Abstract
Spermidine-deficient Saccharomyces cerevisiae cells are much more sensitive to paromomycin than nondeficient cells, resulting in cessation of growth and cell death.
Paromomycin is an aminoglycoside antibiotic that has been used for a variety of clinical infections (5). Paromomycin, like other aminoglycoside antibiotics, is strongly cationic and has been shown to bind to ribosomes and to affect the fidelity of translation both in vitro and in vivo (4, 6, 7). In the present study, we have found that spermidine-deficient mutants of Saccharomyces cerevisiae are much more sensitive to paromomycin than cells that are not deficient.
S. cerevisiae Y344 (ura3-52 his6 leu2 Δspe2-5::LEU2) cannot synthesize spermidine and spermine because of a deletion mutation in the SPE2 gene; this gene codes for S-adenosylmethionine decarboxylase, an essential step in the biosynthesis of these two amines. As previously described (2), when this strain is grown in amine-deficient purified medium, the cells become deficient in spermidine and spermine, and, after complete depletion of the intracellular amines, growth stops. Y344/pSPE2 (2) contains a multicopy plasmid with the gene for yeast S-adenosylmethionine decarboxylase; Y344/YEp352 contains only the vector plasmid.
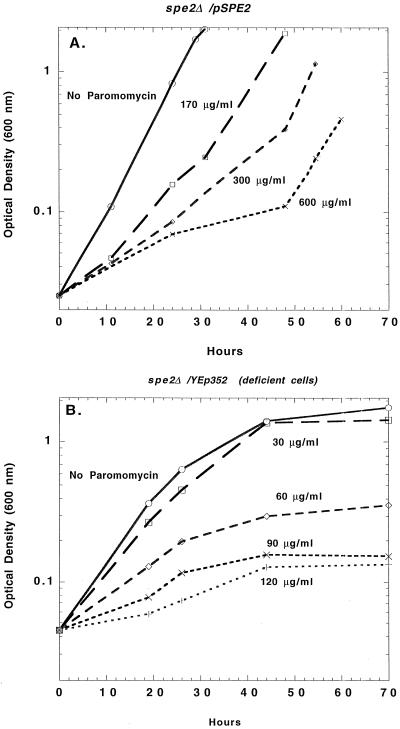
Strain Y344/YEp352 and strain Y344/pSPE2 were grown overnight in SD medium (6.7 g of yeast nitrogen base [Difco], 20 g of dextrose, 10 mmol of K2HPO4 [each per liter]) containing 0.1 mM spermidine. These cultures were then diluted 1:400 in amine-free SD medium and incubated for another 24 h. These partially depleted cultures were then diluted in the same medium to an optical density at 600 nm of 0.02 (ca. 2 × 105 cells per ml) and dispensed into a number of glass tubes, each containing a different concentration of paromomycin. The tubes were incubated at 30°C with shaking, and the optical density at 600 nm was measured periodically. The results are shown in Fig. 1 and show that the amine-deficient Y344/YEp352 cells (Fig. 1B) were considerably more sensitive to the antibiotic than the Y344/pSPE2 cells (Fig. 1A), which are able to make spermidine and spermine.
FIG. 1.
Polyamine deficiency increases the inhibitory effect of paromomycin on growth, as determined by optical density measurements. (A) spe2Δ cells containing pSPE2; (B) spe2Δ cells containing the control plasmid YEp352.
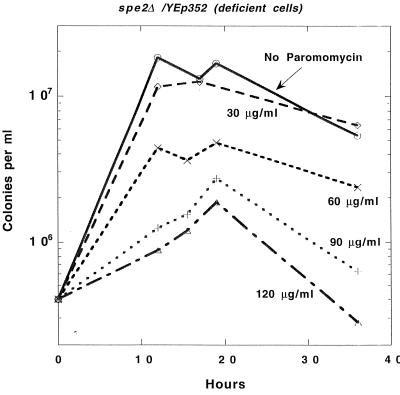
In another comparable set of experiments, we measured the viable cell counts in the spermidine-deficient cells after treatment with various concentrations of paromomycin. As shown in Fig. 2, concentrations of paromomycin (90 to 120 μg/ml) that had little or no effect on the growth of nondeficient cells not only inhibited the growth of the amine-deficient cells but eventually resulted in their death.
FIG. 2.
Polyamine deficiency increases the inhibitory effect of paromomycin on growth, as measured by cell counts, and results in cell death.
Essentially the same results (as measured by optical density; results not shown) were obtained in other experiments using a strain (spe1Δ spe2Δ mutant) that was deficient in putrescine as well as spermidine and spermine, indicating that the increased toxicity of paromomycin was not affected by the intracellular putrescine levels.
