Abstract
The MICs of 17 antimicrobial agents for 181 Streptococcus pneumoniae strains were determined by the E-test. Overall, 69.1% were penicillin resistant (MIC > 0.06 μg/ml). Resistance to ciprofloxacin (MIC > 2 μg/ml), levofloxacin (MIC > 2 μg/ml), or trovafloxacin (MIC > 1 μg/ml) was found in 12.1, 5.5, or 2.2% of the strains, respectively. These high rates of resistance raise concerns for the future.
The resistance of Streptococcus pneumoniae to β-lactams, macrolides, tetracyclines, and chloramphenicol has been increasing rapidly in southeast Asia (1). Some of the newer fluoroquinolones, by virtue of their excellent in vitro activity against S. pneumoniae, oral bioavailability, and tissue penetration, hold promise as the drugs of choice for therapy of respiratory tract infections due to multiple drug-resistant S. pneumoniae in this region (6). We have therefore conducted a study on the in vitro activities of 3 fluoroquinolones and 13 other antimicrobial agents.
A total of 181 consecutive, nonduplicate isolates of S. pneumoniae were obtained from four regional laboratories (61 isolates from laboratory A, 41 isolates from laboratory B, 31 isolates from laboratory C, and 49 isolates from laboratory D) during the second half of 1998. These four laboratories provide microbiology service to seven public hospitals, serving a population of about 3 million in the Hong Kong island (south and west), Kowloon (central), and the New Territory (south and north) regions of Hong Kong. The strains were isolated from throat (5), nose (4), eye (5), sputum (143), tracheal aspirate (4), and blood (21). Strains were identified as S. pneumoniae by Gram stain, colony morphology, optochin susceptibility, and bile solubility.
The MICs of ciprofloxacin, levofloxacin, trovafloxacin, penicillin, ampicillin, ticarcillin-clavulanate, piperacillin-tazobactam, cefuroxime, cefpodoxime, ceftibuten, ceftriaxone, cefepime, meropenem, erythromycin, azithromycin, clindamycin, and vancomycin were determined by the E-test (AB Biodisk, Solna, Sweden) following the manufacturer’s instructions. All susceptibility testings were done in the University of Hong Kong by one technician. Susceptibility tests were performed from a bacterial inoculum whose turbidity was equivalent to that of a McFarland standard of 0.5. From this suspension, E-tests were performed on Mueller-Hinton agar with 5% sheep blood (Micro Diagnostics Incorporated, Lombard, Ill.). The plates were incubated at 35°C in 5% CO2 for 20 to 24 h (10). MICs falling between two marks on the E-test strip were rounded up to the next higher twofold dilution, as recommended in the instructions. Quality control strains (S. pneumoniae ATCC 49619, Staphylococcus aureus ATCC 29213, and Escherichia coli ATCC 25922) were included with each run. Interpretation of results was performed according to recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) (8). Because the NCCLS breakpoints for microbroth dilution appear to have limited applicability to E-test results for macrolides versus S. pneumoniae, modified breakpoints as recommended by the manufacturer were used (2). For erythromycin, the breakpoints were as follows: susceptible, ≤0.5 μg/ml; intermediate, 1 μg/ml; and resistant, ≥2 μg/ml. For azithromycin, the following breakpoints were used: susceptible, ≤4 μg/ml; intermediate, 8 μg/ml; and resistant, ≥16 μg/ml. No reference breakpoints for cefpodoxime, ceftibuten, ticarcillin-clavulanate, piperacillin-tazobactam, and ciprofloxacin are available. Multiple drug resistance is defined as resistance to at least one member from each of the three classes of antimicrobial agents including the β-lactams, macrolides, and fluoroquinolones. The chi-square, Fisher exact, or Kruskal-Wallis test was used for statistical analysis.
