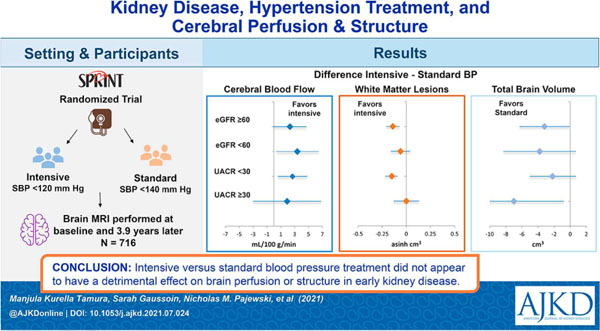
Abstract
Rationale and Objective:
The safety of intensive blood pressure (BP) targets is controversial for persons with chronic kidney disease (CKD). We studied the effects of hypertension treatment on cerebral perfusion and structure in those with and without CKD.
Study Design:
Neuroimaging substudy of a randomized trial.
Setting & Participants:
A subset of participants in the Systolic Blood Pressure Intervention Trial who underwent brain MRI studies. Presence of baseline CKD was assessed by estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (UACR).
Intervention:
Participants were randomly assigned to intensive (systolic BP <120 mm Hg) versus standard (systolic BP <140 mm Hg) BP lowering.
Outcomes:
The magnetic resonance imaging outcome measures were the four-year change in global cerebral blood flow, white matter lesion (WML) volume, and total brain volume.
Results:
A total of 716 randomized participants with mean age of 68 years were enrolled; follow-up imaging occurred after a median 3.9 years. Among participants with eGFR <60 ml/min/1.73m2 (N=234), the effects of intensive versus standard BP treatment on change in global cerebral blood flow, WMLs and total brain volume were 3.38 mL/100 g/min (95% CI 0.32, 6.44), −0.06 cm3 (asinh transformed, 95% CI −0.16, 0.04), and −3.8 cm3 (95% CI −8.3, 0.7), respectively. Among participants with UACR >30 mg/g (N=151), the effects of intensive versus standard BP treatment on change in global cerebral blood flow, WMLs and total brain volume were 1.91 ml/100g/min (95% CI −3.01, 6.82), 0.003 cm3 (asinh transformed, 95% CI −0.13, 0.13), and −7.0 cm3 (95% CI −13.3, −0.3), respectively. The overall treatment effects on cerebral blood flow and total brain volume were not modified by baseline eGFR or UACR; however the effect on WMLs was attenuated in participants with albuminuria (interaction p-value 0.04).
Limitations:
Measurement variability due to multi-site design.
Conclusions:
Among hypertensive adults with primarily early kidney disease, intensive versus standard blood pressure treatment did not appear to have a detrimental effect on brain perfusion or structure. The findings support the safety of intensive blood pressure treatment targets on brain health in persons with early kidney disease.
Funding:
The Systolic Blood Pressure Intervention Trial was funded by the National Institutes of Health (including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke), and this substudy was funded by the National Institutes of Diabetes and Digestive and Kidney Diseases.
Trial Registration:
SPRINT was registered at ClinicalTrials.gov with the study number NCT01206062.
Index words: hypertension, blood pressure, chronic kidney disease, albuminuria, white matter lesions
Graphical Abstract
Plain Language Summary
The Systolic blood PRessure INtervention Trial (SPRINT) found that intensive versus standard blood pressure treatment reduced mortality, major cardiovascular events, mild cognitive impairment, and progression of brain white matter lesions, but the cognitive benefits were attenuated in persons with kidney disease. This analysis evaluated the effects of intensive versus standard blood pressure treatment on brain perfusion and structure in participants with kidney disease. Among hypertensive adults with primarily early kidney disease, intensive versus standard blood pressure treatment did not appear to have a detrimental effect on brain perfusion or structure. The findings support the safety of intensive blood pressure treatment targets on brain health in persons with early kidney disease.
Introduction
Chronic kidney disease (CKD), characterized by a reduction in glomerular filtration rate (GFR) or the presence of excess albumin in the urine, affects more than 25 million US adults.1 Persons with CKD are at 2- to 7-fold higher risk for stroke and dementia, with varying risk according to level of estimated GFR (eGFR) and albuminuria.2–5 Cerebral small vessel ischemic disease is highly prevalent in persons with CKD and considered a major contributor to stroke and dementia in this high risk population.6,7
Hypertension is also common in persons with CKD, and it is a modifiable risk factor for stroke, dementia and cerebral small vessel ischemic disease in the general population. CKD is associated with vascular stiffness, inflammation, and small vessel remodeling in several vascular beds;8 these factors could impair local regulation of blood flow and contribute to cerebral ischemia. Cohort studies have identified a J-shaped association between blood pressure (BP) and stroke risk in persons with CKD.9 Studies in persons with dialysis-dependent CKD have demonstrated that BP instability is associated with white matter injury and cognitive impairment.10,11 These observations have prompted concerns about the safety of intensive BP treatment targets in the CKD population.
Randomized trials of BP treatment intensity have not provided conclusive evidence about stroke or cognitive end-points in CKD. The largest of these trials, the Systolic blood PRessure INtervention Trial (SPRINT), found that intensive versus standard BP treatment reduced mortality, major cardiovascular events, mild cognitive impairment, and progression of white matter lesions (WMLs), a marker of small vessel ischemic disease.12,13 Pre-specified subgroup analyses in SPRINT found no evidence for effect modification of intensive BP lowering by baseline eGFR on the main cardiovascular outcomes. However, eGFR modified the effect of intensive treatment on mild cognitive impairment such that the benefit of intensive BP treatment was driven by participants with eGFR >60 ml/min/1.73m2, and attenuated among participants with reduced eGFR.14
Better understanding of the physiologic effects of the degree of BP lowering on cerebral perfusion and structure in hypertensive adults with CKD could inform treatment targets. Whereas both kidney markers have been independently associated with risk for stroke and dementia, albuminuria is more consistently associated with cerebral small vessel ischemic disease and reduced eGFR is more consistently associated with perfusion abnormalities. These observations suggest that the effects of BP treatment might differ for adults with CKD characterized by reduced eGFR versus albuminuria.
In this report, we characterize the effect of intensive treatment on global cerebral blood flow (CBF), WML volume, and total brain volume (TBV) according to eGFR and urine albumin to creatinine ratio (UACR) at study entry. Additionally, we assess the independent association between baseline eGFR and UACR with longitudinal changes in cerebral perfusion and structure.
