Abstract
Objective
To determine whether high-dose dexamethasone increases the number of ventilator-free days (VFD) among patients with acute respiratory distress syndrome (ARDS) caused by COVID-19.
Design
Multicenter, randomized, open-label, clinical trial.
Participants
Consecutive patients with confirmed COVID-19-related ARDS were enrolled from June 17, 2020, to March 27, 2021, in four intensive care units (ICUs) in Argentina
Intervention
16 mg of dexamethasone intravenously daily for five days followed by 8 mg of dexamethasone daily for five days or 6 mg of dexamethasone intravenously daily for 10 days.
Main Outcome and Measures
The primary outcome was ventilator-free days during the first 28 days. The secondary outcomes were all-cause mortality at 28 and 90 days, infection rate, muscle weakness, and glycemic control in the first 28 days.
Results
Data from 98 patients who received at least one dose of dexamethasone were analyzed. The trial was prematurely terminated due to low enrollment rate. At 28 days after randomization, there was no difference between high- and low-dose dexamethasone groups in VFD (median, 0 [interquartile range [IQR] 0-14] vs. 0 [IQR 0-1] days; P = .231), or in the mean duration of mechanical ventilation (19 ± 18 vs. 25 ± 22 days; P = .078). The cumulative hazard of successful discontinuation from mechanical ventilation was increased by the high-dose treatment (adjusted sub-distribution hazard ratio: 1.84; 95% CI: 1.31 to 2.5; P < .001). None of the prespecified secondary and safety outcomes showed a significant difference between treatment arms.
Conclusions
Among patients with ARDS due to COVID-19, the use of higher doses of dexamethasone compared with the recommended low-dose treatment did not show an increase in VFD. However, the higher dose significantly improved the time required to liberate them from the ventilator
Keywords: acute respiratory distress syndrome, coronavirus, dexamethasone, randomized controlled trial, viral pneumonia
In December 2019, an outbreak of coronavirus disease 2019 (COVID-19), which was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), broke out in Wuhan, China.1,2 The World Health Organization (WHO) declared it a significant threat to international health. COVID-19 mainly affected the respiratory system with some patients rapidly progressing to acute respiratory distress syndrome (ARDS). Many of them will require mechanical ventilation for a long time, overcrowding the health system. The COVID-19-related ARDS main pathological pattern is diffuse alveolar damage,3 and there is evidence that the dysregulated inflammatory response may worsen the prognosis.
Corticosteroids might exert an effect in controlling this exacerbated response. Over the last decades, many clinical studies have tested the utility of corticosteroids in critically ill patients with ARDS having inconsistent findings.4–6 Nonetheless, several trials evaluated the role of corticosteroids for ARDS treatment in non–COVID-197,8 and COVID-19 patients,9 suggesting a decrease in 28-day mortality in patients with oxygen needs or mechanical ventilation. Although the benefit was considered a general class effect of glucocorticoids, various dose regimens were used, leaving the question of a dose–effectiveness relationship less definitively answered.9
The dexamethasone for COVID-19-related ARDS randomized clinical trial was conducted to evaluate the effectiveness and safety of high- versus low-dose dexamethasone in patients with ARDS. The hypothesis was that high-dose dexamethasone would increase the number of ventilator-free days (VFD) during the first 28 days.
Methods
Study Design and Oversight
We conducted an investigator-initiated, multicenter, randomized, open-label clinical trial in four intensive care units (ICUs) in Argentina. The trial protocol and the statistical analysis plan had been previously published.10 The study was approved at the Research Ethics Committee of CEMIC (Buenos Aires, Argentina) and by the Argentine Society of Intensive Care Medicine (SATI) Ethics Committee. Either a written or an electronic informed consent (REDCap electronic consent framework) was obtained from the legal representative of each patient. The study was registered in PRIISA.BA (code 1264) and in ClinicalTrials.gov on 16 May 2020 (NCT04395105).
Patients
We enrolled patients aged 18 years old or more, who had ARDS according to the Berlin Definition criteria,11 who had confirmed SARS-CoV-2 infection by reverse transcription polymerase chain reaction and were receiving mechanical ventilation for less than 72 h. The exclusion criteria were pregnant or breastfeeding women, terminal disease, therapeutic limitation, severe immunosuppression, chronic treatment with glucocorticoids, participation in another randomized clinical trial, prior use of dexamethasone for COVID-19 (>5 days), or consent refusal (e-Methods Appendix 1).
Trial Procedures
Treatment allocation was performed through an online web-based system (REDCap)12 using a permuted random block sequence stratified by center.
