90 (14.40)*,‡‡ | 68.53 (15.78)*,§ | 58.07 (17.52)§,‡‡ | <0.001¶ |
| Median (IQRs) | 63.00 (50.00, 75.00) | 73.00 (62.00, 82.00) | 70.00 (59.00, 80.00) | 59.00 (45.00, 71.00) | |
| Gender | |||||
| Female | 3933 (50.89%) | 229 (51.12%) | 1100 (48.74%)§ | 2604 (51.84%)§ | 0.049† |
| Male | 3795 (49.11%) | 219 (48.88%) | 1157 (51.26%) | 2419 (48.16%) | |
| Race (n = 7713/448/2255/5010) | |||||
| Black or African American | 1921 (24.91%) | 84 (18.75%)‡‡ | 464 (20.58%)§ | 1373 (27.41%)§,‡‡ | <0.001† |
| White or Caucasian | 5335 (69.17%) | 337 (75.22%) | 1667 (73.92%) | 3331 (66.49%) | |
| Other | 457 (5.93%) | 27 (6.03%) | 124 (5.50%) | 306 (6.11%) | |
| BMI, kg/m2 (n = 7509/438/2184/4887) | |||||
| Mean | 31.17 (8.67) | 29.27 (7.37)*,‡‡ | 30.63 (8.31)*,§ | 31.58 (8.90)§,‡‡ | <0.001¶ |
| Median | 29.63 (25.28, 35.44) | 28.25 (24.17, 34.01) | 29.22 (24.90, 34.70) | 29.95 (25.69, 35.94) | |
| Pre-existing comorbidities | |||||
| ESRD | 315 (4.08%) | 61 (13.62%)*,‡‡ | 140 (6.20%)*,§ | 114 (2.27%)§,‡‡ | <0.001† |
| Immunocompromised | 1105 (14.30%) | 132 (29.46%)*,‡‡ | 404 (17.90%)*,§ | 569 (11.33%)§,‡‡ | <0.001† |
| Elixhauser weighted score | |||||
| Mean | 10.54 (12.18) | 13.53 (12.96)*,‡‡ | 11.90 (12.86)*,§ | 9.66 (11.69)§,‡‡ | <0.001¶ |
| Median | 10.00 (1.00, 18.00) | 12.00 (3.00, 22.00) | 10.00 (2.00, 20.00) | 9.00 (0.00, 17.00) | |
| In-hospital therapies | |||||
| O2 therapy | 5529 (71.55%) | 269 (60.04%)‡‡ | 1463 (64.82%)§ | 3797 (75.59%)§,‡‡ | <0.001† |
| Nasal cannula/non-rebreather | 3080 (39.86%) | 180 (40.18%) | 917 (40.63%) | 1983 (39.48%) | 0.644† |
| High flow O2 | 1138 (14.73%) | 30 (6.70%)‡‡ | 202 (8.95%)§ | 906 (18.04%)§,‡‡ | <0.001† |
| Non-invasive ventilation | 633 (8.19%) | 37 (8.26%) | 198 (8.77%) | 398 (7.92%) | 0.473† |
| Mechanical ventilation | 678 (8.77%) | 22 (4.91%)‡‡ | 146 (6.47%)§ | 510 (10.15%)§,‡‡ | <0.001† |
| Vasopressor | 694 (8.98%) | 33 (7.37%) | 168 (7.44%)§ | 493 (9.81%)§ | 0.002† |
| ICU-level care | 1084 (14.03%) | 56 (12.50%) | 271 (12.01%)§ | 757 (15.07%)§ | 0.001† |
| Length of stay, hours | |||||
| Mean | 184.13 (183.16) | 164.31 (169.23)‡‡ | 171.15 (158.81)§ | 191.73 (193.83)§,‡‡ | <0.001¶ |
| Median | 128.00 (72.00, 234.00) | 113.50 (62.00, 207.50) | 126.00 (71.00, 213.00) | 134.00 (74.00, 241.50) | |
| Outcomes | |||||
| Death | 908 (11.75%) | 32 (7.14%)*,‡‡ | 232 (10.28%)*,§ | 644 (12.82%)§,‡‡ | <0.001† |
| Discharge disposition location (n = 6820/416/2025/4379) | |||||
| Home | 5846 (85.72%) | 319 (76.68%) | 1620 (80.00%) | 3907 (89.22%) | |
| Rehab | 100 (1.47%) | 13 (3.12%) | 36 (1.78%) | 51 (1.16%) | |
| SNF | 786 (11.52%) | 72 (17.31%) | 342 (16.89%) | 372 (8.50%) | |
| Hospice | 50 (0.73%) | 11 (2.64%) | 17 (0.84%) | 22 (0.50%) | |
| Other | 38 (0.55%) | 1 (0.24%) | 10 (0.49%) | 27 (0.62%) | |
Abbreviations: FV&B=fully vaccinated and boosted; FV=fully vaccinated; UV=unvaccinated; BMI=body mass index; ESRD=end-stage renal disease; ICU=intensive care unit; Rehab=rehabilitation; SNF=skilled nursing facility.
