To the Editor,
Bastard et al. reported that autoantibodies neutralizing type I interferons (IFN)s are found in at least 18% of deceased COVID-19 patients [1]. They also showed that the prevalence of neutralizing anti-type I IFN antibodies in the general population increases with age, with antibodies to IFN-α2 and/or IFN-ω present in 0.17% of people < 70 years and in at least 4% of people over 80 years [1], suggesting that the autoantibodies predate COVID-19 [1]. In four [1] and two [2] COVID-19 patients for whom pre–COVID-19 samples were available, autoantibodies against IFN-α2 and/or IFN-ω were present before SARS-CoV-2 infection.
It is, however, unknown whether SARS-CoV-2 infection enhances antibody formation to type I IFNs in patients with pre-existing antibodies or might even trigger antibody formation in patients without pre-existing antibodies. Comparatively, little is known about the fluctuation of antibody levels over time. In order to address these questions, we studied anti-IFN-α2 antibodies in controls and in patients with severe COVID-19 [disease severity class IV (according to reference [3]): respiratory failure requiring mechanical ventilation, shock, organ failure that requires ICU care] for whom pre- and post-COVID-19 left-over serum samples (from routine laboratory analyses) were available. The study was approved by the Ethics Committee of the University Hospitals Leuven, Belgium (S64799, S63708, S63881).
Anti-IFN-α2 antibodies were measured by a Luminex bead-based assay as previously described [4] with minor modifications (See Supplemental Data). In order to evaluate whether the autoantibodies neutralized IFN-α2, CD14-stained blood (200 µL) from a healthy control was incubated with 10% healthy control or patient serum and unstimulated or stimulated with 10 ng/mL IFN-α2 (Miltenyi Biotec) for 15 min at 37 °C. Cells were fixed, permeabilized and stained for intracellular phosphorylated STAT1 [using PE-labeled mouse anti-PSTAT1 (pY701), BD]. Cells were acquired on a BD Lyric instrument with gating on CD14-positive monocytes.
Anti-IFN-α2 antibodies were measured in samples from 15 healthy non-infected individuals (median age 31, age range 25–61, males/females ratio 6/9) and in 52 patients with severe COVID-19 (median age 67, age range 38–91, males/females ratio 38/14). In this latter population, 8/52 had anti-IFN-α2 antibodies. From the 52 patients, pre- and post-SARS-CoV-2 infection samples from eight patients, irrespective of having anti-IFN-α2 antibodies, were selected based on sample availability. In addition, post-infection samples from two patients with severe COVID-19 and IFN-α2 antibodies were included for whom no pre-infection samples were available. The median age of ten included patients was 68 years (age range 50–83 years old) and the male/female ratio 6/4.
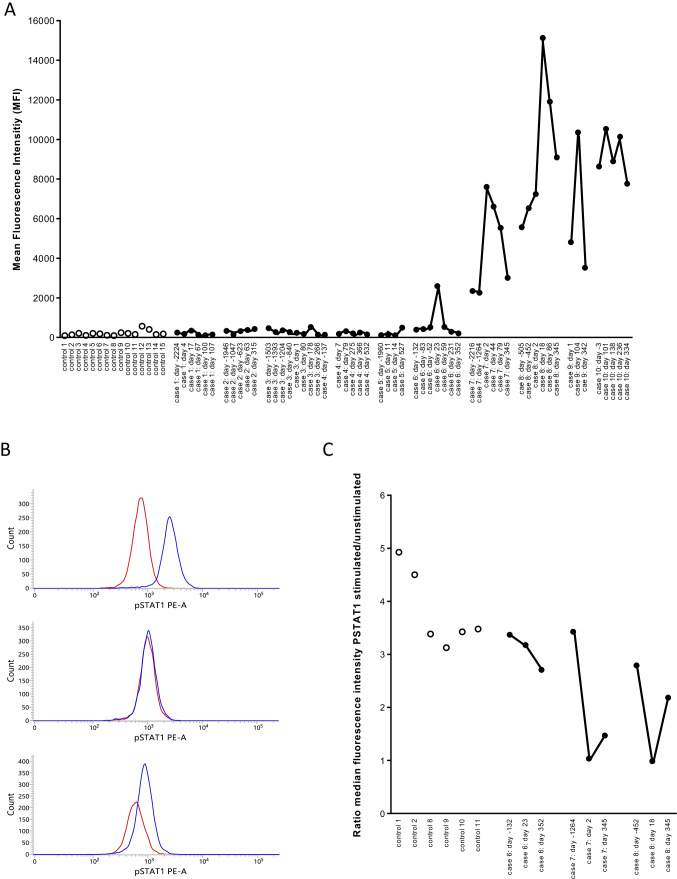
In two patients with pre-existing IFN-α2 autoantibodies (case 7 and case 8), higher antibody levels were observed during COVID-19 compared to pre-COVID-19. In both patients the antibody levels started to decline + / − 6–12 weeks after SARS-CoV-2 infection and further declined up to 11 months after infection (Fig. 1A). In both patients, the capacity of the serum to neutralize IFN-α2 was higher during infection than pre-infection, with complete abolishment of STAT1 phosphorylation in whole blood by serum obtained during acute COVID-19 infection (Fig. 1B, C). In the pre-infection samples, the neutralizing capacity was comparable to or marginally higher than the neutralizing capacity of the controls. It should be noted, however, that our conditions for detecting neutralizing autoantibodies (neutralization of 10 ng/mL IFN-α2) were more stringent than the conditions recently validated by Bastard et al. (neutralization of 100 pg/mL IFN-α2) [1]. It is thus possible that patients who had neutralizing activity pre-COVID-19 similar to controls may have antibodies capable of neutralizing lower but more physiologic levels of IFN-α2.
