Abstract
Background
Magnetic sphincter augmentation (MSA) is a modern surgical anti-reflux technique with proven efficacy and low postoperative morbidity in patients with acidic reflux. The aim of this retrospective review study was to evaluate the symptomatic outcome of MSA in patients with weakly acidic reflux.
Methods
From a prospectively collected clinical database, comprising all 327 patients that underwent MSA at our institution, a total of 67 patients with preoperative weakly acidic reflux measured in the 24-h impedance-pH-metry were identified. Postoperative gastrointestinal symptoms, proton pump inhibitor intake (PPI), GERD Health-Related Quality-of-Life (GERD-HRQL), alimentary satisfaction (AS), and patients’ overall satisfaction were evaluated within highly standardized follow-up appointments. Furthermore, outcome of these patients was compared to the postoperative outcome of a comparable group of patients with a preoperative acidic reflux.
Results
At a median follow-up of 24 months, none of the patients with weakly acidic reflux presented with persistent dysphagia, or underwent endoscopic dilatation or reoperation. The postoperative GERD-HRQL score was significantly reduced (2 vs. 20; p = 0.001) and the median AS was 9/10. Preoperative daily heartburn, regurgitations, and respiratory complaints were improved in 95%, 95%, and 96% of patients, respectively. A total of 10% of the patients continued to use PPIs postoperatively. No significant difference was observed in terms of postoperative outcome or quality of life when comparing weakly acidic reflux patients with those diagnosed with preoperative acidic reflux.
Conclusion
Magnetic sphincter augmentation significantly improves GERD-related symptoms and quality of life in patients with weakly acidic reflux with very low postoperative morbidity.
Keywords: GERD, Magnetic sphincter augmentation, Weakly acid, Hiatal hernia
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common upper gastrointestinal (GI) tract disorders with a worldwide high prevalence and yearly increasing incidence rates.1–3 Patients not only suffer from a variety of burdensome symptoms that lead to a reduced quality of life, but also are at an increased risk of developing Barrett’s esophagus (BE) and esophageal adenocarcinoma.4–9 The combination of clinical evaluation, upper GI endoscopy, and esophageal function testing, including high-resolution manometry as well as 24-h impedance-pH-metry, is applied to diagnose GERD.10,11 Depending on the measured pH in the esophagus, GERD can be divided into acid (pH < 4) or weakly acidic or non-acidic (pH ≥ 4) reflux, both of which cause GERD symptoms and most commonly occur together.7,12–15 First-line treatment consists of lifestyle changes and pharmacotherapy with proton pump inhibitors (PPIs).16 Suppressing acid production and increasing the pH of the gastric refluxate PPIs effectively eliminate symptoms in approximately 60% of GERD patients. However, medical treatment does not improve the dysfunctional lower esophageal sphincter (LES) and still allows for non-acidic reflux to occur.17 Furthermore, not only has mixed acidic and bile reflux been associated with the most severe mucosal injury and deterioration of esophageal function,18 but also medical treatment of the acid component has shown to provide only short-term symptom control in patients and not long-term protection of developing BE. The biliary component by itself can cause BE and its degeneration to adenocarcinoma.19,20
The gold standard of anti-reflux surgery—the laparoscopic fundoplication (LF) —was shown to have long-term efficacy and safety.21–26 Nevertheless, GERD surgery rates have been decreasing in the third millennium, likely due to fear of possible adverse effects, such as dysphagia and gas bloat syndrome.27–30 Magnetic sphincter augmentation (MSA) represents an alternative, less invasive surgical option, developed in an effort to decrease side effects associated with LF, while still achieving effective symptom control.17,22–24,31–33 The MSA device (LINX® Reflux Management System; Torax Medical, Maple Grove, MN) consists of magnetic beads connected by a flexible titanium ring, which is placed around the LES, meaning to enhance its barrier function and prevent reflux but allow for physiological bolus transport.34 Studies have shown the LINX® implantation not only to be as safe and effective as the Nissen fundoplication, but also superior in terms of postoperative gas bloating and increased ability to belch/vomit.17,31–33,35,36 Due to the novelty of this procedure, long-term outcome studies of more than 5 years are still needed. Furthermore, one of the questions still unanswered is whether MSA has a comparably good outcome in patients suffering from weakly acidic or non-acidic reflux. Non-acidic reflux is known to cause PPI refractory symptoms in the absence of acid exposure, which makes conservative treatment challenging and underlines the importance of surgical anti-reflux therapy.
The aim of this study was to evaluate the long-term postoperative outcomes of patients with preoperative weakly acidic/biliary reflux undergoing MSA in a high-volume specialized reflux center.
Methods
Preoperative Assessment
All patients received a standardized interview, clinical examination, an upper GI endoscopy, a video esophagogram, and esophageal functioning tests consistent of a high-resolution manometry and a 24-h impedance-pH-metry. Patients with the following characteristics were included in our study:
Acidic pH < 4 percentage time > 4.2% in the 24-h impedance-pH-metry and/or
Increased total reflux episodes > 40 in the 24-h impedance-pH-metry and/or
Typical symptoms responsive to PPIs.
Hiatal hernias (HH) were diagnosed with high precision using both upper GI endoscopy and high-resolution manometry. During EGD, a HH was considered if the level of the rise of the endoscopically visible rugal folds dislocated ≥ 1.0 cm above the level of diaphragmatic impression. Weakly acidic reflux was diagnosed in the 24-h impedance-pH-metry by a reduced total acidic (pH < 4) percentage time (< 4.2%) and an increased number of total reflux episodes (> 40). All patients needed to discontinue their PPI therapy 14 days prior undergoing the 24-h impedance-pH-metry.