‡For continuous variables, medians (interquartile ranges, IQRs) and means (standard deviation, SD) were presented. For categorical variables, frequencies (percentage) were presented.
¶Kruskal-Wallis test.
†Chi-squared or Fisher's exact test.
*p < 0.05 between FV&B and FV¶¶.
§p < 0.05 between FV and UV¶¶.
‡‡p < 0.05 between FV&B and UV¶¶.
¶¶In multiple comparisons test, post hoc Holm-Bonferroni procedure and the Tukey-Kramer method was used for categorical and numerical variables, respectively. P-values are available in Supplementary Table 1.
In a sub-analysis comparing FV&B (n = 320), FV (n = 1449), and UV (n = 1885) patients >65 years old, outcome differences between groups were more apparent. Specifically, in-hospital mortality was 9.1% in FV&B, which was significantly lower than both the FV group (13.6%; p = 0.035) and UV group (21.2%; p < 0.001). (Table 2 and Supplementary Table 1)
Table 2.
Demographics, pre-existing comorbidities, in-hospital therapies, and outcomes among FV&B, FV, and UV individuals age 65 and older.
| Vaccination Status |
|||||
|---|---|---|---|---|---|
| Variables‡ | All, Age 65+ | FV&B, Age 65+ | FV, Age 65+ | UV, Age 65+ | p value |
| N | 3654 | 320 (8.76%) | 1449 (39.66%) | 1885 (51.59%) | |
| Demographics | |||||
| Age, years | |||||
| Mean (SD) | 76.97 (8.47) | 78.21 (8.27)‡‡ | 77.97 (8.65)§ | 75.99 (8.25)§,‡‡ | <0.001¶ |
| Median (IQRs) | 76.00 (70.00, 83.00) | 78.00 (71.00, 84.25) | 77.00 (71.00, 85.00) | 74.00 (69.00, 82.00) | |
| Gender | |||||
| Female | 1939 (53.07%) | 162 (50.62%) | 710 (49.00%)§ | 1067 (56.60%)§ | <0.001† |
| Male | 1715 (46.93%) | 158 (49.38%) | 739 (51.00%) | 818 (43.40%) | |
| Race (n = 3652/320/1448/1884) | |||||
| Black or African American | 628 (17.20%) | 51 (15.94%) | 212 (14.64%)§ | 365 (19.37%)§ | <0.003† |
| White or Caucasian | 2838 (77.71%) | 249 (77.81%) | 1170 (80.80%) | 1419 (75.32%) | |
| Other | 186 (5.09%) | 20 (6.25%) | 66 (4.56%) | 100 (5.31%) | |
| BMI, kg/m2 (n = 3540/312/1398/1830) | |||||
| Mean | 28.91 (7.22) | 28.30 (6.99) | 29.15 (7.12) | 28.84 (7.33) | 0.108¶ |
| Median | 27.80 (24.11, 32.49) | 27.12 (23.49, 33.14) | 28.22 (24.21, 32.90) | 27.53 (24.13, 32.28) | |
| Pre-existing comorbidities | |||||
| ESRD | 174 (4.76%) | 43 (13.44%)*,‡‡ | 84 (5.80%)*,§ | 47 (2.49%)§,‡‡ | <0.001† |
| Immunocompromised | 555 (15.19%) | 89 (27.81%)*,‡‡ | 233 (16.08%)*,§ | 233 (12.36%)§,‡‡ | <0.001† |
| Elixhauser weighted score | |||||
| Mean | 14.24 (12.70) | 15.62 (13.25) | 14.25 (13.00) | 13.99 (12.36) | 0.093¶ |
| Median | 13.00 (5.00, 22.00) | 16.00 (5.00, 25.00) | 13.00 (5.00, 23.00) | 13.00 (5.00, 22.00) | |
| In-hospital therapies | |||||
| O2 therapy | 2735 (74.85%) | 213 (66.56%)‡‡ | 1023 (70.60%)§ | 1499 (79.52%)§,‡‡ | <0.001† |
| Nasal cannula/non-rebreather | 1508 (41.27%) | 144 (45.00%) | 628 (43.34%)§ | 736 (39.05%)§ | 0.016† |
