Abstract
Rapid ventricular pacing (RVP) is commonly employed during transcatheter aortic valve replacement (TAVR); however, frequent TAVR is associated with worse prognoses. The retrograde INOUE-BALLOON® (IB) allows balloon aortic valvuloplasty (BAV) without RVP. The aim of this study was to evaluate the feasibility of retrograde IB for TAVR preparation. The study population included 178 consecutive patients (mean age, 84 ± 5 years; male, 47%) who underwent retrograde BAV before prosthetic valve replacement via the transfemoral approach. Patients were divided into a retrograde IB group without RVP (n = 74) and a conventional balloon (CB) group with RVP (n = 104). The primary endpoint was prolonged hypotension after BAV (reduced systolic pressure < 80 mmHg for over 1 min or vasopressor drug requirement). The incidence of prolonged hypotension after BAV was significantly lower in the IB group compared with the CB group (4% vs. 16%, p = 0.011). Balloons were able to penetrate and expand the aortic valve in both groups. RVP was used less for total TAVR in the IB group compared with the CB group. The aortic valve area-index after BAV was not significantly different between the two groups (0.72 ± 0.14 cm2/m2 vs. 0.71 ± 0.12 cm2/m2; p = 0.856). Multivariate analysis demonstrated that IB use was associated with avoidance of prolonged hypotension (OR, 0.27 [0.059–0.952]; p = 0.041). In conclusion, BAV using retrograde IB without RVP is both safe and feasible. More stable hemodynamics were achieved using retrograde IB by avoiding RVP during TAVR.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12928-021-00789-0.
Keywords: Aortic stenosis, Hemodynamics, Prolonged hypotension
Introduction
Methods for transcatheter aortic valve replacement (TAVR) are rapidly evolving with major refinements in technology, procedural techniques, patient selection, and biomedical engineering. In recent years, TAVR has become much simpler and safer, and, with expanding indications, an increasing number of younger patients are being referred for TAVR. Although TAVR has been used extensively, the development of safer and more precise procedures is essential. Rapid ventricular pacing (RVP), a common step during TAVR, is associated with prolonged hypotension and unstable hemodynamics. Frequent RVP is associated with worse prognoses [1–3]. In addition, balloon aortic valvuloplasty (BAV) before TAVR is not necessary for all patients but some patients require balloon dilation before prosthetic valve replacement.
The INOUE-BALLOON® (IB) was first reported in 1984 as a device for percutaneous transvenous mitral commissurotomy (PTMC) [4]. The volume-controlled hourglass shape of the IB ensures proper positioning at the stenosis site, prevents migration of the catheter, and provides optimal dilation. Subsequently, the IB designed for PTMC was also applied for antegrade BAV and demonstrated favorable clinical outcomes [5] despite technically complex strategies. According to these clinical experiences, a modified IB (Toray Industries Inc., Tokyo, Japan) for retrograde BAV was developed and launched in Japan in 2018. The retrograde IB enables BAV without RVP during TAVR; RVP is required when using a conventional balloon [6]. Reduced intraoperative RVP using the retrograde IB was expected to further stabilize hemodynamics during TAVR. However, few reports focus on the use of the retrograde IB for TAVR [6]. Accordingly, this study was designed to verify the safety and feasibility of this new technology during the TAVR procedure.