Methods
Study Participants
The trial design and primary outcomes of SPRINT have been reported (NCT01206062).15,16 In brief, 9361 adult participants with hypertension and increased risk for cardiovascular disease were enrolled from 102 clinical sites between November 2010 and March 2013, and randomly assigned to a systolic BP target of <120 mm Hg or <140 mm Hg. Individuals were considered at increased risk for cardiovascular disease if they had an eGFR 20–59 ml/min/1.73m2, a 10-year Framingham risk score ≥15%, were ≥75 years of age, or had evidence of clinical or subclinical cardiovascular disease. Major exclusion criteria included diabetes mellitus, proteinuria >1 g/day, polycystic kidney disease, prior stroke, symptomatic heart failure, and known left ventricular ejection fraction <35%. SPRINT participants accessible to one of seven designated MRI centers were screened for the Magnetic Resonance Imaging (MRI) substudy. In 2012, four centers were added to recruit additional participants with CKD for the MRI substudy.17 Exclusion criteria for the SPRINT MRI substudy included claustrophobia or an MR incompatible metal or electrical device implant. The study was approved by the institutional review board at all participating centers and all participants provided written informed consent. For these analyses, we used data from all participants who completed a baseline or follow-up MRI that passed quality control, and also had baseline eGFR or UACR measurements.
Markers of kidney disease and other measurements
At the baseline visit, participants completed questionnaires ascertaining age, sex, race/ethnicity, education, medical history and health behaviors. Antihypertensive medications, including use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, were recorded at the baseline visit. Education was categorized as less than high school, high school diploma, post high-school, and college degree. History of cardiovascular disease was defined as self-report or clinical evidence of current or history of coronary artery disease or peripheral arterial disease.15 Smoking status was characterized as current, former, or never smokers; and alcohol use was characterized as non-drinker, light drinker, moderate drinker, or heavy drinker.
Serum creatinine as well as urine albumin and creatinine concentrations were measured at the central study laboratory using blood and random urine samples collected at the baseline visit. Serum creatinine was measured with a method traceable to isotope dilution mass spectrometry. Urine creatinine was measured with the Siemens ProSpec nephelometric analyzer. Hematocrit was measured at the central study laboratory using blood samples collected at the study close-out visit. Estimated GFR was calculated using the Modification of Diet in Renal Disease (MDRD) 4-variable equation,18 and categorized as ≥60 ml/min/1.73m2 or <60 ml/min/1.73m2.19 The UACR was calculated from a spot urine sample and categorized as <30 mg/g and ≥30 mg/g; the latter is hereafter referred to as albuminuria.20 BP was measured at baseline and follow-up visits using standardized techniques.21 Visit BP was the mean of 3 recordings.
MRI measurements
The MRI protocol has previously been reported.13,17 The primary outcome was change in WML volume. Change in CBF and TBV were secondary outcomes. The protocol specified that baseline MRIs should be performed within three months of randomization, and follow-up MRIs 48 months after randomization. Due to the early termination of the trial intervention, some participants received the follow-up MRI prior to the 48-month visit.
The standardized MRI protocol was performed on 3 Tesla scanners and included sagittal 3D FLAIR, T2-, and T1-weighted sequences with whole brain coverage, and a pseudocontinuous arterial spin labeling (pcASL) perfusion sequence.13,17 The scanners had identical field strength but were from three different manufacturers (Siemens, Phillips, General Electric). Similar MRI pulse sequences were run on all scanners, except for pcASL, for which two manufacturers (Siemens or Philips scanners in 10 centers) utilized a 2D sequence while one General Electric scanner utilized a 3D sequence. Each participating MRI center performed quarterly phantom scans to assess scanner stability and image distortion using phantoms. MRI scanner performance across the centers was stable over the duration of the study.
Image analysts were blinded to BP treatment group. Structural MRIs were first preprocessed by applying an automated pipeline for correcting inhomogeneity22 and extraction of intracranial brain tissue.23 Image analysis was based on the multi-atlas label fusion method to segment brain tissue into supratentorial gray matter and white matter, with the sum defining TBV.24 As reported, WMLs were identified from FLAIR images using a deep learning-based method.13,25 WML segmentations were inspected for quality by a neuroradiologist blinded to treatment group. Arterial spin labeling imaging was transformed into CBF following a consensus recommended approach.26 CBF processing included motion correction, CBF quantification and denoising based on a structural correlation with Robust Bayesian criteria.27,28 CBF maps with poor quality were excluded based on an automated quality evaluation index. The CBF maps were registered to the high resolution T1 images and then mean CBF in whole brain, gray matter and white matter were extracted for statistical analysis.
Statistical Analyses
Due to the skewed distribution of WML volumes, values were transformed by applying an inverse hyperbolic sine (asinh) to accommodate values of zero.29 Linear mixed models incorporating all baseline and follow-up imaging were used to estimate the change in each MRI outcome measure from baseline to follow-up and for comparison between the treatment groups, stratified by baseline eGFR and by baseline UACR. Interactions between treatment group and baseline kidney markers were assessed with a likelihood ratio test. The models included intracranial volume and days from randomization at the time of MRI acquisition as covariates, with MRI site and participant included as random effects. Because anemia is more common in persons with CKD and the quantification of CBF by arterial spin labeling may be affected by anemia,30 we assessed whether correction of CBF values for hematocrit affected between group comparisons. Correction for hematocrit did not materially affect between-group comparisons, therefore uncorrected CBF values are presented in the analysis (Supplement Table S1). To facilitate interpretation and comparison of the effect sizes, in complementary analyses we also analyzed the outcomes as standardized z-scores.
We conducted several sensitivity analyses. First, we estimated GFR using the Chronic Kidney Disease Epidemiology equation instead of the MDRD equation.19 Second, we included participants whose baseline scan was performed between three and 12 months after randomization (N=26). We also evaluated the association of baseline eGFR and UACR with change in each MRI outcome measure, using linear mixed models with adjustments for treatment group, age, sex, race/ethnicity, history of cardiovascular disease, and smoking status. All analyses were performed using SAS version 9.4 (SAS, Cary, NC) and the R Statistical Computing Environment (http://www.r-project.org). All of the hypothesis tests were two-sided, and P values of less than 0.05 were considered to indicate statistical significance. Adjustments were not made for multiple comparisons.
Data Availability
Deidentified participant data will be available in the BioLinCC repository (https://biolincc.nhlbi.nih.gov/studies/sprint).
Results
Participant Characteristics
A total of 718 participants completed a baseline or follow-up MRI that met quality control requirements, and of these, 716 had baseline eGFR and 690 had baseline UACR measurements (Figure 1). Participants who did not complete a follow-up scan included 88 who were unwilling to participate, 32 who withdrew or were lost to follow-up, 32 who died, and 71 for other reasons. Participants who did not have a follow-up MRI had similar eGFR and higher UACR levels compared to those who did complete follow-up (Supplemental Table S2). Baseline characteristics of participants in the MRI substudy were well balanced by treatment arm (Table 1). There were 234 participants (32.8%) who had an eGFR <60 ml/min/1.73m2 and 151 (22.0%) with albuminuria at baseline.
Figure 1.