| High flow O2 | 502 (13.74%) | 21 (6.56%)*,‡‡ | 150 (10.35%)*,§ | 331 (17.56%)§,‡‡ | <0.001† |
| Non-invasive ventilation | 373 (10.21%) | 29 (9.06%) | 139 (9.59%) | 205 (10.88%) | 0.373† |
| Mechanical ventilation | 352 (9.63%) | 19 (5.94%)‡‡ | 106 (7.32%)§ | 227 (12.04%)§,‡‡ | <0.001† |
| Vasopressor | 380 (10.40%) | 25 (7.81%)‡‡ | 119 (8.21%)§ | 236 (12.52%)§,‡‡ | <0.001† |
| ICU-level care | 551 (15.08%) | 44 (13.75%) | 192 (13.25%)§ | 315 (16.71%)§ | 0.017† |
| Length of stay, hours | |||||
| Mean | 204.63 (181.11) | 185.23 (177.25)‡‡ | 187.16 (161.96)§ | 221.35 (193.75)§,‡‡ | <0.001¶ |
| Median | 148.00 (91.00, 266.75) | 133.50 (76.00, 242.00) | 144.00 (85.00, 240.00) | 159.00 (96.00, 292.00) | |
| Outcomes | |||||
| Death | 626 (17.13%) | 29 (9.06%)*,‡‡ | 197 (13.60%)*,§ | 400 (21.22%)§,‡‡ | <0.001† |
| Discharge disposition location (n = 3028/291/1252/1485) | |||||
| Home | 2257 (74.54%) | 201 (69.07%) | 904 (72.20%) | 1152 (77.58%) | |
| Rehab | 73 (2.41%) | 13 (4.47%) | 28 (2.24%) | 32 (2.15%) | |
| SNF | 641 (21.17%) | 66 (22.68%) | 302 (24.12%) | 273 (18.38%) | |
| Hospice | 45 (1.49%) | 11 (3.78%) | 14 (1.12%) | 20 (1.35%) | |
| Other | 12 (0.40%) | 0 (0.00%) | 4 (0.32%) | 8 (0.54%) | |
Abbreviations: FV&B=fully vaccinated and boosted; FV=fully vaccinated; UV=unvaccinated; BMI=body mass index; ESRD=end-stage renal disease; ICU=intensive care unit; Rehab=rehabilitation; SNF=skilled nursing facility.
‡For continuous variables, medians (interquartile ranges, IQRs) and means (standard deviation, SD) were presented. For categorical variables, frequencies (percentage) were presented.
¶Kruskal-Wallis test.
†Chi-squared or Fisher's exact test.
*p < 0.05 between FV&B and FV¶¶.
§p < 0.05 between FV and UV¶¶.
‡‡p < 0.05 between FV&B and UV¶¶.
¶¶In multiple comparisons test, post hoc Holm-Bonferroni procedure and the Tukey-Kramer method was used for categorical and numerical variables, respectively. P-values are available in Supplementary Table 1.
A second sub-analysis was performed on patients requiring ICU-level care including FV&B group (n = 56), FV group (n = 271), and UV group (n = 757). The FV&B group had a higher proportion of immunocompromised individuals (41.1%) compared to the FV group (25.1%; p = 0.024) and UV group (12.4%; p < 0.001). There was no difference in median Elixhauser weighted comorbidity between the FV&B (22.5 (IQR 11, 31)) and FV (22 (IQR 11, 31)) (p = 0.999), although both were higher than the UV group (17 (IQR 7,26)) (p = 0.050 and p < 0.001, respectively). There was no difference in in-hospital mortality between the FV&B (37.5%) and FV (52.4%) (p = 0.119) or the FV and UV (58.5%) (p = 0.119), however, in-hospital mortality was significantly lower among the FV&B compared to the UV (p = 0.010) (Table 3 and Supplementary Table 1).
Table 3.