The study was originally designed before RECOVERY trial publication, and the control group had not included corticosteroids.13 Soon after the pre-publications of these results, we amended the protocol to include a low-dose dexamethasone in the control arm. Thereafter, eligible patients were randomly assigned in a 1:1 ratio to receive high- or low-dose dexamethasone plus standard care. The former was 16 mg dexamethasone administered intravenously once daily for five days, followed by 8 mg intravenously administered once daily for additional five days. The low-dose group received 6 mg of dexamethasone per day for 10 days according to the RECOVERY trial. The investigators who assessed the outcomes were not blinded for the assigned treatment.
Standard care was not regulated by the protocol. Nonetheless, it was suggested to treat the patients according to their institutional protocols or the international guidelines for ARDS,6,14 antibiotics, and hemodynamic support for COVID-19 infection.6,15 The ventilator liberation protocol was defined by each site. Nevertheless, it was recommended to daily evaluate the eligibility of the patients to perform a spontaneous breathing trial.15
Clinical and Laboratory Data
Data on demographic characteristics, physiological variables, severity scores, timing from ARDS diagnosis to randomization, corticosteroid use, COVID-19 therapies, and other clinical and laboratory data were collected. The use of sedatives, neuromuscular blocking agents, prone positioning, vasopressors, renal replacement therapy, and extracorporeal membrane oxygenation (ECMO) were registered daily throughout the first 28 days since randomization or until ICU discharge. For that period, we collected information on the use of mechanical ventilation, respiratory monitoring, and other oxygen supportive therapies in the case of extubation, such as high flow nasal oxygen, venturi mask support, or noninvasive ventilation. Data regarding infections, glycemic control, muscle dysfunction, and delirium were also collected as a safety measurement (e-Methods Appendix 1).
The patients were followed up for 28 days after randomization or until hospital discharge, whichever occurred first. The vital status was assessed 28 and 90 days after randomization when needed by a phone interview.
Outcomes
The primary outcomes were VFD during the first 28 days, defined as the number of days alive and free from mechanical ventilation up to the 28th day from randomization. For the patients who died, the number of VFD was set as 0. As a co-primary outcome, the time to complete and successful discontinuation of mechanical ventilation or death was calculated from randomization. The former was defined as the difference in time between randomization and the last day spent on mechanical ventilation without further invasive respiratory support.16
The secondary outcomes were all-cause mortality at 28 and 90 days, the rate of nosocomial infections, the daily value of glucose and insulin dose, muscle strength score, and the frequency of delirium within 28 days of randomization (e-Methods Appendix 1).
Statistical Analysis
No reliable data on ARDS caused by COVID-19 were available at the trial design to allow for an accurate sample size calculation. Therefore, we employed the data from a recently published multicenter randomized trial of non–COVID-19 ARDS7 for our sample size calculation. The sample size was calculated at 142 patients in each group to detect a difference of three VFD between groups, assuming a mean and a standard deviation of 9 days with a two-sided α level of .05 and a power of 80%.
The quantitative variables were expressed as mean and standard deviation or median (25th to 75th percentile range) for normal or non-normal data distribution assessed with the Shapiro–Wilks normality test. The comparison of these variables between experimental treatments was performed using a t-test or a Wilcoxon rank sum test. Furthermore, the proportions were compared with the Fisher exact or chi-squared tests.
The main outcome variable analysis (VFD at day 28) was compared between treatments as stated above. As the Wilcoxon rank sum test does not provide a measure of effect, we calculated the basic bootstrap 95% confidence interval of the difference between treatment arm medians as an exploratory analysis.17 To further explore the potential effect of the treatments, a time-to-event analysis for competing risks was performed to evaluate the time to the complete and successful discontinuation of mechanical ventilation within 28 days. In this analysis, death was considered the competing event, and cumulative incidence curves according to the treatment allocations were constructed. Furthermore, a competing-risks regression model for clustered data was utilized to estimate the effect of treatment on the sub-distribution hazard adjusted for acute physiology and chronic health evaluation (APACHE) II and ARDS severity.18 Each ICU was included as a cluster in the model.16
The probability of survival at 90 days was evaluated with a Kaplan–Meier analysis, and the log-rank test was used for comparison between treatments. A Cox proportional hazards regression model was fit to adjust the treatment effect with APACHE II and ARDS severity.
The rate of infection observed within 28 days of inclusion was calculated using a Poisson regression model, and the experimental treatment was used as a predictor. Additionally, the incidence rate ratio for high-dose dexamethasone treatment and its 95% confidence interval were calculated. Mixed effects linear models were also employed to evaluate the interaction between the treatment allocation and the time after the inclusion of glucose blood levels and insulin doses. To avoid pseudo-replications, each subject was used as a random effect.