The susceptibilities of the 181 pneumococcal isolates to 17 antimicrobial agents are summarized in Table 1. Rates of resistance to penicillin (MIC > 0.06 μg/ml) were 60.7% (37 of 61) for laboratory A, 80.5% (33 of 41) for laboratory B, 54.8% (17 of 31) for laboratory C, and 79.2% (38 of 48) for laboratory D (P = 0.05 for median MICs, Kruskal-Wallis test). Penicillin resistance rates were similar for children (age, ≤12 years) and adults (age, >12 years) (73.8 versus 66.4%, respectively; P > 0.05) but penicillin resistance was less common for blood isolates than for isolates from other sites (33.3 versus 73.8%, respectively; P < 0.01). Thirty-four (18.8%) isolates were highly resistant to penicillin with MICs of >2 μg/ml. For the most resistant isolate, the MIC was 6 μg/ml. The MICs of ampicillin and piperacillin-tazobactam for most strains were within one dilution difference of that of penicillin. However, the MIC of ticarcillin-clavulanate increased disproportionately with an increasing level of resistance to penicillin (data not shown). Ceftriaxone had the lowest MIC50 (MIC at which 50% of isolates were inhibited) and MIC90 among the cephalosporins, followed by cefepime, cefpodoxime, and cefuroxime. Multiple drug resistance was found in 12.1% (22 of 181) of the isolates.
TABLE 1.
Susceptibilities of 181 isolates of S. pneumoniae stratified by penicillin susceptibility
| Antimicrobial agent and penicillin susceptibility (n)a | Breakpoint (μg/ml)b | MIC (μg/ml)c
|
No. (%) of isolates not susceptible to indicated antimicrobial agentb | ||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
| Penicillin | >0.06 | ||||
| S (56) | 0.023–0.064 | 0.032 | 0.064 | 0 (0) | |
| I (27) | 0.094–1 | 0.19 | 1 | 27 (100) | |
| R (98) | 1.5–6 | 2 | 4 | 98 (100) | |
| Total (181) | 0.023–6 | 1.5 | 3 | 125 (69.1) | |
| Cefuroxime | >0.5 | ||||
| S | 0.023–0.19 | 0.023 | 0.064 | 0 (0) | |
| I | 0.064–3 | 0.38 | 4 | 12 (44.4) | |
| R | 2–16 | 4 | 6 | 98 (100) | |
| Total | 0.023–16 | 3 | 4 | 110 (60.8)d | |
| Cefpodoxime | NA | ||||
| S | 0.023–0.94 | 0.032 | 0.064 | NA | |
| I | 0.05–2 | 0.38 | 2 | NA | |
| R | 1–8 | 3 | 4 | NA | |
| Total | 0.023–8 | 2 | 3 | NA | |
| Ceftibuten | NA | ||||
| S | 2–48 | 4 | 12 | NA | |
| I | 6–>256 | 96 | >256 | NA | |
| R | >256 | >256 | >256 | NA | |
| Total | 2–>256 | >256 | >256 | NA | |
| Ceftriaxone | >0.5 | ||||
| S | 0.012–0.19 | 0.023 | 0.032 | 0 (0) | |
| I | 0.032–0.5 | 0.19 | 0.5 | 0 (0) | |
| R | 0.25–3 | 0.75 | 1 | 80 (81.6) | |