Surgery
All procedures were performed by the same specialized upper gastrointestinal surgical team. The surgical approach was laparoscopic in all cases. All procedures were standardized regarding the surgeon’s and patient’s positions (anti-Trendelenburg), trocar sites, and instruments used. A hiatoplasty was performed in 81% of patients. Since the year 2014, hiatoplasty was performed in all patients in principle. These procedures were conducted by hiatal dissections and crural closures with 2–5 stitches using non-absorbable sutures. All cases were performed without the use of an esophageal bougie.
Magnetic Sphincter Augmentation
MSA was performed as previously described:17 briefly after the mobilization of the esophagogastric junction and identifying the vagal nerve and excluding it, the adequate ring size was measured with the sizing tool and the magnetic ring was wrapped around the lower end of the lower esophageal sphincter.
Sizing of the Device
The sizing tool was placed around the esophagus without applying any tension or compression. It was then closed until it popped off. To make sure that the esophagus was not squeezed, the sizing tool was wiggled. If measurement yielded 10 to 12 beads, then we added 3 beads. If measurement yielded 13, we added 3 or 2, depending when squeezing by the sizing tool occurred. If measurement yielded 14 or 15, we added 2, if 17 beads were available. If measurement yielded 16, we used this size if no squeezing by the sizing tool occurred. If measurement yielded 16 and squeezing by the sizing tool occurred, the procedure would not be performed.
Postoperative Care
Postoperatively, all patients received an unrestricted diet, putting an emphasis on regular intake of foods every 2 h, to avoid the development of dysphagia due to formation of scar tissue surrounding the device.
On the first postoperative day, a contrast swallow with iopamidol was performed in all patients. When showing no abnormality, patients were discharged on the first postoperative day per hospital protocol.
Postoperative Assessment
The median follow-up time was 24 months (IQR, 10 – 41). Long-term follow-up was performed by the same physician using a standardized interview that assessed postoperative gastrointestinal symptoms, proton pump inhibitor intake, and GERD Health-related Quality-of-Life score (GERD-HRQL). The frequency and severity of postoperative dysphagia were assessed using the classification of Saeed et al., where the ability to swallow can be scored from 0 to 5, where 0 implies the inability to swallow and 5 indicates normal swallowing.37
Adverse effects such as complications, hospital readmission, emergency surgery, or elective re-operation were documented. Patients with recurrent symptoms received upper GI endoscopy as well as esophageal functioning tests in selected patients.
Postoperative outcome including symptom relief, PPI intake, dysphagia rates, and quality of life was additionally compared between patients with acidic reflux and those with weakly acidic reflux.
Statistical Analysis
Statistical analysis was performed using SPSS® statistics 20.0 (IBM, Armonk, NY). The data was described using median (interquartile range) or mean (range). Statistical analysis appropriate for non-parametric data was used. Categorical variables were assessed using the Fisher exact test and continuous data using the Wilcoxon rank test as appropriate. Statistical significance was defined as a p-value < 0.05.
This study (2293/2017) was approved by the Institutional Review Board of the Medical University of Vienna, Austria. Methods were carried out in accordance with relevant guidelines and regulations.
Results
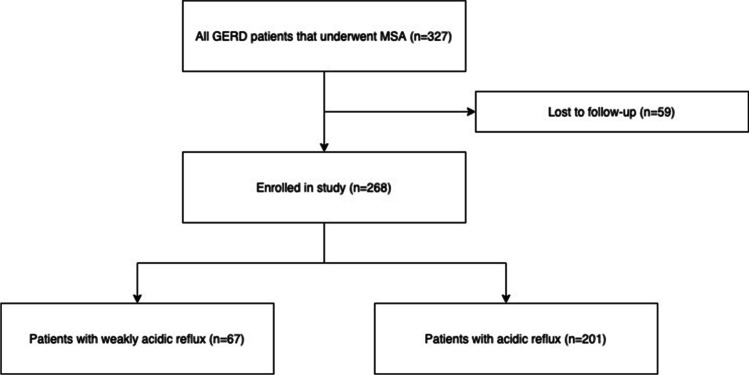
A total of 327 patients underwent MSA for chronic gastroesophageal reflux disease in a period of 8 years (2012–2020) in our specialized upper gastrointestinal surgery center. Fifty-nine patients were lost to follow-up, leaving a total number of 268 (100%) patients in our study. Finally, a total of 67 (31 female and 36 male) out of 268 of the individuals were found to have weakly acidic reflux, while 201 (64 female and 137 male) out of 268 individuals revealed acidic reflux in the preoperative 24-h impedance-pH-metry. A study flowchart is shown in Fig. 1.
Fig. 1.
Study flowchart showing all eligible and included patients
Weakly Acidic Reflux Patients (n = 67)
The median age was 44 years (IQR, 19, range 23 – 67) and the median preoperative BMI was 25 (IQR, 4.1). Demographics and preoperative findings are shown in Table 1.
Table 1.
