Abstract
Background
Hepatosteatosis is the earliest stage in the pathogenesis of nonalcoholic fatty (NAFLD) and alcoholic liver disease (ALD). As NAFLD is affecting 10–24% of the general population and approximately 70% of obese patients, it carries a large economic burden and is becoming a major reason for liver transplantation worldwide. ALD is a major cause of morbidity and mortality causing 50% of liver cirrhosis and 10% of liver cancer related death. Increasing evidence has accumulated that gut-derived factors play a crucial role in the development and progression of chronic liver diseases.
Methods
A selective literature search was conducted in Medline and PubMed, using the terms “nonalcoholic fatty liver disease,” “alcoholic liver disease,” “lipopolysaccharide,” “gut barrier,” and “microbiome.”
Results
Gut dysbiosis and gut barrier dysfunction both contribute to chronic liver disease by abnormal regulation of the gut-liver axis. Thereby, gut-derived lipopolysaccharides (LPS) are a key factor in inducing the inflammatory response of liver tissue. The review further underlines that endotoxemia is observed in both NAFLD and ALD patients. LPS plays an important role in conducting liver damage through the LPS-TLR4 signaling pathway. Treatments targeting the gut microbiome and the gut barrier such as fecal microbiota transplantation (FMT), probiotics, prebiotics, synbiotics, and intestinal alkaline phosphatase (IAP) represent potential treatment modalities for NAFLD and ALD.
Conclusions
The gut-liver axis plays an important role in the development of liver disease. Treatments targeting the gut microbiome and the gut barrier have shown beneficial effects in attenuating liver inflammation and need to be further investigated.
Keywords: NAFLD, ALD, LPS, Gut barrier, Microbiome
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a pathologic condition defined by the deposition of triglycerides in the liver at greater than 5% of the total liver weight in the absence of excessive alcohol consumption.1 The term NAFLD encompasses a spectrum of pathologic conditions ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), a progressive form of fatty liver disease that may lead to fibrosis, cirrhosis, hepatocellular carcinoma, and death.2 NAFLD is now a burgeoning health burden, and it is estimated that the prevalence of NAFLD in the general population is 24% (20–29%).3 Around 20–30% of NAFLD patients develop NASH, with only some of them further evolving to fibrosis and cirrhosis.4 NAFLD carries a large economic burden, contributes to a decreased quality of life, and is becoming a major reason for liver transplantation worldwide.5
Alcoholic liver disease (ALD) is a major cause of morbidity and mortality among people who abuse alcohol.6 Similar to NAFLD, the spectrum of ALD ranges from simple steatosis to alcoholic steatohepatitis (ASH), fibrosis, cirrhosis, and ultimately hepatocarcinoma.7 Alcohol causes 50% of liver cirrhosis and 10% of liver cancer related death.8 Besides the direct effects of alcohol on liver injury, gut microbiota plays an important role in liver damage. Alcohol intake can lead to changes in gut microbiota composition, even before the onset of liver disease.9
Lipopolysaccharide (LPS), also known as endotoxin, is a component of the outer cell wall of Gram-negative bacteria.10 In humans, the gut microbiota is the major source of LPS.11 LPS is generally known for its role in the induction of sepsis, septic shock, and multiple organ failure.12 Recently, LPS is found to be related to several other diseases, especially to metabolic disorders such as type 2 diabetes mellitus and atherosclerosis.13,14 Furthermore, the role of LPS in neurologic and mental disorders like Alzheimer’s disease and autism has been well described,15,16 linking gut microbiota to the homeostasis of the entire body.
The connection between gut microbiota and chronic liver disease has been first noticed in the 1980s when NASH was encountered as a common complication of jejunoileal bypass surgery for morbid obesity that could be reversed by treatment with metronidazole.17 Bacterial overgrowth in the blind loop has been considered to be responsible for liver damage. Today, the human intestinal microbiota has emerged as an important mediator of the development and progression of chronic liver diseases.
This manuscript briefly reviews the role of gut-derived LPS in the development and progression of NAFLD and ALD. Gut barrier dysfunction and dysbiosis are highlighted as they are the two major mechanisms of endotoxemia as well as potential therapeutic targets.
The Intestinal Barrier and LPS Translocation
The intestinal barrier serves as a physical and functional barrier deterring translocation of potentially harmful luminal antigens into circulation. The four layers of the intestinal barrier include luminal intestinal alkaline phosphatase (IAP) released from the intestinal epithelial cells, surface mucus, epithelial layer, and immune defense. The epithelial cell layer and the mucin layer constitute the physical barrier; IAP and antibacterial proteins secreted by Paneth cells represent the functional barrier.18
IAP dephosphorylates substrates such as LPS and other pathogen-associated molecular patterns. Its role in reducing local intestinal inflammation and maintaining gut barrier function is reviewed later. The intestinal mucosal layer represents the first physical barrier. The small intestine harbors a single, tightly attached mucus layer, whereas in the colon, the mucus is composed of two layers: the essentially sterile inner layer which is firmly attached to the epithelial cells and the outer layer which contains commensal bacteria that prevent the entry of pathogenic bacteria. The epithelium is constituted of a single layer of different intestinal epithelial cells (IECs), and cells within the epithelial layer are sealed by tight junction proteins including claudins, zonula occludens-1 (ZO-1), and occluding, preventing paracellular transport.19 The tight junctions (TJs) constitute the major determinant of the intestinal physical barrier and can prevent the paracellular passage of large molecules through the epithelium.20
Disruption of the intestinal barrier can cause LPS translocation and cause endotoxemia in systemic circulation and chronic liver inflammation. The permeability of the epithelium is determined by the composition and abundance of different components of the TJs.21 Many factors can alter intestinal permeability, such as diet, alcohol intake, medication, and physiological factors such as age and stress.22–24 Changes in intestinal microbiota composition can influence intestinal permeability, as a study showing that a high-fat diet changed the gut microbiota content and could increase intestinal permeability. More importantly, this effect was completely restored by antibiotic treatment.25 And vice versa, probiotic bacteria and probiotic mixture are shown to have beneficial effects in reestablishing intestinal homeostasis and preserving epithelial barrier function.26 Bacterial metabolites, such as short-chain fatty acids (SCFAs), are also reported to play an important role in maintaining both intestinal immune functions and regulating gut barrier functions.27