| Total | 0.012–3 | 0.5 | 1 | 80 (44.2)d | |
| Cefepime | >0.5 | ||||
| S | 0.023–0.94 | 0.047 | 0.094 | 1 (1.8) | |
| I | 0.094–1.5 | 0.5 | 1.5 | 12 (44.4) | |
| R | 0.75–3 | 1.5 | 2 | 98 (100)d | |
| Total | 0.023–3 | 1.5 | 2 | 111 (61.3) | |
| Meropenem | >0.25 | ||||
| S | 0.012–0.12 | 0.012 | 0.02 | 0 (0) | |
| I | 0.023–0.38 | 0.064 | 0.38 | 5 (18.5) | |
| R | 0.094–0.75 | 0.5 | 0.75 | 95 (96.9) | |
| Total | 0.012–0.75 | 0.38 | 0.5 | 100 (55.2)d | |
| Erythromycin | >0.25; >0.5 | ||||
| S | 0.032–>256 | 0.13 | >256 | 22 (39.3); 22 (39.3) | |
| I | 0.13–>256 | >256 | >256 | 23 (85.2); 23 (85.2) | |
| R | 0.13–>256 | 4 | >256 | 97 (98.9); 97 (98.9) | |
| Total | 0.032–>256 | 4 | >256 | 142 (78.5); 142 (78.5)d | |
| Azithromycin | >0.5; >4 | ||||
| S | 0.094–>256 | 1.5 | >256 | 45 (80.3); 22 (39.3) | |
| I | 0.75–>256 | >256 | >256 | 27 (100); 23 (85.2) | |
| R | 1–>256 | 24 | >256 | 98 (100); 91 (92.9) | |
| Total | 0.094–>256 | 24 | >256 | 170 (93.9); 136 (75.1)d | |
| Clindamycin | >0.25 | ||||
| S | 0.023–>256 | 0.19 | >256 | 12 (21.4) | |
| I | 0.094–>256 | >256 | >256 | 17 (62.9) | |
| R | 0.064–>256 | 0.19 | >256 | 16 (16.3) | |
| Total | 0.023–>256 | 0.19 | >256 | 45 (24.7) | |
| Ciprofloxacin | NA | ||||
| S | 0.38–8 | 1 | 2 | NA | |
| I | 0.5–>32 | 1 | 4 | NA | |
| R | 0.38–>32 | 1 | 12 | NA | |
| Total | 0.38–>32 | 1 | 6 | NA | |
| Levofloxacin | >2 | ||||
| S | 0.5–1.5 | 0.75 | 1 | 0 (0) | |
| I | 0.5–24 | 0.75 | 1.5 | 1 (3.7) | |
| R | 0.38–>32 | 1 | 3 | 9 (9.2) | |
| Total | 0.38–>32 | 1 | 1.5 | 10 (5.5)d | |
| Trovafloxacin | >1 | ||||
| S | 0.094–0.38 | 0.19 | 0.25 | 0 (0) | |
| I | 0.094–0.38 | 0.19 | 0.25 | 0 (0) | |
| R | 0.064–>32 | 0.19 | 0.5 | 4 (4) | |
| Total | 0.064–>32 | 0.19 | 0.38 | 4 (2.2) | |
| Vancomycin | >1 | ||||
| S | 0.38–1 | 0.5 | 0.75 | 0 (0) | |
| I | 0.38–0.75 | 0.75 | 0.75 | 0 (0) | |
| R | 0.25–1 | 0.5 | 0.75 | 0 (0) | |
| Total | 0.25–1 | 0.5 | 0.75 | 0 (0) | |
S, susceptible; I, intermediate; R, resistant.
Breakpoints are given in accordance with NCCLS standard M100-S (10) or guidelines from the manufacturer of the E-test. The breakpoints and interpretations according to the recommendations of the manufacturer of the E-test are underlined. Both intermediate and resistant strains were included as nonsusceptible.
50% and 90%, MICs required to inhibit 50 and 90% of the isolates, respectively.
Penicillin-susceptible versus penicillin-nonsusceptible strains. P < 0.05 by chi-square or Fisher exact test.