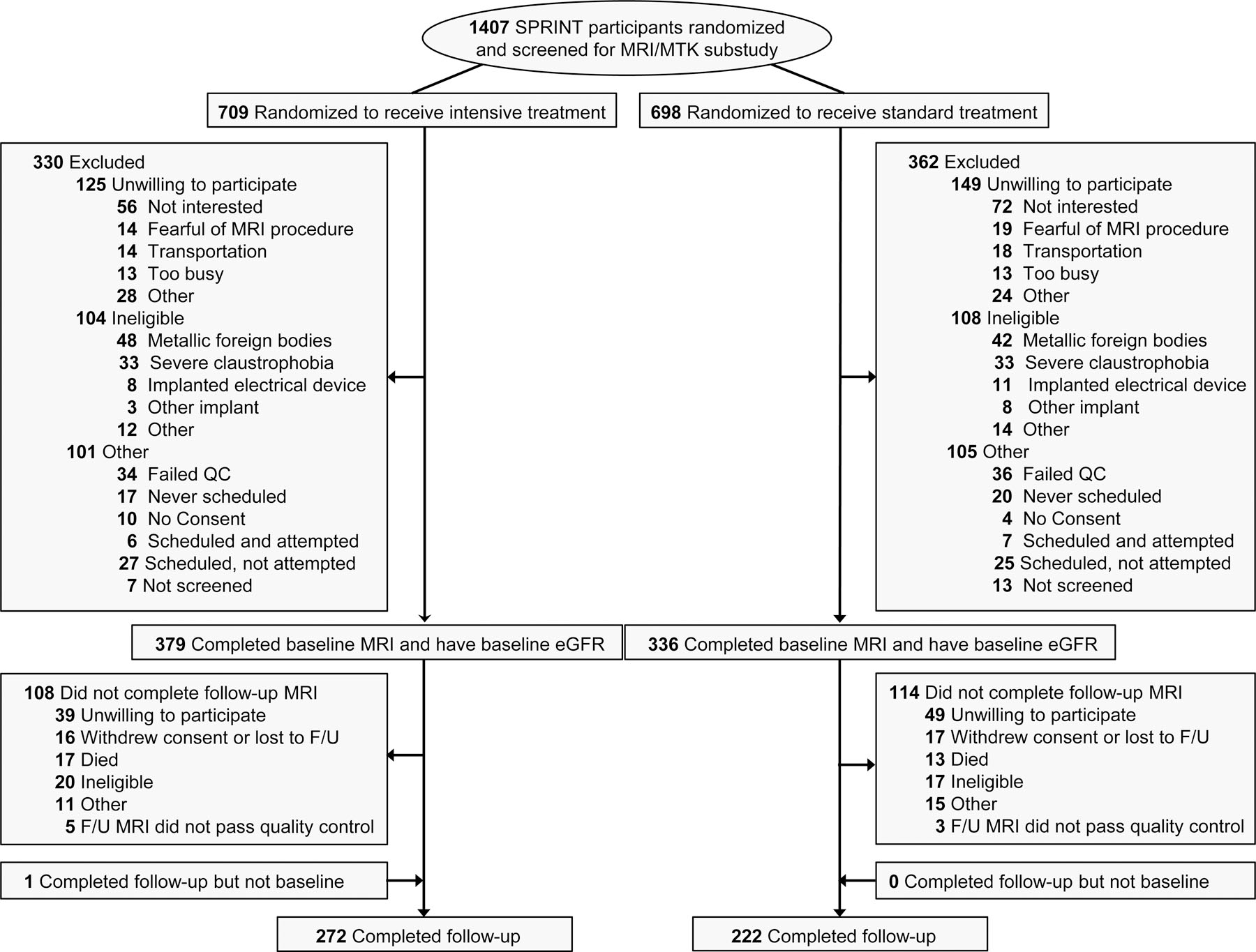
Flowchart for inclusion of participants
Table 1.
Baseline characteristics of trial participants by treatment arm
| Characteristic | Intensive Treatment (N=380) |
Standard Treatment (N=336) |
p-value |
|---|---|---|---|
| Age | 68.3 (8.5) | 67.5 (8.7) | 0.2 |
|
| |||
| Female Sex | 160 (42.1%) | 123 (36.6%) | 0.2 |
|
| |||
| Race | 0.4 | ||
| White | 239 (62.9%) | 208 (61.9%) | |
| Black | 120 (31.6%) | 100 (29.8%) | |
| Hispanic | 14 (3.7%) | 22 (6.6%) | |
| Other | 7 (1.8%) | 6 (1.8%) | |
|
| |||
| Education | 0.8 | ||
| < high school | 26 (6.8%) | 27 (8.0%) | |
| High school diploma | 58 (15.3%) | 45 (13.4%) | |
| Post high school | 134 (35.3%) | 119 (35.4%) | |
| College degree | 162 (42.6%) | 145 (43.2%) | |
|
| |||
| Smoking | 0.5 | ||
| Never | 165 (43.4%) | 160 (47.6%) | |
| Former | 167 (44.0%) | 138 (41.1%) | |
| Current | 48 (12.6%) | 38 (11.3%) | |
|
| |||
| Alcohol | 0.1 | ||
| Non-drinker | 146 (40.7%) | 141 (44.9%) | |
| Light Drinker | 92 (25.6%) | 71 (22.6%) | |
| Moderate Drinker | 84 (23.4%) | 57 (18.2%) | |
| Heavy Drinker | 37 (10.3%) | 45 (14.3%) | |
|
| |||
| History of CVD | 52 (13.7%) | 49 (14.6%) | 0.7 |
|
| |||
| ACE Inhibitor/ARB | 229 (60.3%) | 186 (55.4%) | 0.2 |
|
| |||
| Systolic BP, mm Hg | 138.6 (17.5) | 138.0 (15.3) | 0.5 |
|
| |||
| Diastolic BP, mm Hg | 77.2 (11.1) | 78.2 (12.1) | 0.3 |
|
| |||
| eGFR, ml/min/1.73m2 | 70.1 (20.6) | 70.8 (21.6) | 0.7 |
|
| |||
| UACR, mg/g | 9.7 (5.4, 25.9) | 10.2 (5.8, 22.3) | 0.7 |
|
| |||
| Hematocrit* | 41.0 (3.9) | 41.6 (4.5) | 0.1 |
Results are presented as N and (%), mean (standard deviation), or median (interquartile range). P-values based on Chi-square or t-test with the exception of urine albumin to creatinine ratio which is based on Wilcoxon rank sum test
Measured at follow-up visit.