LPS translocate the intestinal barrier mainly through the transcellular pathway, and chylomicrons can also bind and facilitate the absorption of LPS.28 Translocation of LPS can occur in physiological states.29 Under pathological conditions when there is an increase in gut barrier permeability, paracellular pathway of LPS is increased. The liver is the first organ in the body to encounter gut-derived bacteria and pathogen associated molecular patterns (PAMPs). Chronic exposure to increased levels of PAMPs has been linked to liver diseases.30
LPS Conducts Liver Injury Through TLR4 Signaling Pathway
As the gut is considered to be the first barrier against bacteria, the liver is the second barrier based on the fact that the liver and the gut share both anatomical and functional relations and proximities; their close interaction is also described as the gut-liver axis.31 The portal venous system sits at the interface between the host and the inflammatory mediators that exist within the gut. The most important gut-derived inflammatory mediator, LPS, enters the liver through the portal vein and is detoxified in the liver. Only few LPS passes through the gut barrier and finally arrives in the liver under physiologic conditions. However, small intestinal bacteria overgrowth (SIBO) or an increase in intestinal permeability leads to translocation of bacteria and its by-products such as LPS. After arriving in the liver, LPS is taken up by hepatocytes and Kupffer cells and is excreted into the bile duct in further process.32 In patients with chronic liver disease, high LPS portal/peripheral concentrations have been observed.33 LPS binds to LPS-binding protein (LBP), and the LBP-LPS complex is transferred to membrane bound or soluble cluster of differentiation 14 (CD14), thereby specifically binds to toll-like receptor 4 (TLR4) and induces the interaction of TLR4 with adaptor molecule myeloid differentiation factor 88 (MyD88). MyD88 further activates downstream mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-kB). Stimulation of the LPS-TLR4 signaling pathway eventually leads to the release of proinflammatory mediators like tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6).34 LPS also mediates signaling through MyD88-independent pathway, but the activation of MAPK and NF-kB occurs in a delayed manner.35 In both ALD and NAFLD progression, TLR4 signaling is considered a key pathway, and, very interestingly, it is reported that mice deficient in TLR4 are resistant to both alcohol-induced liver injury and NAFLD.36,37
TLR4 is expressed on all types of liver cells, including Kupffer cells, hepatocytes, hepatic stellate cells (HSCs), and also cholangiocytes. Its expression on these cells is correlated with activation of fibrogenic cells and the stage of fibrosis.32 In normal liver, hepatic cells express minimal TLRs; therefore, the liver has a high tolerance to TLR ligands. Hepatocytes directly clear LPS as data showed that fluorescence was revealed in hepatocytes 5 min after injection of fluorescein isothiocyanate (FITC)-LPS into the portal vein and then rapidly secreted into the bile.38 LPS promotes TNF-α production in Kupffer cells. The inflammatory mediator TNF-α is considered a central mediator in the pathogenesis of both ALD and NAFLD.39 Despite the fact that Kupffer cells are the main targets of LPS in the liver, it is the HSCs to promote TLR4-dependent fibrosis.40 LPS can activate HSCs in vitro and in vivo, and Kupffer cells strongly enhance this process by producing transforming growth factor beta (TGF-β) and increasing the sensitivity of HSCs to TGF-β. Recent studies have pointed out that platelets can also play a role in liver injury as biopsy results showed that the number of platelets is associated with disease severity in NAFLD.33 Platelets passing through hepatic sinusoids can be activated by LPS and the number of TLR4+ platelets is positively correlated with serum LPS level in NAFLD patients, suggesting that LPS may activate platelets through TLR4 signaling pathway. LPS is capable of activating platelets via TLR4-mediated over-production of eicosanoids, and mice given aspirin showed a decrease of liver fibrosis.41,42
Histology of ALD and NAFLD
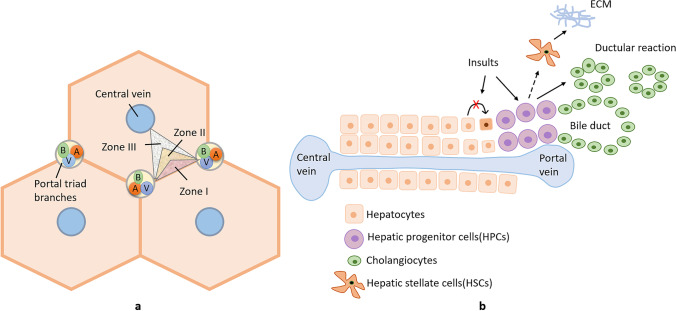
ALD shares histological similarities to NAFLD,43 indicating that there may be a common pathway for liver injury in both diseases. The hallmark of NAFLD and ALD is the excessive fat accumulation in the hepatocytes, which may be an isolated event (simple steatosis) or accompanied by evidence of inflammation and cell injury with or without fibrosis (NASH and ASH)44. The histological changes in ALD or NAFLD are lobulocentric (typically affecting zone 3) but not portal-based,45 including hepatocyte ballooning, Mallory bodies, zone 3 inflammation, and perisinusoidal fibrosis. Recent studies have identified the importance of portal fibrosis in predicting the subgroup of NAFLD patients that develop progressive liver disease and liver-related mortality.46 Portal inflammation is associated with portal-based changes, such as ductular reaction (DR), a reactive lesion at the portal tract interface comprising small biliary ductules with an accompanying complex of stroma and inflammatory cells.47 The underlying mechanism of DR is the activation of a secondary proliferative pathway of hepatic progenitor cells (HPCs) during the replacement of necrotic and apoptotic hepatocytes.48 Under regular conditions, the primary pathway of liver regeneration is maintained by the replication of adjacent hepatocytes within the lobules. With an insult of toxins, viral infection, alcohol, etc., the primary pathway is blocked and replaced by the secondary pathway: HPCs proliferate and differentiate into hepatocytes and bile ductal epithelia; the by-product is the DR (Figure 1).
Fig. 1.
Histological zones of liver lobules (A) and the ductular reaction (B). A The liver can be divided functionally into three zones. Zone I is located around the portal triad, which is the most nutrient-oxygenated region. Zone III is located around the central vein, where oxygenation is poor. Zone II is located in between. B In chronic liver injury, hepatocyte regeneration is impaired and is replaced by the second pathway of HPCs activation. HPCs are bipotential cells and can differentiate into hepatocytes and cholangiocytes. The latter process causes the ductular reaction (DR). The HPCs can also interact with hepatic stellate cells, which are the primary
source of the extracellular matrix (ECM) and the key players of the liver fibrogenic response
Odena et al.49 proved that the LPS-TLR4 pathway stimulates the expansion of ductular reaction in alcoholic hepatitis (AH). The study found that the keratin 23 (KRT23) gene, which is expressed in the ductular reaction cells, is the most upregulated gene in AH compared to NASH and normal livers. They discovered that LPS administration markedly induced KRT23 expression in mice. This effect can be attenuated in TLR4-deficient mice, further indicating that the LPS-TLR4 pathway mediates the development of ductular reaction in chronic liver injury.
Another study by Vespasiani-Gentilucci et al.32 provided evidence that serum LPS levels correlated with portal/interface inflammation, the activity of portal/septal myofibroblasts, and fibrosis in NAFLD patients. Using immunohistochemistry, TLR4 expression was observed in hepatic progenitor cells, biliary cells, and portal/septal macrophages. TLR4-positive hepatic progenitor cells and bile ducts/ductules correlate with portal/interface inflammation, activation of fibrogenic cells, and fibrosis, implicating that the LPS-TLR4 pathway is associated with inflammation and fibrosis progression in NAFLD.