Demographic data and results of preoperative diagnostics of patients with weakly acidic and acidic reflux
| Weakly acidic reflux patients | Acidic reflux patients | ||
|---|---|---|---|
| Total number | n = 67 (25%) | n = 201 (75%) | |
| Sex (M vs. F) | 36 (54%) vs. 31 (46%) | 137 (68%) vs. 64 (32%) | p = 0.03 |
| Median age (IQR) | 44 (19) | 51 (21) | p = 0.09 |
| Median BMI (IQR) | 25 (4.1) | 25.5 (5.5) | p = 0.122 |
| HH present | 57 (85%) | 174 (87%) | p = 0.584 |
| HH > 3 cm | 19 (28%) | 43 (21%) | p = 0.409 |
| Median total pH < 4% (IQR) | 2.2 (1.125) % | 7.5 (10.6) % | p = 0.00 |
| Median total reflux episodes (IQR) | 68 (31.75) | 60 (44.5) | p = 0.292 |
| Median LES resting pressure (IQR) | 20 (16.45) mmHg | 16.9 (13.725) mmHg | p = 0.059 |
| Median IRP (IQR) | 9 (7.75) | 9 (6.5) | p = 0.465 |
| Presence of IEM | 6 (9%) | 12 (6%) | p = 0.403 |
| Presence of BE | 7 (10%) | 22 (11%) | p = 0.801 |
| Use of PPIs | 60 (90%) | 175 (87%) | p = 0.507 |
Data were obtained and statistics applied, as described in “Methods” Abbreviations: HH hiatal hernia, LES lower esophageal sphincter, IRP integrated relaxation pressure, IEM ineffective esophageal motility, BE Barrett’s esophagus
Preoperative Symptoms
The three most common typical and atypical GERD-associated preoperative symptoms in our patients were heartburn (n = 60/67, 90%), regurgitations (n = 31/67, 46%), and respiratory symptoms (n = 22/67, 32%). A total of 60 out of 67 (90%) of the patients reported the use of PPIs prior to surgery.
Surgery
The median OR time was 30 min (range, 9 – 52). The surgical approach was laparoscopic in all patients. No perioperative complications were seen. The median MSA device size implanted was 14 (range, 12 – 16). Fifty-two out of 67 (81%) individuals received additional crural closure. The median hospital stay was 1 day (IQR, 1).
Postoperative Symptom Control
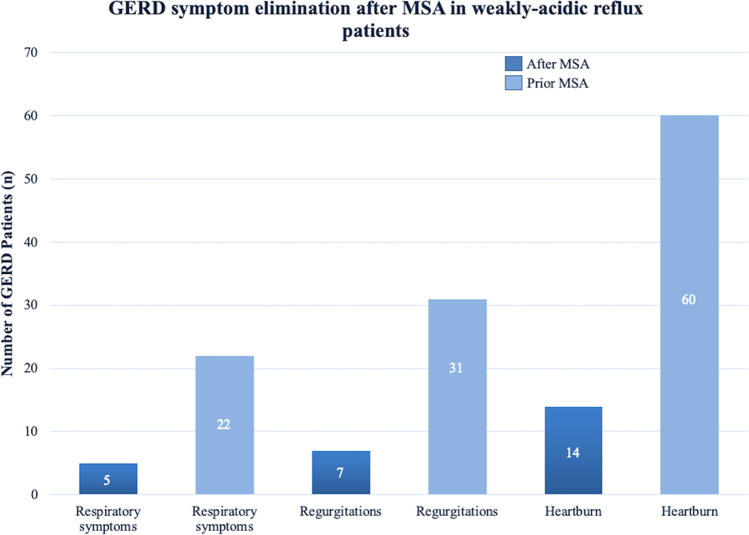
The median follow-up time was 24 months (IQR, 31). Heartburn, regurgitations, and respiratory symptoms were fully eliminated in 46 out of 60 (77%, p = 0.0001), 24 out of 31 (78%, p = 0.0001), and 17 out of 22 (77%, p = 0.0001) of the patients and improved in 56 out of 60 (93%, p = 0.0001), 29 out of 31 (94%, p = 0.0001), and 20 out of 22 (91%, p = 0.0001) patients, respectively. A graphic comparison of the three most reported symptoms before and after MSA is shown in Fig. 2. Only 7 out of 67 (10%, p = 0.0001) patients reported a need for use of PPIs postoperatively.
Fig. 2.
Comparison of GERD-related symptoms before and after MSA in weakly acidic reflux patients
Postoperative Adverse Effects
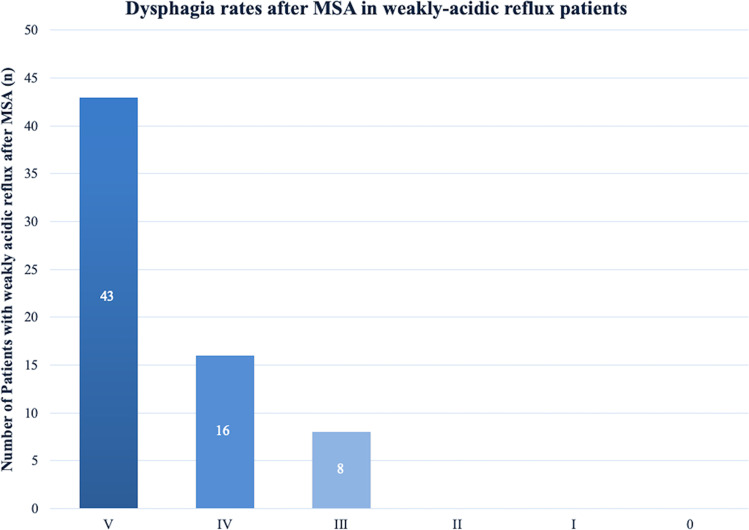
After MSA, a total of 43 out of 67 (64%) individuals reported absolutely no difficulty swallowing with solids or liquids. Rarely difficulties swallowing with solids only was reported by 16 out of 67 (24%) the patients, while 8 out of 67 (12%) patients had occasional difficulties swallowing with solids. Finally permanent dysphagia, defined as not being able to swallow solids or/and liquids, was not seen in any of the patients at time of follow-up. A graphic depiction of postoperative dysphagia is shown in Fig. 3.
Fig. 3.