A modified intention-to-treat approach was used for the analysis, including only data from those patients who received at least one dose of dexamethasone after inclusion. A two-sided P value of less than.05 was considered statistically significant, and all the analyzes were performed using R software version 3.6.1.19
Premature Trial Termination
During the recruitment period, the use of dexamethasone in the early course of COVID-19 was widely recommended by several trials,13,20–22 which implied that an increasing number of patients were admitted to the ICU with complete corticosteroid treatment. Moreover, the use of high-flow nasal cannula oxygen therapy for severe COVID-19 pneumonia was encouraged,6,23 thereby delaying the initiation of mechanical ventilation in some patients. Therefore, the recruitment rate lowered substantially. By the end of March 2021, we estimated that the time required to achieve the calculated sample size would be greater than three years (e-Figure 1 and e-Figure 2 Appendix 1). As COVID-19 management is rapidly evolving, the research question would probably be obsolete at the end of this time. Due to this estimation, the investigators decided to prematurely terminate the trial on April 5, 2021. No interim data analysis of efficacy or safety was performed before this decision.
Results
Patients
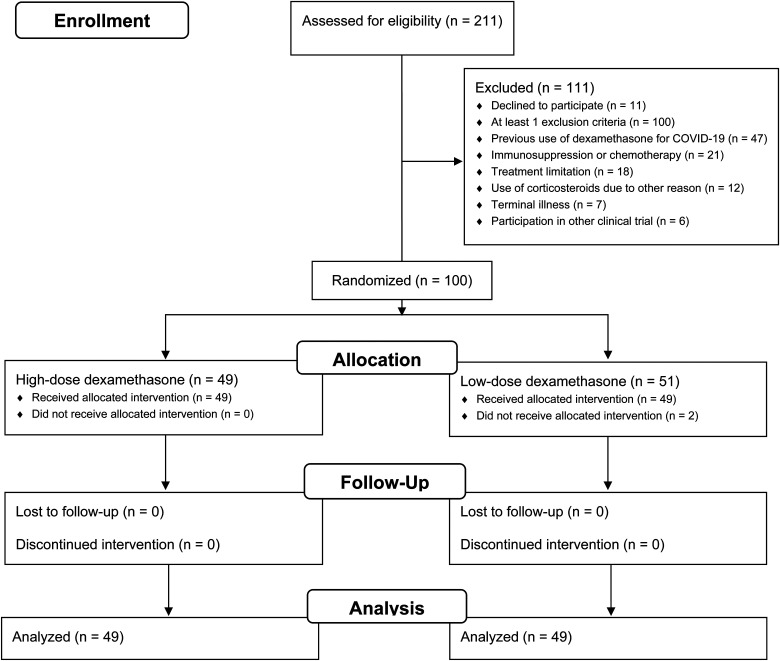
Between June 17, 2020, and March 27, 2021, 211 ARDS patients were screened. One hundred were enrolled, of whom 49 were randomized to the high-dose dexamethasone and 51 to the control group (Figure 1). The data from the first two participants from the control group were not analyzed as they did not receive any dexamethasone. The baseline characteristics were well balanced between groups (Table 1), except for APACHE II and time in mechanical ventilation before inclusion.
Figure 1.
Flow diagram of the study.
Abbreviations: COVID-19, Coronavirus disease 2019.
Table 1.
Baseline Patients’ Characteristicsa.
| Low-dose dexamethasone | High-dose dexamethasone | |
|---|---|---|
| n = 49 | n = 49 | |
| Age (y) | 60.04 ± 13.08 | 63.57 ± 13.59 |
| Gender (female) | 16 (33%) | 13 (26%) |
| Weight (kg) | 97 (80-110) | 92 (80-110) |
| BMI (kg/cm2) | 33.8 (28.3-38.9) | 31.1 (28.3-38.1) |
| APACHE IIb | 12.1 ± 4.6 | 14.7 ± 5.5 |
| SOFAc | 6 (3-7) | 5 (3-6) |
| Charlson's comorbidity indexd | 0 (0-1) | 1 (0-1) |
| Symptoms (days from onset) | 10 (8-13) | 9 (7-12) |
| Hospital length of stay (days)e | 3 (2-6) | 4 (2-8) |
| Length of MV (hours)e | 22 (10-31) | 31 (18-49) |
| ARDS severity | ||
| Mild | 1 (2%) | 4 (8%) |
| Moderate | 30 (61%) | 30 (61%) |
| Severe | 18 (37%) | 15 (31%) |
| Ventilator setting and monitoring | ||
| PEEP (cmH2O) | 13.1 ± 2.9 | 12.7 ± 2.6 |
| Tidal volume (mL/kg PBW) | 6.3 (6-6.9) | 6.3 (6-6.9) |
| Respiratory rate (bpm) | 25 (24-27) | 25 (24-27) |
| Plateau pressure (cmH2O) | 24 (22-27) | 24 (22-27) |
| Driving pressure (cmH2O) | 11 (10-12) | 11 (9-14) |
| Compliance (mL/cmH2O) | 35 (30-40) | 37 (32-45) |
| FiO2 (%) | 50 (45-60) | 50 (45-60) |
| Gas exchange | ||
| pH | 7.31 ± .07 | 7.33 ± .06 |
| PaCO2 (mm Hg) | 47 (41-53) | 47 (40-51) |
| PaO2/FiO2 ratio (mm Hg) | 182 ± 55 | 207 ± 73 |
| Prone positioning (%) | 12 (24%) | 8 (16%) |
| NMBD use (%) | 35 (71%) | 39 (80%) |
| Vasoactive drugs (%) | 35 (71%) | 25 (51%) |
| Renal replacement (%) | 0 (0%) | 1 (2.04%) |
Ventilator setting, monitoring and gas exchange variables were acquired at the time of inclusion in the study.