LPS in the Pathogenesis of NAFLD
NAFLD is the liver manifestation of metabolic syndrome and is characterized by massive ectopic triglyceride accumulation in the liver in the absence of any other liver disease or significant alcohol consumption.50 The etiology of NAFLD is previously referred to as a “two-hit” hypothesis:51 The first hit involves lipid accumulation in the hepatocytes, making the liver more vulnerable to toxins. Further insults like ethanol and LPS can act as the second hit and amplify the initial stress, causing oxidative stress and inflammatory response within the liver, resulting in the development of steatohepatitis (Figure 2). The “two-hit” hypothesis suggests that NASH is generally preceded by simple steatosis. But this view seems to be too simple to elucidate the complexity of the pathogenesis of NAFLD. Recently, it became widely accepted that the pathogenesis of NAFLD is a “multiple parallel hits” process.52 In this hypothesis, various hits (genetic and environmental factors) may occur parallelly, leading to liver inflammation. Among these hits, gut-derived factors appear to play a central role. Although traditional views consider that simple steatosis and NASH are consecutive changing processes, emerging evidence indicates that these two diseases can arise as independent conditions.53,54 Studies have found that the patients with simple steatosis will not always develop NASH and that the two diseases have a huge difference in prognosis in long term follow-up. This evidence further demonstrates that steatosis may not always precede inflammation.
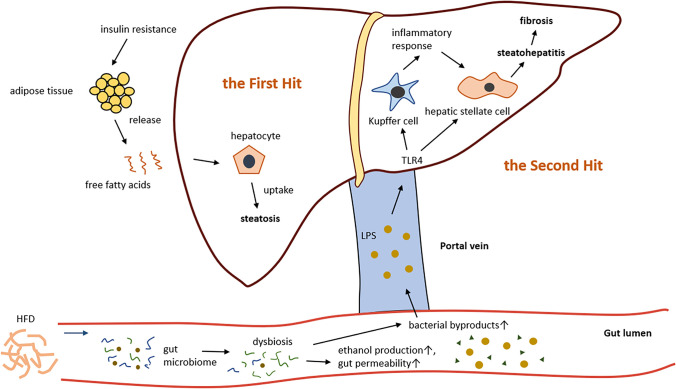
Fig. 2.
LPS acts as the second hit in the pathogenesis of NAFLD. Insulin resistance is the major factor for the progression of NAFLD, leading to an increase in free fatty acids (FFAs) in the circulating blood. Excessive uptake of FFAs by hepatocytes results in steatosis, making the liver more vulnerable to further insults, which is considered the “first hit.” A high-fat diet (HFD) could lead to gut dysbiosis, which further causes an increase in bacterial by-product production and increased gut permeability. Lipopolysaccharide (LPS) translocates the gut barrier, enters the liver through the portal vein, and activates Kupffer cells and hepatic stellate cells (HSCs) through the LPS-TLR4 pathway, resulting in an inflammatory response which leads to steatohepatitis and eventually fibrosis
Insulin resistance (IR) has been recognized as a major factor in the development of NAFLD.55 IR is thought to contribute to NAFLD by enhancing lipolysis of the adipose tissue, resulting in the increase of free fatty acids flux into the liver.56 High-fat, high cholesterol diets can result in endotoxemia and low-grade inflammation in both human and animal models.57,58 Lifestyle modifications such as diet, physical exercise, and weight loss are advocated. The Mediterranean diet characterized by reduced carbohydrate intake and increased monounsaturated and omega-3 fatty acid intake has a beneficial effect in overweight patients with NAFLD.59 A previous study showed that even short-term diet modification could reduce liver steatosis and steatohepatitis.60
Endotoxin plasma level is significantly higher in NAFLD patients, and it seems that endotoxin level is associated with the severity of hepatic steatosis.61 Gut dysbiosis is causative for the enhanced secretion of LPS and the resulting inflammation in NAFLD development.62 Generally, dysbiosis is defined as an imbalance or alteration in the microbiota that can have an unfavorable effect on the host.63 A previous study investigated taxonomic compositions of gut microbiota within the spectrum of NALFD lesions and found that more serious NAFLD lesions (NASH and significant fibrosis) associate with gut dysbiosis.64 The study also found that increased levels of Bacteroides were independently associated with NASH and increased Ruminococcus abundance with fibrosis, linking these two taxa of bacteria to the severity of NAFLD.
The gut microbiota composition of an individual is a kind of a fingerprint highly influenced by the type of diet.65 It has been shown that microbiota play an important role in nutrition intake and patients with obesity or metabolic disorder appear to have an intestinal microbiota signature that can harvest more energy than their healthy counterparts.66 Changes in abundance and diversity of the gut microbiota in NAFLD have also been characterized. Though many studies have reported conflicting results of the microbiome signatures in NAFLD, an increase in Firmicutes and a reduction in Bacteroidetes is found in most studies.67 Thus, a higher Firmicutes/Bacteroidetes ratio is frequently cited as a hallmark of obesity.68 Furthermore, the amount of ethanol-producing bacteria (e.g., Escherichia coli) was reported to be increased in NAFLD, which may cause increased intestinal permeability and increased translocation of endotoxins from the intestinal lumen to the portal blood.69 The impact of microbiota on liver disease is further supported by experiments during which bacterial transfer from the human gut to germ-free (GF) mice resulted in metabolic diseases70,71
Guo and collegues72 found that LPS per se can cause an increase in intestinal epithelial tight junction permeability in vitro and in vivo. This effect was mediated by an increase in enterocyte TLR4 expression and a TLR4-dependent increase in membrane CD14 expression. Studies have shown that physiologically relevant concentrations of LPS (0 to 2000 pg/mL) cause an increase in intestinal epithelial TJ permeability.73,74 These findings indicate that LPS is an important pathogenic factor in the intestinal inflammatory process.
The gut microbiota is influenced by various factors, including the genetic background of the host, type of diet, age, and medication. How these factors exactly affect gut microbiota composition and function is not fully understood with several studies presenting contradictory data. The regulation of gut microbiota provides a new insight into the treatment of NAFLD.
LPS in the Pathogenesis of ALD
ALD is associated with high morbidity and mortality rates. Most chronic heavy drinkers develop steatosis, but only 35% develop advanced liver disease.75 A previous study showed that intestinal hyperpermeability occurs only in alcoholics with ALD and not in those without liver disease.76 Furthermore, chronic alcohol administration has been shown to increase gut-derived endotoxin levels in the portal circulation.77 This indicates that alcohol plays a crucial role in promoting intestinal hyperpermeability resulting in endotoxemia, systemic inflammation, and liver damage. Alcohol consumption destroys the integrity of the intestinal barrier, disturbs the gut microbiota, and is associated with an increase in the abundance of endotoxin-producing bacterial types.78 Metagenomic analysis of the intestinal microbiome of individuals with a history of chronic alcohol abuse has revealed reduced bacterial diversity and a lower proportion of Bacteroidaceae and probiotic bacteria such as Lactobacillus 79,80. Patients with alcoholic cirrhosis have an increased relative abundance of Enterobacteriaceae.81 Alcohol consumption affects bacterial composition within specific phyla. A study by Llopis et al.82 found that mice harboring the intestinal microbiota from patients with severe alcoholic hepatitis developed more severe liver inflammation, indicating that individual susceptibility to ALD is substantially driven by the intestinal microbiome.