Methods
Study population and design
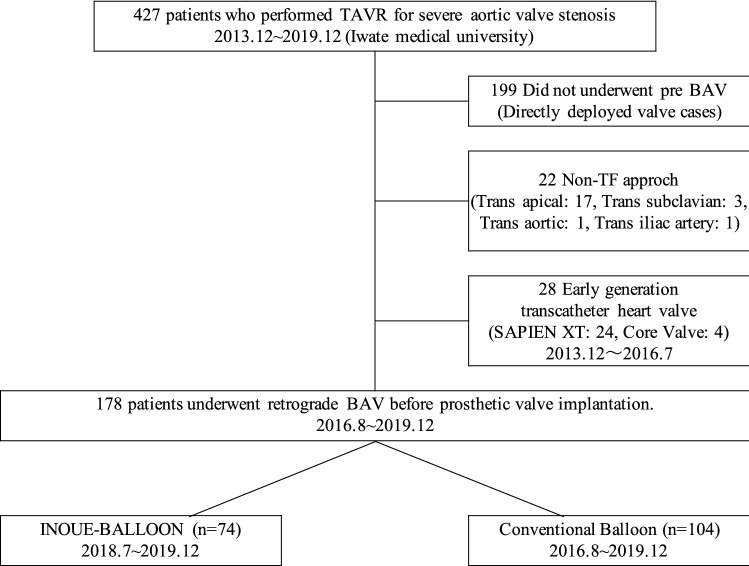
This is a single-center, retrospective, and observational study. The manufacturer of the retrograde IB, Toray, had no role in data collection, analysis, or manuscript drafting, and did not provide any financial support for the study. Consecutive patients with symptomatic severe aortic stenosis (AS) who underwent transfemoral TAVR from 2013 to 2019 at Iwate Medical University were included in the analysis (n = 427), retrospectively. After excluding 199 patients who did not undergo BAV, 22 patients who underwent TAVR with a non-TF approach and 28 patients who implanted early transcatheter heart valves (SAPIEN XT: 24, Core Valve: 4), 178 patients were enrolled in the study (Fig. 1). No patients underwent emergent or urgent TAVR during the study period. Eligibility for TAVR was established based on the consensus of a multidisciplinary heart team. Coronary angiography was exclusively performed before TAVR and percutaneous coronary intervention (PCI) was performed in patients who required coronary revascularization before TAVR. Regarding BAV, either the retrograde IB or the conventional balloon (CB) was used for the aortic valve; CBs included the Edwards Transfemoral Balloon Catheter (Edwards Lifesciences; Irvine), OSPYKA VACS® II (Osypka AG; Germany), and Z-MED II™ (NuMED Inc.; Canada). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and prior approval by the Human Research Committee of our institution was obtained. Written informed consent for data collection was obtained from each patient before TAVR (MH2018-503).
Fig. 1.
Patient recruitment flowchart
Retrograde INOUE-BALLOON device
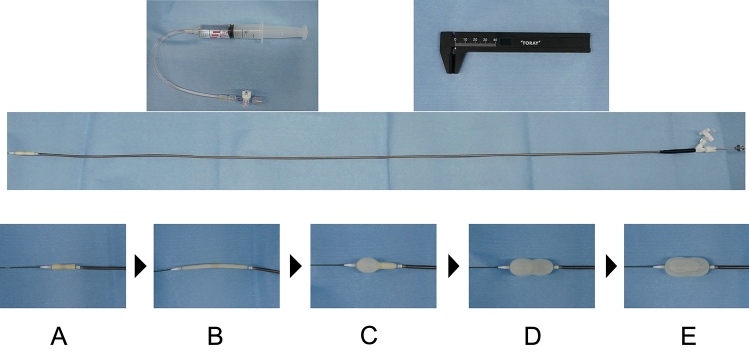
The retrograde IB for BAV is designed to prevent the balloon from slipping through the central waist during biphasic inflation. The retrograde IB allows for balloon expansion without RVP. The IB has a long, thin shaft compatible with 0.035ʺ, and the tip of the balloon has an elliptical shape to match the rigid guidewire and calcified valve. The catheter is manufactured of polyvinyl chloride, with a balloon attached to the distal end. The balloon is composed of two latex layers, with polyester micromesh between the two layers. The catheter is 9F in diameter, and the inflated balloon length is 35 mm, which is the ideal length to prevent migration. The special inflated shape of the balloon enables the IB to minimize slipping at the aortic valve. As a result, significant dives into the left ventricle are rare, and the IB reduces the risk of balloon injury to the left ventricular muscle. Five types of balloons, with maximum expansion diameters of 20 mm, 22 mm, 24 mm, 26 mm, and 28 mm, are available. A syringe is used to manually inflate the balloon, and the balloon diameter is measured with a ruler, very similar to the traditional IB for mitral valve commissurotomy (Fig. 2). The retrograde IB has the advantages of stable fixation and multistage expansion without RVP. The balloon is characterized by reliable valve expansion as a result of its special expansion shape, in addition to speedy expansion and contraction, and gradual valve expansion.
Fig. 2.
Balloon structure and expansion process. The IB catheter is an over-the-wire system with a lateral lumen for balloon dilation. Diluted contrast medium is injected with an accessory syringe to inflate the balloon. The balloon diameter is measured with the accessory caliper. While the catheter is stabilized to the aortic valve, the balloon is inflated with a prescribed amount of diluted contrast medium to dilate the aortic valve from a dumbbell to cylinder type. A Balloons in normal condition. B Balloon-extension state: Catheter insertion into the sheath. C Only one side of the balloon is inflated when the balloon is positioned at the aortic valve. D Dumbbell-shaped balloon, the central taper of which fits into the aortic valve. E The balloon is fully inflated during aortic valve dilation
Definitions and outcomes
Coronary revascularization was defined as patients who had previously undergone PCI or coronary artery bypass graft (CABG). Acute kidney injury was classified according to the AKIN criteria [7]. Vascular complications, bleeding, and stroke were defined and classified by the Valve Academic Research Consortium-2 definition [8]. Balloon slippage was defined as the complete extrusion of the balloon from the aortic valve during inflation. The full disclosure of invasive blood pressure recordings during the entire TAVR procedure was reviewed retrospectively. The number of RVP, prolonged hypotension, and duration of the procedure were determined by referring to the surgical records of the anesthesiologists. RVP in this study included pacing for pre-dilatation, post-dilatation, and valve deployment. RVP for the threshold and output testing of the pacemaker were excluded.