Abbreviations: GFR – glomerular filtration rate, CVD – cardiovascular disease, ACE – angiotensin converting enzyme, ARB – angiotensin receptor blocker, BP – blood pressure, UACR – urine albumin to creatinine ratio
Through the end of the active intervention phase of the trial, the mean systolic BP among participants in the MRI substudy was 122.3 (SD=14.9) mm Hg in the intensive treatment group and 135.2 (SD=13.3) mm Hg in the standard treatment group. Achieved systolic BP during the intervention phase did not differ by eGFR in either treatment group or by UACR in the standard group (Supplement Figures S1–S2). In the intensive treatment group, however, systolic BP was 4.6 mm Hg higher in the subgroup with UACR ≥30 mg/g compared to those with UACR <30 mg/g. Following the termination of the trial intervention, participants transitioned to management of their BP by their primary care provider. During this transitional period, mean systolic BP increased in both treatment groups. The between group difference in systolic BP was sustained across eGFR and UACR strata. Follow-up MRIs were performed a median of 3.9 years (range 2.8–4.7 years) after randomization.
Effect of BP intervention on MRI outcomes by baseline kidney markers
The effects of intensive treatment on global CBF, WML volume, and TBV were not modified by eGFR (Table 2 and Figure 2). Among participants with eGFR <60 ml/min/1.73m2, intensive versus standard BP treatment resulted in an increase in global CBF (3.38 ml/100 g/min, 95% CI 0.32, 6.44), attenuated progression of WMLs (−0.06 asinh cm3, 95% CI −0.16, 0.04), and a larger decrease in TBV (−3.8 cm3, 95% CI −8.3, 0.7). Expressed as z-scores, intensive versus standard BP treatment resulted in a difference of 0.32, −0.06, and −0.03 on global CBF, WML volume, and TBV, respectively, in the subgroup with eGFR <60 ml/min/1.73m2 (Supplemental Figure 3). Among participants with albuminuria, intensive vs. standard treatment also resulted in a numerical increase in global CBF (1.91 ml/100 g/min, 95% CI −3.01, 6.82) and a larger decrease in TBV (−7.0 cm3, 95% CI −13.3, −0.8) (Table 3 and Figure 2), corresponding to z-scores differences of 0.18 and −0.05, respectively (Supplemental Figure 3). The effects of intensive treatment on global CBF and TBV were not modified by baseline UACR; however UACR modified the effect of intensive treatment on WML volume (interaction p-value =0.04). Among participants with albuminuria, there was no difference in change in WML volume with intensive versus standard BP treatment, whereas in those without albuminuria, intensive versus standard BP treatment led to a significantly smaller increase in WML volume.
Table 2.
Effect of intensive treatment on change in cerebral blood flow, white matter lesion volume, and total brain volume, stratified by baseline estimated glomerular filtration rate.
| MRI measure | Intensive | Standard | Difference in Change | P-value for interaction | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Baseline (95% CI) |
Follow-up (95% CI) |
Change (95% CI) |
Baseline (95% CI) |
Follow-up (95% CI) |
Change (95% CI) |
(95% CI) | ||
|
Total cerebral blood flow, (mL/100 g/min)
| ||||||||
| eGFR ≥60 | 36.64 (33.32, 39.96) | 38.23 (34.72, 41.74) | 1.58 (−0.08, 3.25) | 35.36 (32.02, 38.7) | 34.63 (31.11, 38.15) | −0.73 (−2.46, 1) | 2.32 (−0.07, 4.7) | 0.6 |
| eGFR <60 | 36.89 (32.93, 40.84) | 37.84 (33.68, 42.01) | 0.96 (−1.12, 3.04) | 37.78 (33.72, 41.83) | 35.35 (31.02, 39.69) | −2.42 (−4.67, −0.17) | 3.38 (0.32, 6.44) | |
|
WML volume, asinh (cm 3 ) | ||||||||
| eGFR ≥60 | 1.89 (1.75, 2.04) | 2.02 (1.87, 2.17) | 0.13 (0.08, 0.17) | 1.86 (1.71, 2.02) | 2.13 (1.98, 2.29) | 0.27 (0.22, 0.32) | −0.14 (−0.21, −0.07) | 0.2 |
| eGFR <60 | 2.28 (2.1, 2.46) | 2.49 (2.31, 2.68) | 0.21 (0.14, 0.28) | 2.12 (1.93, 2.3) | 2.39 (2.2, 2.58) | 0.27 (0.19, 0.34) | −0.06 (−0.16, 0.04) | |
|
Total brain volume, (cm 3 ) | ||||||||
| eGFR ≥60 | 1139.9 (1129.1, 1150.8) | 1109.5 (1098.6, 1120.4) | −30.4 (−32.5, −28.3) | 1138.9 (1127.9, 1149.9) | 1111.7 (1100.6, 1122.6) | −27.2 (−29.5, −24.9) | −3.2 (−6.3, −0.1) | 0.8 |
| eGFR <60 | 1112.2 (1097.8, 1126.6) | 1083.2 (1068.7, 1097.7) | −28.9 (−32.1, −25.9) | 1111.8 (1096.9, 1126.6) | 1086.6 (1071.7, 1101.5) | −25.2 (−28.4, −21.9) | −3.8 (−8.3, 0.7) | |
Interaction p-value is testing the difference in change between eGFR groups. Estimates are based on a linear mixed model adjusting for intracranial volume (except in CBF models) and days since randomization, with random effects for participant and MRI facility. Estimates represent least squares means, with follow-up estimated computed at 1452 days (3.98 years) post-randomization. For change estimates, negative values denote decreases from baseline, while positive values indicating increases from baseline. Difference in change represents intensive treatment group minus standard treatment group.
There were N=671, 713, and 716 participants included in the cerebral blood flow, WML volume, and brain volume analyses, respectively.
Abbreviations: asinh inverse hyperbolic sine transformation, f(x) = log(x + (x2 + 1)0.5), eGFR – estimated glomerular filtration rate, MRI – magnetic resonance imaging, SE - standard error, CI - confidence interval, WML - white matter lesion eGFR is expressed as ml/min/1.73m2
Figure 2.