Both chronic alcohol consumption and acute alcohol intake (binge drinking) can impair the intestinal barrier and increase serum levels of bacterial products.76,83 Endotoxemia in ALD was first recognized by the detection of antibodies against Escherichia coli in the plasma of patients with ALD.84 Humans and animals with chronic alcohol consumption develop a “leaky gut,” as evidenced by higher levels of plasma endotoxin. The plasma endotoxin levels are also associated with the severity of disease.85 Alcohol damages specific components of the intestinal barrier such as proteins involved in innate antibacterial defense. Animal experiments have shown that chronic exposure to ethanol can cause gut-barrier disruption featured by a decrease in tight junction protein ZO-1 and occludin expression.86,87
Besides ethanol, the by-product acetaldehyde from the metabolization of alcohol by gut microbiota appears to play a crucial role in gut barrier dysfunction. At concentrations ranging from 99 to 760μM, acetaldehyde increases the paracellular permeability of Caco-2 cell monolayer.88 The underlying mechanism includes the dissociation of ZO-1 from the junctions in acetaldehyde-treated cell monolayer, thus leading to disrupted TJ structure and the increase in paracellular permeability.89 The study further revealed that acetaldehyde increases tyrosine phosphorylation of ZO-1, E-cadherin, and β-catenin, resulting in disruption of the TJs.
Chronic alcohol consumption is also associated with changes in bile acid profiles. ALD patients have an increased secondary bile acid formation.90 A study by Xie et al.91 showed that ethanol consumption could lead to a substantial decrease in taurine-conjugated (hydrophilic and less toxic) bile acids, resulting in impaired lipid emulsification and liver steatosis in mice.
Intestinal Targeted Therapy
While simple steatosis is considered a “benign disease,” treatment of NAFLD and ALD should focus on NASH and ASH. Currently, there is no targeted and thus effective drug therapy for the treatment of NASH or ASH. Especially, the long-term intake of traditional treatments for chronic liver diseases such as antibiotics and corticosteroids is associated with various side effects for patients. There is accumulating evidence that the interplay between the gut microbiota and the liver is critical in the pathogenesis of chronic liver disease. Hence, there is a large interest in modulating the microbial community to achieve a therapeutic effect or even reverse liver fibrosis. The manipulation of the gut microbiome by fecal microbiota transplantation (FMT), probiotics, prebiotics, and synbiotics was shown to have a beneficial effect in improving liver phenotype in patients with chronic liver disease. IAP as a naturally occurring brush boarder enzyme that detoxifies LPS and preserves microbial homeostasis and gut barrier integrity appears to be a promising candidate for treatment of liver fibrosis in patients with NAFLD or ALD.
Fecal Microbiota Transplantation
This procedure involves the transfer of processed feces to restore a “healthy microbiome.” FMT has been successfully used to treat recurrent Clostridium difficile infection (rCDI) for years92,93 and possesses potential in treating gastrointestinal as well as extraintestinal diseases.
FMT has shown an effect in alleviating high-fat diet-induced steatohepatitis in mice.94 The therapeutic effect may be caused by an increase in “beneficial” gut microbiota, improving the tight junction of small intestinal and lowering the LPS levels. Ferrere et al.95 performed FMT from alcohol-resistant donor mice (alcohol-fed mice did not develop alcohol-induced liver lesions) to alcohol-sensitive receiver mice (alcohol-fed mice developed liver lesions) and found that FMT protected the alcohol-sensitive mice from alcohol-induced depletion of Bacteroides. FMT treatment was also found to prevent steatosis in alcohol-fed mice, indicating the beneficial effect of FMT in preventing ALD development. In a small-scale pilot study conducted by Philips et al.,96 8 patients with steroid-ineligible severe alcoholic hepatitis received FMT treatment from healthy donors. The results showed that indices of liver disease severity, including ascites, hepatic encephalopathy, and mean bilirubin, significantly improved after FMT. FMT treatment also showed an effect on modulation of gut microbiota and improved prognosis in these patients. In a recent randomized trial, twenty patients with liver cirrhosis were randomly given FMT capsules or placebo. Patients receiving FMT capsules showed an improved gut microbial function and significantly reduced systemic inflammation markers (IL-6 and LBP).97
FMT contributes to restore a balanced gut microbiota composition and has emerged as a therapeutic option for chronic liver disease. Although FMT shows its beneficial effect in animal models and clinical trials of non-alcoholic and alcoholic liver steatosis, it has not been approved for clinical use. Further research is needed to verify the safety and effectiveness of FMT in chronic liver disease.
Probiotics
Probiotics are defined by the Food and Agriculture Organization (FAO)/World Health Organization (WHO) as living microorganisms that confer a health benefit on the host when administered in adequate amounts.98 They exhibit characteristics including tolerance to gastrointestinal conditions, ability to adhere to the gastrointestinal mucosa and competitive exclusion of pathogens.99 The strains most frequently used as probiotic bacteria belong to the Bifidobacterium and Lactobacillus genera and are widely used in yogurts and other dairy products.100 Probiotics are generally considered to have a beneficial effect on the human intestine. Induction of probiotics may control the growth of pathologic organisms. Studies have proven that probiotics are efficient in treating various diseases such as antibiotic-associated diarrhea (ADD), inflammatory bowel diseases (IBD), and neuropsychiatric disorders. 101–103
Probiotics have been proven to lower the plasma triglyceride (TG) levels in animal models of metabolic syndrome.104 Plaza-Diaz et al.105 demonstrated the reduction in liver steatosis of obese mice fed certain probiotic strains. This effect was associated with lower serum LPS levels as matched trends were observed in LPS serum concentration and liver TG content.
Clinical trials have confirmed the beneficial effects of probiotics in nonalcoholic liver diseases both in adults and children.106–108 Probiotic treatment has been shown to play a role in improving liver function. The intake of probiotics leads to reduced levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamine transferase (GGT), total cholesterol (TC), TG, and improved liver histological marker such as NAS (NAFLD Activity Score).107 Probiotic supplementation is also able to improve biomarkers of inflammation (e.g., TNF-α, IL-6) and steatosis (e.g., arginase, prolidase).109
Similar to NAFLD, patients with alcoholic liver disease can also benefit from a probiotic treatment. In a Russian pilot study110, Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 were randomly given to patients diagnosed with alcoholic psychosis. The treatment led to the alterations in bowel flora in alcoholic patients and liver enzyme reduction, indicating that probiotics can improve alcohol-induced liver injury. Other randomized-controlled trials of Lactobacillus casei supplements in patients with alcoholic liver injury support this finding.111
In general, the mechanisms by which probiotics prevent chronic liver injury include: reducing gut-derived microbial LPS by restoring the bowel flora, repairing the intestinal mucosa and barrier function, modulation of the immune system, and reducing inflammatory cytokine levels.112
Prebiotics and Synbiotics
A prebiotic is a non-viable food component that can confer a health benefit on the host that is based on the modulation of the intestinal microbiota.113 Main prebiotics include primarily short- and long-chain fructans (fructo-oligosaccharides (FOS) and inulin), galacto-oligosaccharides (GOS), and lactulose.114 These substances possess features including non-digestibility, fermentation by intestinal microflora, and selective stimulation of growth and activity of intestinal bacteria.115
Prebiotics have beneficial effects on the gastrointestinal tract, which include prevention of pathogen damage or immune system modulation, improvement of gut barrier function, reduction in the pathogenic bacteria population, and the production of SCFAs.116 Prebiotics are used mostly as a selective medium for the growth of a probiotic strain, and Lactobacilli and Bifidobacteria are the usual target genera for prebiotics. Large bundles of studies have shown that prebiotics are able to increase the composition and/or activity of Lactobacillus and Bifidobacterium populations.117–120 In lipid metabolism, prebiotics exhibit serum or hepatic lipid-lowering properties, and are now considered a potential dietary adjunct in reducing the risks of cardiovascular diseases (CVD) with minimal side effects121. A randomized placebo-controlled trial carried out in Canada showed that, after 16 weeks of consumption of oligofructose-enriched inulin, there was a significant decrease in body weight z-score, percent body fat, and percent trunk fat and serum level of IL-6 in healthy children with overweight or obesity compared to the placebo control group.122 The study also observed a significantly higher abundance of Bifidobacterium spp. in the prebiotic consumption group, indicating that prebiotic consumption could selectively alter gut microbiota.