Frequency and degree of postoperative dysphagia in weakly acidic patients based on the classification of Saeed et al. Columns from up to down: 0 = Unable to swallow (0). I = Swallowing liquids with difficulty, solids impossible (0). II = Swallowing liquids without difficulty, solids impossible (0). III = Occasionally difficulty swallowing with solids (8). IV = Rarely difficulty swallowing with solids (16). V = Swallowing normally (43)
A total of 55 out of 67 (82%) of the patients retained their ability to belch/vomit and only 5 out of 67 (8%) complained about increased daily gas bloating.
None of the individuals needed revision surgery or balloon dilatations. No erosion or migration of the device was seen. Postoperative outcomes are shown in Table 2.
Table 2.
Comparison of postoperative outcome measures in weakly acidic reflux vs. acidic reflux patients after magnetic sphincter augmentation (MSA)
| Weakly acidic reflux patients | Acidic reflux patients | ||
|---|---|---|---|
| Total n = 268 (100%) | n = 67 (25%) | n = 201 (75%) | |
| Persistent dysphagia | 0 | 0 | |
| Endoscopic dilatation | 0 | 3 (1.5%) | p = 0.574 |
| Reoperation | 0 | 6 (3%) | p = 0.198 |
| Device removal | 0 | 2 (1%) | |
| Re-hiatoplasty | 0 | 4 (2%) | |
| Gas bloating syndrom | 5 (7%) | 11 (6%) | p = 0.556 |
| Ability to belch/vomit | 55 (82%) | 167 (83%) | p = 0.727 |
| Median total GERD-HRQL score | 2 (5) | 2 (4) | p = 0.787 |
| Median AS | 9 (2) | 9 (2) | p = 0.855 |
| Use of PPIs | 7 (10%) | 18 (9%) | p = 0.808 |
Data were obtained and statistics applied, as described in “Methods” Abbreviations: AS alimentary satisfaction
Quality of Life
Prior to surgery, 31 out of 67 (46%) patients had completed the GERD-HRQL score. The preoperative median GERD-HRQL score was 20 (IQR, 16, range 5–36). MSA led to a significant reduction of the GERD-HRQL total score (2 vs. 20, p = 0.001). Moreover, the median alimentary satisfaction (AS) of the 67 patients was rated 9 (IQR, 2). When asked if they would be willing to undergo the same surgery, in the same circumstances, 53 out of 67 (80%) patients said yes. Quality-of-Life results are presented in Table 2.
Acidic Reflux Patients (n = 201)
The median age was 51 years (IQR, 21, range 19 – 81) and the median preoperative BMI was 25.6 (IQR, 5.5). Further demographics and preoperative findings are also shown in Table 1.
Preoperative Symptoms
The three most common typical and atypical GERD-associated preoperative symptoms in our patients were heartburn (n = 181/201, 90%), regurgitations (n = 107/201, 53%), and respiratory symptoms (n = 75/201, 37%). A total of 175 out of 201 (87%) patients reported the use of PPIs prior to surgery.
Surgery
The median OR time was 30 min (range, 10 – 95). The surgical approach was laparoscopic in all patients. No perioperative complications were seen. The median MSA device size implanted was 15 (range, 12 – 16). A total of 164 out of 201 (81%) individuals received additional crural closure. The median hospital stay was 1 day (IQR, 1).
Postoperative Symptom Control
The median follow-up time was 22 months (IQR, 24). Heartburn, regurgitations, and chronic cough were fully eliminated in 148 out of 181 (81%, p = 0.0001), 88 out of 107 (82%, p = 0.0001), and 61out of 75 (81%, p = 0.0001) patients and improved in 172 out of 181 (95%, p = 0.0001), 102 out of 107 (95%, p = 0.0001), and 72 out of 75 (96%, p = 0.0001) patients, respectively. Only 18 out of 201 (9%, p = 0.0001) individuals reported a need for use of PPIs postoperatively.
Postoperative Adverse Effects
After MSA, a total of 127 out of 201 (63%) patients reported absolutely no difficulty swallowing with solids or liquids. Rarely difficulties in swallowing with solids only was reported by 52 out of 201 (26%) of the patients, while 22 out of 201 (11%) patients had occasional difficulties swallowing with solids. Finally, permanent dysphagia was not seen in any of the individuals at time of follow-up.
A whole of 167 out of 201 (83%) of the patients retained their ability to belch/vomit and only 11 out of 201 (5%) complained about increased daily gas bloating.
In 3 out of 201 (1%) patients, dysphagia was successfully managed by endoscopic balloon dilatation. Six out of 201 (3%) individuals needed revision surgery: Two of the patients underwent explant of the MSA device due to unclear pain in the chest area, while the other four patients developed paraesophageal herniation of the gastric fundus and underwent re-hiatoplasty. No erosion or migration of the device was seen. Postoperative outcomes are shown in Table 2.
Quality of Life
Prior to surgery, 102 out of 201 (51%) patients had completed the GERD-HRQL score. The preoperative median GERD-HRQL was 19 (IQR, 13, range, 2 – 39). MSA led to a significant reduction of the GERD-HRQL total score (2 vs. 29, p = 0.001). Also, the median alimentary satisfaction (AS) of all 201 patients was rated 9 (IQR, 2). When asked if they would be willing to undergo the same surgery, in the same circumstances, 162 out of 201 (81%) individuals said yes. Quality of life results are presented in Table 2.
Comparison Between Weakly Acidic and Acidic Reflux
As abovementioned, we observed 67 out of 268 (25%) patients with weakly acidic reflux and 201 out of 268 (75%) patients with acidic reflux measured in the 24-h impedance-pH-metry prior to MSA. As demonstrated in Table 1, no statistically significant difference was seen between the groups other than the preoperative acid exposure.