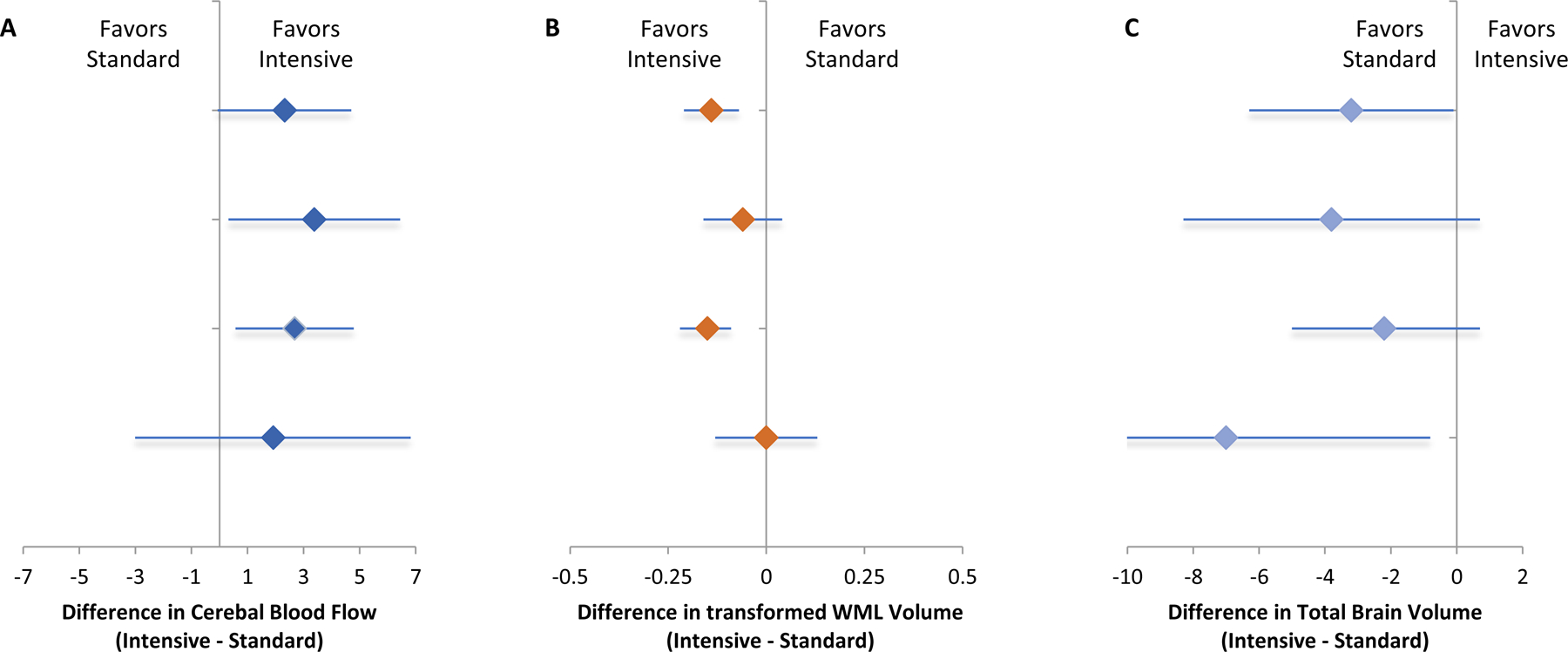
Effect of intensive versus standard BP treatment on change in cerebral blood flow (Panel A), white matter lesion volume (Panel B), and total brain volume (Panel C), by baseline estimated glomerular filtration rate and urine albumin to creatinine ratio. Note: Units are expressed as ml/100 g/min for cerebral blood flow, asinh (cm3) for white matter lesion volume, and cm3 for total brain volume.
Table 3.
Effect of intensive treatment on change in cerebral blood flow, white matter lesion volume, and total brain volume, stratified by baseline urine ACR.
| MRI measure | Intensive | Standard | Difference in Change | P-value for interaction by UACR | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Baseline (95% CI) | Follow-up (95% CI) | Change (95% CI) | Baseline (95% CI) | Follow-up (95% CI) | Change (95% CI) | (95% CI) | ||
| Total cerebral blood flow (mL/100 g/min) | ||||||||
| UACR <30 | 36.02 (32.9, 39.14) | 37.73 (34.48, 40.98) | 1.71 (0.26, 3.15) | 35.83 (32.67, 38.99) | 34.86 (31.54, 38.17) | −0.97 (−2.53, 0.58) | 2.68 (0.57, 4.79) | 0.8 |
| UACR ≥30 | 38.71 (34.3, 43.13) | 38.02 (33.01, 43.02) | −0.69 (−4.12, 2.73) | 36.7 (32.18, 41.22) | 34.1 (28.99, 39.21) | −2.6 (−6.14, 0.93) | 1.91 (−3.01, 6.82) | |
|
WML volume, asinh (cm3) | ||||||||
| UACR <30 | 1.93 (1.82, 2.04) | 2.06 (1.95, 2.17) | 0.13 (0.08, 0.17) | 1.88 (1.76, 2) | 2.16 (2.03, 2.28) | 0.28 (0.23, 0.33) | −0.15 (−0.22, −0.09) | 0.04 |
| UACR ≥30 | 2.34 (2.11, 2.57) | 2.57 (2.33, 2.81) | 0.23 (0.14, 0.32) | 2.25 (2, 2.5) | 2.48 (2.22, 2.74) | 0.23 (0.13, 0.32) |
0.003 (−0.13, 0.13) | |
|
Total brain volume (cm3) | ||||||||
| UACR <30 | 1129.7 (1116.5, 1142.8) | 1099.7 (1086.5, 1112.9) | −29.9 (−31.8, −28.0) | 1126.5 (1113.1, 1139.9) | 1098.8 (1085.3, 1112.2) | −27.8 (−29.9, −25.6) | −2.2 (−5.0, 0.7) | 0.1 |
| UACR ≥30 | 1115.5 (1095,0 1136.1) | 1085.9 (1065.2, 1106.7) | −29.6 (−33.8, −25.4) | 1116.8 (1095.7, 1137.9) | 1094.2 (1072.9, 1115.5) | −22.6 (−27.1, −18.0) | −7.0 (−13.3, −0.8) | |
Interaction p-value is testing the difference in change between UACR groups. Estimates are based on a linear mixed model adjusting for intracranial volume (except in CBF models) and days since randomization, with random effects for participant and MRI facility. Estimates represent least squares means, with follow-up estimated computed at 1452 days (3.98 years) post-randomization. For change estimates, negative values denote decreases from baseline, while positive values indicating increases from baseline. Difference in change represents intensive treatment group minus standard treatment group.
There were N=645, 687, and 690 participants included in the cerebral blood flow, WML volume, and brain volume analyses, respectively.
Abbreviations: asinh inverse hyperbolic sine transformation, f(x) = log(x + (x2 + 1)0.5), eGFR – estimated glomerular filtration rate, MRI – magnetic resonance imaging, SE - standard error, CI - confidence interval, WML - white matter lesion UACR is expressed as mg/g
Baseline kidney markers and change in MRI outcomes
In models adjusted for BP treatment group, intracranial volume, age, sex, race, history of cardiovascular disease, smoking, MRI scanner, and the baseline MRI value, lower baseline eGFR was not significantly associated with longitudinal changes in global CBF, WML volume or TBV (Table 4). Similarly, albuminuria was not significantly associated with longitudinal changes in global CBF, WML volume or TBV.
Table 4.