Fig. 1.
Antibodies to IFN-α2 in controls and in COVID-19 patients. Panel A. Antibodies to IFN-α2 were measured in 15 healthy controls, in 6 patients with pre-COVID-19 antibody levels that were comparable to the antibody levels in the 15 controls (cases 1–6), in 2 patients with pre-COVID anti-IFN-α2 antibodies (cases 7–8) and in 2 patients with anti-IFN-α2 antibodies but for whom with no pre-COVID samples were available (cases 9–10). In COVID-19 patients, antibody levels were measured in pre-COVID-19 samples, during and post-COVID-19. The results are from a representative experiment out of 2 experiments. Controls are represented by open symbols, cases by filled symbols. In the X-axis, the number of days before infection or the number of days post-infection are indicated (first positive PCR for SARS-CoV-2 is day 0). Panel B. STAT-1 phosphorylation in the presence of 10% serum in unstimulated (red line) or stimulated (10 ng/mL IFN-α2) cells (blue line). The distribution of the fluorescence intensities is shown. The results for patient case 7 are shown with serum obtained before (day − 1264), during (day 2) and after (day 345) SARS-CoV-2 infection. Panel C. Neutralizing capacity of 10% serum on whole blood STAT-1 phosphorylation. STAT-1 phosphorylation was evaluated in 6 controls and three patients (cases 6, 7, and 8). For each patient, three serum samples (pre-infection, during infection, and post-infection) were evaluated. The ratio of the median fluorescence intensity in IFN-α2-stimulated to unstimulated cells was calculated. A ratio of 1 indicates that IFN-α2-induced STAT1 phosphorylation was abolished, suggesting the presence of neutralizing antibodies. The days before or after the first positive PCR for SARS-CoV-2 are indicated (first positive PCR is day 0)
Six COVID-19 patients had pre-COVID-19 antibody levels that were comparable to the antibody levels in the 15 controls. SARS-CoV-2 infection (documented by positive PCR) did not induce anti-IFN-α2 antibodies in five (cases 1–5) of the six patients, whereas it did in one patient (case 6). In this patient, antibodies to IFN-α2 were increased 23 days after the first positive PCR for SARS-CoV-2 and declined to pre-COVID-19 levels 59 days after the first positive PCR. The serum obtained 23 days after the first positive PCR did not neutralize IFN-α2 (Fig. 1C).
Finally, we also evaluated two COVID-19 patients with anti-IFN-α2 antibodies for whom only post-COVID-19 serum samples were available. In one of these patients (case 9) a transient increase in anti-IFN-α2 antibody levels was observed upon SARS-CoV-2 infection, whereas in the other patient (case 10), antibody levels remained high over a long period (11 months). This patient presented with acute respiratory distress syndrome and required 83 days of intensive care.
The clinical features of the patients with increased anti-IFN-α2 antibodies are summarized in Supplemental Data Table 1. Interestingly, different auto-immune features were observed in two patients: one was known with auto-immune hepatitis under corticosteroid therapy and the other was diagnosed with myasthenia gravis during hospitalization. Of note, antibodies to IFN-α2 have previously been described in myasthenia gravis [5].
Our finding that anti-IFN-α2 antibodies transiently increase during COVID-19 infection is consistent with the observation of Shaw et al. [6] that binding levels and neutralization activity of autoantibodies to type I IFNs were highest during acute COVID-19 and decreased afterwards. Shaw et al. [6] also reported more stable fluorescence intensity of autoantibodies neutralizing type I IFNs in patients with autoimmune polyendocrine syndrome type-1, thymoma, and RAG deficiency and in patients with only anti-IFN-ω autoantibodies [6].
