Hundreds of peptide antibiotics have been described in the past half-century (20, 28, 35). These fall into two classes, non-ribosomally synthesized peptides, such as the gramicidins, polymyxins, bacitracins, glycopeptides, etc., and ribosomally synthesized (natural) peptides. The former are often drastically modified and are largely produced by bacteria, whereas the latter are produced by all species of life (including bacteria) as a major component of the natural host defense molecules of these species. The former have been well described to date (28, 35) and will be briefly summarized here with emphasis on their clinical importance, similarities in function to the natural peptides, and future prospects. The latter represent a new opportunity for the medicinal chemist and will be described in more detail with emphasis on the role in natural host defenses (as nature’s antibiotics) and the clinical potential of peptides derived from these natural peptides.
NONRIBOSOMALLY SYNTHESIZED PEPTIDES
Introduction.
Nonribosomally synthesized peptides can be described as peptides elaborated in bacteria, fungi, and streptomycetes that contain two or more moieties derived from amino acids (28, 35). By definition even the longer peptidic molecules in this class are made on multienzyme complexes rather than being synthesized, in the normal method of proteins, on ribosomes (as pre-pro-proteins in the case of the ribosomally synthesized peptides considered below). By this definition, many of the antibiotics used in our society are peptide derived. For example, the natural penicillins can be dissected into residues of monosubstituted acetic acid, l-cysteine and d-valine, while cephalosporin C, the basic building block of many semisynthetic cephalosporins comprises d-α-aminoadipic acid, l-cysteine, α,β-dehydrovaline, and acetic acid. Also, the glycopeptide class of antibiotics, including vancomycin and teicoplanin, have sugar-substituted peptide backbones. However, given the enormous volume of literature on these and the large number of peptides that are not used in the clinic, we are restricting ourselves here to the high-molecular-weight peptide antibiotics which have been used clinically.
Biosynthesis.
A large amount of information has shown that nonribosomal peptide synthesis is performed according to the multiple-carrier thiotemplate mechanism (40). In this template-driven assembly, a series of very large multifunctional peptide synthetases, with a modular arrangement, perform the peptide synthesis in an ordered fashion. A single peptide synthetase gene (e.g., grsB of the gramicidin S biosynthetic operon [38]) can be as large as 13 kb (4,300 amino acids) and contain four to six modules (resulting in the addition of four to six residues). Each module contains the basic ability to recognize a residue, activate it, modify it as necessary, and add it to the growing peptide chain. The minimal module is capable of activating one amino acid or hydroxyacid residue, stabilizing the activated residue as a thioester, and polymerizing it in its correct sequence to the previously added residue with the aid of a covalently attached cofactor, 4′-phosphopantotheine. This basic mechanism can result in a great chemical variety of peptide products containing hydroxy-, l-, d-, or unusual amino acids, which can be further modified by N methylation, acylation, glycosylation, or heterocyclic ring formation. More than 300 different residues are known to be incorporated into these peptide secondary structures. The structures of three antibiotics—bacitracin, gramicidin S, and polymyxin B—that are used clinically are listed in Table 1.
TABLE 1.
Examples of primary amino acid sequences of natural antimicrobial peptides
| Peptide | Structurea |
|---|---|
| Gramicidin S | Cyclic (LOVPFdLOVPFd) |
| Bacitracin | Cyclized I(C)LEdI(KOdIFHD)Dd-NH2 |
| Polymyxin B | Cyclized isooctanoyl BTBB(BFdLBBT) |
| Rabbit α-defensin (NP-1) | VVC1AC2RRALC3LPRERRAGFC3RIRGRIHLC2 C1RR |
| Human β-defensin 1 | DHYNC1VSSGQC2LYSAC3PIFTKIQGTC2YRGKAKC1C3K |
| Crab tachyplesin | RRWC1FRVC2YRGFC2YRKC1R |
| Cattle bactenecin | RLC1RIVVIRVC1R |
| Silk moth cecropin A | KWKFKKIEKMGRNIRDGIVKAGPAIEVIGSAKAI |
| Cattle indolicidin | ILPWKWPWWPWRR |
| Bacterial nisin | IXA1IULA1Z2PGA2KZ3GLAMGA3NMKZ4AZ5A4HA5SIHVUK |
One-letter amino acid code with the following additions. Positively charged residues at neutral pH are boldfaced. Parentheses indicate amino acids that are cyclized. Superscript d represents the d-enantiomer; all other amino acids are l-form. The subscript numbers represent amino acids that are joined by either cysteine disulfides or (for nisin) thioether bridges. O, ornithine; B, diaminobutyrate; X, 2,3-didehydrobutyrine; U, 2,3-didehydroalanine; Z, α-aminobutyrate.
Activities and mechanisms of action.
Two of the peptides described in Table 1 are cationic in nature, with polymyxin B having a net charge of +5 and gramicidin S having a charge of +2. Polymyxins tend to be rather gram negative selective. In contrast, gramicidin S has traditionally been considered gram positive selective. However, we recently showed that, if MIC measurements are done in the correct fashion, gramicidin S has excellent activity against gram-negative bacteria and the fungus Candida albicans (29). With this caveat, the accumulated data suggests that these cationic antibiotics act in exactly the same way on cells as the cationic antimicrobial peptides described below (i.e., self-promoted uptake across the cytoplasmic membrane followed by interference with the cytoplasmic membrane barrier).