Abbreviations: APACHE, acute physiology and chronic health evaluation; ARDS, acute respiratory distress syndrome; BMI, body mass index; FiO2, fraction of inspired oxygen; MV, mechanical ventilation; NMBD, neuromuscular blockade drugs; PaCO2, partial pressure of arterial oxygen; PaO2, partial pressure of arterial carbon dioxide; PaO2/FiO2 ratio, partial pressure of arterial oxygen to the fraction of inspired oxygen ratio; PBW, predicted body weight; PEEP, positive end expiratory pressure; SOFA, Sequential Organ Failure Assessment.
Continuous variables are presented as median (25th to 75th interquartile range) or mean ± SD.
The Acute Physiology and Chronic Health Evaluation II ranges from 0 to 71, with higher scores indicating a higher risk of death. It is calculated from 14 variables within 24 h of admission to the intensive care unit.
The Sequential Organ Failure Assessment was measured in six organ systems (cardiovascular, hematologic, gastrointestinal, renal, pulmonary, and neurologic), with each organ having a score from 0 to 4, resulting in an aggregated score that ranges from 0 to 24, with higher scores indicating greater dysfunction.
Charlsońs comorbidity index predicts the one-year mortality for a patient who may have a range of comorbid conditions, from a total of 22. Each condition is assigned a score of 1, 2, 3, or 6, depending on the risk of dying associated with each one. Scores are summed to provide a total score to predict mortality.
Times until randomization.
At randomization, ventilator settings, respiratory system mechanics, and gas exchange parameters were not different between the treatment groups (Table 1). The baseline laboratories and additional treatments did not differ between groups (e-Table 1 and e-Table 2 Appendix 1).
Interventions
The durations of dexamethasone treatment were 10 (7-10) and 9 (7-10) days in the high- and low-dose groups, respectively (P = .339). After the intervention phase, 20 (20.4%) patients received corticosteroids, mainly hydrocortisone, due to septic shock (10 in each group, P > .999).
Primary Outcomes
The VFD within 28 days of the inclusion in the trial was not different between the study groups (Table 2; 0 [0-14] vs. 0 [0-1] days for the high- and low-dose dexamethasone groups; P = .231). The difference between these medians was 0 (bootstrap 95% CI: 0 to 2) days. The times spent on mechanical ventilation after randomization were 12 (6-26) or 18.8 ± 18.3 versus 19 (9-32) or 24.7 ± 21.9 days for the high- and low-dose groups (P = .078). The difference was −7 (bootstrap 95% CI: −17 to 3) days. When only patients discharged alive without mechanical ventilation were considered, these times were 14 (8-26) or 23.2 ± 21.1 versus 27 (10-31.5) or 26 ± 17.5 days for the high- and the low-dose of the dexamethasone groups (P = .154), and the within-median difference was −13 (bootstrap 95% CI: −31 to −6) days.
Table 2.
Primary and Secondary Outcomes According to the Treatment Allocation.