Association of eGFR and albuminuria with change in cerebral blood flow, white matter lesion volume, and total brain volume.
| Kidney marker | Cerebral blood flow (ml/100 g/min) | Transformed WML volume asinh (cm3) | Total brain volume (cm3) | ||||
|---|---|---|---|---|---|---|---|
| eGFR | N | Estimate (95% CI) |
p-value | Estimate (95% CI) |
p-value | Estimate (95% CI) |
p-value |
| eGFR (≥60 ml/min/1.73m2) | 326 | 0.49 (−0.68, 1.66) | 0.4 | 0.19 (0.15, 0.22) | 0.1 | −29.04 (−30.57, −27.51) | 0.2 |
| <60 ml/min/1.73m2 | 162 | −0.46 (−2.12, 1.2) | 0.24 (0.19, 0.29) | −27.19 (−29.43, −24.95) | |||
| eGFR (≥90 ml/min/1.73m2) | 79 | −0.16 (−2.56, 2.23) | 0.2 | 0.21 (0.14, 0.28) | 0.2 | −29.00 (−32.08, −25.93) | 0.2 |
| 60 to<90 ml/min/1.73m2 | 247 | 0.68 (−0.66, 2.01) | 0.18 (0.14, 0.22) | −29.05 (−30.82, −27.29) | |||
| 45 to <60 ml/min/1.73m2 | 115 | 0.50 (−1.48, 2.47) | 0.22 (0.15, 0.28) | −27.36 (−30.02, −24.69) | |||
| <45 ml/min/1.73m2 | 47 | −2.57 (−5.59, 0.44) | 0.31 (0.21, 0.40) | −26.84 (−31.00, −22.68) | |||
| Albuminuria | Estimate (95% CI) |
p-value | Estimate (95% CI) |
p-value | Estimate (95% CI) |
p-value | |
| UACR<30 mg/g Cr | 380 | 0.54 (−0.55, 1.63) | 0.08 | 0.19 (0.16, 0.23) | 0.3 | −28.99 (−30.42, −27.55) | 0.1 |
| ≥30 mg/g Cr | 90 | −1.63 (−3.85, 0.59) | 0.23 (0.16, 0.30) | −26.44 (−29.35, −23.54) | |||
Estimates based on a linear mixed model, adjusting for intracranial volume (except in CBF models), treatment assignment, age, sex, race, history of CVD, smoking and days since randomization, with random effects for participant and MRI facility. Estimates represent least squares means, with follow-up estimated computed at 1452 days (3.98 years) post-randomization. For change estimates, negative values denote decreases from baseline, while positive values indicate increases from baseline.
Abbreviations: asinh inverse hyperbolic sine transformation, f(x) = log(x + (x2 + 1)0.5), eGFR – estimated glomerular filtration rate, MRI – magnetic resonance imaging, SE - standard error, CI - confidence interval, WML - white matter lesion
Sensitivity Analyses
When we used the Chronic Kidney Disease Epidemiology equation to estimate GFR, the findings in the subgroup with reduced eGFR were similar for global CBF and TBV; however, effect modification on WML volume was present (p-value interaction 0.03). Specifically, among participants with eGFR <60 ml/min/1.73m2, there was no difference in change in WML volume with intensive versus standard BP treatment (−0.02, 95% CI −0.12, 0.09, Table S3). The findings were similar when participants who completed the baseline MRI outside the three month window were included in the analysis (Supplement Tables S4–S5)
Discussion
In a randomized trial of BP treatment intensity, intensive versus standard BP treatment resulted in an increase in global CBF and a decrease in TBV, with similar effects among those with reduced eGFR and those with albuminuria. The effect of intensive versus standard BP treatment on WML volume was diminished among individuals with albuminuria, and in sensitivity analyses, among those with reduced eGFR. After accounting for treatment group, center, and participant demographic and clinical characteristics, there was no significant association between baseline values of either marker of kidney disease with longitudinal changes in global CBF, WML volume, or TBV.
The link between CKD and cerebrovascular disease is hypothesized to reflect similar anatomic and hemodynamic features of their vascular beds. The brain and kidney are high-flow low-resistance end-organs. Their small vessel beds are exposed to high pulsatile pressure, making them uniquely susceptible to fluctuations in upstream pressure and flow as well as conditions that impair endothelial function.8,31 Reduced eGFR and albuminuria have differential associations with stroke and cognitive impairment, as well as brain structure and perfusion. At the clinical level, reduced eGFR is principally associated with ischemic stroke and global cognitive impairment.2,32 In neuroimaging studies, reduced eGFR is associated with hemodynamic impairment and inconsistently associated with WMLs.17,33,34 Albuminuria is associated with an increased risk for ischemic and hemorrhagic stroke, and more consistently associated with WMLs which are principally associated with executive dysfunction.35,36 These complementary kidney markers may thus reflect different aspects of systemic impairment in vascular function.
The effect size of intensive BP treatment on CBF in the albuminuria and reduced eGFR subgroups was equivalent to 0.18 to 0.32 SDs respectively, or a 10% increase, suggesting these effects could be clinically meaningful. In participants without either marker of kidney disease, an increase in CBF was accompanied by reduced progression of WML volume, a marker of small vessel ischemic disease. Persons with chronic hypertension are thought to have cerebrovascular autoregulation shifted to the right. The observed increase in CBF and absence of WML progression with intensive BP lowering suggest that cerebrovascular autoregulation also normalized. Intensive treatment had no effect on WML volume among participants with albuminuria and a diminished effect among participants with reduced eGFR; consequently, the significance of the increase in CBF in these subgroups is less clear. The relationship between global perfusion and tissue ischemia is complex. In addition to blood flow, tissue ischemia depends on metabolic demand, development of collateral circulation, and arterial oxygen content.
The lack of effect on WML volume may be a chance finding, as the interaction p-values were not corrected for multiple comparisons and dependent on the method used to estimate GFR. However, this result is consistent with the lack of benefit of intensive treatment on cognitive impairment in the subgroup of participants with reduced eGFR.14 This result could also reflect diminished BP separation between the intensive and standard treatment arms in the albuminuria subgroup. Intensive treatment also resulted in a statistically significant larger decrease in TBV; however, the effect size was small, equivalent to 0.05 SDs, and may not be clinically meaningful. Importantly, we found no evidence that intensive BP treatment resulted in a decrease in cerebral perfusion or an increase in WML volume among participants with reduced eGFR or albuminuria, the primary hypothesized detrimental effects of intensive BP treatment.
Our findings inform the debate about the safety of intensive BP treatment in persons with CKD. In persons with dialysis-dependent CKD, intra-dialytic hemodynamic instability is associated with white matter injury and cognitive impairment.10,37 MacEwen et al demonstrated wide variation in autoregulatory thresholds in this population, and that autoregulation is absent in a significant fraction of patients. The implication is that it is not possible to predict safe BP treatment levels for individual patients. This heterogeneity in autoregulatory thresholds may be reflected in the wider range of CBF values among participants in SPRINT with versus without CKD.
Hemodialysis induced cerebral ischemia is an extreme example of hemodynamically mediated cognitive impairment. Pharmacologic BP lowering may differ from hemodialysis treatment in the intensity, frequency and variability of BP lowering that can provoke cerebral ischemia. In addition, compared to persons with primarily mild or moderate CKD in SPRINT, persons with dialysis-dependent CKD may have more pronounced vascular stiffness and remodeling, making them more vulnerable to end-organ ischemia with hypotensive episodes. SPRINT had relatively few participants with eGFR <45 ml/min/1.73m2 or albuminuria >300 mg/g; thus we cannot exclude the possibility that intensive BP treatment may have different effects on cerebral perfusion and structure in more advanced CKD.