In contrast, the gram-positive-specific antibiotic bacitracin works by inhibiting the transfer of cytoplasmically synthesized peptidoglycan precursors to bactoprenol pyrophosphate. Other antibiotic peptides of nonribosomal origin, the streptogramins, are protein synthesis inhibitors.
Clinical applications.
Colimycin, the methosulfate derivative of the cationic lipopeptide colistin (polymyxin E), has been utilized quite successfully in an aerosol formulation against Pseudomonas aeruginosa lung infections (25). Colimycin appears to be well tolerated. The major reason for chemically modifying the natural lipopeptide is to decrease systemic toxicity. Such toxicity may be partially due to the lipid tail appended to the nonapeptide, but it is our understanding that even the deacylated derivative of polymyxin (polymyxin B nonapeptide) tends to be too toxic for human systemic use. Indeed, the nonacylated cyclic decapeptide gramicidin S is also quite toxic, causing erythrocyte lysis at concentrations only threefold higher than the MIC for many bacteria (29, 30). For this reason such peptides are restricted to topical applications. Polymyxin B, together with gramicidin S and bacitracin, is a very highly utilized topical preparation. Aerosol applications of colistin are also under active consideration.
Future prospects.
Although most of the nonribosomal antimicrobial peptides described here have been known for decades, many others with antibiotic activity have been described in the literature, and these peptides offer a potentially rich source of novel antimicrobials. Three types of approaches are being undertaken. The first involves modification of existing peptides (and presumably also isolation of novel peptides from nature and modification of these). For example, the streptogramins are a family of cyclic peptides discovered in the 1950s, which are quite potent but rather insoluble. Recent work has resulted in two water-soluble, semisynthetic streptogramins, dalfopristin and quinupristin. These peptides have just completed phase III clinical trials as a combination parenteral agent (Synercid) against resistant gram-positive bacteria. A second, rather exciting approach involves the modular nature of synthesis of the nonribosomal peptide antibiotics. Schneider et al. (38) have demonstrated that one can put together a novel combination of peptide synthesis modules and arrive at a novel structure. Thus, there is great potential for obtaining significant chemical diversity in the backbone amino acids or their modifications, and a combinatorial approach to generating diversity (i.e., mixing and matching modules) is possible. The third approach is to use these structures as templates for chemical synthesis and diversity. The gramicidins are one example of this approach. Variants of gramicidin S with altered ring size, charge, amino acid sequences, hydrophobicity, etc., have been constructed and shown to have greater selectivity for bacteria than for mammalian cells (30).
RIBOSOMALLY SYNTHESIZED PEPTIDES
Frog skin has been used for medicinal purposes for centuries and is still used today in South American countries. It was not until 1962 that Kiss and Michl (27) noted the presence of antimicrobial and hemolytic peptides in the skin secretions of Bombina variegata, and this led to the isolation of a 24-amino-acid antimicrobial peptide named “bombinin” (12). In 1972, an antimicrobial and hemolytic peptide, melittin, was isolated from bee venom (17) and became the basis for extensive research into the structure and mechanism of action of this type of cationic antimicrobial peptide. While the hemolytic nature of melittin prevented its exploitation as a new antimicrobial, it has led to the isolation of numerous naturally occurring cationic peptides with antimicrobial activity and limited, or no, hemolytic activity (6, 8, 10, 16, 20, 31).
Antimicrobial, ribosomally synthesized, cationic peptides have been recognized only recently as an important part of innate immunity (6, 8, 21) found throughout the evolutionary tree. However, examination of these peptides has shown general trends but little sequence homology, and this suggests that each peptide has evolved (probably convergently) to act optimally in the environment in which it is produced and against local microorganisms. The lack of sequence homology makes it difficult to predict the activities of the peptides in vivo and makes it challenging to design potent synthetic antimicrobial peptides which have the desired in vivo activities. The potential of synthetic peptides as novel chemotherapeutic agents will be discussed.
Distribution of naturally occurring antimicrobial peptides.
Antimicrobial peptides are so widespread that they are likely to play an important protective role. This section will focus on a selected range of peptides from mammals, amphibians, insects, plants, bacteria, and even viruses, highlighting the similarities and differences between these peptides. By comparing peptides from all the different organisms (20), one can examine if there is a common consensus and whether this could be used to design potent peptides targeted against specific organisms. However, although certain peptide structural groups occur (β-structures stabilized by disulfide bonds, amphipathic alpha-helices, extended structures, and loops [8, 18]), and these structures tend to be amphipathic (with a polar face and a hydrophobic face), no positional conservation of even classes of amino acids occurs. While some weakly charged (usually bacterium-derived) peptides exist, antimicrobial peptides generally have two to nine excess, positively charged amino acids (arginine or lysine). Most antimicrobial peptides have at least 50% hydrophobic amino acid residues and a low proportion of both neutral polar and negatively charged amino acids. Although peptides with antifungal activity show a higher proportion of polar neutral amino acids, there seem to be few other similarities. Since no specific rules are evident, it is probable that synthetic peptides will be necessary to determine which factors are important for antimicrobial activity within individual groups. These then will provide potential candidates for development as antimicrobials.