| Low-dose dexamethasone | High-dose dexamethasone | All Patients | P | ||||
|---|---|---|---|---|---|---|---|
| Statistic | n | Statistic | n | Statistic | N | ||
| Primary Outcome | |||||||
| 28-VFD (days) | 0 (0-1) | 49 | 0 (0-14) | 49 | 0 (0-8.75) | 98 | .231 |
| Total time on MV (days) | |||||||
| All | 19 (9-32) | 49 | 12 (6-26) | 49 | 15.5 (7-30) | 98 | .078 |
| Discharged without MV | 27 (10-31.5) | 23 | 14 (8-26) | 25 | 18 (8-30) | 58 | .154 |
| Intensive care unit outcome | |||||||
| Mortality (%) | 24 (49%) | 49 | 21 (43%) | 49 | 45 (46%) | 98 | .685 |
| Length of stay (days) | |||||||
| All | 24 (10-36) | 49 | 15 (9-28) | 49 | 17 (9.25-35) | 98 | .137 |
| Survivors | 33 (16-43) | 25 | 18.5 (13-44) | 28 | 27 (14-43) | 53 | .397 |
| Hospital outcome | |||||||
| Mortality (%) | 24 (49%) | 49 | 22 (45%) | 49 | 46 (47%) | 98 | .840 |
| Length of stay (days) | |||||||
| All | 25 (11-41) | 49 | 22 (11-43) | 49 | 23.5 (11-42.5) | 98 | .365 |
| Survivors | 36 (25-46) | 25 | 30 (20.5-54) | 27 | 36 (21-52) | 52 | .905 |
| Mortality rate | |||||||
| 28-day (%) | 19 (39%) | 49 | 20 (41%) | 49 | 39 (40%) | 98 | >.999 |
| 90-day (%) | 23 (47%) | 49 | 23 (47%) | 49 | 46 (47%) | 98 | >.999 |
Abbreviations: MV, mechanical ventilation; VFD: ventilator free days.
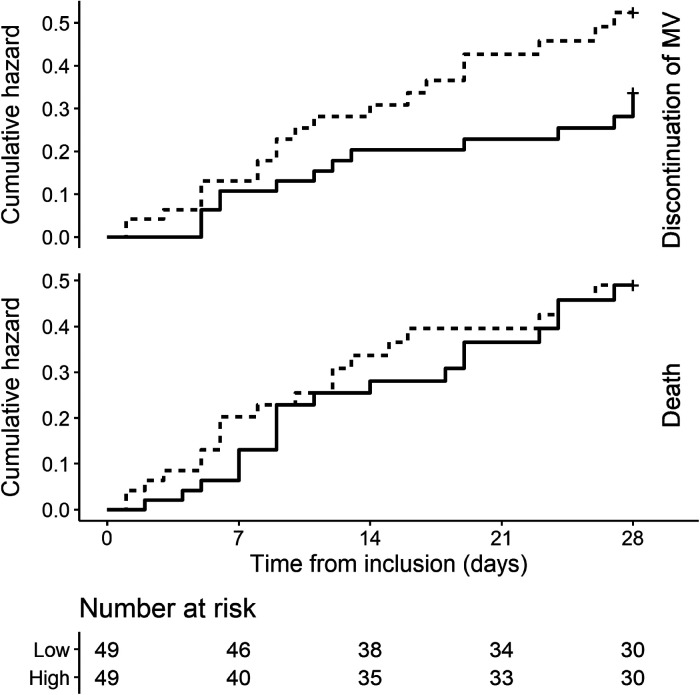
Figure 2 illustrates the cumulative incidence curves for successful discontinuation from mechanical ventilation and death within 28 days according to the treatment group. The unadjusted sub-distribution hazard ratio for the former of high-dose dexamethasone compared with the low-dose was 1.6 (95% CI: 1.1 to 2.33, P = .013). After adjustment with APACHE II and ARDS severity (Table 3), this ratio was 1.84 (95% CI: 1.31 to 2.59, P < .001).
Figure 2.
Probability of the successful discontinuation of mechanical ventilation or death within 28 days of the randomization.
Cumulative incidence curves of the successful discontinuation of mechanical ventilation (upper panel) and death (lower panel) according to the treatment allocation within 28 days of randomization. Low and high-dose dexamethasone are represented as solid and dashed lines, respectively. The unadjusted sub-distribution hazard ratio for the discontinuation of the mechanical ventilation and death of high-dose dexamethasone were 1.6 (95% CI: 1.1 to 2.33, P = .013) and 1.05 (95% CI: .77 to 1.45, P = .739) respectively.
Abbreviation: MV, mechanical ventilation; CI, confidence interval.
Table 3.
Summaries of the Competing-Risks Regression for Time to the Successful Discontinuation of Mechanical Ventilation at 28 Days and the Cox Regression Model for 90-Day Mortality.
| Predictor | Discontinuation of MV | 90-day mortality | ||||
|---|---|---|---|---|---|---|
| SHR | 95% CI | P | HR | 95% CI | P | |
| High dose dexamethasone | 1.84 | 1.31 to 2.59 | <.001 | .9 | .79 to 1.02 | .10 |
| APACHE II | .94 | .91 to .98 | .005 | 1.07 | 1.05 to 1.1 | <.001 |
| ARDS severity | .823* | .699* | ||||
| Mild | ref | ref | ||||
| Moderate | 1.05 | .31 to 3.59 | 1.4 | .39 to 5.06 | ||
| Severe | .82 | .12 to 5.84 | 1.08 | .32 to 3.63 | ||
Abbreviations: APACHE, acute physiology and chronic health evaluation; ARDS, acute respiratory distress syndrome; CI, confidence interval; HR, hazard ratio; MV, mechanical ventilation; SHR, sub-distribution hazard ratio.