The primary endpoint of this study was prolonged hypotension immediately after BAV. Prolonged hypotension was defined as reduced systolic pressure to < 80 mmHg for over 1 min or requirement for the administration of vasopressor drugs. All suspected events were adjudicated by a blinded interventional cardiologist. The secondary endpoints were the successful passage and dilation of the retrograde IB, the number of RVP during the procedure, and the index of aortic valve area (AVA-I) after BAV by echocardiography. Two independent and blinded observers (a cardiac ultrasound specialist and a TAVR specialist) measured the AVA-I by planimetry in the transesophageal echocardiography.
Statistical analysis
All statistical analyses were performed using JMP® 13 (SAS Institute Inc., Cary, NC, USA). Continuous variables are expressed as mean ± SD or median and interquartile range as appropriate. Qualitative variables are expressed as numbers and percentages. Normality was checked using the Shapiro–Wilk test. Differences between means were evaluated using paired and unpaired (for independent group comparisons) Student t-tests for normally distributed data. The Mann–Whitney or Wilcoxon signed-rank tests were used to evaluate non-parametric data. The chi-square test was used for categorical variables and the Fisher’s exact test was used for categorical variables with low frequencies (expected cell count < 5). Pearson correlation coefficients were used to investigate the relationship between cardiac reverse remodeling parameters and baseline parameters. A two-tailed p value < 0.05 was considered statistically significant. Univariate logistic regression was used to evaluate the association between high-risk categories and prolonged hypotension post-BAV. Multivariate logistic regression analysis was performed, accounting for significant independent predictors of prolonged hypotension post-BAV. The factors indicated as significant in the univariate analysis were determined as parameters for the final model in the multivariate analysis.
Results
Baseline characteristics
Patients were divided into two groups according to pre-BAV type: Retrograde IB (n = 74, 42%) or CB (n = 104, 58%). No significant differences were observed between the groups in terms of baseline characteristics, including age, sex, serum, history of hypertension, history of diabetes, Society of Thoracic Surgery score, New York Heart Association class 3–4, permanent pacemaker, cerebrovascular disease, and cardiac artery disease (Table 1). The numbers of patients who underwent coronary revascularization were not significantly different between the two groups. Three patients underwent CABG. Baseline echocardiography showed no significant differences in peak aortic velocity, mean pressure gradient, ejection fraction, severe aortic regurgitation (AR), and severe mitral regurgitation between the two groups, while the AVA-I was significantly smaller in the IB group.
Table 1.
Baseline patient and procedural characteristics
| INOUE (n = 74) | Conventional (n = 104) | p value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 83 ± 5 | 84 ± 5 | 0.109 |
| Male, n (%) | 35 (47) | 39 (38) | 0.191 |
| NYHA > III, n (%) | 13 (18) | 15 (14) | 0.491 |
| Dyslipidemia, n (%) | 29 (39) | 49 (47) | 0.294 |
| Diabetes, n (%) | 19 (25) | 25 (24) | 0.803 |
| Hypertension, n (%) | 59 (80) | 87 (84) | 0.502 |
| Chronic renal failure, n (%) | 40 (54) | 56 (54) | 0.978 |
| Atrial fibrillation, n (%) | 12 (16) | 25 (24) | 0.205 |
| Previous MI, n (%) | 2 (3) | 3 (3) | 0.942 |
| Coronary revascularization, n (%) | 23 (31) | 21 (20) | 0.582 |
| Permanent pacemaker, n (%) | 4 (5) | 6 (6) | 1.000 |
| Cerebrovascular disease, n (%) | 12 (16) | 30 (29) | 0.051 |
| COPD, n (%) | 1 (1) | 7 (7) | 0.088 |
| STS score, % | 6.5 ± 3.7 | 5.6 ± 2.8 | 0.075 |
| Clinical frailty scale | 3.7 ± 0.8 | 3.3 ± 0.8 | 0.003 |
| Laboratory data | |||