Mammalian peptides.
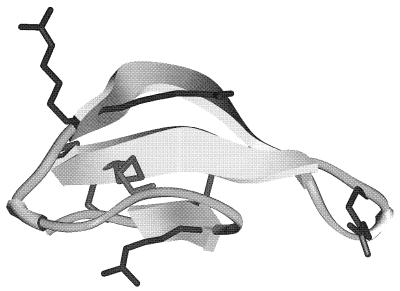
Antimicrobial peptides isolated from mammals can be present within the granules of neutrophils, in mucosal or skin secretions from epithelial cells, or as the degradation products of proteins (8). Neutrophils, which have a dedicated antimicrobial function, contain a range of antimicrobial proteins and peptides including bactericidal/permeability-increasing protein, cationic antimicrobial proteins, lysozyme, lactoferrin, bactenecins, defensins, indolicidins, and cathelicidins (16, 20). Other cell types including epithelial cells (which produce β-defensins) and platelets (which produce platelet microbicidal proteins), etc., also produce antimicrobial substances. The most researched mammalian peptides are the defensins (16). Defensins have been categorized into two groups, β-defensins and classical (sometimes called α-) defensins (Fig. 1; Table 1). Both contain three pairs of disulfide-linked cysteines and a high arginine content, but the location and connectivity of the cysteines are different between the two groups and there are also differences in other conserved amino acids. The structure of classical defensins has been shown to consist of a triple-stranded β-sheet connected by a loop with a β-hairpin hydrophobic finger (Fig. 1), and we assume that β-defensins will have a similar structure. In mammals, the classical defensins are present primarily within neutrophils and Paneth cells, and the β-defensins are isolated from epithelial cells, neutrophils, and leukocytes. Defensins are also found in the fat body in insects and the seeds of plants (8, 10). They have a range of activities, and mammalian defensins have activities against bacteria, fungi, and viruses (16). The proteolytic degradation of cationic proteins is thought also to contribute to the formation of antimicrobial peptides. Antimicrobial regions on lactoferrin (a protein with a primary function as an iron carrier) have been liberated upon gastric pepsin digestion of lactoferrin, and an 11-amino-acid peptide in human lactoferrin has been shown to be responsible for its antimicrobial activity (34). In these cases, the antimicrobial region may play a role in bacterial killing, both within the whole protein and as a more potent free peptide. Lactoferrin is currently being used as a nutritional supplement, which can liberate active peptides upon gastric digestion. It is able to reach the lower gastrointestinal tract, where it exerts its effect (whereas oral administration of peptides by themselves would probably result in peptide degradation, thus rendering the peptide inactive).
FIG. 1.
Molecular model of a defensin, human neutrophil peptide (HNP-1), based on the two-dimensional nuclear magnetic resonance-derived structure. The ribbon structures are β-strands connected by tubes of β-turn and random structures and stabilized by three intrachain disulfide bridges. The side chains of the positively charged lysine residues are shown as extended Y-shaped structures.
Amphibian peptides.
The isolation of bombinin (12) and subsequently the magainins from Xenopus species (48) led to the investigation and discovery of peptides throughout the amphibian species. For example, within Xenopus over a dozen antibiotic peptides, which are expressed not only within the granular glands of the skin but also in the cells of the gastric mucosa and intestinal tract, have been discovered (31). In the frog Phylomedusa sauvagii, the dermaseptins, a family of five antimicrobial peptides, are present, and they are notable for their good antifungal activities (33). For peptides from both amphibians, synergy is observed with combinations of the peptides. Peptides from amphibians tend to have little sequence homology. Indeed, it has been commented that no two amphibians have homologous peptides and that even within the same species there is a high degree of variation (31). However, all amphibian peptides either have been shown or predicted to form cationic amphipathic alpha-helices, e.g., magainins, dermaseptins, and buforin II, or are cysteine-disulfide loop peptides, e.g., ranalexin and the brevinins from the Rana frog species (31, 48).
Insect peptides.
Insect antimicrobial peptides have been isolated from two sources. They are secreted either within the insect (e.g., the cecropins, which are found within the hemolymph of the cecropia moth [23]) or outside the body (e.g., venoms such as bee melittin [17]). Although both classes are antimicrobial, the venoms tend to have cytotoxic activities. The cecropins have a high degree of homology and are active primarily against gram-negative bacteria (8). The discovery of a porcine cecropin (8) in the upper intestinal tract indicates that this type of peptide may be more broadly distributed.
Insects can express different peptides depending on the invading microorganism. For example, Drosophila has at least seven different antimicrobial peptides in its hemolymph (22). Some of these peptides are inducible upon infection, and one subset of peptides is induced by the same types of signalling pathways (22) as those used in mammals to induce both peptides and elements of the immune response (i.e., the Tol1 signalling pathway, which results in activation of the transcriptional factor NF-κB). Interestingly, although the peptides do not have the exquisite specificity of the immune response, Drosophila can discriminate between different types of invading organisms and produce the appropriate peptide. For instance, Drosophila naturally infected by entomopathogenic fungi exhibits an adaptive response by producing only antifungal peptides (32). Thus, antimicrobial peptides are thought to replace the immune response in these more primitive organisms.
Plant peptides.