Secondary Outcomes
By day 28, 20 (41%) patients in the high-dose dexamethasone group and 19 (39%) in the low-dose group had died (P > .999). The ICU and hospital mortality were also similar between groups. In addition, the length of stay in the ICU in the high-dose dexamethasone group was 15 (9-28) days versus 24 (10-36) days for the low-dose group (P = .137), and the difference between these medians was − 9 (bootstrap 95% CI: −20 to 0) days. The hospital length of stay was not affected by the treatment allocation.
Vital status 90 days after randomization was available from all the patients. Forty-six (47%) of them had died, 36 were at home, 11 were in chronic care facilities, and five were still in the hospital. e-Figure 3 shows that the 90-day probability of survival according to treatment allocation was not statistically different (log-rank P = .862). Table 3 exhibits the adjusted hazard ratio of high-dose dexamethasone was .9 (95% CI: .79 to 1.02, P = .10). e-Table 3 displayed mortality according to ARDS stratification.
Protocol-Defined Safety Outcomes
Microbiologically confirmed infections were diagnosed in 72 (73%) patients during the first 28 days. The estimated rate of the infections of the control arm was 2.42 (95% CI: 1.92 to 3) per patient per month, and the incidence rate ratio of the high-dose group was 1.12 (95% CI: .81 to 1.54, P = .502).
Both groups had a comparable peak finger-prick glucose and daily insulin use. The patients from both groups had low measurements of muscle strength and frequently experienced delirium. No differences between treatments were seen in these two outcomes (e-Results Appendix 1).
Discussion
In this multicenter randomized, open-label, clinical trial involving 98 adults mechanically ventilated for ARDS due to COVID-19, a 10-day course of intravenous high-dose dexamethasone compared with low-dose dexamethasone, both in addition to the standard care, did not show an increase in the VFD during the first 28 days, but significantly increased the hazard of the successful discontinuation of mechanical ventilation. High-dose dexamethasone did not affect the probability of survival at 90 days and was not associated with an increased risk of corticosteroid-associated safety issues.
The RECOVERY trial showed a survival advantage for hospitalized COVID-19 patients with respiratory support treated with 6 mg dexamethasone once daily for up to 10 days. The corticosteroid was administered either orally or intravenously. Although dexamethasone pharmacokinetics has not been studied in critically ill patients, its volume of distribution was approximately 1 L/kg of body weight in a study involving hospitalized patients due to community acquired pneumonia.24 Interestingly, this study reported an equivalent area under the curve of serum dexamethasone concentration following a single oral administration of 6 mg or a 4 mg intravenous bolus. As many severe COVID-19 patients are obese or have overt overweight, doses of dexamethasone larger than 6 mg would probably be required in these cases. The initial median dose of intravenous dexamethasone in our patients from the low-dose group was .06 (.05 to .08) mg/kg, which is equivalent to .42 (.36 to .5) mg/kg of prednisone. This dose might be considered low for a critically ill patient suffering from an acute and severe inflammatory lung disease. Conversely, the high-dose dexamethasone arm initially received .17 (.15 to .2) mg/kg of the drug, which is equivalent to 1.16 (.97 to 1.33) mg/kg of prednisone.
Recently published studies evaluated different doses of corticosteroids for severe COVID-19 treatment. Pinzon et al. in a before-and-after study, compared the clinical course of two consecutive cohorts of patients requiring supplemental oxygen treated with low-dose dexamethasone and high-dose methylprednisolone according to the protocolized management in a single center in Colombia.25 The proportion of patients requiring mechanical ventilation (2.9% vs. 19.8%) and the time to recovery (3 [3-4] vs. 6 [5-8] days) were significantly lower in the high-dose cohort. The mortality was not statistically different between cohorts. The COVID STEROID226 Trial Group reported the results of a multicenter, randomized, blinded trial involving 1000 patients with severe and critical COVID-19 requiring at least a supplemental oxygen flow of 10 liters per minute. Most of them (79%) were not ventilated at the inclusion. They were allocated to a high- and low-dose dexamethasone regimen. The main outcome (days without life support after 28 days) was not affected by the treatment. Other secondary outcomes, including 90-days mortality, and adverse events were not significantly different between arms.