The prebiotic inulin is proven to be effective in preventing NAFLD in animal experiments.123,124 However, the effect of inulin in human trials remains controversial. Chambers et al.125 explored the effects of dietary supplementation with inulin in adults with NAFLD and found that inulin consumed at 20 g/d increased intrahepatocellular lipid (IHCL). They speculated that the acetate derived from colonic fermentation of inulin could provide an additional lipogenic substrate to the liver.
A synbiotic is a mixture of 1 or more probiotics and 1 or more prebiotics that beneficially affect the host by promoting the survival and colonization of the live microbes in the gastrointestinal tract.115 It is known that probiotics are active in the small and large intestine and the effect of a prebiotic is mainly in the colon. The combination of the two may have a synergistic effect.126 The intake of synbiotics has been demonstrated to modify the composition of the microbiota, thus protecting against inflammation and hepatocyte damage.127 A recent meta-analysis involving 15 randomized clinical trials in which 8 studies about synbiotics were included showed that synbiotics supplementation can improve TC, TG, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) in patients with NAFLD, indicating that synbiotics could improve lipid profiles in those patients.128 Among these studies, Malaguarnera et al.127 conducted a randomized clinical trial in which 66 NASH patients were enrolled and randomly given Bifidobacterium longum with fructo-oligosaccharides or placebo. The results showed that synbiotic treatment improved liver histology in NASH patients and had a beneficial effect in reducing inflammation markers like C-reactive protein (CRP) and TNF-α. These effects may be due to reduced LPS exposure to the liver.
Though studies of the relationship between synbiotics and ALD are much lesser, the beneficial effect of synbiotics in attenuating chronic alcohol intake induced liver injury is observed in mice.129,130 Synbiotic supplementation was also shown to decrease serum LPS levels in high-risk alcoholic participants and in patients with alcohol-related cirrhosis.131,132
Intestinal Alkaline Phosphatase
IAP is ubiquitously expressed by enterocytes in the proximal small intestine and exists in high concentrations within luminal vesicles secreted by enterocytes on the brush border of the microvilli.133 IAP is secreted bilaterally and is also released in small amounts into the blood.134 IAP plays an important anti-inflammatory role by dephosphorylating potentially pro-inflammatory ligands such as adenosine triphosphate (ATP), uridine diphosphate (UDP), unmethylated cytosine-guanosine dinucleotides (CpG), and LPS.135 Luminal IAP can prevent and reduce intestinal inflammation and bacterial translocation and is considered the first layer of the intestinal barrier.18 The anti-inflammatory effect makes it a potential treatment for various diseases. Previous studies had found decreased levels of IAP protein expression in inflamed colonic mucosa in children with IBD and decreased IAP mRNA levels in inflamed tissue of adults with IBD.136,137 Exogenous IAP supplementation showed a beneficial effect in alleviating inflammation in a rat model of IBD.138 In a phase II trial, intravenous injection of IAP improved renal function in patients with severe sepsis and septic shock.139 And another phase II trial demonstrated that administration of bovine IAP could reduce post-surgical inflammatory response in patients undergoing coronary artery bypass grafting (CABG).140
IAP is an important enzyme to maintain the integrity of the gut barrier. By dephosphorylating luminal ATP, IAP can act as a component of the ecto-purinergic signaling system to regulate ATP-dependent HCO3− secretion and localized extracellular pH (pH0).141 Liu et al.142 demonstrated that IAP can directly regulate TJ protein levels as its gene depletion in mouse embryonic fibroblasts resulted in significantly lower levels of ZO-1, ZO-2, and occludin expression, and IAP overexpression in Caco-2 and T84 cells resulted in increases in mRNA levels of ZO-1 and ZO-2 (Figure 3). Another study showed a similar result that the intestinal tissue of IAP-KO mice presents a significant decrease in TJ proteins; moreover, oral supplementation with IAP restores TJ protein expression.143
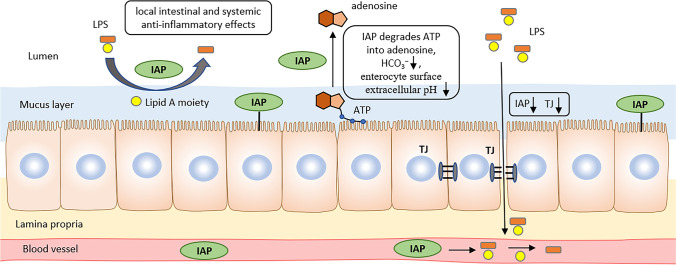
Fig. 3.
The role of IAP in preventing local inflammation, preventing LPS translocation, and regulation of enterocyte surface extracellular pH. IAP is highly expressed in the brush border membrane of duodenal epithelial cells and is secreted bilaterally into the gut lumen and the blood. IAP can detoxify LPS, resulting in amelioration of intestinal and systemic inflammation. ATP serves as a substrate for brush border IAP. In the gut lumen, the presence of ATP increases HCO3—secretion. IAP decreases luminal ATP concentration and diminish this pathway. IAP also plays a role in regulating tight junction protein levels, preserving gut integrity and preventing translocation of bacterial by-products
IAP also plays a crucial role in regulating the gut microbiota. Malo et al.144 examined the status of the gut microbiota in IAP knockout (IAP-KO) mice and found dramatically fewer microbes in their stools compared with wild-type (WT) mice when fed a high-fat diet. Moreover, oral supplementation of calf IAP (cIAP) promoted the restoration of the normal gut microbiota following antibiotic treatment. Kühn et al.145 observed the beneficial effect of IAP supplementation in preserving the homeostasis of gut microbiota during aging in mice. IAP has been shown to prevent chronic liver disease in 2 different mice models. Liu et al. 146 demonstrated that fecal IAP activity decreases in humans with liver cirrhosis and oral supplement of IAP attenuated liver fibrosis in mice. Hamarneh et al.147 demonstrated that pretreatment with IAP attenuated the development of alcohol-induced fatty liver, decreased hepatic pro-inflammatory cytokines, as well as serum LPS levels, and prevented alcohol-induced gut barrier dysfunction in mice, indicating that oral IAP supplementation could present a novel therapy to prevent alcoholic-related liver disease. Though the beneficial effect of IAP in chronic liver diseases and maintaining gut barrier function has been observed in various preclinical studies, the therapeutic value of IAP in patients with chronic liver disease is not known.