Thionins were the first antimicrobial peptides to be isolated from plants (15). They are toxic towards both gram-positive and gram-negative bacteria, fungi, yeast, and various mammalian cell types (10). Other antimicrobial peptides were isolated which were found to be structurally related to insect and mammal defensins and have been named “plant defensins” (10). Whereas most antimicrobial peptides from animals and bacteria have antibacterial activity, plant defensins have a high antifungal activity (10), reflecting the relative importance of fungal as opposed to bacterial pathogens in the plant world. The plant defensins with antifungal activity can be divided into two groups: those that inhibit fungal growth through morphological distortions of the fungal hyphae and those that inhibit fungal growth without morphological distortion (10). It has been shown that these peptides can be induced in the leaves of the radish upon challenge with a fungal pathogen (again via a conserved signalling pathway), highlighting the importance of peptides in the plant defense system. Studies on the plant defensin from the seeds of Heuchera sanguinea have shown that specific, high-affinity binding sites are present on Neurospora crassa hyphae and microsomal membranes (43). Binding was shown to be competitive, reversible, and saturable. A similarity in binding affinity was found between hyphae and microsomal membrane interactions which indicates that binding sites reside on the plasma membrane. Competition studies showed that structurally related plant defensins were able to compete, but structurally unrelated antimicrobial peptides were not. Evidence suggests that binding of plant defensins to their receptor sites is linked to their antifungal effects (43).
Bacterial peptides.
Antimicrobial peptides, including both cationic and neutral peptides, are secreted from both gram-positive and gram-negative bacteria. These have been classified within the bacteriocins (which also include proteins [1, 2, 24]). Bacteriocins are generally able to kill specific bacterial competitors while causing little or no harm to the host bacterium, due to posttranscriptional modification and/or specific immunity mechanisms (2). Some peptide bacteriocins, including the Escherichia coli 7-amino-acid peptide microcin C7, which inhibits protein synthesis, and the Lactococcus peptide mersacidin, which inhibits peptidoglycan biosynthesis, have specific mechanisms which inhibit bacterial functions. However, most of these peptides, e.g., nisin and epidermidin, are thought to permeabilize target cell membranes (2, 45).
Viral peptides.
Viral peptides were first identified, through protein modelling, as two positively charged, highly amphipathic helices within the cytoplasmic tail of the envelope protein of HIV-1 (13). Further studies have shown these peptides, and peptides derived from other lentivirus transmembrane proteins, to have antimicrobial and cytolytic activities (42). All of these peptides have a high proportion of arginines and no lysines, but a difference in selectivity between the peptides has been observed (42).
Synthetic peptides.
To permit full exploitation of peptides as new antimicrobial agents, it is important to determine their mode of action. To this end, synthetic peptides have been made by systematic variation of naturally occurring peptides, by variation of model peptide sequences predicted to form amphipathic alpha-helices, or, more rarely, by random processes. By basing a synthetic peptide on a naturally occurring peptide, it is possible to improve antibacterial activity and at the same time give insight into the mechanism of action. As an early example of this, analogues with improved antibacterial activity and low cytotoxicity were found for the cecropins, and cecropin-melittin hybrids were developed (9). Many other analogue studies appear only in the patent literature.
Bessalle et al. (5) synthesized a number of peptides named “modellins,” of different lengths and hydrophobicities. They found that amphipathic peptides composed of 16 and 17 amino acids with highly hydrophobic (Trp and Phe) and hydrophilic (Lys) amino acids on opposite faces had high antibacterial and hemolytic activities. By replacing Trp or Phe with Leu, thereby reducing the hydrophobic nature of the peptide, a drastic reduction in hemolytic activity was seen, but bioactivity was only slightly decreased. They also observed that smaller peptides of only 9 or 10 amino acids had a lower antimicrobial activity and that they have a much lower alpha-helical content than the longer peptides. This led to the suggestion that smaller peptides may have a different mechanism of killing than the larger peptides. However, it is important to note that 12- to 14-amino-acid peptides like bactenecin and indolicidin derivatives (14, 47) and 10-amino-acid peptides like gramicidin S (30) can have excellent broad-spectrum antimicrobial activities. Thus, structure is more important than size. Analogous modification experiments have been undertaken to design peptides based on both sequence and amphipathicity. A model alpha-helical antibacterial peptide was synthesized by determining the most frequent amino acids in the first 20 positions for over 80 different natural sequences (44). As with many other alpha-helical peptides, this peptide was found to be unstructured in water but readily adopts an alpha-helix in a hydrophobic environment.
Synthetic peptides can also be designed to improve factors such as specificity, stability, and toxicity. It was shown that all-d-amino acid magainin exhibited antibacterial activity nearly identical to that of all-l-magainin, as well as being nonhemolytic (4). The presence of d-amino acids would make the peptide highly resistant to proteolysis, and therefore it would theoretically be more stable in vivo.
These studies have been based on naturally occurring peptides. It is also possible to discover potent antimicrobial peptides randomly. Combinatorial libraries allow the systematic examination of millions of peptides. Investigators have identified a number of tetra- and hexapeptides composed of l-, d-, and unnatural amino acids which exhibit antimicrobial activities against Staphylococcus aureus (7).
Activities.