Discussion
MSA represents a novel surgical technique, FDA approved for the treatment of GERD in 2012.34 Over the years, multiple studies have demonstrated the safety and long-term effectiveness of the procedure, making it a highly standardized technique in anti-reflux surgery.38 Nevertheless, patients included in these studies had to adhere to strict inclusion criteria such as increased exposure to esophageal acid in the 24-h pH-metry, not having a large hiatal hernia, an esophagitis LA grade C or D, or dysphagia more than 3 times a week,29 thus leaving out patients with weakly acidic or non-acidic reflux. The importance of such a surgical definite GERD treatment rather than a symptomatic medical treatment lies precisely in the possible prevention of the development of long-term complications through acidic as well as non-acidic or mixed reflux. The aim of this study was to analyze the postoperative outcome of GERD patients with preoperative weakly acidic or non-acidic reflux measured in the 24-h impedance-pH-metry that underwent MSA in our highly specialized reflux center. Moreover, we compared the outcome between patients with weakly acidic reflux and those with acidic reflux.
As mentioned above, we defined weakly acidic reflux by a reduced total acidic (pH < 4) and percentage time (< 4.2%) and an increased number of total reflux episodes (> 40) in the 24-h impedance-pH-metry. This definition was based on the Lyon consensus, proposing that a total acidic (pH < 4) percentage time less than 4.2% and a total number of reflux episodes less than 40 are definitely physiological, and a total acidic (pH < 4) percentage time more than 6% and a total number of reflux episodes more than 80 are definitely pathological, while all the values in between are inconclusive. As the inconclusive values could possibly be pathological, but definitely not physiological, we chose the lower cut-off points.
As Campos et al. showed in his multivariate analyses in 1999, the three most predictive factors for a successful outcome of a Nissen fundoplication were an abnormal 24-h pH score, a typical primary symptom, and symptoms responsive to PPI therapy.39 We expanded our inclusion criteria to also include patients with an increased number of reflux episodes, as it can be a sign of non-acidic reflux and such patients could potentially profit from an anti-reflux operation, possibly less invasive than a Nissen fundoplication.
Regarding symptom control, our study showed promising results in patients with weakly acidic reflux: improvement of daily heartburn, regurgitations, and respiratory complaints were noted in 93% (p = 0.0001), 94% (p = 0.0001), and 91% (p = 0.0001) of the patients, respectively. These results are in line with the outcomes of patients with acidic reflux described: Louie et al. reported a relief of heartburn and regurgitations in 93.9% and 100%, respectively, after MSA.40 Furthermore, in a multicenter prospective observational study, we reported a reduction of sleep-awaking heartburn from 30.2 to 3.5%, moderate to severe regurgitations from 58.3 to 3.1%, and extraesophageal symptoms from 63.9 to 22% at 1-year follow-up.41 Concerning postoperative pharmacotherapy, here we show that 88% (p = 0.0001) of the patients did not need to use PPIs anymore, which is also in concordance with previously published articles.17,41,42 When comparing the improvement of the three most common symptoms (heartburn 93% vs. 95%, p = 0.615; regurgitations 94% vs. 95%, p = 0.723; and respiratory complaints 91% vs. 96%, p = 0.373), we found no difference between patients with preoperative weakly acidic and acidic reflux. Also, no significant difference was observed in the postoperative use of PPIs between patients with the two types of reflux (weakly acidic 7 vs. acid 18, p = 0.808). These findings show that patients with weakly acidic reflux also benefit from MSA, as the main goal of anti-reflux therapy is symptom control and mucosal healing.
At follow-up, 82% of our patients were able to belch/vomit (weakly acidic 55 vs. acidic 167, p = 0.727) and only 8% complained about daily gas bloating (weakly acidic 5 vs. acidic 11, p = 0.555) with no difference between our two groups. Most recently, Bonavina et al. reported a significant difference in excessive gas bloating and the ability to vomit between MSA and LF patients, specifically excessive gas bloating was reported in 10% of MSA patients, compared to 31% of LF patients, while 91% of MSA patients retained their ability to vomit if needed, compared to only 44.4% of LF patients.42
Persistent dysphagia, the most feared complication after anti-reflux surgery did not occur in our cohort, neither in patients with acidic nor weakly acidic reflux, at time of follow-up. However, rarely difficulties swallowing with solids was reported by 16 out of 67 (24%) patients, while 8 out of 67 (12%) patients had occasional difficulties swallowing with solids. This outcome is comparable to previous studies. Bonavina et al. reported one patient needing removal of the device due to postoperative dysphagia, while Ganz et al. found that 6% of the patients had bothersome dysphagia 5 years after MSA in a prospective study of 100 patients.43,44 According to current medical literature on anti-reflux surgery, patients after MSA commonly suffer from early postoperative dysphagia that resolves after 8 weeks, compared to patients after LF who later suffer from dysphagia.38 This can be explained by the different dietary regimes recommended postoperatively, namely a liquid/soft diet for the first 2 weeks with a gradual transition to solid foods in patients undergoing LF, compared to a solid unrestricted diet after MSA. This prevents forming of scaring tissue around the closed magnetic ring. Rarely, in 1.5–5.6% of patients, this bothersome symptom persists, and endoscopic dilatation is needed.32,42,45 None of our patients with weakly acidic reflux underwent endoscopic dilatation, device removal, or revisional surgery; however, the small sample size has to be taken into consideration. A safety profile analysis of the first thousand conducted MSAs showed a re-operation rate of 3.4%, all patients undergoing device removal due to dysphagia and reoccurrence of reflux symptoms.45 Also, a single-center cohort study, focusing on device removal after MSA, found 6.7% of patients undergoing non-emergent reoperation with removal of the MSA device, due to, most commonly, reoccurrence of heartburn or regurgitations. Furthermore, they observed two cases of full thickness erosion of the esophageal wall with partial endoluminal penetration.46 We found no difference in the postoperative rate of dilatation (weakly acidic 0 vs. 3, p = 0.574) or surgical revision (weakly acidic 0 vs. acid 6, p = 0.198) rate between patients with weakly acidic and acidic reflux, further showing the equally positive outcome in selected patients with a negative 24-h pH-metry.