| Hemoglobin, mg/dL | 11.5 ± 1.4 | 11.5 ± 1.6 | 0.740 |
| eGFR, mL/min/1.73m2 | 50 ± 20 | 54 ± 15 | 0.185 |
| BNP, pg/dL | 360 [18–2630] | 356 [17–2834] | 0.922 |
| Echocardiographic data | |||
| AVA-I, cm2/m2 | 0.43 ± 0.10 | 0.45 ± 0.11 | 0.107 |
| Peak aortic velocity, m/s | 5.1 ± 0.6 | 5.1 ± 0.8 | 0.652 |
| Mean pressure gradient, mmHg | 63 ± 17 | 62 ± 22 | 0.960 |
| LVEF, % | 62 ± 11 | 65 ± 8 | 0.021 |
| Severe AR, n (%) | 1 (1.4) | 0 (0) | 0.416 |
| Severe MR, n (%) | 2 (2.7) | 0 (0) | 0.172 |
| Procedural data | |||
| Balloon size, mm | 19.8 ± 1.0 | 18.7 ± 1.8 | < 0.001 |
| RVP during BAV, n (%) | 0 (0) | 104(100) | N/A |
| Balloon-expandable valve, n (%) | 46 (49) | 71 (61) | 0.398 |
| Valve type | |||
| SAPIEN 3, n (%) | 46 (49) | 71 (61) | |
| EvolutR/Pro, n (%) | 28 (46) | 33 (54) | |
AR aortic regurgitation, AVA-I AVA indexed, BNP brain natriuretic peptide, COPD chronic obstructive pulmonary disease, eGFR estimated glomerular filtration rate, LVEF left ventricular ejection fraction, MI myocardial infarction, MR mitral regurgitation, NYHA New York Heart Association, STS Society of Thoracic Surgery
Procedural outcomes and postoperative clinical outcomes within 30 days
The procedural outcomes immediately after BAV and TAVR are shown in Table 2. The balloons exclusively penetrated and expanded the aortic valves in both groups. None of the patients in the IB required RVP during BAV, whereas all patients in the CB group required RVP during BAV. Compared to the CB group, the IB group used fewer RVP during the TAVR procedures. After BAV, the AVA-I on the transesophageal echocardiogram was significantly enlarged in both groups (IB: 0.43 ± 0.10 cm2/m2 vs. 0.72 ± 0.14 cm2/m2, p < 0.001; CB: 0.45 ± 0.11 cm2/m2 vs. 0.71 ± 0.12 cm2/m2, p < 0.001) and the AVA-I after BAV were similar. The frequency of progression to acute severe AR after BAV was similar between the two groups. The TAVR procedure time was shorter in the IB group (62 min vs. 78 min, p < 0.001). Prolonged hypotension after BAV was less frequent in the IB group compared with the CB group (4% vs. 16%, p = 0.011). In the IB group, no cases of cardiopulmonary arrest (CPA) occurred immediately after BAV, whereas three cases of CPA occurred in the CB group. However, compared to the CB group, the IB group had a relatively higher incidence of balloon slippage due to the omission of RVP. No complications due to balloon slips were observed. Furthermore, the incidence of balloon slips did not impact clinical outcomes, with no aortic dissection in the slip group (0% vs. 0.8%, p = 1.000) and a stroke rate of 1.9% (1.9% vs. 2.4%, p = 1.000). No significant differences in maximum creatine kinase levels within 24 h postoperatively between the two groups were observed (IB vs. CB: 107 [43–482] vs. 104 [42–331], p = 0.953). Creatine kinase-MB tended to increase in the CB group (IB vs. CB: 14 [3–70] vs. 16 [4–69], p = 0.064).
Table 2.
Procedural outcomes
| INOUE (n = 74) | Conventional (n = 104) | p value | |
|---|---|---|---|
| Post-BAV | |||
| Balloon slip, n (%) | 36 (49) | 18 (17) | < 0.001 |
| Prolonged hypotension, n (%) | 3 (4) | 17 (16) | 0.011 |
| AVA-I, cm2/m2 | 0.72 ± 0.14 | 0.71 ± 0.12 | 0.856 |
| Progression to acute severe AR, n (%) | 2 (3) | 2 (2) | 0.731 |
| Post-TAVR | |||
| Procedure time, min | 62 [43–485] | 78 [45–355] | < 0.001 |
| Frequency of RVP in TAVR, n | 0.8 ± 0.7 | 2.0 ± 0.8 | < 0.001 |
| Total RVP time, min | 15.6 ± 12.9 | 28.7 ± 13.5 | < 0.001 |
| Post-BAV, n (%) | 11 (11) | 13 (18) | 0.178 |
AR aortic regurgitation, AVA-I AVA indexed, BAV balloon aortic valvuloplasty, TAVR transcatheter aortic valve replacement
Patients in the IB group showed no cases of symptomatic stroke after TAVR. Three patients died within 30 days in the CB group, but none in the IB group. The clinical outcomes after TAVR were not significantly different between the two groups (Table 3).