Cationic antimicrobial peptides have a variety of activities ranging from gram negative selective to gram positive selective to broad spectrum in nature. It is important to measure MICs in the correct fashion (41, 44) since these peptides tend to precipitate at high concentrations and bind to many surfaces. The best peptides have good MICs (1 to 8 μg/ml) against a wide range of bacteria, including some of the most difficult to treat, antibiotic-resistant pathogens. They are bactericidal with very rapid killing kinetics, even around the MIC. It is also very difficult to raise mutants resistant to these cationic peptides, and there are very few naturally resistant bacteria (none are major human infectious agents). As a result of their mechanism of action (see below), some peptides have subsidiary activities that offer added side benefits, including an ability to neutralize endotoxin and synergy with conventional antibiotics especially against resistant mutants. For these reasons they appear to have excellent potential in the fight against antibiotic-resistant bacterial pathogens. Activities in animal models of both topical and systemic infections have been demonstrated (18, 19).
Individual peptides have also been shown to have a variety of interesting activities including antifungal, antiviral, antiparasitic, and anticancer activities and an ability to promote wound healing. In most cases the exact mechanisms behind these activities are not well understood.
Mechanism of action.
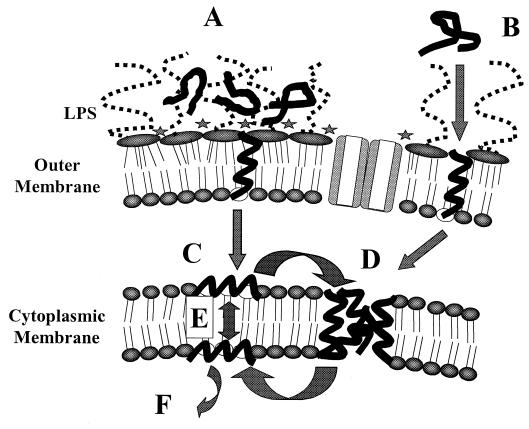
From the sequence alone it can be difficult to predict either the activity of a peptide or the secondary structure that it will form. Most of the peptides without disulfide bridges have random structures in water, and it is only when they bind to a membrane or other hydrophobic environment, or self-aggregate, that these peptides form a structure (3, 14). For example, cecropins and melittin fold into amphipathic alpha-helices in membranous environments. It is known that the dual cationic and hydrophobic nature of the peptides is important for the initial interaction between the peptide and bacterial membrane. Cationicity promotes interaction with bacterial outer and cytoplasmic membranes (20, 47). Also, hydrophobicity is important and, e.g., increasing the hydrophobic moment of magainin analogues causes increased binding of the peptide to the membrane due to increased hydrophobic interactions between lipid acyl chains and the hydrophobic helix core (46). An overview of the proposed interaction of peptides with the cell envelope membranes of gram-negative bacteria is given in Fig. 2.
FIG. 2.
Proposed mechanism of interaction of cationic antimicrobial peptides with the cell envelope of gram-negative bacteria. Passage across the outer membrane is proposed to occur by self-promoted uptake. According to this hypothesis, unfolded cationic peptides are proposed to associate with the negatively charged surface of the outer membrane and either neutralize the charge over a patch of the outer membrane, creating cracks through which the peptide can cross the outer membrane (A), or actually bind to the divalent cation binding sites on LPS and disrupt the membrane (B). Once the peptide has transited the outer membrane, it will bind to the negatively charged surface of the cytoplasmic membrane, created by the headgroups of phosphatidylglycerol and cardiolipin, and the amphipathic peptide will insert into the membrane interface (the region where the phospholipid headgroups meet the fatty acyl chains of the phospholipid membrane) (C). It is not known at which point in this process the peptide actually folds into its amphipathic structure (i.e., during transit across the outer membrane or during insertion into the cytoplasmic membrane). Many peptide molecules will insert into the membrane interface and are proposed to then either aggregate into a micelle-like complex which spans the membrane (D) or flip-flop across the membrane under the influence of the large transmembrane electrical potential gradient (approximately −140 mV) (E). The micelle-like aggregates (D) are proposed to have water associated with them, and this provides channels for the movement of ions across the membrane and possibly leakage of larger water-soluble molecules. These aggregates would be variable in size and lifetime and will dissociate into monomers that may be disposed at either side of the membrane. The net effect of D and E is that some monomers will be translocated into the cytoplasm and can dissociate from the membrane and bind to cellular polyanions such as DNA and RNA (F).
A number of peptides have been shown to bind to lipopolysaccharide (LPS) (or other cell wall components in gram-positive bacteria) and to permeabilize the outer membrane to self-promote uptake into gram-negative bacteria (18). Because cationic peptides have affinities for LPS that are at least 3 orders of magnitude higher than those for the native divalent cations Ca2+ and Mg2+, they competitively displace these ions and, being bulky, disrupt the normal barrier property of the outer membrane. In our experience, however, some peptides have much lower affinities for LPS binding but still are effective permeabilizers, and these presumably permeabilize by a related but distinguishable method (Fig. 1). The affected outer membrane is thought to develop transient “cracks” which permit the passage of a variety of molecules, including the uptake of the peptide itself. After these initial interactions, the mode of bacterial killing is not so clear.