Demographics, pre-existing comorbidities, in-hospital therapies, and outcomes among FV&B, FV, and UV individuals requiring intensive care.
| Vaccination Status |
|||||
|---|---|---|---|---|---|
| Variables‡ | All, ICU | FV&B, ICU | FV, ICU | UV, ICU | p value |
| n | 1084 | 56 (5.17%) | 271 (25.00%) | 757 (69.83%) | |
| Demographics | |||||
| Age, years | |||||
| Mean (SD) | 63.07 (15.79) | 72.05 (13.17)‡‡ | 70.09 (12.60)§ | 59.89 (15.94)§,‡‡ | <0.001¶ |
| Median (IQRs) | 65.00 (53.00, 74.00) | 73.50 (66.00, 81.25) | 71.00 (63.00, 79.50) | 61.00 (49.00, 71.00) | |
| Gender | |||||
| Female | 463 (42.71%) | 32 (57.14%) | 110 (40.59%) | 329 (43.46%) | 0.714† |
| Male | 621 (57.29%) | 24 (42.86%) | 161 (59.41%) | 428 (56.54%) | |
| Race (n = 1081/56/270/755) | |||||
| Black or African American | 222 (20.54%) | 6 (10.71%) | 47 (17.41%) | 169 (22.38%) | 0.155† |
| White or Caucasian | 796 (73.64%) | 47 (83.93%) | 206 (76.30%) | 543 (71.92%) | |
| Other | 63 (5.83%) | 3 (5.36%) | 17 (6.30%) | 43 (5.70%) | |
| BMI, kg/m2 (n = 1068/56/266/746) | |||||
| Mean | 32.96 (9.66) | 29.37 (5.82)‡‡ | 31.67 (9.25)§ | 33.69 (9.93)§,‡‡ | <0.001¶ |
| Median | 31.41 (26.32, 37.59) | 28.87 (25.45, 33.72) | 30.51 (24.99, 36.29) | 31.91 (27.14, 38.61) | |
| Pre-existing comorbidities | |||||
| ESRD | 60 (5.54%) | 13 (23.21%)*,‡‡ | 27 (9.96%)*,§ | 20 (2.64%)§,‡‡ | <0.001† |
| Immunocompromised | 185 (17.07%) | 23 (41.07%)*,‡‡ | 68 (25.09%)*,§ | 94 (12.42%)§,‡‡ | <0.001† |
| Elixhauser weighted score | |||||
| Mean | 18.94 (13.90) | 22.09 (13.69)‡‡ | 21.99 (15.20)§ | 17.61 (13.22)§,‡‡ | <0.001¶ |
| Median | 19.00 (9.00, 28.00) | 22.50 (11.00, 31.25) | 22.00 (11.00, 31.00) | 17.00 (7.00, 26.00) | |
| In-hospital therapies | |||||
| O2 therapy | 1042 (96.13%) | 49 (87.50%)‡‡ | 259 (95.57%) | 734 (96.96%)‡‡ | 0.005† |
| Nasal cannula/non-rebreather | 129 (11.90%) | 10 (17.86%) | 44 (16.24%)§ | 75 (9.91%)§ | 0.008† |
| High flow O2 | 84 (7.75%) | 3 (5.36%) | 19 (7.01%) | 62 (8.19%) | 0.755† |
| Non-invasive ventilation | 212 (19.56%) | 14 (25.00%) | 65 (23.99%) | 133 (17.57%) | 0.042† |
| Mechanical ventilation | 617 (56.92%) | 22 (39.29%)‡‡ | 131 (48.34%)§ | 464 (61.29%)§,‡‡ | <0.001† |
| Vasopressor | 623 (57.47%) | 32 (57.14%) | 146 (53.87%) | 445 (58.78%) | 0.373† |
| Length of stay, hours | |||||
| Mean | 388.93 (297.27) | 370.29 (291.23) | 344.73 (239.55)§ | 406.13 (314.49)§ | 0.014¶ |
| Median | 323.50 (187.75, 514.50) | 292.50 (142.75, 504.00) | 299.00 (168.00, 454.00) | 338.00 (201.00, 534.00) | |
| Outcomes | |||||
| Death | 606 (55.90%) | 21 (37.50%)‡‡ | 142 (52.40%) | 443 (58.52%)‡‡ | 0.004† |
| Discharge disposition location (n = 478/35/129/314) | |||||
| Home | 298 (62.34%) | 18 (51.43%) | 75 (58.14%) | 205 (65.29%) | |
| Rehab | 43 (9.00%) | 6 (17.14%) | 13 (10.08%) | 24 (7.64%) | |
| SNF | 111 (23.22%) | 9 (25.71%) | 36 (27.91%) | 66 (21.02%) | |
| Hospice | 9 (1.88%) | 2 (5.71%) | 3 (2.33%) | 4 (1.27%) | |
| Other | 17 (3.56%) | 0 (0.00%) | 2 (1.55%) | 15 (4.78%) | |
Abbreviations: FV&B=fully vaccinated and boosted; FV=fully vaccinated; UV=unvaccinated; BMI=body mass index; ESRD=end-stage renal disease; ICU=intensive care unit; Rehab=rehabilitation; SNF=skilled nursing facility.