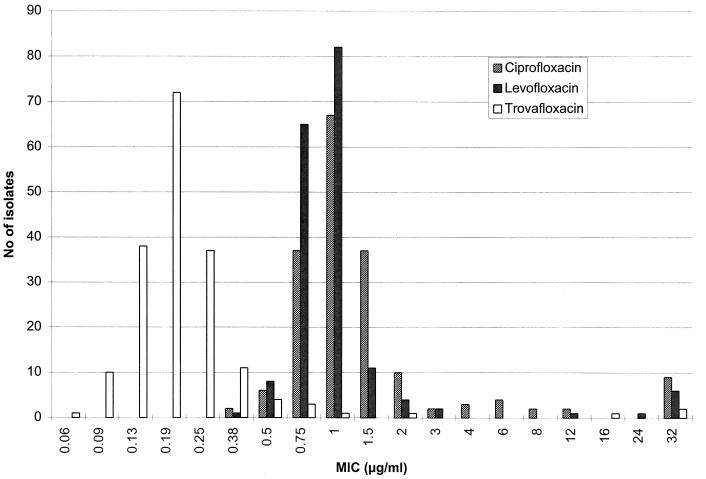
The distribution of MICs for the fluoroquinolones are shown in Fig. 1. Ciprofloxacin was the least active fluoroquinolone with a MIC of >2 μg/ml for 12.1% (22 of 181) of the isolates. Importantly, resistance to levofloxacin and trovafloxacin was only found in the penicillin-resistant isolates. Trovafloxacin was highly active, with similar MIC90s for both penicillin-susceptible and -resistant isolates. However, all four trovafloxacin-resistant isolates were also penicillin and macrolide resistant (Table 2).
FIG. 1.
Distribution of MICs of three fluoroquinolones for 181 strains of S. pneumoniae.
TABLE 2.
Distribution and MICs of four β-lactam–macrolide–fluoroquinolone-resistant pneumococci
| Strain | Laboratory | Date of isolation (mo/day/yr) | Sample | MICa (μg/ml) of:
|
||||
|---|---|---|---|---|---|---|---|---|
| Trovafloxacin | Ciprofloxacin | Levofloxacin | Penicillin | Erythromycin | ||||
| S10B8 | A | 2/11/98 | Sputum | 2 | >32 | >32 | 2 | 3 |
| S7D7 | D | 3/9/98 | Sputum | 16 | >32 | >32 | 2 | 4 |
| S7C5 | C | 27/8/98 | Sputum | >32 | >32 | >32 | 3 | 6 |
| S7E1 | D | 4/9/98 | Sputum | >32 | >32 | >32 | 1.5 | 2 |
See Table 1 for breakpoints.
A marked increase in the overall prevalence of resistance to penicillin (69.1%) was found compared to rates from previous studies in 1993 (18%) and 1995 (28.9%) in Hong Kong (5, 7). While resistance to penicillin among S. pneumoniae isolates is an emerging problem worldwide, this high rate of resistance (>60%) has only been reported in several Asian countries, including Korea (73.4%), Taiwan (71%), Japan (67.7%), and Thailand (63.1%) (1, 12). High rates of cross-resistance to the cephalosporins (64 to 88%) and meropenem (80.2%) among the penicillin-intermediate or -resistant isolates were also found.
The overall rate of resistance to erythromycin also rose dramatically from 10% in 1993 and 39.2% in 1995 to 78.5% in the present study (3). The overall consumption of antimicrobial agents is frequently identified as a risk factor for the rapid emergence of resistance. Notably, the consumption of macrolides in Hong Kong increased by more than twofold from 1994 to 1997 (13). In addition, overcrowdedness may be another factor that contributes to the rapid dissemination of drug-resistant S. pneumoniae in Hong Kong. This is supported by findings from Ip and coworkers (6) that 70% of the penicillin-resistant strains belong to a variant of the Spanish type 23F clone.
The emergence of fluoroquinolone-resistant S. pneumoniae is alarming because this group of antimicrobial agents is at present the only possible option for oral therapy of infection due to β-lactam–macrolide-resistant strains. Within 3 years, the rate of resistance to fluoroquinolones has increased from <0.5% for ofloxacin (5) to 5.5% for levofloxacin (MIC > 2 μg/ml). This is in contrast to the lack of development of resistance reported in other areas (2, 11, 14). Most alarming, 4% of the penicillin-resistant isolates were already highly resistant to trovafloxacin, an agent only registered for use in Hong Kong in October 1998.
In conclusion, we have reported extremely high rates of resistance to β-lactams and macrolides among S. pneumoniae isolates in Hong Kong. Trovafloxacin is highly active in vitro against β-lactam–macrolide-resistant strains. However, ominous resistance to fluoroquinolones is emerging. The global mobility of human populations and the convergence of tourist and business traffic in Hong Kong will likely facilitate the worldwide spread of these very resistant clones (7).