In conclusion, our findings indicate that SARS-CoV-2 infection can enhance pre-existing (neutralizing) anti-IFN-α2 antibodies and, albeit to a lesser extent, induce anti-IFN-α2 antibodies in a patient without pre-existing antibodies. In most cases, the increase was transient and antibody levels tended to return to pre-COVID-19 levels within 2–11 months. Our limited data endorse that the presence of anti-IFN-α2 antibodies can be associated with autoimmune disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Birgit Timmermans and Eliena Jongbeis for expert technical assistance. We thank P. Bastard and JL Casanova for helpful discussions.
Author Contribution
Material preparation, data collection, and analysis were performed by Sophie Steels, Jan Van Elslande, Maaike Cockx, Glynis Frans, Maya Imbrechts, Doreen Dillaerts, Pieter Vermeersch, Els Wauters, and Joost Wauters. The first draft of the manuscript was written by Xavier Bossuyt and Paul De Munter. All the authors commented on the manuscript.
Funding
The study was supported by a COVID-19 grant from KU Leuven (KOOR, Group Biomedical Sciences and University Hospitals Leuven). PV is a senior clinical investigator of the Fund for Scientific Research — Flanders.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval
Ethics approval was granted by the Ethics Committee of the University Hospital Leuven, Belgium (S64799, S63708, S63881).
Informed Consent and Consent for Publication
This was a retrospective study using left-over material of samples submitted to the clinical laboratory (secondary use) for which an informed consent waiver was authorized by the ethics committee (University Hospital Leuven, Belgium) (S64799). Patients were notified of the content and aim of the study. Besides, written informed consent was obtained from six study participants according to the ethical guidelines of the Declaration of Helsinki. The Ethics Committee of the University Hospitals Leuven approved this study (S63881). Healthy controls gave informed consent (S63708).
Competing Interests
The authors declare no competing interests.
Consortium
Leuven COVID-19 Study Group.
Maaike Cockx4, Glynis Frans1, Maya Imbrechts5, Doreen Dillaerts4, Isabelle Meyts6, Nick Geukens5, Els Wauters7, Pieter Vermeersch1, Joost Wauters2,3
1Department of Laboratory Medicine, University Hospitals Leuven, Herestraat 49, B-3000 Leuven, Belgium.
2Laboratory for Clinical Infectious and Inflammatory Disorders, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium.
3Department of Internal Medicine, University Hospitals Leuven, Leuven, Belgium.
4Clinical and Diagnostic Immunology, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium.
5PharmAbs, the KU Leuven Antibody Center, KU Leuven, Leuven, Belgium.
6Inborn Errors of Immunity, Department of Microbiology, Immunology and Transplantation, KU Leuven and Department of Pediatrics, University Hospitals Leuven, Leuven, Belgium.
7Laboratory of Respiratory Diseases and Thoracic Surgery, Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sophie Steels and Jan Van Elslande have equally contributed to this work.
Contributor Information
Xavier Bossuyt, Email: Xavier.bossuyt@uzleuven.be.
Leuven COVID-Study Group:
Maaike Cockx, Glynis Frans, Maya Imbrechts, Doreen Dillaerts, Isabelle Meyts, Nick Geukens, Els Wauters, Pieter Vermeersch, and Joost Wauters
References
- 1.Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol. 2021;6(62):eabl4340. [DOI] [PMC free article] [PubMed]
- 2.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020 23;370(6515):eabd4585. [DOI] [PMC free article] [PubMed]
- 3.Qu J, Wu C, Li X, Zhang G, Jiang Z, Li X, et al. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:2255–2258. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guldager DKR, von Stemann JH, Larsen R, Bay JT, Galle PS, Svenson M, et al. A rapid, accurate and robust particle-based assay for the simultaneous screening of plasma samples for the presence of five different anti-cytokine autoantibodies. J Immunol Methods. 2015;425:62–68. doi: 10.1016/j.jim.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Bello-Rivero I, Cervantes M, Torres Y, Ferrero J, Rodríguez E, Pérez J, García I, Díaz G, López-Saura P. Characterization of the immunoreactivity of anti-interferon alpha antibodies in myasthenia gravis patients. Epitope mapping J Autoimmun. 2004;23(1):63–73. doi: 10.1016/j.jaut.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Shaw ER, Rosen LB, Cheng A, Dobbs K, Delmonte OM, Ferré EMN, Schmitt MM, Imberti L, Quaresima V, Lionakis MS, Notarangelo LD, Holland SM, Su HC. Temporal dynamics of anti-type 1 interferon autoantibodies in COVID-19 patients. Clin Infect Dis. 2021:ciab1002. 10.1093/cid/ciab1002. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.