There was no association between either kidney marker and longitudinal changes in cerebral perfusion and structure after controlling for treatment group and other confounders. This observation is consistent with the concept that kidney disease markers reflect impairments in systemic vascular function. Accordingly, control of traditional vascular risk factors such as hypertension may be most important for stroke and dementia risk reduction in this population. Compared to participants with preserved eGFR, there was a decrease in global CBF over time among participants with baseline eGFR <45 ml/min/1.73m2, and similar findings for albuminuria, but these differences were not statistically significant. Thus, we cannot rule out the possibility that there are independent contributions of CKD on cerebral perfusion that we were underpowered to detect.
This study has several additional limitations. First, the MRI substudy sample was less than 10% of SPRINT participants and may not be representative of the overall trial population. Second, the MRI completion rate was lower than expected. Third, the use of multiple MRI scanners due to the multi-site design likely increased measurement variability. These factors may have biased our findings towards the null. Finally, our study was designed to address longer-term changes in cerebral perfusion and structure and did not capture short-term changes that might have occurred during BP treatment intensification; though significant short-term reductions in CBF might have been expected to increase WML volumes.
In summary, among hypertensive adults, intensive versus standard BP treatment increased global CBF and led to a small decrease in TBV; these effects were similar in participants with primarily mild to moderate CKD. There was no evidence that intensive treatment accelerated the accumulation of WMLs. The results support the safety of more intensive BP treatment targets on brain health in the high risk CKD population.
Supplementary Material
Figure S1. Blood pressure in intensive and standard treatment groups during trial and cohort phases, stratified by estimated glomerular filtration rate.
Figure S2. Blood pressure in intensive and standard treatment groups during trial and cohort phases, stratified by urine albumin to creatinine ratio.
Figure S3. Effect of intensive versus standard BP treatment on change in cerebral blood flow (Panel A), white matter lesion volume (Panel B), and total brain volume (Panel C) expressed as z-scores, by baseline estimated glomerular filtration rate and urine albumin to creatinine ratio.
Table S1. Cerebral blood flow at follow-up, with and without correction for hematocrit, by baseline eGFR and urine albumin to creatinine ratio.
Table S2. Baseline characteristics of trial participants by MRI data completeness
Table S3. Effect of SPRINT intervention on change in cerebral blood flow, white matter lesion volume, and total brain volume, stratified by baseline eGFR using the Chronic Kidney Disease Epidemiology equation.
Table S4. Effect of SPRINT intervention on change in cerebral blood flow, white matter lesion volume, and total brain volume, including individuals scanned outside of baseline window.
Table S5. Effect of SPRINT intervention on change in cerebral blood flow, white matter lesion volume, and total brain volume, stratified by albuminuria, including individuals scanned outside baseline window.
Support:
The Systolic Blood Pressure Intervention Trial was funded by the National Institutes of Health (including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke) under contracts HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. Azilsartan and chlorthalidone (combined with azilsartan) were provided by Takeda Pharmaceuticals International Inc. Computing resources were supported through 1S10OD023495-01 and additional support was provided through the following National Center for Advancing Translational Sciences clinical and translational science awards: UL1TR000439 (awarded to Case Western Reserve University); UL1RR025755 (Ohio State University); UL1RR024134 and UL1TR000003 (University of Pennsylvania); UL1RR025771 (Boston University); UL1TR000093 (Stanford University); UL1RR025752, UL1TR000073, and UL1TR001064 (Tufts University); UL1TR000050 (University of Illinois); UL1TR000005 (University of Pittsburgh); 9U54TR000017-06 (University of Texas Southwestern Medical Center); UL1TR000105-05 (University of Utah); UL1 TR000445 (Vanderbilt University); UL1TR000075 (George Washington University); UL1 TR000002 (University of California, Davis); UL1 TR000064 (University of Florida); and UL1TR000433 (University of Michigan); and by National Institute of General Medical Sciences, Centers of Biomedical Research Excellence award NIGMS P30GM103337 (awarded to Tulane University). The work presented here was also supported by R01DK092241, R01AG055606, and funding from the Alzheimer’s Association. The NIH and the U.S. Department of Veterans Affairs had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; but not in the decision to submit the manuscript for publication. Takeda Pharmaceuticals, did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. Kurella Tamura has received an honorarium from the American Federation for Aging Research outside the submitted work. Dr. Oparil reports grants and support from NIH/National Heart, Lung and Blood Institute (NHLBI) during the conduct of the study and outside the submitted work, has received personal fees from Preventric Diagnostics, CinCor, and currently serves as Editor-in-Chief for Current Hypertension Reports. Dr. Auchus reports grants from the University of Mississippi Medical Center during the conduct of the study. Dr. Roumie reports grants from the NIH during the conduct of the study. Dr. Beddhu reports grants from NHLBI and NIDDK during the conduct of the study, support from Bayer, Boehringer Ingelheim, Novo Nortis, and UpToDate outside the submitted work. Dr. Zaharchuk reports grants from the NIH during the conduct of the study. Dr. Freedman reports grants from the NIH during the conduct of the study. Dr. Williamson reports grants from the NIH, Biogen and Alzheimer’s Association outside the submitted work. Dr. Nasrallah reports grants from NIH during the conduct of the study and fees from Biogen outside the submitted work. The remaining authors declare that they have no relevant financial interests.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
Peer Review: Received March 2, 2021. Evaluated by 4 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form July 28, 2021. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Vart P, Powe NR, McCulloch CE, et al. National Trends in the Prevalence of Chronic Kidney Disease among Racial/Ethnic and Socioeconomic Status Groups, 1988–2016. JAMA Netw Open 2020;3(7):e207932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoodi BK, Yatsuya H, Matsushita K, et al. Association of Kidney Disease Measures with Ischemic Versus Hemorrhagic Strokes: Pooled Analyses of 4 Prospective Community-Based Cohorts. Stroke 2014;45(7):1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low Glomerular Filtration Rate and Risk of Stroke: Meta-Analysis. Bmj 2010;341:c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar MI, O’Meara ES, Seliger S, et al. Albuminuria and the Risk of Incident Stroke and Stroke Types in Older Adults. Neurology 2010;75(15):1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate Renal Impairment and Risk of Dementia among Older Adults: The Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004;15(7):1904–1911. [DOI] [PubMed] [Google Scholar]