Model membrane studies have often shown that the peptides can permeabilize liposomes at very high peptide-to-lipid ratios. Other ex vivo studies have indicated that the defensins, cecropins, bacteriocins, and indolicidin all form voltage-dependent ion-permeable “channels” in planar lipid bilayer membranes (14, 26). These channels usually are extremely heterogeneous in size and lifetime (although exceptions do occur). Generally speaking, these data have been interpreted according to the barrel stave mechanism, first proposed to explain similar behavior with the peptide alamethicin (reviewed in reference 20). This mechanism involves binding of monomers to the membrane and insertion into the membrane to form a pore (with individual monomeric peptides forming the staves of the barrel-like pore), followed by progressive recruitment of additional monomers to increase the pore size. However, even the fact that these peptides form channels has been disputed, and alternative models have been proposed, e.g., the carpet model (39), in which peptide molecules saturate the surface of the cytoplasmic membrane before causing a wholesale disruption of the membrane permeability barrier. Another possibility that we favor is that local aggregations of varied numbers of peptide molecules occur within the membrane and provide a route for passage of ions (Fig. 2). To try to resolve such a dilemma arising from model membrane studies, we have devised an assay based on measurement of the effects of peptides on the transcytoplasmic membrane potential gradient (47, 47a). This assay showed that only certain peptides completely depolarize the cytoplasmic membrane of E. coli at their MICs. However, they cause partial collapse of membrane potential at concentrations well below their MICs (an observation that contradicts the carpet model, which suggests that when peptides achieve a threshold concentration, the membrane is destroyed—a phenomenon that is also not visible in electron micrographs). Still other peptides (e.g., indolicidin and bactenecin) do not permeabilize the cytoplasmic membrane to any great extent at their MICs, and a separate mechanism of action is suggested. For different cationic peptides this has been proposed to be an action on the nucleic acids of bacteria or a triggering of autolysis (summarized in reference 47a).
The bactericidal effects of these peptides tend to be extremely fast (i.e., 3 log order of killing within a couple of minutes at the MIC), and therefore it is difficult to monitor the stages of bacterial killing. Human lactoferrin peptides have a relatively slow action, and for these peptides it has been shown that membrane potential collapses, followed by membrane integrity, resulting in cell lysis (11). It has also been observed that the structures of human lactoferrin peptides alter with time once the peptides are bound to bacterial cell wall constituents and that the peptide does not form pores (unpublished data).
The mechanism by which antimicrobial peptides act has become a complex issue. It is important to understand how the peptides act to fully exploit the use of peptides as antimicrobial agents. Small sequence changes can lead to major changes in activity (summarized in reference 20). Not only is antimicrobial activity difficult to predict, but so are cytotoxic activities. Indolicidin has been observed to kill autoimmune T cells but not a number of other cell lines (37) including neuronal cells, whereas bactenecin is cytotoxic to neuronal and glial cells (36). Other peptides are selective for tumor over normal host cells. It is also very difficult to predict which peptides will be active in vivo based on in vitro MICs. However, many peptides do have reasonable activities in animal models without obvious toxicity (18, 19) and thus have been considered for potential use in the clinic.
Clinical applications.
Despite several preclinical studies by small biotechnology companies on the host defense peptides (19), there are unanswered concerns about production costs, lability to proteases in vivo, and unknown toxicities (see references 18, 19, and 21 for discussions of these concerns). Since there are no published preclinical studies, we have had to rely on company press releases for information (18, 19). The cationic protein rBPI21 (Neuprex; Xoma Corp., Berkeley, Calif.) has provided the greatest amount of information (16a). Although it is a cationic protein (more than 200 amino acids) rather than a peptide, we discuss it here because small cationic peptide portions of rBPI21 have the same activities as the intact molecule. In a phase II/III clinical trial of therapy against meningococcemia, rBPI21 given intravenously along with other supportive therapies resulted in a dramatic decrease in deaths. rBPI21 has excellent antiendotoxin activity but a somewhat lower antibacterial activity. Thus, it is undergoing a range of clinical trials in which endotoxin is indicated as an important factor.
Another well-studied peptide is the magainin derivative MSI-78 (Locilex; Magainin Pharmaceuticals Inc., Plymouth Meeting, Pa.). In phase III trials of 926 patients, topical MSI-78 has been found to show equivalence to oral ofloxacin against polymicrobic diabetic foot ulcers. However, it should be mentioned that oral antibiotics work poorly against such infections because of poor perfusion, so this comparison may be inappropriate. Indeed, a Food and Drug Administration panel recently rejected this drug. A previous phase III study of MSI-78 (called Cytolex in that study) against impetigo failed because of a very large placebo effect associated with merely washing the infected site.
Both nisin (a lantibiotic cationic peptide produced by AMBI, Purchase, N.Y.) and IB-367 (a protegrin-like cationic peptide from Intrabiotics, Mountain View, Calif.) have undergone phase I (safety) clinical trials successfully. They are being considered for stomach ulcers due to Helicobacter pylori (nisin) and oral mucositis (IB-367), although other indications are being considered. Indeed, a phase I safety trial of aerosolized IB-367 has been initiated in healthy adults with the objective of using this peptide against chronic Pseudomonas aeruginosa lung infections in cystic fibrosis patients. Micrologix Biotech Inc. (Vancouver, Canada) recently entered two phase I clinical trials for catheter-associated infections and serious acne infections.