‡For continuous variables, medians (interquartile ranges, IQRs) and means (standard deviation, SD) were presented. For categorical variables, frequencies (percentage) were presented.
¶Kruskal-Wallis test.
†Chi-squared or Fisher's exact test.
*p < 0.05 between FV&B and FV¶¶.
§p < 0.05 between FV and UV¶¶.
‡‡p < 0.05 between FV&B and UV¶¶.
¶¶In multiple comparisons test, post hoc Holm-Bonferroni procedure and the Tukey-Kramer method was used for categorical and numerical variables, respectively. P-values are available in Supplementary Table 1.
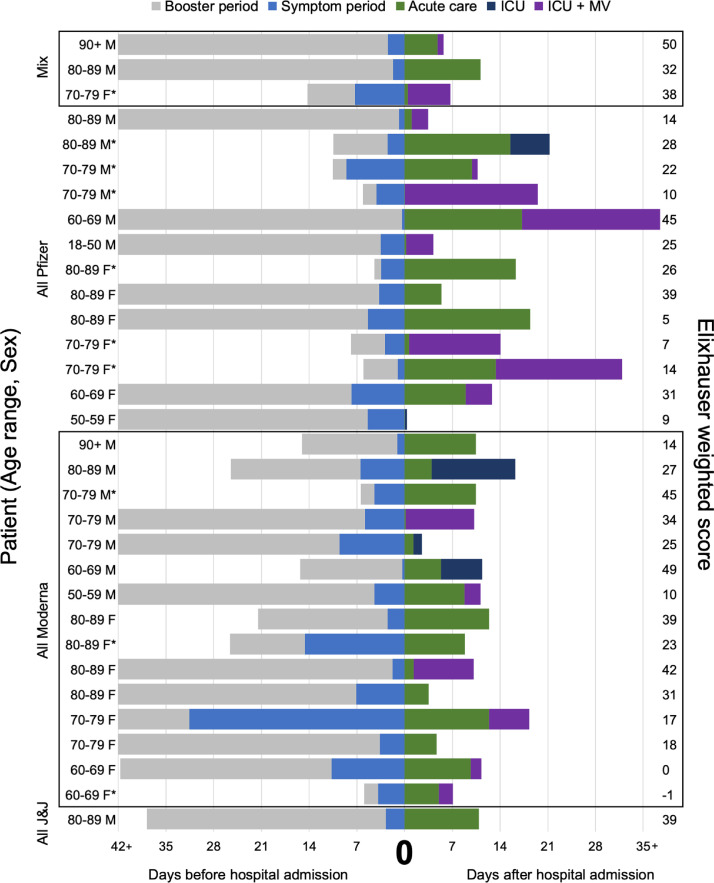
There were 32 (7.1%) patients in the FV&B cohort that died. Among these patients, three had received a mix of mRNA vaccine types (Pfizer and Moderna), 13 received three doses of the Pfizer vaccine and 15 received three doses of the Moderna vaccine, while one patient had received two doses of the viral vector vaccine (Janssen). The median number of days from booster vaccination to symptom onset was 38 (IQR 7.8, 66) with 10 subjects (31.3%) experiencing COVID-19 symptoms less than 12 days from booster administration (Figure 2).
Figure 2.
Characteristics and comorbidities of 32 fully vaccinated and boosted patients who expired from COVID-19.
Demographic/clinical data includes age range, sex, Elixhauser weighted score, vaccine type, timing of booster, and timing of symptom onset. Outcomes data includes mechanical ventilation and mortality.
Abbreviations: ICU=Intensive care unit; MV=Mechanical ventilation; J&J=Janssen.