The improved outcomes regarding symptom control are in line with the positive results regarding quality of life in our study. A significant drop in the median postoperative total GERD-HRQL score was seen in weakly acidic reflux patients after MSA (20 vs. 2, p = 0.001). This proves a substantial increase in quality of life. Moreover, the median alimentary satisfaction of our patients was 9, also showing an overall satisfaction in our cohort. These findings are in line with publications so far, all indicating that not only does the GERD-related quality of life improve after MSA, but overall patient satisfaction is high.41,42,47,48
Our study shows that patients with weakly acidic reflux, measured with a 24-h impedance-pH-metry, would profit from MSA, as this surgical technique is associated with a significant reduction in daily bothersome heartburn, regurgitations, and respiratory complaints, with favorably low morbidity and side effect rate as well as improvement in quality of life in patients. It also underlines the importance of a preoperative 24-h impedance-pH-metry where not only the total pH percentage time can be measured, as with the BRAVO capsule, but also a total number of reflux episodes as well as symptom correlation. Although the BRAVO capsule has shown to be more tolerable with comparable sensitivity to the 24-h impedance-pH-metry, reflux diagnostic centers should not fully give up on the 24-h wired catheter and patients tested negative with the wireless capsule should undergo further testing with an 24-h impedance-pH-metry.49,50 This method allows us to identify a high number of patients with negative total pH percentage time, but increased reflux episodes, exhibiting weakly acidic reflux, who would also potentially benefit from MSA.
Nevertheless, certain limitations of our study, such as the retrospective design, should be taken into consideration. Also, standardized objective postoperative testing with EFTs was not conducted due to the logistics of asymptomatic patients undergoing invasive diagnostic testing. Further prospective studies with larger sample sizes, as well as objective postoperative testing, would be of great value for further research.
Conclusion
MSA leads to a significant reduction in daily, bothersome GERD symptoms, with low postoperative morbidity and increase in GERD-related quality of life, as well as alimentary satisfaction in patients with weakly acidic reflux. Furthermore, the importance of a preoperative 24-h impedance-pH-metry in detecting patients with weakly acidic reflux, who would benefit from MSA, should not be underestimated.
Author Contribution
All the authors listed above contributed substantially to the conception or design of the work and the acquisition, analysis, or interpretation of data for the work; and all the authors contributed to the drafting of the work or revising it critically for important intellectual content and the final approval of the version to be published; and all the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Open access funding provided by Medical University of Vienna.
Declarations
Conflict of Interest
S F. Schoppmann received unrestricted grant from Thorax Medical. Nikolic M., Feka J., Matic A., Gensthaler L., Kristo I., Osmokrovic B., Martin R., and Mosleh B. have no conflict of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Danilova DA, Bazaev AV, Gorbunova LI. Current aspects of surgical treatment of gastro-esophageal reflux disease. Khirurgiia (Mosk) 2020;2:89–94. doi: 10.17116/hirurgia202002189. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus G: The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006, 101(8):1900–1920; quiz 1943. [DOI] [PubMed]
- 3.Bakhos CT, Abbas AE, Petrov RV. Tailoring Endoscopic and Surgical Treatments for Gastroesophageal Reflux Disease. Gastroenterol Clin North Am. 2020;49(3):467–480. doi: 10.1016/j.gtc.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Butt I, Kasmin F: Esophageal pH Monitoring. In: StatPearls. edn. Treasure Island (FL); 2020. [PubMed]
- 5.Moore M, Afaneh C, Benhuri D, Antonacci C, Abelson J, Zarnegar R. Gastroesophageal reflux disease: A review of surgical decision making. World J Gastrointest Surg. 2016;8(1):77–83. doi: 10.4240/wjgs.v8.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes MA, Herbella FAM. Pathophysiology of Gastroesophageal Reflux Disease. World J Surg. 2017;41(7):1666–1671. doi: 10.1007/s00268-017-3952-4. [DOI] [PubMed] [Google Scholar]
- 7.Zikos TA, Clarke JO. Non-acid Reflux: When It Matters and Approach to Management. Curr Gastroenterol Rep. 2020;22(9):43. doi: 10.1007/s11894-020-00780-4. [DOI] [PubMed] [Google Scholar]
- 8.Kurtom S, Kaplan BJ. Esophagus and Gastrointestinal Junction Tumors. Surg Clin North Am. 2020;100(3):507–521. doi: 10.1016/j.suc.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasoma P. New evidence defining the pathology and pathogenesis of lower esophageal sphincter damage. European Surgery. 2019;51(6):282–290. doi: 10.1007/s10353-019-00616-0. [DOI] [Google Scholar]
- 10.Talley NJ, Zand Irani M: Optimal management of severe symptomatic gastroesophageal reflux disease. J Intern Med 2020. [DOI] [PubMed]