Acknowledgments
This study was supported by grants from the Committee on Research and Conference Grant, The University of Hong Kong, and the Pfizer Corporation, Hong Kong.
We thank Allan Ronald for a critical reading of the manuscript.
REFERENCES
- 1.Chiou C C, Liu Y C, Huang T S, Hwang W K, Wang J H, Lin H H, Yen M Y, Hsieh K S. Extremely high prevalence of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae among children in Kaohsiung, Taiwan. J Clin Microbiol. 1998;36:1933–1937. doi: 10.1128/jcm.36.7.1933-1937.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunha B A, Qadri S M, Ueno Y, Walters E A, Domenico P. Antibacterial activity of trovafloxacin against nosocomial Gram-positive and Gram-negative isolates. J Antimicrob Chemother. 1997;39(Suppl. B):29–34. doi: 10.1093/jac/39.suppl_2.29. [DOI] [PubMed] [Google Scholar]
- 3.Fasola E L, Bajaksouzian S, Appelbaum P C, Jacobs M R. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob Agents Chemother. 1997;41:129–134. doi: 10.1128/aac.41.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerardo S H, Citron D M, Claros M C, Goldstein E J C. Comparison of Etest to broth microdilution method for testing Streptococcus pneumoniae susceptibility to levofloxacin and three macrolides. Antimicrob Agents Chemother. 1996;40:2413–2415. doi: 10.1128/aac.40.10.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho P L, Yuen K Y, Yam W C, Wong S S Y, Luk W K. Changing patterns of susceptibilities of blood, urinary and respiratory pathogens in Hong Kong. J Hosp Infect. 1995;31:305–317. doi: 10.1016/0195-6701(95)90209-0. [DOI] [PubMed] [Google Scholar]
- 6.Ip M, Lyon D J, Yung R W H, Chan C, Cheng A F. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Molecular epidemiology of penicillin- and multidrug-resistant Streptococcus pneumoniae in Hong Kong, abstr. C20; p. 74. [Google Scholar]
- 7.Kam K M, Luey K Y, Fung S M, Yiu P P, Harden T J, Cheung M M. Emergence of multiple-antibiotic-resistant Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1995;39:2667–2670. doi: 10.1128/aac.39.12.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klugman K P, Gootz T D. In-vitro and in-vivo activity of trovafloxacin against Streptococcus pneumoniae. J Antimicrob Chemother. 1997;39(Suppl. B):51–55. doi: 10.1093/jac/39.suppl_2.51. [DOI] [PubMed] [Google Scholar]
- 9.Munoz R, Coffey T J, Daniels M, Dowson C G, Laible G, Casal J, et al. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991;164:302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing, 8th informational supplement. M100-S8. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 11.Plouffe J F. Levofloxacin in vitro activity against bacteremic isolates of Streptococcus pneumoniae. Franklin County Pneumonia Study Group Diagn Microbiol Infect Dis. 1996;25:43–45. doi: 10.1016/0732-8893(96)00068-5. [DOI] [PubMed] [Google Scholar]
- 12.Song J H the Asian Network for Surveillance of Resistant Pneumococci (ANSORP) Study Group. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Prevalence of drug-resistant pneumococci in 11 Asian countries, abstr. C48; p. 54. [Google Scholar]
- 13.Tam C Y. Extremely high rate of resistance to the macrolides in Hong Kong. Ming Pao Post. 1998;1998(Nov. 25):A2. [Google Scholar]
- 14.Thornsberry C, Ogilvie P, Kahn J, Mauriz Y. Surveillance of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States in 1996–1997 respiratory season. The Laboratory Investigator Group. Diagn Microbiol Infect Dis. 1997;29:249–257. doi: 10.1016/s0732-8893(97)00195-8. [DOI] [PubMed] [Google Scholar]