Table 3.
Postoperative clinical outcome within 30 days
| INOUE (n = 74) | Conventional (n = 104) | p value | |
|---|---|---|---|
| All-cause death, n (%) | 0 (0) | 3 (3) | 0.267 |
| Adverse events | |||
| Minor vascular complications, n (%) | 0 (0) | 3 (3) | 0.267 |
| Major vascular complications, n (%) | 2 (3) | 0 (0) | 0.172 |
| Minor bleeding, n (%) | 4 (5) | 13 (13) | 0.129 |
| Major bleeding, n (%) | 1 (1) | 3 (3) | 0.642 |
| Life-threatening bleeding, n (%) | 1 (1) | 0 (0) | 0.416 |
| Permanent pacemaker, n (%) | 2 (3) | 6 (6) | 0.472 |
| Stroke, n (%) | 0 (0) | 2 (2) | 0.512 |
| Acute kidney injury, n (%) | 1.000 | ||
| Stage 1 | 13 (10) | 5 (5) | |
| Stage 2 | 0 (0) | 0 (0) | |
| Stage 3 | 2 (3) | 1 (1) | |
| New atrial fibrillation, n (%) | 3 (4) | 5 (4) | 1.000 |
Logistic regression analysis of predictors for prolonged hypotension post-BAV
In the univariate analysis with logistic regression models, BAV with the IB, RVP > 35 ms, and history of atrial fibrillation (AF) were independent predictors of prolonged hypotension after BAV (IB: OR, 0.22 [0.061–0.768]; p = 0.018; RVP > 35 ms: OR, 2.84 [1.034–7.510]; p = 0.043; AF: OR, 2.97 [1.111–7.920]; p = 0.023). The multivariate analysis with logistic regression models indicated that a BAV with the IB was an independent predictor of prolonged hypotension after BAV (OR, 0.27 [0.059–0.952]; p = 0.041) (Table 4).
Table 4.
Univariate and multivariate logistic regression analyses of predictors for prolonged hypotension post-BAV
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Male | 1.40 | 0.578–3.731 | 0.419 | |||
| Age | 1.02 | 0.930–1.123 | 0.645 | |||
| NYHA > 3 | 0.91 | 0.250–3.367 | 0.896 | |||
| Hypertension | 0.46 | 0.162–1.306 | 0.145 | |||
| Diabetes | 2.26 | 0.858–5.952 | 0.099 | |||
| Atrial fibrillation | 2.97 | 1.111–7.920 | 0.023 | 2.53 | 0.886–6.929 | 0.081 |
| Coronary revascularization | 1.96 | 0.727–5.307 | 0.183 | |||
| eGFR | 0.99 | 0.964–1.019 | 0.517 | |||
| BNP | 1.00 | 0.999–1.000 | 0.969 | |||
| LVEF (Simpson) | 0.98 | 0.941–1.029 | 0.488 | |||
| AVA-I | 0.77 | 0.032–18.946 | 0.137 | |||
| LVEDV | 0.99 | 0.970–1.012 | 0.356 | |||
| LVESV | 1.01 | 0.978–1.035 | 0.671 | |||
| Balloon size | 0.98 | 0.941–1.029 | 0.488 | |||
| RVP time | 1.03 | 0.992–1.060 | 0.140 | |||
| Long RVP (> 35 s) | 2.84 | 1.034–7.510 | 0.043 | 1.59 | 0.534–4.594 | 0.396 |
| INOUE-BALLOON without RVP | 0.22 | 0.061–0.768 | 0.018 | 0.27 | 0.059–0.952 | 0.041 |
AVA-I AVA indexed, eGFR estimated glomerular filtration rate, LVEDV left ventricular end-diastolic volume, LVEF left ventricular ejection fraction, LVESV left ventricular end-systolic volume, MI myocardial infarction, NYHA New York Heart Association, RVP rapid ventricular pacing
Discussion
The present cohort study is the first to demonstrate the safety and feasibility of the retrograde IB for pre-dilatation of TAVR. The major findings are as follows: (1) retrograde IB was successfully performed without RVP for all patients, (2) retrograde IB provided a similar capability for aortic valve expansion to CB, and (3) retrograde IB was less likely to cause systemic hypotension immediately after balloon dilation.