- 11.Falk GL, Gooley SC, Church NG, Rangiah DS. How effective is the control of laryngopharyngeal reflux symptoms by fundoplication? Symptom score analysis. European Surgery. 2020;52(3):123–126. doi: 10.1007/s10353-020-00632-5. [DOI] [Google Scholar]
- 12.Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, Zhang X, Adhami T, Murray J, Peters J, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99(6):1037–1043. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 13.Zerbib F, des Varannes SB, Roman S, Pouderoux P, Artigue F, Chaput U, Mion F, Caillol F, Verin E, Bommelaer G et al: Normal values and day-to-day variability of 24-h ambulatory oesophageal impedance-pH monitoring in a Belgian-French cohort of healthy subjects. Aliment Pharmacol Ther 2005, 22(10):1011–1021. [DOI] [PubMed]
- 14.Smout AJ. Review article: The measurement of non-acid gastro-oesophageal reflux. Aliment Pharmacol Ther. 2007;26(Suppl 2):7–12. doi: 10.1111/j.1365-2036.2007.03527.x. [DOI] [PubMed] [Google Scholar]
- 15.Mainie I, Tutuian R, Shay S, Vela M, Zhang X, Sifrim D, Castell DO. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut. 2006;55(10):1398–1402. doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badillo R, Francis D. Diagnosis and treatment of gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5(3):105–112. doi: 10.4292/wjgpt.v5.i3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwameis K, Nikolic M, Morales Castellano DG, Steindl A, Macheck S, Kristo I, Zorner B, Schoppmann SF: Results of Magnetic Sphincter Augmentation for Gastroesophageal Reflux Disease. World J Surg 2018. [DOI] [PMC free article] [PubMed]
- 18.Oh DS, Hagen JA, Fein M, Bremner CG, Dunst CM, Demeester SR, Lipham J, Demeester TR: The impact of reflux composition on mucosal injury and esophageal function. J Gastrointest Surg 2006, 10(6):787–796; discussion 796–787. [DOI] [PubMed]
- 19.Bruzzi M, Chevallier JM, Czernichow S. One-Anastomosis Gastric Bypass: Why Biliary Reflux Remains Controversial? Obes Surg. 2017;27(2):545–547. doi: 10.1007/s11695-016-2480-x. [DOI] [PubMed] [Google Scholar]
- 20.Kahrilas PJ, Howden CW, Hughes N: Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011, 106(8):1419–1425; quiz 1426. [DOI] [PubMed]
- 21.Nikolic M, Schwameis K, Kristo I, Paireder M, Matic A, Semmler G, Semmler L, Schoppmann SF. Ineffective Esophageal Motility in Patients with GERD is no Contraindication for Nissen Fundoplication. World J Surg. 2020;44(1):186–193. doi: 10.1007/s00268-019-05229-y. [DOI] [PubMed] [Google Scholar]
- 22.Nikolic M, Schwameis K, Paireder M, Kristo I, Semmler G, Semmler L, Steindl A, Mosleh BO, Schoppmann SF. Tailored modern GERD therapy - steps towards the development of an aid to guide personalized anti-reflux surgery. Sci Rep. 2019;9(1):19174. doi: 10.1038/s41598-019-55510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabach L, Saad AR, Velanovich V: How to choose among fundoplication, magnetic sphincter augmentation or transoral incisionless fundoplication. Curr Opin Gastroenterol 2019. [DOI] [PubMed]
- 24.Gyawali CP, Fass R. Management of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154(2):302–318. doi: 10.1053/j.gastro.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 25.Falk GL, D’Netto TJ, Little SC. Endoscopic atlas of fundoplication. European Surgery. 2019;52(1):48–52. doi: 10.1007/s10353-019-00615-1. [DOI] [Google Scholar]
- 26.Falk GL, Archer L, Gooley SC. Is fundoplication advisable in repair of para-oesophageal hernia? “Little operation” or “big operation”? European Surgery. 2020;52(6):277–281. doi: 10.1007/s10353-020-00652-1. [DOI] [Google Scholar]
- 27.Wang YR, Dempsey DT, Richter JE. Trends and perioperative outcomes of inpatient antireflux surgery in the United States, 1993–2006. Dis Esophagus. 2011;24(4):215–223. doi: 10.1111/j.1442-2050.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 28.Richter JE: Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol 2013, 11(5):465–471; quiz e439. [DOI] [PubMed]
- 29.Ganz RA, Peters JH, Horgan S. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368(21):2039–2040. doi: 10.1056/NEJMc1303656. [DOI] [PubMed] [Google Scholar]
- 30.Khan F, Maradey-Romero C, Ganocy S, Frazier R, Fass R. Utilisation of surgical fundoplication for patients with gastro-oesophageal reflux disease in the USA has declined rapidly between 2009 and 2013. Aliment Pharmacol Ther. 2016;43(11):1124–1131. doi: 10.1111/apt.13611. [DOI] [PubMed] [Google Scholar]
- 31.Schwameis K, Nikolic M, Castellano DGM, Steindl A, Macheck S, Riegler M, Kristo I, Zorner B, Schoppmann SF. Crural Closure improves Outcomes of Magnetic Sphincter Augmentation in GERD patients with Hiatal Hernia. Sci Rep. 2018;8(1):7319. doi: 10.1038/s41598-018-24322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonavina L, Saino G, Lipham JC, Demeester TR. LINX((R)) Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Therap Adv Gastroenterol. 2013;6(4):261–268. doi: 10.1177/1756283X13486311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schizas D, Mastoraki A, Papoutsi E, Giannakoulis VG, Kanavidis P, Tsilimigras D, Ntourakis D, Lyros O, Liakakos T, Moris D. LINX((R)) reflux management system to bridge the "treatment gap" in gastroesophageal reflux disease: A systematic review of 35 studies. World J Clin Cases. 2020;8(2):294–305. doi: 10.12998/wjcc.v8.i2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonavina L, Saino GI, Bona D, Lipham J, Ganz RA, Dunn D, DeMeester T. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg. 2008;12(12):2133–2140. doi: 10.1007/s11605-008-0698-1. [DOI] [PubMed] [Google Scholar]
