Abstract
A limited number of studies have been conducted on the relationship between serum vitamin levels and pulmonary function, particularly in the elderly population. This study attempted to confirm the association between serum vitamin levels (folate, vitamin A, and vitamin E) and pulmonary function in the elderly population of Korea. A total of 1166 subjects (528 men and 637 women) participated in the Korean National Health and Nutrition Examination Survey from 2016 to 2018. Serum levels of folate, vitamin A, and vitamin E were measured in the subjects. The subjects’ pulmonary function measurement items were as follows: forced vital capacity (FVC), forced expiratory volume in one second (FEV1), forced expiratory volume in one second/forced vital capacity (FEV1/FVC), forced expiratory flow at 25% and 75% of the pulmonary volume (FEF25–75%), forced expiratory volume in 6 s (FEV6), and peak expiratory velocity (PEV). We performed regression analysis considering FEV1, PEV, FVC, FEF25–75%, and FEV1/FVC and FEV6 as dependent variables. Serum vitamin A levels were not associated with pulmonary function. In elderly men, serum vitamin E levels were negatively correlated with FVC [B = − 0.012, 95% confidence interval (CI) − 0.022 to − 0.003, p = 0.012] and FEV1 (B = − 0.010, 95% CI − 0.115 to − 0.007, p = 0.028). We confirmed a positive correlation of the serum folate level with FEV1 (B = 0.017, 95% CI 0.004–0.030, p = 0.009), FEV1/FVC (B = 0.003, 95% CI 0.001–0.005, p = 0.007), and FEF25–75% (B = 0.031, 95% CI 0.010–0.053, p = 0.005) in elderly men. This study confirmed that high serum folate levels were positively associated with pulmonary function in elderly men in Korea. Further studies are needed to understand the longitudinal effect of folate and its biological mechanism in pulmonary function.
Subject terms: Nutrition, Respiratory signs and symptoms
Introduction
The improving economic levels and rising interest in health-related vitamins in the modern society has resulted in an increased intake of vitamins in adults1. As a result, sales of supplements such as vitamins has steadily increased to 30 billion dollars, and has a widespread impact on public health in the United States2. Among these supplements, antioxidant vitamins such as vitamin C and vitamin E have been reported to capture organic free radicals and reduce cardiovascular disease by preventing the formation of atherosclerotic plaques3. Additionally, an inverse relationship has been observed between the incidence of type 2 diabetes and vitamin levels4. In addition, a significant difference in glycated hemoglobin (HbA1c) levels has been observed between the group taking vitamins C and E and the group not taking vitamins C and E5.
Studies have revealed that an increased intake of antioxidant vitamins, such as vitamin C, can improve pulmonary function and protect human lungs6,7. In particular, it has been reported that low antioxidant vitamin intake increases the risk of chronic constructive pulmonary disease (COPD), especially in men who smoke8. Recently, several studies have further shown the association between folate and pulmonary function. A study by Jung et al. reported that a lack of folate intake was associated with the development of airflow limitation9. In addition, a previous study has indicated that the intake of appropriate folates may reduce the incidence of airway diseases such as COPD10.
However, only a few studies have reported the association between various serum vitamins and pulmonary function in the elderly population. Therefore, this study attempted to analyze the association between various serum vitamins and pulmonary function in the elderly population aged 60 to 80 years using data from the Korean National Health and Nutrition Examination Survey (KNHANES) (2016–2018). In particular, this study attempted to analyze the association between serum folate, vitamin A, and vitamin E levels and pulmonary function.
Results
We analyzed a total of 1,166 elderly individuals aged 60–80 years (528 men and 637 women) in this study. There was no significant difference in the mean age between men (68.1 ± 5.9) and women (68.3 ± 6.3). The mean serum levels of folate [7.1 (± 3.5) vs. 9.1 (± 3.9); p < 0.001] and vitamin E [13.9 (± 5.2) vs. 15.2 (± 5.8); p < 0.001] were significantly higher in women than in men. On the contrary, the mean serum levels of vitamin A were higher in men than in women [13.9 (± 5.2) vs. 15.2 (± 5.8); p < 0.001]. The Cohen’s D values of folate, vitamin A, and vitamin E were 0.527, 0.408, and 0.247, respectively. Therefore, it is considered that the value of folate was a relatively larger difference between men and women compared to vitamin A and vitamin E. The mean values of the pulmonary function test items for all participants are as follows: FVC: 3.1 (± 0.8) L, FEV1: 2.3 (± 0.6) L, FEV1/FVC: 0.7 (± 0.1), FEF 25–75%: 1.9 (± 0.8) L/second, FEV6: 2.9 (± 0.7) L, PEV: 6.0 (± 1.9) L/min. The mean FVC, FEV1, FEV6, and PEV values were higher in men than in women (Table 1).