BAV is often required before valve replacement to facilitate passage of the valve with high calcification or a very narrow valve area. Retrograde IB was developed for BAV without RVP during TAVR. This study showed that penetration of the balloon through the stenotic aortic valve was similarly accomplished with either the IB or CB. In addition, despite the smaller baseline AVA-I with the retrograde IB, the AVA-I after pre-dilatation was almost identical.
RVP is necessary for BAV with a CB [9, 10]. However, it is well known that RVP decreases microvascular tissue perfusion [11] and leads to right ventricular dysfunction [12]. Furthermore, RVP is associated with myocardial damage [3, 13, 14], prolonged hypotension, and unfavorable prognoses [1, 15, 16]. In contrast, stable balloon fixation and multistage expansion with an hourglass shape, even without RVP, ensures that the calcified aortic valve is opened and prevents the collapse of hemodynamics (Fig. 3). In the present study, the low frequency of prolonged hypotension after expansion with retrograde IB may be strongly associated with the absence of RVP. The retrograde IB group had significantly lower total RVP during TAVR, which may have increased intraoperative hemodynamic stability. The clinical implication of the use of IB is that it reduces intraoperative anxiety factors by avoiding prolonged hypotension, leading to a safer TAVR procedure. Moreover, stroke events and 30-day deaths are more commonly associated with the valve replacement procedure than balloon pre-dilatation. The decrease in the total number of RVP and the hemodynamic stability after pre-dilatation may potentially improve clinical outcomes in the IB group. Moreover, in this study, prolonged hypotension after BAV was observed in many patients with low-flow, low-gradient aortic stenosis (LFLG AS), suggesting that LFLG AS is prone to hemodynamic instability after BAV. In patients with LFLG AS, IB may be used to safely prepare for valve implantation. However, further investigation in more patients is needed.
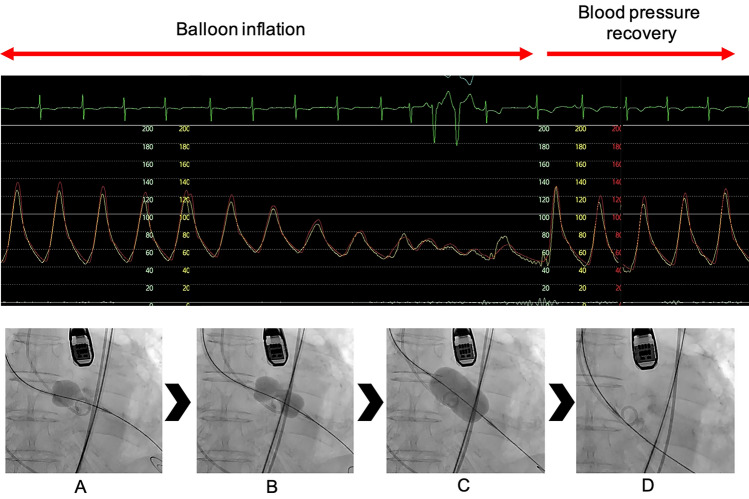
Fig. 3.
Aortic pressure during INOUE-BALLOON inflation. The balloon is inflated in three steps. Although blood pressure falls during full inflation, it recovers immediately after deflation. A First step: half balloon dilation; there is no decrease in blood pressure. B Second step: dumbbell-shaped balloon expansion; the aortic valve is anchored, and the blood pressure gradually decreases. C Third step: full balloon dilation and change to transient low pulse pressure. D Balloon deflation: the blood pressure immediately recovers to the baseline level after inflation
The higher frequencies of balloon slippage during expansion in the IB group, primarily caused by the omission of RVP, did not appear to be problematic, and no impact on hemodynamic stability or left ventricular injury was observed in this study. However, myocardial damage due to balloon dives into the left ventricle and vascular damage, including aortic dissection due to slippage into the aortic side, should be monitored. As a result of our experiences with the retrograde IB, we developed specific tips to prevent the IB from slipping, including anchoring the initially inflated portion of the balloon to the native aortic balloon (push or pull the balloon slightly to fix). Furthermore, both the proximal and distal portions can be inflated earlier, which might help to determine the initial longitudinal balloon position relative to the native aortic valve.