The primary endpoint of our study was 28-day VFD, which has frequently been utilized as a failure-free outcome in the critical care literature. It is a composite variable, which combines the time required for the liberation of mechanical ventilation and the risk of death. Thus, a certain value could arise from the divergent combinations of its components. When the former is too long or the latter too high, an excess of zeros may preclude the detection of any effect related to the intervention with standard statistics.27 This explains why the median 28-day VFD of our patients was 0. To overcome these issues, we also decided to use a competing-risks analysis to evaluate the effect of the intervention in our statistical plan. Using this approach, even if we could not attain the planned sample size, a high-dose dexamethasone independently reduced the time required for liberating patients from the ventilator. In fact, in an exploratory comparison of the duration of ventilatory support, this time was 13 (95% CI: 6 to 31) days lower in survivors of the high-dose dexamethasone group. To our knowledge, this is the first study that displays a shorter time of invasive mechanical ventilation with dexamethasone doses higher than those recommended. Tomazzini et al.20 reported results from the CoDEX trial, which compared a high-dose dexamethasone versus no corticosteroids in a sample of patients with baseline clinical characteristics like ours. They did not observe a difference between the mean duration of mechanical ventilation in the overall population (12.5 vs. 13.9 days). Contrarily, Villar et al.7 showed a mean difference of 5.3 days (14.2 ± 13.2 vs. 19.5 ± 13.2) in the survivors of ARDS non-related to COVID-19 treated with high dexamethasone versus usual care.
Survival probability was not affected by the treatment allocation. We also found a 90-day mortality of 47%, which is within the range of mortalities recently reported by the REVA Network and the COVID-ICU investigators.28 In this large epidemiological study, mortality varied between 30% and 50% according to ARDS severity.
Although the hospital length of stay was not affected by the experimental treatment, there was a trend to a shorter time in the ICU in the high-dose group (P = .137). While this could be explained by chance, the finding might be related to a briefer requirement of ventilatory support in patients treated with high-dose dexamethasone. One interesting finding of our trial is that 16% of our patients were still hospitalized either in the primary hospital or in a chronic care/rehabilitation facility 90 days after inclusion.
We found a significant burden of safety issues potentially related to corticosteroids, including nosocomial infections, hyperglycemia, muscle weakness, and delirium. The occurrence of these problems seemed not to be increased by the high-dose treatment. However, this should be interpreted with caution as our trial was not powered to detect minor differences in these safety outcomes.
This study has many limitations. First, it is an open-label study. A double-blind design would be desirable but, given the urgent need for evidence required by the pandemic, we were unable to do it differently. Nevertheless, we believe that our data may provide some useful and interesting findings. Second, the early and unplanned termination of the study due to poor recruitment after nine months prevented us from reaching our target sample size. The reasons for this premature termination were related to the fast-changing dynamics of the pandemic and were not anticipated by us during the trial design. The smaller size probably reduced the power of our study to detect differences in the VFD and other secondary outcomes. Additionally, this might explain a minor difference in two baseline variables (APACHE II and the time from intubation to inclusion in the study) between groups. Third, the lack of shared procedures for liberation from mechanical ventilation in this multicenter open-label trial produces a potential bias. However, international guidelines commonly used were suggested. Finally, the investigators reported the results and conducted the analysis. Nevertheless, to prevent bias, the analysis was conducted as planned at the writing of the protocol, following such procedures after the termination of the data recollection.
Conclusions
This open-label multicenter clinical trial did not show difference in ventilator-free days between high- and low-dose dexamethasone in patients with ARDS due to COVID-19. However, suggests that a 10-day course of high-dose intravenous dexamethasone improved the time required to liberate these patients from the ventilator compared with the recommend low-dose treatment. This was not associated with a benefit in 90-day mortality or a higher frequency of safety issues. The reduction in the time of mechanical ventilation, even in the absence of a demonstrated treatment effect on mortality, might be critical considering the shortage of ICU resources reported worldwide.