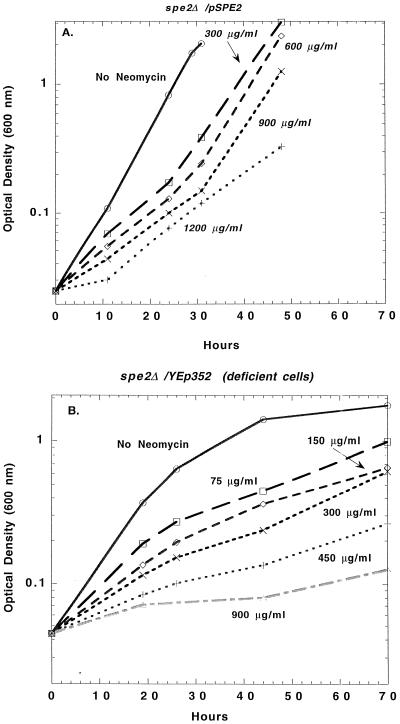
In other experiments (Fig. 3), we found that the spermidine-deficient cells (Fig. 3B) were also more sensitive to neomycin than the cells that were not deficient (Fig. 3A), but the effects were not as striking as those with paromomycin.
FIG. 3.
Polyamine deficiency increases the inhibitory effect of neomycin on growth, as determined by optical density measurements. (A) spe2Δ cells containing pSPE2; (B) spe2Δ cells containing the control plasmid YEp352.
The mechanism for the increased toxicity of paromomycin in the deficient cells is unclear, but one possibility is that the antibiotic is able to bind more tightly to its effector site (such as the ribosomes) in the absence of a competing polycation such as spermidine. Another possible explanation for the increased toxicity of the paromomycin in the deficient cells is that there might be increased uptake of paromomycin in the polyamine-deficient cells.
We and others have previously obtained evidence in both Escherichia coli and yeast for effects of polyamine deficiency on protein biosynthesis and on the fidelity of translation (1, 3, 8, 9). In view of our previous demonstration that frameshifting is increased in spermidine-deficient cells (1), we first speculated that the increased toxicity might be related to a greater effect on frameshifting. However, this explanation seems unlikely since in our previous experiments on the effect of spermidine deficiency on increasing frameshifting, an increased level of intracellular putrescine as well as a decreased level of intracellular spermidine was needed, and such an effect of putrescine was not seen in the present study on paromomycin toxicity.
The increased sensitivity of polyamine-deficient cells to paromomycin has proven to be a useful tool for distinguishing spermidine-deficient from nondeficient cells in various genetic studies, such as genetic crosses or transformation experiments, in which there is a need to characterize the polyamine phenotype of the various isolates. The usefulness of this method was demonstrated in an experiment in which a suspension of nondeficient cells and a suspension of spermidine-deficient cells were placed in alternate rows of a 96-well plate. These wells were then replicated onto agar plates containing SD medium and onto the same medium containing 100 μg of paromomycin per ml. Without paromomycin, there is still considerable growth in the wells with the deficient cells. In contrast, the deficient cells showed essentially no growth in the plate containing the paromomycin.
The increased toxicity of paromomycin in polyamine-deficient cells suggests the possible chemotherapeutic value of combining paromomycin therapy with one of the numerous inhibitors of polyamine biosynthesis that have been described previously (3).
REFERENCES
- 1.Balasundaram D, Dinman J D, Wickner R B, Tabor C W, Tabor H. Spermidine deficiency increases +1 ribosomal frameshifting efficiency and inhibits Ty1 retrotransposition in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1994;91:172–176. doi: 10.1073/pnas.91.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasundaram D, Tabor C W, Tabor H. Spermidine or spermine is essential for the aerobic growth of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:5872–5876. doi: 10.1073/pnas.88.13.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S S. A guide to the polyamines. Oxford, United Kingdom: Oxford University Press; 1998. [Google Scholar]
- 4.Fourmy D, Recht M I, Blanchard S C, Puglisi J D. Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science. 1996;274:1367–1371. doi: 10.1126/science.274.5291.1367. [DOI] [PubMed] [Google Scholar]
- 5.Hardman J G G, Gilman A, Limbird L L. Goodman & Gilman’s the pharmacological basis of therapeutics. 9th ed. New York, N.Y: McGraw-Hill Book Co.; 1996. [Google Scholar]
- 6.Palmer E, Wilhelm J M, Sherman F. Phenotypic suppression of nonsense mutants in yeast by aminoglycoside antibiotics. Nature. 1979;277:148–150. doi: 10.1038/277148a0. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Ursic D, Davies J. Phenotypic suppression and misreading in Saccharomyces cerevisiae. Nature. 1979;277:146–148. doi: 10.1038/277146a0. [DOI] [PubMed] [Google Scholar]
- 8.Tabor H, Tabor C W. Polyamine requirement for efficient translation of amber codons in vivo. Proc Natl Acad Sci USA. 1982;79:7087–7091. doi: 10.1073/pnas.79.23.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabor H, Tabor C W, Cohn M S, Hafner E W. Streptomycin resistance (rpsL) produces an absolute requirement for polyamines for growth of an Escherichia coli strain unable to synthesize putrescine and spermidine [Δ(speA-speB) ΔspeC] J Bacteriol. 1981;147:702–704. doi: 10.1128/jb.147.2.702-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]