Table 1.
Demographic data, clinical parameters, serum folate, vitamin values, and pulmonary function of subjects.
| Total (N = 1166) | Men (n = 528) | Women (N = 637) | p value | |
|---|---|---|---|---|
| Age (years)$ | 68.2 ± 6.1 | 68.1 ± 5.9 | 68.3 ± 6.3 | 0.521 |
| Height (cm)$ | 158.8 ± 8.8 | 167.2 ± 5.8 | 153.7 ± 5.7 | < 0.001 |
| Weight (Kg)$ | 62.3 ± 9.8 | 67.6 ± 5.8 | 57.9 ± 8.0 | < 0.001 |
| Alcohol Hx* | ||||
| No | 244 | 40 | 204 | < 0.001 |
| Yes | 922 | 489 | 433 | |
| Smoking Hx# | ||||
| No | 703 | 99 | 604 | < 0.001 |
| less than 5packs | 9 | 8 | 1 | |
| More than 5 packs | 454 | 422 | 32 | |
| Folate (ng/mL)$ | 8.2 ± 3.8 | 7.12 ± 3.46 | 9.06 ± 3.87 | < 0.001 |
| Vitamin A (mg/L)$ | 0.5 ± 0.2 | 0.59 ± 0.20 | 0.51 ± 0.17 | < 0.001 |
| Vitamin E (mg/L)$ | 14.6 ± 5.6 | 13.86 ± 5.16 | 15.23 ± 5.83 | < 0.001 |
| FVC (L)$ | 3.1 ± 0.8 | 3.67 ± 0.69 | 2.54 ± 0.46 | < 0.001 |
| FEV1 (L)$ | 2.3 ± 0.6 | 2.60 ± 0.61 | 1.97 ± 0.38 | < 0.001 |
| FEV1/FVC (%)$ | 0.7 ± 0.1 | 0.71 ± 0.9 | 0.77 ± 0.06 | < 0.001 |
| FEF25–75% (L/s)$ | 1.9 ± 0.8 | 1.93 ± 0.93 | 1.88 ± 0.67 | 0.240 |
| FEV6 (L)$ | 2.9 ± 0.7 | 3.47 ± 0.67 | 2.49 ± 0.44 | < 0.001 |
| PEV (L/s)$ | 6.0 ± 1.9 | 7.21 ± 1.99 | 5.03 ± 1.19 | < 0.001 |
Demographic data of study population were presented as appropriate.
Alcohol history and smoking history—number of each group; Height, weight, folate, vitamin A, vitamin E, FVC, FEV1, FEV/FVC, FEF25–75%, FEV6, PEV—presented with mean ± standard deviation.
*Using chi square test.
#Using linear by linear association.
$Using student t-test.
We performed unadjusted linear regression analysis between each pulmonary function test and each serum vitamin level. Results showed very different results between each vitamin and lung function results depending on the target group. (Supplemental Tables 1, 2, and 3). Therefore, we decided to include three vitamins and variables such as weight, height, and smoking history, which were known to be correlated with lung function in previous studies, in multivariate linear regression.