In addition to the selection of the BAV balloon, this study indicated that history of atrial fibrillation (AF) is independently associated with prolonged depression of blood pressure after BAV. In terms of the pre-existing risk of AF, the mechanisms used to prevent prolonged hypotension immediately after BAV are unclear. Atrial kick is defined as the force produced by the atria contracting before ventricular systole or at the end of ventricular diastole. In patients with AF, cardiac output is reduced by the loss of atrial kick [17]. The contribution of the atrial kick depends on the heart rate and heart structure. During tachycardia, the ventricular diastole period is reduced leading to increased dependence of the ventricular filling on the atrial kick. Patients with increased ventricular stiffness and decreased relaxation function (ventricular diastolic dysfunction) have decreased rapid filling and early diastole and become more dependent on atrial kick [18]. Most patients with AS show left ventricular hypertrophy, impaired relaxation, and reduced left ventricular capacity. Therefore, patients with AS might be more likely to have prolonged hypotension immediately after RVP. Furthermore, recent studies suggest that AF is associated with impaired baroreflex and that restoration of sinus rhythm improves baroreflex gain [19]. In addition, a long RVP time may also prolong the time of myocardial blood flow reduction and delay myocardial recovery after RVP. RVP may have a temporary adverse effect on the myocardium [12, 20]. Furthermore, prolonged RVP has been reported to worsen prognosis after TAVR [1]. RVP is an inseparable tool for TAVR, but frequent and prolonged RVP should be avoided. The current experience with IB remains limited and further research is required to establish its advantages, as well as to share tips and tricks for the optimal use of this technology.
Our study has several limitations. First, this is a relatively small, single-center, retrospective study of patients in a relatively high-volume center. Future studies with larger sample sizes are needed and should ideally be conducted in a randomized fashion. Second, the enrollment periods of the two groups were different; the retrograde IB was predominantly used in the second half of the study because it has been used since 2018. Furthermore, following the accumulation of experience, TAVR devices are likely to improve, which might impact the clinical outcomes at each time point. The statistically significant differences in procedural times between the two groups may be due to differences in hemodynamic conditions during pre-dilatation; however, these results may also be affected by potential differences in technical skills and experience of the heart team between the enrollment periods. Finally, there is no established rule to determine the balloon size; however, all balloon sizes were determined following an extensive discussion between the members of the experienced heart team, considering aortic valve measurements and the amount of calcification detected on cardiac computed tomography scan and echocardiography. The balloon size required for the preparation of the prosthetic valve implantation was usually selected.
In conclusion, BAV using retrograde IB without RVP is both safe and feasible. Furthermore, the use of retrograde IB has led to more stable hemodynamics by omitting the need for RVP during TAVR.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP 20K17093. The authors are deeply grateful to the laboratory members Yumiko Okuyama (research nurse), Kayoko Fujiwara, and Kanako Omiya (secretaries).
Abbreviations
- AF
Atrial fibrillation
- AR
Aortic regurgitation
- AS
Aortic stenosis
- AVA-I
Aortic valve area-index
- BAV
Balloon aortic valvuloplasty
- CPA
Cardiopulmonary arrest
- CABG
Coronary artery bypass graft
- CB
Conventional balloon
- IB
INOUE-BALLOON
- PTMC
Percutaneous transvenous mitral commissurotomy
- RVP
Rapid ventricular pacing
- TAVR
Transcatheter aortic valve replacement
Funding
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors. Dr. Morino received educational grants from Edwards Lifescience and lecture fees from Medtronic and Edwards Lifesciences. Dr. Fusazaki serves as a consultant for Medtronic. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Declarations
Conflict of interest
None of the authors have any conflicts of interest or financial disclosures.