Supplemental Material
Supplemental material, sj-docx-1-jicm-10.1177_08850666211066799 for High- Versus Low-Dose Dexamethasone for the Treatment of COVID-19-Related Acute Respiratory Distress Syndrome: A Multicenter, Randomized Open-Label Clinical Trial by Luis Patricio Maskin, Ignacio Bonelli, Gabriel Leonardo Olarte, Fernando Palizas, Agostina E Velo, María Fernanda Lurbet, Pablo Lovazzano, Sophia Kotsias, Shiry Attie, Ignacio Lopez Saubidet, Natalio D Baredes, Mariano Setten and Pablo Oscar Rodriguez in Journal of Intensive Care Medicine
Supplemental material, sj-docx-2-jicm-10.1177_08850666211066799 for High- Versus Low-Dose Dexamethasone for the Treatment of COVID-19-Related Acute Respiratory Distress Syndrome: A Multicenter, Randomized Open-Label Clinical Trial by Luis Patricio Maskin, Ignacio Bonelli, Gabriel Leonardo Olarte, Fernando Palizas, Agostina E Velo, María Fernanda Lurbet, Pablo Lovazzano, Sophia Kotsias, Shiry Attie, Ignacio Lopez Saubidet, Natalio D Baredes, Mariano Setten and Pablo Oscar Rodriguez in Journal of Intensive Care Medicine
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
ORCID iD: Luis Patricio Maskin https://orcid.org/0000-0001-7912-7810
Pablo Oscar Rodriguez https://orcid.org/0000-0002-3254-2213
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, Confalonieri M, Pastores SM, Meduri GU. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2(4):e0111. doi: 10.1097/cce.0000000000000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri D, Sasaki K, Karkar A, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521-537. doi: 10.1007/s00134-021-06394-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID - 19). Springer; 2020. doi: 10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267-276. doi: 10.1016/S2213-2600(19)30417-5 [DOI] [PubMed] [Google Scholar]
- 8.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200-2211. doi: 10.1007/s00134-020-06192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA - J Am Med Assoc. 2020;324(13):1292-1295. doi: 10.1001/jama.2020.16747 [DOI] [PubMed] [Google Scholar]
- 10.Maskin LP, Olarte GL, Palizas F, et al. High dose dexamethasone treatment for acute respiratory distress syndrome secondary to COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):20-22. doi: 10.1186/s13063-020-04646-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA: J Am Med Assoc. 2012;307(23):2526-2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95(December 2018):103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papazian L Aubron C, Brochard L, et al . Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ARDSNetwork. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308. doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez PO, Tiribelli N, Fredes S, et al. Prevalence of reverse triggering in early ARDS: results From a multicenter observational study. Chest. 2021;159(1):186-195. doi: 10.1016/j.chest.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 17.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000; 19(9):1141-1164. doi: [DOI] [PubMed] [Google Scholar]
- 18.Zhou B, Fine J, Latouche A, Labopin M. Competing risks regression for clustered data. Biostatistics. 2012;13(3):371-383. doi: 10.1093/biostatistics/kxr032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. R: A Language and Environment for Statistical Computing. Published online 2019. https://www.r-project.org/
- 20.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA - J Am Med Assoc. 2020;324(13):1307-1316. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA - J Am Med Assoc. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA - J Am Med Assoc. 2020;324(13):1317-1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellado-Artigas R, Ferreyro BL, Angriman F, et al. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care. 2021;25(1):1-10. doi: 10.1186/s13054-021-03469-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spoorenberg SMC, Deneer VHM, Grutters JC, et al. Pharmacokinetics of oral versus intravenous dexamethasone in patients hospitalized with community-acquired pneumonia. Br J Clin Pharmacol. 2014;78(1):78-83. doi: 10.1111/bcp.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinzón MA, Ortiz S, Holguín H, et al. Dexamethasone versus methylprednisolone high dose for covid-19 pneumonia. PLoS One. 2021;16(5 May). doi: 10.1371/journal.pone.0252057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell L, Uhre KR, Lindgaard ALS, et al. Effect of 12 mg versus 6 mg of dexamethasone on the number of days alive without life support in adults With COVID-19 and severe hypoxemia. JAMA. 2021;326(18):1807. doi: 10.1001/jama.2021.18295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828-836. doi: 10.1164/rccm.201810-2050CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M, Hajage D, Demoule A, et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60-73. doi: 10.1007/s00134-020-06294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jicm-10.1177_08850666211066799 for High- Versus Low-Dose Dexamethasone for the Treatment of COVID-19-Related Acute Respiratory Distress Syndrome: A Multicenter, Randomized Open-Label Clinical Trial by Luis Patricio Maskin, Ignacio Bonelli, Gabriel Leonardo Olarte, Fernando Palizas, Agostina E Velo, María Fernanda Lurbet, Pablo Lovazzano, Sophia Kotsias, Shiry Attie, Ignacio Lopez Saubidet, Natalio D Baredes, Mariano Setten and Pablo Oscar Rodriguez in Journal of Intensive Care Medicine
Supplemental material, sj-docx-2-jicm-10.1177_08850666211066799 for High- Versus Low-Dose Dexamethasone for the Treatment of COVID-19-Related Acute Respiratory Distress Syndrome: A Multicenter, Randomized Open-Label Clinical Trial by Luis Patricio Maskin, Ignacio Bonelli, Gabriel Leonardo Olarte, Fernando Palizas, Agostina E Velo, María Fernanda Lurbet, Pablo Lovazzano, Sophia Kotsias, Shiry Attie, Ignacio Lopez Saubidet, Natalio D Baredes, Mariano Setten and Pablo Oscar Rodriguez in Journal of Intensive Care Medicine