Conclusion
Accumulating evidence has pointed out the importance of the gut-liver axis in the development of liver disease. Translocation of bacterial by-products such as LPS results in chronic inflammation in the liver. Treatments targeting the gut microbiome such as FMT, probiotics, prebiotics, and synbiotics have shown an effect in attenuating liver inflammation. IAP possessing an anti-inflammatory effect by dephosphorylating LPS and maintaining gut barrier function is also showing therapeutic potential. Current evidence demonstrates that maintaining gut barrier integrity and microbiome homeostasis is of great significance in treating liver diseases.
Author Contribution
All authors have contributed substantially to the conception of this review and devised or revised the manuscript for intellectual content and have approved the final version for publication. In addition, all authors agree to be accountable for all aspects of the work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17(5):774. doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–654 e641–649; quiz e639–640. [DOI] [PMC free article] [PubMed]
- 5.Sherif ZA, Saeed A, Ghavimi S, et al. Global Epidemiology of Nonalcoholic Fatty Liver Disease and Perspectives on US Minority Populations. Dig Dis Sci. 2016;61(5):1214–1225. doi: 10.1007/s10620-016-4143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113(2):175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391(11):1249–1264. doi: 10.1515/bc.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avila MA, Dufour JF, Gerbes AL, et al. Recent advances in alcohol-related liver disease (ALD): summary of a Gut round table meeting. Gut. 2020;69(4):764–780. doi: 10.1136/gutjnl-2019-319720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(4):235–246. doi: 10.1038/s41575-018-0099-1. [DOI] [PubMed] [Google Scholar]
- 10.Tuin A, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K. On the role and fate of LPS-dephosphorylating activity in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G377–385. doi: 10.1152/ajpgi.00147.2005. [DOI] [PubMed] [Google Scholar]
- 11.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4(11):430–435. doi: 10.1016/0966-842X(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 12.Opal SM. Endotoxins and other sepsis triggers. Contrib Nephrol. 2010;167:14–24. doi: 10.1159/000315915. [DOI] [PubMed] [Google Scholar]
- 13.Gomes JMG, Costa JA, Alfenas RCG. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism. 2017;68:133–144. doi: 10.1016/j.metabol.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients. 2020;12(10). [DOI] [PMC free article] [PubMed]
- 15.Zhan X, Stamova B, Sharp FR. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer's Disease Brain: A Review. Front Aging Neurosci. 2018;10:42. doi: 10.3389/fnagi.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srikantha P, Mohajeri MH. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int J Mol Sci. 2019;20(9):2115. doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drenick EJ, Fisler J, Johnson D. Hepatic steatosis after intestinal bypass–prevention and reversal by metronidazole, irrespective of protein-calorie malnutrition. Gastroenterology. 1982;82(3):535–548. doi: 10.1016/S0016-5085(82)80403-4. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Yannie PJ, Wang J, Ghosh SS. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. Journal of the Endocrine Society. 2020;4(2):bvz039. [DOI] [PMC free article] [PubMed]
- 19.Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11(9):821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130(1):55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullin JM, Valenzano MC, Verrecchio JJ, Kothari R. Age- and diet-related increase in transepithelial colon permeability of Fischer 344 rats. Dig Dis Sci. 2002;47(10):2262–2270. doi: 10.1023/A:1020191412285. [DOI] [PubMed] [Google Scholar]
- 23.Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. 2017;312(6):G559–G571. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- 24.Jennison E, Byrne CD. The role of the gut microbiome and diet in the pathogenesis of non-alcoholic fatty liver disease. Clin Mol Hepatol. 2021;27(1):22–43. doi: 10.3350/cmh.2020.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 26.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286(4):G613–626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerville M, Boudry G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G1–G15. doi: 10.1152/ajpgi.00098.2016. [DOI] [PubMed] [Google Scholar]
- 29.Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128(5):1258–1267. doi: 10.1053/j.gastro.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. 2015;421:44–53. doi: 10.1016/j.jim.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malaguarnera G. Gut microbiota in alcoholic liver disease: Pathogenetic role and therapeutic perspectives. World Journal of Gastroenterology. 2014;20(44):16639. doi: 10.3748/wjg.v20.i44.16639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vespasiani-Gentilucci U, Carotti S, Perrone G, et al. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver Int. 2015;35(2):569–581. doi: 10.1111/liv.12531. [DOI] [PubMed] [Google Scholar]
- 33.Carpino G, Del Ben M, Pastori D, et al. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology. 2020;72(2):470–485. doi: 10.1002/hep.31056. [DOI] [PubMed] [Google Scholar]
- 34.Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28(Suppl 1):38–42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Zhuang ZJ, Bian DX, et al. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin Exp Pharmacol Physiol. 2014;41(7):482–488. doi: 10.1111/1440-1681.12241. [DOI] [PubMed] [Google Scholar]
- 37.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 38.Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109(6):1969–1976. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 39.Kawaratani H, Tsujimoto T, Douhara A, et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. doi: 10.1155/2013/495156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 41.Li CJ, Yang ZH, Shi XL, Liu DL. Effects of aspirin and enoxaparin in a rat model of liver fibrosis. World J Gastroenterol. 2017;23(35):6412–6419. doi: 10.3748/wjg.v23.i35.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nocella C, Carnevale R, Bartimoccia S, et al. Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb Haemost. 2017;117(8):1558–1570. doi: 10.1160/TH16-11-0857. [DOI] [PubMed] [Google Scholar]
- 43.Rakha EA, Adamson L, Bell E, et al. Portal inflammation is associated with advanced histological changes in alcoholic and non-alcoholic fatty liver disease. J Clin Pathol. 2010;63(9):790–795. doi: 10.1136/jcp.2010.079145. [DOI] [PubMed] [Google Scholar]
- 44.Higuera-de la Tijera F, Servin-Caamano AI. Pathophysiological mechanisms involved in non-alcoholic steatohepatitis and novel potential therapeutic targets. World J Hepatol. 2015;7(10):1297–1301. doi: 10.4254/wjh.v7.i10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 46.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53(6):1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 47.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54(5):1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 48.Bria A, Marda J, Zhou J, et al. Hepatic progenitor cell activation in liver repair. Liver Res. 2017;1(2):81–87. doi: 10.1016/j.livres.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odena G, Chen J, Lozano JJ, et al. LPS-TLR4 Pathway Mediates Ductular Cell Expansion in Alcoholic Hepatitis. Sci Rep. 2016;6:35610. doi: 10.1038/srep35610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekstedt M, Nasr P, Kechagias S. Natural History of NAFLD/NASH. Curr Hepatol Rep. 2017;16(4):391–397. doi: 10.1007/s11901-017-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 52.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 53.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 55.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 56.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13(26):3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 58.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86(5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 59.Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Reeves JG, Suriawinata AA, Ng DP, Holubar SD, Mills JB, Barth RJ., Jr Short-term preoperative diet modification reduces steatosis and blood loss in patients undergoing liver resection. Surgery. 2013;154(5):1031–1037. doi: 10.1016/j.surg.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Nier A, Huber Y, Labenz C, Michel M, Bergheim I, Schattenberg JM. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2020;12(3):699. doi: 10.3390/nu12030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saltzman ET, Palacios T, Thomsen M, Vitetta L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-alcoholic Fatty Liver Disease. Front Microbiol. 2018;9:61. doi: 10.3389/fmicb.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016;22(5):1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambertz J, Weiskirchen S, Landert S, Weiskirchen R. Fructose: A Dietary Sugar in Crosstalk with Microbiota Contributing to the Development and Progression of Non-Alcoholic Liver Disease. Front Immunol. 2017;8:1159. doi: 10.3389/fimmu.2017.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15(13):1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 67.Nier A, Huber Y, Labenz C, Michel M, Bergheim I, Schattenberg JM. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2020;12(3):699. doi: 10.3390/nu12030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12(5):1474. [DOI] [PMC free article] [PubMed]
- 69.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 70.Fei N, Bruneau A, Zhang X, et al. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio. 2020;11(1):e03263-19. [DOI] [PMC free article] [PubMed]
- 71.Chiu CC, Ching YH, Li YP, et al. Nonalcoholic Fatty Liver Disease Is Exacerbated in High Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients. 2017;9(11):1220 [DOI] [PMC free article] [PubMed]
- 72.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182(2):375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen SW, Wang PY, Zhu J, et al. Protective effect of 1,25-dihydroxyvitamin d3 on lipopolysaccharide-induced intestinal epithelial tight junction injury in caco-2 cell monolayers. Inflammation. 2015;38(1):375–383. doi: 10.1007/s10753-014-0041-9. [DOI] [PubMed] [Google Scholar]
- 74.Nighot M, Rawat M, Al-Sadi R, Castillo EF, Nighot P, Ma TY. Lipopolysaccharide-Induced Increase in Intestinal Permeability Is Mediated by TAK-1 Activation of IKK and MLCK/MYLK Gene. Am J Pathol. 2019;189(4):797–812. doi: 10.1016/j.ajpath.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osna NA, Donohue TM, Jr, Kharbanda KK. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Re. 2017;38(2):147–161. [PMC free article] [PubMed] [Google Scholar]
- 76.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94(1):200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 77.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142(2):367–373. [PMC free article] [PubMed] [Google Scholar]
- 78.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gurwara S, Dai A, Ajami NJ, et al. Alcohol use alters the colonic mucosa-associated gut microbiota in humans. Nutr Res. 2020;83:119–128. doi: 10.1016/j.nutres.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lachar J, Bajaj JS. Changes in the Microbiome in Cirrhosis and Relationship to Complications: Hepatic Encephalopathy, Spontaneous Bacterial Peritonitis, and Sepsis. Semin Liver Dis. 2016;36(4):327–330. doi: 10.1055/s-0036-1593881. [DOI] [PubMed] [Google Scholar]
- 82.Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65(5):830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 83.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9(5):e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Staun-Olsen P, Bjorneboe M, Prytz H, Thomsen AC, Orskov F. Escherichia coli antibodies in alcoholic liver disease. Correlation to alcohol consumption, alcoholic hepatitis, and serum IgA. Scand J Gastroenterol. 1983;18(7):889–896. doi: 10.3109/00365528309182111. [DOI] [PubMed] [Google Scholar]
- 85.Fujimoto M, Uemura M, Nakatani Y, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24(4 Suppl):48S–54S. doi: 10.1111/j.1530-0277.2000.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 86.Gimenez-Gomez P, Perez-Hernandez M, O'Shea E, et al. Changes in brain kynurenine levels via gut microbiota and gut-barrier disruption induced by chronic ethanol exposure in mice. FASEB J. 2019;33(11):12900–12914. doi: 10.1096/fj.201900491RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32(2):355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 88.Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22(8):1724–1730. doi: 10.1111/j.1530-0277.1998.tb03972.x. [DOI] [PubMed] [Google Scholar]
- 89.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280(6):G1280–1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 90.Kakiyama G, Hylemon PB, Zhou H, et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306(11):G929–937. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie G, Zhong W, Li H, et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013;27(9):3583–3593. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859. [PubMed] [Google Scholar]
- 93.Kelly CR, Khoruts A, Staley C, et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann Intern Med. 2016;165(9):609–616. doi: 10.7326/M16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou D, Pan Q, Shen F, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7(1):1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferrere G, Wrzosek L, Cailleux F, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66(4):806–815. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 96.Philips CA, Pande A, Shasthry SM, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15(4):600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 97.Meighani A, Alimirah M, Ramesh M, Salgia R. Fecal Microbiota Transplantation for Clostridioides Difficile Infection in Patients with Chronic Liver Disease. Int J Hepatol. 2020;2020:1874570. doi: 10.1155/2020/1874570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Indian Council of Medical Research Task F. Co-ordinating Unit I. Co-ordinating Unit DBT ICMR-DBT guidelines for evaluation of probiotics in food. Indian J Med Res. 2011;134:22–25. [PMC free article] [PubMed] [Google Scholar]
- 99.Zielinska D, Kolozyn-Krajewska D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. Biomed Res Int. 2018;2018:5063185. doi: 10.1155/2018/5063185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol. 2011;89(5):685–695. doi: 10.1189/jlb.1109753. [DOI] [PubMed] [Google Scholar]
- 101.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 102.Hedin C, Whelan K, Lindsay JO. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. Proc Nutr Soc. 2007;66(3):307–315. doi: 10.1017/S0029665107005563. [DOI] [PubMed] [Google Scholar]
- 103.Morkl S, Butler MI, Holl A, Cryan JF, Dinan TG. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr Nutr Rep. 2020;9(3):171–182. doi: 10.1007/s13668-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park DY, Ahn YT, Huh CS, McGregor RA, Choi MS. Dual probiotic strains suppress high fructose-induced metabolic syndrome. World J Gastroenterol. 2013;19(2):274–283. doi: 10.3748/wjg.v19.i2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Plaza-Diaz J, Gomez-Llorente C, Abadia-Molina F, et al. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS One. 2014;9(5):e98401. doi: 10.1371/journal.pone.0098401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang W, Shi LP, Shi L, Xu L. Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease. Zhonghua Nei Ke Za Zhi. 2018;57(2):101–106. doi: 10.3760/cma.j.issn.0578-1426.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 107.Cai GS, Su H, Zhang J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine (Baltimore) 2020;99(32):e21464. doi: 10.1097/MD.0000000000021464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J Pediatr Gastroenterol Nutr. 2017;64(3):413–417. doi: 10.1097/MPG.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 109.Tenorio-Jimenez C, Martinez-Ramirez MJ, Tercero-Lozano M, et al. Evaluation of the effect of Lactobacillus reuteri V3401 on biomarkers of inflammation, cardiovascular risk and liver steatosis in obese adults with metabolic syndrome: a randomized clinical trial (PROSIR) BMC Complement Altern Med. 2018;18(1):306. doi: 10.1186/s12906-018-2371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirpich IA, Solovieva NV, Leikhter SN, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42(8):675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X, Liu Y, Guo X, Ma Y, Zhang H, Liang H. Effect of Lactobacillus casei on lipid metabolism and intestinal.microflora in patients with alcoholic liver injury. Eur J Clin Nutr. 2021;75(8):1227-1236. 10.1007/s11605-021-0518 [DOI] [PMC free article] [PubMed]