Thus, there is a considerable drive to try to examine clinical situations in which the assets of antimicrobial peptides will be efficacious. However, it is very difficult to assess the success of such ventures due to a dearth of information currently available.
ACKNOWLEDGMENTS
R.E.W.H. is a Medical Research Council of Canada Distinguished Scientist and acknowledges the Canadian Bacterial Diseases Network and the Canadian Cystic Fibrosis Foundation for funding his own peptide research. D.S.C. thanks the Special Trustees of St. Thomas’ Hospital for financial support.
D.S.C. thanks R. W. Evans and C. L. Joannou for technical assistance and helpful discussions.
REFERENCES
- 1.Abee T. Pore-forming bacteriocins of gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol Lett. 1995;129:1–10. doi: 10.1016/0378-1097(95)00137-T. [DOI] [PubMed] [Google Scholar]
- 2.Baba T, Schneewind O. Instruments of microbial warfare: bacteriocin synthesis, toxicity and immunity. Trends Microbiol. 1998;6:66–71. doi: 10.1016/S0966-842X(97)01196-7. [DOI] [PubMed] [Google Scholar]
- 3.Bello J, Bello H R, Granados E. Conformation and aggregation of melittin: dependence on pH and concentration. Biochemistry. 1982;21:461–465. doi: 10.1021/bi00532a007. [DOI] [PubMed] [Google Scholar]
- 4.Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. All-d-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990;274:151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- 5.Bessalle R, Gorea A, Shalit I, Metzger J W, Dass C, Desiderio D M. Structure-function studies of amphiphilic antibacterial peptides. J Med Chem. 1993;36:1203–1209. doi: 10.1021/jm00061a011. [DOI] [PubMed] [Google Scholar]
- 6.Bevins C. Antimicrobial peptides as agents of mucosal immunity. Ciba Found Symp. 1994;186:250–269. doi: 10.1002/9780470514658.ch15. [DOI] [PubMed] [Google Scholar]
- 7.Blondelle S E, Perez-Paya E, Houghton R A. Synthetic combinatorial libraries: novel discovery strategy for identification of antimicrobial agents. Antimicrob Agents Chemother. 1996;40:1067–1071. doi: 10.1128/aac.40.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 9.Boman H G, Wade D, Boman I A, Wahlin B, Merrifield R B. Antibacterial and antimalarial properties of peptides that are cecropinA-melittin hybrids. FEBS Lett. 1989;259:103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 10.Broekaert W F, Cammue B P A, Debolle M F C, Thevissen K, Desamblanx G W, Osborn R W. Antimicrobial peptides from plants. Crit Rev Plant Sci. 1997;16:297–323. [Google Scholar]
- 11.Chapple D S, Mason D J, Joannou C L, Odell E W, Gant V A, Evans R W. Structure-function relationship of antibacterial synthetic peptides homologous to a helical surface region on human lactoferrin against Escherichia coli serotype O111. Infect Immun. 1998;66:2434–2440. doi: 10.1128/iai.66.6.2434-2440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csordas A, Michl H. Isolation and structure of a haemolytic polypeptide from the defensive secretion of European Bombina species. Monatsh Chem. 1970;101:182–189. [Google Scholar]
- 13.Eisenberg D, Wesson M. The most highly amphiphilic alpha-helices include two amino acid segments in human immunodeficiency virus glycoprotein. Biopolymers. 1990;41:171–177. doi: 10.1002/bip.360290122. [DOI] [PubMed] [Google Scholar]
- 14.Falla T J, Karunaratne D N, Hancock R E W. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez de Caleya R, Gonzalez-Pascual B, Garcia-Olmedo F, Carbonero P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Microbiol. 1972;23:998–1000. doi: 10.1128/am.23.5.998-1000.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganz T, Lehrer R I. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 16a.Giroir B, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N I, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997;360:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 17.Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 18.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 19.Hancock R E W. Therapeutic potential of cationic peptides. Expert Opin Investig Dis. 1998;7:167–174. doi: 10.1517/13543784.7.2.167. [DOI] [PubMed] [Google Scholar]
- 20.Hancock R E W, Falla T, Brown M H. Cationic bactericidal peptides. Adv Microb Physiol. 1995;37:135–175. doi: 10.1016/s0065-2911(08)60145-9. [DOI] [PubMed] [Google Scholar]
- 21.Hancock R E W, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman J A, Reichart J-M. Drosophila immunity. Cell Biol. 1997;7:309–316. doi: 10.1016/S0962-8924(97)01087-8. [DOI] [PubMed] [Google Scholar]
- 23.Hultmark D, Steiner H, Rasmusson T, Boman H G. Insect immunity: purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980;106:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 24.Jack R W, Jung G. Natural peptides with antimicrobial activity. Chimia. 1998;52:48–55. [Google Scholar]