Footnotes
The original online version of this article was revised due to there was an error in table 1, table 4 and Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/12/2021
A Correction to this paper has been published: 10.1007/s12928-021-00816-0
References
- 1.Fefer P, Bogdan A, Grossman Y, Berkovitch A, Brodov Y, Kuperstein R, et al. Impact of rapid ventricular pacing on outcome after transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7:e009038. doi: 10.1161/JAHA.118.009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iritakenishi T, Kamibayashi T, Torikai K, Maeda K, Kuratani T, Sawa Y, et al. Predictors of Prolonged hemodynamic compromise after valve deployment during transcatheter aortic valve implantation. J Cardiothorac Vasc Anesth. 2015;29:868–874. doi: 10.1053/j.jvca.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Kahlert P, Al-Rashid F, Plicht B, Wild C, Westhölter D, Hildebrandt H, et al. Myocardial injury during transfemoral transcatheter aortic valve implantation: an intracoronary Doppler and cardiac magnetic resonance imaging study. EuroIntervention. 2016;11:1401–1408. doi: 10.4244/EIJY15M05_10. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. doi: 10.1016/S0022-5223(19)37390-8. [DOI] [PubMed] [Google Scholar]
- 5.Sakata Y, Syed Z, Salinger MH, Feldman T. Percutaneous balloon aortic valvuloplasty: antegrade transseptal vs. conventional retrograde transarterial approach. Catheter Cardiovasc Interv. 2005;64:314–321. doi: 10.1002/ccd.20300. [DOI] [PubMed] [Google Scholar]
- 6.Moriki T, Tobaru T, Higuchi R, Shimizu J, Takanashi S, Takayama M. The brand-new Inoue balloon for retrograde approach: first experience in Japan. Cardiovasc Interv Ther. 2019;34:293–294. doi: 10.1007/s12928-018-0550-9. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Mok M, Dumont E, Doyle D, Rodés-Cabau J. Transcatheter aortic valve implantation using the slow balloon inflation technique: making balloon-expandable valves partially repositionable. J Card Surg. 2012;27:546–548. doi: 10.1111/j.1540-8191.2012.01492.x. [DOI] [PubMed] [Google Scholar]
- 10.Witzke C, Don CW, Cubeddu RJ, Herrero-Garibi J, Pomerantsev E, Caldera A, et al. Impact of rapid ventricular pacing during percutaneous balloon aortic valvuloplasty in patients with critical aortic stenosis: should we be using it? Catheter Cardiovasc Interv. 2010;75:444–452. doi: 10.1002/ccd.22289. [DOI] [PubMed] [Google Scholar]
- 11.Selle A, Figulla HR, Ferrari M, Rademacher W, Goebel B, Hamadanchi A, et al. Impact of rapid ventricular pacing during TAVI on microvascular tissue perfusion. Clin Res Cardiol. 2014;103:902–911. doi: 10.1007/s00392-014-0728-9. [DOI] [PubMed] [Google Scholar]
- 12.Axell RG, White PA, Giblett JP, Williams L, Rana BS, Klein A, et al. Rapid pacing-induced right ventricular dysfunction is evident after balloon-expandable transfemoral aortic valve replacement. J Am Coll Cardiol. 2017;69:903–904. doi: 10.1016/j.jacc.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Barbash IM, Dvir D, Ben-Dor I, Badr S, Okubagzi P, Torguson R, et al. Prevalence and effect of myocardial injury after transcatheter aortic valve replacement. Am J Cardiol. 2013;111:1337–1343. doi: 10.1016/j.amjcard.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 14.Paradis JM, Maniar HS, Lasala JM, Kodali S, Williams M, Lindman BR, et al. Clinical and functional outcomes associated with myocardial injury after transfemoral and transapical transcatheter aortic valve replacement: a subanalysis from the PARTNER trial (Placement of aortic transcatheter valves) JACC Cardiovasc Interv. 2015;8:1468–1479. doi: 10.1016/j.jcin.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapadia S, Agarwal S, Miller DC, Webb JG, Mack M, Ellis S, et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER Trial (Placement of Aortic Transcatheter Valves) Circ Cardiovasc Interv. 2016;9:e002981. doi: 10.1161/CIRCINTERVENTIONS.115.002981. [DOI] [PubMed] [Google Scholar]
- 16.Kleiman NS, Maini BJ, Reardon MJ, Conte J, Katz S, Rajagopal V, et al. Neurological events following transcatheter aortic valve replacement and their predictors: a report from the CoreValve trials. Circ Cardiovasc Interv. 2016;9:e003551. doi: 10.1161/CIRCINTERVENTIONS.115.003551. [DOI] [PubMed] [Google Scholar]
- 17.McNicholas T, Tobin K, O'Callaghan S, Kenny RA. Is orthostatic hypotension more common in individuals with atrial fibrillation?—findings from the Irish Longitudinal Study on Ageing (TILDA) Age Ageing. 2017;46:1006–1010. doi: 10.1093/ageing/afx096. [DOI] [PubMed] [Google Scholar]
- 18.Namana V, Gupta SS, Sabharwal N, Hollander G. Clinical significance of atrial kick. QJM. 2018;111:569–570. doi: 10.1093/qjmed/hcy088. [DOI] [PubMed] [Google Scholar]
- 19.Field ME, Wasmund SL, Page RL, Hamdan MH. Restoring sinus rhythm improves baroreflex function in patients with persistent atrial fibrillation. J Am Heart Assoc. 2016;5:e002997. doi: 10.1161/JAHA.115.002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okitsu K, Iritakenishi T, Imada T, Iwasaki M, Shibata SC, Fujino Y. A longer total duration of rapid ventricular pacing does not increase the risk of postprocedural myocardial injury in patients who undergo transcatheter aortic valve implantation. Heart Vessels. 2017;32:1117–1122. doi: 10.1007/s00380-017-0965-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.