* <12 days from booster vaccination to symptom onset. The median number of days from booster vaccination to symptom onset was 38 (IQR 7.8, 66).
Discussion
Our study is one of the first investigations of the real-world effectiveness of the SARS-CoV-2 booster vaccine in preventing severe disease among hospitalized COVID-19 patients in the United States. Despite their older age (median 73 vs 70 years old (p = 0.018)), higher rate of pre-existing ESRD (13.6% vs 6.2% (p < 0.001)), higher proportion of immunocompromised state (29.5% vs 17.9% (p < 0.001)), and a higher risk of in-hospital death (Elixhauser weighted score 12 vs 10 (p < 0.026)), in-hospital mortality was lower in the FV&B compared to the FV group (7.1% vs 10.3% (p = 0.045)). While it is unsurprising that our FV&B cohort was older and had more immunocompromised individuals given the staged rollout of the booster vaccine by age and risk factors, it is notable that they experienced a lower rate of in-hospital mortality despite being higher risk for death at baseline. Previous evidence suggested that amongst elderly individuals with multiple comorbidities, vaccination status did not provide a significant mortality benefit once breakthrough infection requiring hospitalization had occurred.21 However, in our current analysis, among the subgroup of individuals over the age of 65, we found that not only did FV individuals have a lower incidence of in-hospital death when compared to UV individuals (9.1% vs 21.2% (p < 0.001)), FV&B individuals saw a further reduction in death when compared to FV patients (9.1% vs 13.6% (p = 0.035)). These findings suggest that even among elderly patients with significant comorbidities, a booster vaccine may provide additional protection against the progression toward severe disease despite breakthrough infection requiring hospitalization. This current analysis suggests an additional value of the booster not seen in previous literature on fully vaccinated patients and severe disease.21,22
The relationship between the timing of booster administration and the onset of infectious symptoms is notable in the FV&B population that died. In general, COVID-19 was diagnosed in close proximity to receiving the dose. Specifically, 31.3% (10 out of 32) of the deceased group received the booster dose <12 days from onset of symptoms suggesting that FV&B patients who required ICU care may not have had enough time since their booster dose to confer the full benefit of the additional vaccination. The relevance of the timing of booster administration is supported by data from an Israeli study assessing the benefit of BNT162b2 booster. In that analysis, the authors found that 12 days post-booster immunization, there was a significant decrease in the rate of breakthrough infection when compared to the control, un-boosted, group.23 Our data suggests that the mortality benefit for the FV&B group may be even greater as the full potency of the booster was likely unrecognized in a substantial minority of the deceased.
It is unclear if an immunocompromised state is an independent predictor of severe COVID-19 disease in boosted patients. In our study sample, the proportion of individuals who were identified as immunocompromised was much higher in the FV&B group compared to both the FV and UV groups, 29.5%, 17.9%, and 11.3%, respectively (all p < 0.001). Additionally, among patients who required ICU level care, this trend continued, with FV&B patients having the highest proportion of immunocompromised individuals (41.1%) when compared to FV (25.1% (p = 0.024)) and UV individuals (12.4% (p < 0.001)). Previous evidence has shown that immunocompromised patients may have a tempered immune response to vaccination.24,25 However, additional doses may increase the likelihood of immune response.26 Unfortunately, none of the deceased in the FV&B cohort had laboratory antibody testing results within their health records to explore this variable further.
This study has several limitations. First, the sample size of the FV&B group was small. This was due to a smaller proportion of the population that was eligible for the booster dose during the study period. Further, given that our study only evaluated patients who sought emergency care and subsequently required hospitalization, with presumed strengthened immunity from the additional dose, there are likely a number of breakthrough SARS-CoV-2 infections that were not captured in our cohort. Given the staged approval of the booster doses starting with high-risk individuals, our sample population was biased towards individuals ≥ 65 years old as well as those with a pre-existing immunocompromised state. As with all retrospective studies, we were unable to control for confounders that may have affected hospital outcomes when comparing FV&B, FV, and UV individuals. Lastly, given our small sample size and the heterogeneity of vaccination sequences we were unable to determine if one vaccine, or sequence of vaccines, has benefit over another.
Conclusions
In this study of hospitalized patients with COVID-19, we observed that the cohort of FV&B patients had a lower rate of in-hospital mortality compared to both FV and UV individuals, despite being higher risk for in-hospital mortality at baseline. While vaccination provides a layer of protection against mortality compared to no vaccination, the booster dose enhances this defense and confers an additional mortality benefit. As COVID-19 continues to spread, more extensive studies are needed to further identify risk factors for severe outcomes among the FV&B population.