- 25.Jensen T, Pedersen S S, Garne S, Heilmann C, Hoiby N, Koch C. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother. 1987;19:831–838. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 26.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiss G, Michl H. On the venomous skin secretion of the orange speckled frog Bombina variegata. Toxicon. 1962;1:33–39. [Google Scholar]
- 28.Kleinkauf H, von Dohren H. Peptide antibiotics, β-lactams and related compounds. Crit Rev Biotechnol. 1988;8:1–32. doi: 10.3109/07388558809150536. [DOI] [PubMed] [Google Scholar]
- 29.Kondejewski L H, Farmer S W, Wishart D S, Hancock R E W, Hodges R S. Gramicidin S is active against both gram-positive and gram-negative bacteria. Int J Pept Protein Res. 1996;47:460–466. doi: 10.1111/j.1399-3011.1996.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 30.Kondejewski L H, Farmer S W, Wishart D S, Kay C M, Hancock R E W, Hodges R S. Effect of ring size of gramicidin S analogs on structure, antibacterial and hemolytic activity. J Biol Chem. 1996;271:25261–25268. doi: 10.1074/jbc.271.41.25261. [DOI] [PubMed] [Google Scholar]
- 31.Kreil G. Antimicrobial peptides from amphibian skin: an overview. Ciba Found Symp. 1994;186:77–90. doi: 10.1002/9780470514658.ch5. [DOI] [PubMed] [Google Scholar]
- 32.Lemaitre B, Reichhart J, Hoffman J A. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mor A, Nicolas P. Isolation and structure of novel defensive peptides from frog skin. Eur J Biochem. 1994;219:145–154. doi: 10.1111/j.1432-1033.1994.tb19924.x. [DOI] [PubMed] [Google Scholar]
- 34.Odell E W, Sarra R, Foxworthy M, Chapple D S, Evans R W. Antibacterial activity of peptides homologous to a loop region in human lactoferrin. FEBS Lett. 1996;382:175–178. doi: 10.1016/0014-5793(96)00168-8. [DOI] [PubMed] [Google Scholar]
- 35.Perlman D, Bodansky M. Biosynthesis of peptide antibiotics. Annu Rev Biochem. 1971;40:449–464. doi: 10.1146/annurev.bi.40.070171.002313. [DOI] [PubMed] [Google Scholar]
- 36.Radermacher S W, Schoop V M, Schluesener H J. Bactenecin, a leukocyte antimicrobial peptide, is cytotoxic to neuronal and glial cells. J Neurosci Res. 1993;36:657–662. doi: 10.1002/jnr.490360606. [DOI] [PubMed] [Google Scholar]
- 37.Schluesener H J, Radermacher S W, Melms A, Jung S. Leukocyte antimicrobial peptides kill autoimmune T-cells. J Neuroimmun. 1993;47:199–202. doi: 10.1016/0165-5728(93)90030-3. [DOI] [PubMed] [Google Scholar]
- 38.Schneider A, Stachelhaus T, Mahariel M A. Targetted alteration of the substrate specificity of peptide synthetases by rational module swapping. Mol Gen Genet. 1998;257:308–318. doi: 10.1007/s004380050652. [DOI] [PubMed] [Google Scholar]
- 39.Shai Y. Molecular recognition between membrane-spanning polypeptides. Trends Biochem Sci. 1995;20:460–464. doi: 10.1016/s0968-0004(00)89101-x. [DOI] [PubMed] [Google Scholar]
- 40.Stein T, Vater J, Kruft V, Otto A, Wittmann-Liebold B, Franke P, Panico M, McDowell R, Morris H R. The multiple carrier model of nonribosomal peptide biosynthesis at modular multienzyme templates. J Biol Chem. 1996;271:15428–15435. doi: 10.1074/jbc.271.26.15428. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg D A, Hurst M A, Fujii C A, Kung A H, Ho J F, Cheng F C, Loury D J, Fiddles J C. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tencza S B, Douglass J P, Creighton D J, Montelaro R C, Mietzner T A. Novel antimicrobial peptides derived from human immunodeficiency virus type 1 and other lentivirus transmembrane proteins. Antimicrob Agents Chemother. 1997;41:2394–2398. doi: 10.1128/aac.41.11.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thevissen K, Osborn R W, Acland D P, Broekaert W F. Specific, high affinity binding sites for an antifungal plant defensin on Neurospora crassa hyphae and microsomal membranes. J Biol Chem. 1997;272:32176–32181. doi: 10.1074/jbc.272.51.32176. [DOI] [PubMed] [Google Scholar]
- 44.Tossi A, Tarantino C, Romeo D. Design of synthetic antimicrobial peptides based on sequence analogy and amphipathicity. Eur J Biochem. 1997;250:549–558. doi: 10.1111/j.1432-1033.1997.0549a.x. [DOI] [PubMed] [Google Scholar]
- 45.van Belkum M J, Kok J, Venema G, Holo H, Nes I F, Konings W N, Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein mediated manner. J Bacteriol. 1991;173:7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wieprecht T, Dathe M, Krause E, Beyermann M, Molloy W L, MacDonald D L, Bienert M. Modulation of membrane activity of amphipathic, antibacterial peptides by slight modifications of the hydrophobic moment. FEBS Lett. 1997;417:135–140. doi: 10.1016/s0014-5793(97)01266-0. [DOI] [PubMed] [Google Scholar]
- 47.Wu M, Hancock R E W. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- 47a.Wu, M., E. Maier, R. Benz, and R. E. W. Hancock. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry, in press. [DOI] [PubMed]
- 48.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of precursor. Proc Natl Acad Sci USA. 1987;84:5449–5454. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]