- 6.Khatri M, Wright CB, Nickolas TL, et al. Chronic Kidney Disease Is Associated with White Matter Hyperintensity Volume: The Northern Manhattan Study (Nomas). Stroke 2007;38(12):3121–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney Function Is Related to Cerebral Small Vessel Disease. Stroke 2008;39(1):55–61. [DOI] [PubMed] [Google Scholar]
- 8.O’Rourke MF, Safar ME. Relationship between Aortic Stiffening and Microvascular Disease in Brain and Kidney: Cause and Logic of Therapy. Hypertension 2005;46(1):200–204. [DOI] [PubMed] [Google Scholar]
- 9.Weiner DE, Tighiouart H, Levey AS, et al. Lowest Systolic Blood Pressure Is Associated with Stroke in Stages 3 to 4 Chronic Kidney Disease. J Am Soc Nephrol 2007;18(3):960–966. [DOI] [PubMed] [Google Scholar]
- 10.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L. Relationship between Hypotension and Cerebral Ischemia During Hemodialysis. J Am Soc Nephrol 2017;28(8):2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polinder-Bos HA, Garcia DV, Kuipers J, et al. Hemodialysis Induces an Acute Decline in Cerebral Blood Flow in Elderly Patients. J Am Soc Nephrol 2018;29(4):1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of Intensive Vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019;321(6):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of Intensive Vs Standard Blood Pressure Control with Cerebral White Matter Lesions. JAMA 2019;322(6):524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurella Tamura M, Gaussoin SA, Pajewski NM, et al. Kidney Disease, Intensive Hypertension Treatment, and Risk for Dementia and Mild Cognitive Impairment: The Systolic Blood Pressure Intervention Trial. J Am Soc Nephrol 2020;31(9):2122–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosius WT, Sink KM, Foy CG, et al. The Design and Rationale of a Multicenter Clinical Trial Comparing Two Strategies for Control of Systolic Blood Pressure: The Systolic Blood Pressure Intervention Trial (Sprint). Clinical trials 2014;11(5):532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright JT Jr., Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive Versus Standard Blood-Pressure Control. N Engl J Med 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurella Tamura M, Pajewski NM, Bryan RN, et al. Chronic Kidney Disease, Cerebral Blood Flow, and White Matter Volume in Hypertensive Adults. Neurology 2016;86(13):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Annals of internal medicine 2013;158(11):825–830. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KC, Whelton PK, Cushman WC, et al. Blood Pressure Measurement in Sprint (Systolic Blood Pressure Intervention Trial). Hypertension 2018;71(5):848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tustison NJ, Avants BB, Cook PA, et al. N4itk: Improved N3 Bias Correction. IEEE Trans Med Imaging 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-Atlas Skull-Stripping. Academic radiology 2013;20(12):1566–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doshi J, Erus G, Ou Y, et al. Muse: Multi-Atlas Region Segmentation Utilizing Ensembles of Registration Algorithms and Parameters, and Locally Optimal Atlas Selection. Neuroimage 2016;127:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doshi J, Erus G, Ou Y, Davatzikos C. Ensemble-Based Medical Image Labeling Via Sampling Morphological Appearance Manifolds. Paper presented at: MICCAI Challenge Workshop on Segmentation: Algorithms, Theory and Applications (“SATA”)2013. [Google Scholar]
- 26.Alsop DC, Detre JA, Golay X, et al. Recommended Implementation of Arterial Spin-Labeled Perfusion Mri for Clinical Applications: A Consensus of the Ismrm Perfusion Study Group and the European Consortium for Asl in Dementia. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2015;73(1):102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolui S, Wang Z, Shinohara RT, Wolk DA, Detre JA, Alzheimer’s Disease Neuroimaging I. Structural Correlation-Based Outlier Rejection (Score) Algorithm for Arterial Spin Labeling Time Series. J Magn Reson Imaging 2017;45(6):1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolui S, Wolk D, Detre J. Scrub: A Structural Correlation and Empirical Robust Bayesian Method for Asl Data. Paper presented at: International Society of Magnetic Resonance in Medicine2016; Singapore. [Google Scholar]
- 29.Burbridge JB, Magee L, Robb AL. Alternative Transformations to Handle Extreme Values of the Dependent Variable. Journal of the American Statistical Association 1988;83(401):123–127. [Google Scholar]
- 30.Liu HS, Jawad AF, Laney N, Hartung EA, Furth SL, Detre JA. Effect of Blood T1 Estimation Strategy on Arterial Spin Labeled Cerebral Blood Flow Quantification in Children and Young Adults with Kidney Disease. J Neuroradiol 2019;46(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell GF. Effects of Central Arterial Aging on the Structure and Function of the Peripheral Vasculature: Implications for End-Organ Damage. Journal of applied physiology 2008;105(5):1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner DE, Gaussoin SA, Nord J, et al. Cognitive Function and Kidney Disease: Baseline Data from the Systolic Blood Pressure Intervention Trial (Sprint). Am J Kidney Dis 2017;70(3):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedaghat S, Vernooij MW, Loehrer E, et al. Kidney Function and Cerebral Blood Flow: The Rotterdam Study. J Am Soc Nephrol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepping RJ, Montgomery RN, Sharma P, et al. Normalization of Cerebral Blood Flow, Neurochemicals, and White Matter Integrity after Kidney Transplantation. J Am Soc Nephrol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sink KM, Divers J, Whitlow CT, et al. Cerebral Structural Changes in Diabetic Kidney Disease: African American-Diabetes Heart Study Mind. Diabetes care 2015;38(2):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, Cognitive Functioning, and White Matter Hyperintensities in Homebound Elders. Am J Kidney Dis 2009;53(3):438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Findlay MD, Dawson J, Dickie DA, et al. Investigating the Relationship between Cerebral Blood Flow and Cognitive Function in Hemodialysis Patients. J Am Soc Nephrol 2019;30(1):147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Blood pressure in intensive and standard treatment groups during trial and cohort phases, stratified by estimated glomerular filtration rate.
Figure S2. Blood pressure in intensive and standard treatment groups during trial and cohort phases, stratified by urine albumin to creatinine ratio.
Figure S3. Effect of intensive versus standard BP treatment on change in cerebral blood flow (Panel A), white matter lesion volume (Panel B), and total brain volume (Panel C) expressed as z-scores, by baseline estimated glomerular filtration rate and urine albumin to creatinine ratio.
Table S1. Cerebral blood flow at follow-up, with and without correction for hematocrit, by baseline eGFR and urine albumin to creatinine ratio.
Table S2. Baseline characteristics of trial participants by MRI data completeness
Table S3. Effect of SPRINT intervention on change in cerebral blood flow, white matter lesion volume, and total brain volume, stratified by baseline eGFR using the Chronic Kidney Disease Epidemiology equation.
Table S4. Effect of SPRINT intervention on change in cerebral blood flow, white matter lesion volume, and total brain volume, including individuals scanned outside of baseline window.
Table S5. Effect of SPRINT intervention on change in cerebral blood flow, white matter lesion volume, and total brain volume, stratified by albuminuria, including individuals scanned outside baseline window.
Data Availability Statement
Deidentified participant data will be available in the BioLinCC repository (https://biolincc.nhlbi.nih.gov/studies/sprint).