Contributors
AB, NM, and SJ designed the study, had full access to the data, and take responsibility for the integrity and accuracy of the data analysis. AB, NM, and SJ contributed to subject enrollment, data collection, data and statistical analysis. All authors contributed to the writing and editing of the manuscript. All authors contributed to data acquisition, analysis and interpretation, and all reviewed and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing statement
The data that support the findings of this study are available via a data access agreement. Please contact the corresponding author (AB) for this request.
Funding statement
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Declaration of interests
All authors declare no relevant conflicts of interest relevant to this work.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100227.
Appendix. Supplementary materials
References
- 1.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMOA2001191/SUPPL_FILE/NEJMOA2001191_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine | FDA. Accessed June 18, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
- 3.FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine | FDA. Accessed June 18, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
- 4.FDA issues emergency use authorization for third COVID-19 vaccine | FDA. Accessed June 18, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
- 5.Provisional COVID-19 deaths by week, sex, and age | Data | Centers for Disease Control and Prevention. Accessed December 13, 2021. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-by-Week-Sex-and-Age/vsak-wrfu
- 6.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021 doi: 10.1056/nejmc2104974. Published online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernal JL, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. https://doi.org/101056/NEJMoa2108891. Published online July 21, 2021:NEJMoa2108891. doi:10.1056/NEJMOA2108891
- 8.Daniel W., Nivet M., Warner J., Podolsky D.K. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med. 2021;384(20):1962–1963. doi: 10.1056/nejmc2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redmond S.N., Jones L.D., Sadri N., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated and unvaccinated healthcare personnel in a veterans affairs healthcare system. Infect Control Hosp Epidemiol. 2021:1–6. doi: 10.1017/ice.2021.256. Published online May 27, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selby L., Hewlett A., Cawcutt K., et al. Effect of SARS-CoV-2 mRNA vaccination in healthcare workers with high risk COVID-19 exposure. Infect Control Hosp Epidemiol. 2021:1–2. doi: 10.1017/ice.2021.193. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. https://doi.org/101056/NEJMoa2109072. Published online July 28, 2021. doi:10.1056/NEJMOA2109072
- 12.Sheikh A., McMenamin J., Taylor B., Robertson C. SARS-CoV-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet North Am Ed. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg Y., Mandel M., Bar-On Y.M., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronavirus (COVID-19) update: FDA expands eligibility for COVID-19 vaccine boosters | FDA. Accessed December 13, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters
- 15.FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations | FDA. Accessed December 13, 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations
- 16.Centers for Disease Control and Prevention. COVID-19 vaccine booster shots. Published December 9, 2021. Accessed December 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
- 17.Michigan Care Improvement Registry. Michigan Immunization Portal for Citizens 18 years and older. Published December 1, 2021. Accessed December 23, 2021. https://mcir.org/2021/12/01/michigan-immunization-portal-for-citizens-18-years-and-older/
- 18.Moore B.J., White S., Washington R., Coenen N., Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ elixhauser comorbidity index. Med Care. 2017;55(7):698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality. APPENDIX I: immunocompromised state diagnosis and procedure codes. AHRQ QITM ICD-10-CM/PCS Specification v2019. Published July 2019. Accessed December 21, 2021. https://qualityindicators.ahrq.gov/Downloads/Modules/PSI/V2019/TechSpecs/PSI_Appendix_I.pdf
- 20.Holm S. A simple sequentially rejective multiple test procedure a simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 21.Bahl A., Johnson S., Maine G., et al. Vaccination reduces need for emergency care in breakthrough COVID-19 infections: a multicenter cohort study. Lancet Reg Health Am. 2021;4 doi: 10.1016/j.lana.2021.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butt A.A., Nafady-Hego H., Chemaitelly H., et al. Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination. Int J Infect Dis. 2021;110:353–358. doi: 10.1016/J.IJID.2021.08.008. IJID : official publication of the International Society for Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMOA2114255/SUPPL_FILE/NEJMOA2114255_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/JAMA.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin E.G., Lustig Y., Cohen C., et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMOA2114583/SUPPL_FILE/NEJMOA2114583_DATA-SHARING.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMC2108861/SUPPL_FILE/NEJMC2108861_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.