- 35.Bell R, Lipham J, Louie B, Williams V, Luketich J, Hill M, Richards W, Dunst C, Lister D, McDowell-Jacobs L et al: Laparoscopic magnetic sphincter augmentation versus double-dose proton pump inhibitors for management of moderate-to-severe regurgitation in GERD: a randomized controlled trial. Gastrointestinal endoscopy 2019, 89(1):14–22 e11. [DOI] [PubMed]
- 36.Buckley FP, Havemann B, Chawla A. Magnetic sphincter augmentation: Optimal patient selection and referral care pathways. World J Gastrointest Endosc. 2019;11(8):472–476. doi: 10.4253/wjge.v11.i8.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeed ZA, Winchester CB, Ferro PS, Michaletz PA, Schwartz JT, Graham DY. Prospective randomized comparison of polyvinyl bougies and through-the-scope balloons for dilation of peptic strictures of the esophagus. Gastrointestinal endoscopy. 1995;41(3):189–195. doi: 10.1016/S0016-5107(95)70336-5. [DOI] [PubMed] [Google Scholar]
- 38.Ayazi S, Zheng P, Zaidi AH, Chovanec K, Chowdhury N, Salvitti M, Komatsu Y, Omstead AN, Hoppo T, Jobe BA. Magnetic Sphincter Augmentation and Postoperative Dysphagia: Characterization, Clinical Risk Factors, and Management. J Gastrointest Surg. 2020;24(1):39–49. doi: 10.1007/s11605-019-04331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campos GM, Peters JH, DeMeester TR, Oberg S, Crookes PF, Tan S, DeMeester SR, Hagen JA, Bremner CG. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg. 1999;3(3):292–300. doi: 10.1016/S1091-255X(99)80071-7. [DOI] [PubMed] [Google Scholar]
- 40.Louie BE, Farivar AS, Shultz D, Brennan C, Vallieres E, Aye RW: Short-term outcomes using magnetic sphincter augmentation versus Nissen fundoplication for medically resistant gastroesophageal reflux disease. The Annals of thoracic surgery 2014, 98(2):498–504; discussion 504–495. [DOI] [PubMed]
- 41.Riegler M, Schoppman SF, Bonavina L, Ashton D, Horbach T, Kemen M. Magnetic sphincter augmentation and fundoplication for GERD in clinical practice: one-year results of a multicenter, prospective observational study. Surg Endosc. 2015;29(5):1123–1129. doi: 10.1007/s00464-014-3772-7. [DOI] [PubMed] [Google Scholar]
- 42.Bonavina L, Horbach T, Schoppmann SF, DeMarchi J: Three-year clinical experience with magnetic sphincter augmentation and laparoscopic fundoplication. Surg Endosc 2020. [DOI] [PMC free article] [PubMed]
- 43.Bonavina L, DeMeester T, Fockens P, Dunn D, Saino G, Bona D, Lipham J, Bemelman W, Ganz RA. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg. 2010;252(5):857–862. doi: 10.1097/SLA.0b013e3181fd879b. [DOI] [PubMed] [Google Scholar]
- 44.Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, DeVault KR, Horgan S, Jacobsen G, Luketich JD, Smith CC, et al. Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol. 2016;14(5):671–677. doi: 10.1016/j.cgh.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Lipham JC, Taiganides PA, Louie BE, Ganz RA, DeMeester TR. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus. 2015;28(4):305–311. doi: 10.1111/dote.12199. [DOI] [PubMed] [Google Scholar]
- 46.Asti E, Siboni S, Lazzari V, Bonitta G, Sironi A, Bonavina L. Removal of the Magnetic Sphincter Augmentation Device: Surgical Technique and Results of a Single-center Cohort Study. Ann Surg. 2017;265(5):941–945. doi: 10.1097/SLA.0000000000001785. [DOI] [PubMed] [Google Scholar]
- 47.Asti E, Aiolfi A, Lazzari V, Sironi A, Porta M, Bonavina L. Magnetic sphincter augmentation for gastroesophageal reflux disease: review of clinical studies. Updates Surg. 2018;70(3):323–330. doi: 10.1007/s13304-018-0569-6. [DOI] [PubMed] [Google Scholar]
- 48.Saino G, Bonavina L, Lipham JC, Dunn D, Ganz RA. Magnetic Sphincter Augmentation for Gastroesophageal Reflux at 5 Years: Final Results of a Pilot Study Show Long-Term Acid Reduction and Symptom Improvement. Journal of laparoendoscopic & advanced surgical techniques Part A. 2015;25(10):787–792. doi: 10.1089/lap.2015.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ang D, Teo EK, Ang TL, Ong J, Poh CH, Tan J, Fock KM. To Bravo or not? A comparison of wireless esophageal pH monitoring and conventional pH catheter to evaluate non-erosive gastroesophageal reflux disease in a multiracial Asian cohort. J Dig Dis. 2010;11(1):19–27. doi: 10.1111/j.1751-2980.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 50.Pandolfino JE, Zhang Q, Schreiner MA, Ghosh S, Roth MP, Kahrilas PJ. Acid reflux event detection using the Bravo wireless versus the Slimline catheter pH systems: why are the numbers so different? Gut. 2005;54(12):1687–1692. doi: 10.1136/gut.2005.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]