- 112.Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect. 2014;44(1):1–8. doi: 10.1016/j.medmal.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Pineiro M, Asp NG, Reid G, et al. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S156–159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 114.Guarino MPL, Altomare A, Emerenziani S, et al. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients. 2020;12(4):1037. doi: 10.3390/nu12041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kolida S, Gibson GR. Synbiotics in health and disease. Annu Rev Food Sci Technol. 2011;2:373–393. doi: 10.1146/annurev-food-022510-133739. [DOI] [PubMed] [Google Scholar]
- 116.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12(5):303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- 118.Costabile A, Deaville ER, Morales AM, Gibson GR. Prebiotic Potential of a Maize-Based Soluble Fibre and Impact of Dose on the Human Gut Microbiota. PLoS One. 2016;11(1):e0144457. doi: 10.1371/journal.pone.0144457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ramnani P, Costabile A, Bustillo AG, Gibson GR. A randomised, double- blind, cross-over study investigating the prebiotic effect of agave fructans in healthy human subjects. J Nutr Sci. 2015;4:e10. doi: 10.1017/jns.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tandon D, Haque MM, Gote M, et al. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci Rep. 2019;9(1):5473. doi: 10.1038/s41598-019-41837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olas B. Probiotics, Prebiotics and Synbiotics-A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int J Mol Sci. 2020;21(24):9737. doi: 10.3390/ijms21249737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nicolucci AC, Hume MP, Martinez I, Mayengbam S, Walter J, Reimer RA. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology. 2017;153(3):711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 123.Bao T, He F, Zhang X, et al. Inulin Exerts Beneficial Effects on Non-Alcoholic Fatty Liver Disease via Modulating gut Microbiome and Suppressing the Lipopolysaccharide-Toll-Like Receptor 4-Mpsi-Nuclear Factor-kappaB-Nod-Like Receptor Protein 3 Pathway via gut-Liver Axis in Mice. Front Pharmacol. 2020;11:558525. doi: 10.3389/fphar.2020.558525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bao T, Wang Z, Zhu L, et al. Inulin increases the proportion of monocytic myeloid-derived suppressor cells in peripheral blood, liver, spleen and regulates the secretion of plasma inflammatory cytokines in mice with non-alcoholic fatty liver disease. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36(3):228–235. [PubMed] [Google Scholar]
- 125.Chambers ES, Byrne CS, Rugyendo A, et al. The effects of dietary supplementation with inulin and inulin-propionate ester on hepatic steatosis in adults with non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21(2):372–376. doi: 10.1111/dom.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Macfarlane GT, Cummings JH. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ. 1999;318(7189):999–1003. doi: 10.1136/bmj.318.7189.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Malaguarnera M, Greco F, Barone G, Gargante MP, Malaguarnera M, Toscano MA. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 2007;52(11):3259–3265. doi: 10.1007/s10620-006-9687-y. [DOI] [PubMed] [Google Scholar]
- 128.Liu L, Li P, Liu Y, Zhang Y. Efficacy of Probiotics and Synbiotics in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Dig Dis Sci. 2019;64(12):3402–3412. doi: 10.1007/s10620-019-05699-z. [DOI] [PubMed] [Google Scholar]
- 129.Roychowdhury S, Glueck B, Han Y, Mohammad MA, Cresci GAM. A Designer Synbiotic Attenuates Chronic-Binge Ethanol-Induced Gut-Liver Injury in Mice. Nutrients. 2019;11(1):97. doi: 10.3390/nu11010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han Y, Glueck B, Shapiro D, Miller A, Roychowdhury S, Cresci GAM. Dietary Synbiotic Supplementation Protects Barrier Integrity of Hepatocytes and Liver Sinusoidal Endothelium in a Mouse Model of Chronic-Binge Ethanol Exposure. Nutrients. 2020;12(2):373. [DOI] [PMC free article] [PubMed]
- 131.Sittiprapaporn P, Sirilun S, Chaiyasut C, et al. The effect of synbiotics supplement on alcohol use disorders identification test and biochemical parameters, gamma glutamyl transferase, lipopolysaccharide and immunoglobulin a levels, in high risk alcoholics. Asian Journal of Medical Sciences. 2020;11(1):1–6. doi: 10.3126/ajms.v11i1.26497. [DOI] [Google Scholar]
- 132.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39(5):1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 133.Kühn F, Duan R, Ilmer M, et al. Targeting the Intestinal Barrier to Prevent Gut-Derived Inflammation and Disease: A Role for Intestinal Alkaline Phosphatase. Visceral Medicine. 2021;5(6):1–11. doi: 10.1159/000515910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eliakim R, Mahmood A, Alpers DH. Rat intestinal alkaline phosphatase secretion into lumen and serum is coordinately regulated. Biochim Biophys Acta. 1991;1091(1):1–8. doi: 10.1016/0167-4889(91)90213-H. [DOI] [PubMed] [Google Scholar]
- 135.Moss AK, Hamarneh SR, Mohamed MM, et al. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am J Physiol Gastrointest Liver Physiol. 2013;304(6):G597–604. doi: 10.1152/ajpgi.00455.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Molnar K, Vannay A, Szebeni B, et al. Intestinal alkaline phosphatase in the colonic mucosa of children with inflammatory bowel disease. World J Gastroenterol. 2012;18(25):3254–3259. doi: 10.3748/wjg.v18.i25.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tuin A, Poelstra K, de Jager-Krikken A, et al. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58(3):379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 138.Ramasamy S, Nguyen DD, Eston MA, et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm Bowel Dis. 2011;17(2):532–542. doi: 10.1002/ibd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heemskerk S, Masereeuw R, Moesker O, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med. 2009;37(2):417–423, e411. [DOI] [PubMed]
- 140.Kats S, Brands R, Seinen W, et al. Anti-inflammatory effects of alkaline phosphatase in coronary artery bypass surgery with cardiopulmonary bypass. Recent Pat Inflamm Allergy Drug Discov. 2009;3(3):214–220. doi: 10.2174/187221309789257388. [DOI] [PubMed] [Google Scholar]
- 141.Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol. 2009;587(Pt 14):3651–3663. doi: 10.1113/jphysiol.2009.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu W, Hu D, Huo H, et al. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J Am Coll Surg. 2016;222(6):1009–1017. doi: 10.1016/j.jamcollsurg.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hamarneh SR, Mohamed MMR, Economopoulos KP, et al. A Novel Approach to Maintain Gut Mucosal Integrity Using an Oral Enzyme Supplement. Annals of Surgery. 2014;260(4):706–715. doi: 10.1097/SLA.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Malo MS, Alam SN, Mostafa G, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59(11):1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 145.Kühn F, Adiliaghdam F, Cavallaro PM, et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight. 2020;5(6):e134049. [DOI] [PMC free article] [PubMed]
- 146.Liu Y, Cavallaro PM, Kim B-M, et al. A role for intestinal alkaline phosphatase in preventing liver fibrosis. Theranostics. 2021;11(1):14–26. doi: 10.7150/thno.48468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hamarneh SR, Kim BM, Kaliannan K, et al. Intestinal Alkaline Phosphatase Attenuates Alcohol-Induced Hepatosteatosis in Mice. Dig Dis Sci. 2017;62(8):2021–2034. doi: 10.1007/s10620-017-4576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]