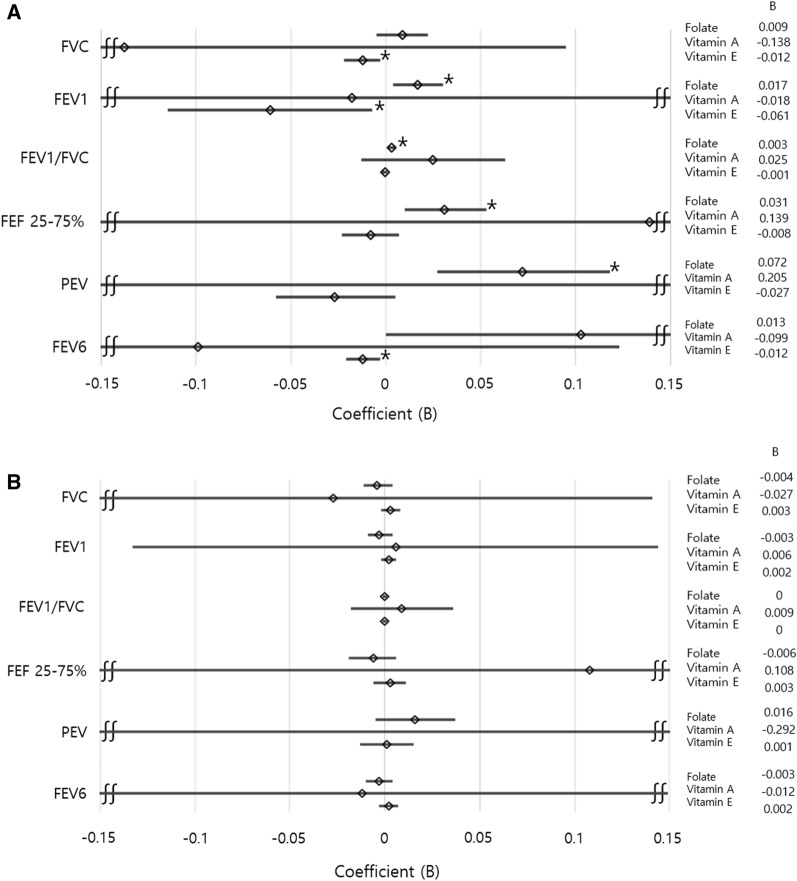
When considering FVC as a dependent variable, the serum folate level did not show a significant difference in men [B = 0.009, 95% confidence interval (CI) − 0.005 to 0.022, p = 0.231] and women (B = − 0.004, 95% CI − 0.011 to 0.004, p = 0.331). However, with respect to the serum vitamin E level, a negative correlation was observed in men (B = − 0.012, 95% CI − 0.022 to − 0.003, p = 0.012) (Table 2). When FEV1 was considered as a dependent variable, we confirmed that the serum folate level was positively correlated in men (B = 0.017, 95% CI 0.004–0.030, p = 0.009), while the serum vitamin E level was negatively correlated with FEV1 (B = − 0.010, 95% CI − 0.115 to − 0.007, p = 0.028) (Table 3). When FEV1/FVC was considered as a dependent variable, we confirmed that serum folate level was positively correlated in men (B = 0.003, 95% CI 0.001–0.005, p = 0.007) (Table 4). When FEF 25–75% was considered as a dependent variable, the serum folate level tended to be positively correlated in men (B = 0.031, 95% CI 0.010–0.053, p = 0.005) (Table 5). When PEV was considered as a dependent variable, serum folate level showed a positive correlation in men (B = 0.072, 95% CI 0.027–0.118, p = 0.002) (Supplemental Table 1). When FEV6 was considered as a dependent variable, serum vitamin E level showed a negative correlation in men (B = − 0.012, 95% CI − 0.021 to − 0.03, p = 0.009) (Supplemental Table 2).
Table 2.
Multivariate linear regression analysis considering FVC as dependent variable.
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| Sex | − 0.468 | − 0.574 to − 0.362 | < 0.001 | ||||||
| Age | − 0.028 | − 0.033 to − 0.024 | < 0.001 | − 0.035 | − 0.043 to − 0.027 | < 0.001 | − 0.025 | − 0.030 to − 0.020 | < 0.001 |
| Alcohol Hx | 0.038 | − 0.031 to 0.108 | 0.280 | 0.125 | − 0.053 to 0.304 | 0.168 | 0.042 | − 0.020 to 0.104 | 0.189 |
| Smoking Hx | 0.011 | − 0.032 to 0.054 | 0.620 | 0.013 | − 0.046 to 0.073 | 0.662 | 0.012 | − 0.052 to 0.076 | 0.716 |
| Height | 0.047 | 0.042 to 0.053 | < 0.001 | 0.061 | 0.051 to 0.071 | < 0.001 | 0.037 | 0.031 to 0.042 | < 0.001 |
| Weight | − 0.001 | − 0.005 to 0.002 | 0.504 | − 0.002 | − 0.008 to 0.004 | 0.597 | − 0.004 | − 0.008 to 0.000 | 0.047 |
| Folate | 0.000 | − 0.007 to 0.008 | 0.937 | 0.009 | − 0.005 to 0.022 | 0.231 | − 0.004 | − 0.011 to 0.004 | 0.331 |
| Vitamin A | − 0.062 | − 0.207 to 0.083 | 0.401 | − 0.138 | − 0.370 to 0.095 | 0.246 | − 0.027 | − 0.195 to 0.141 | 0.755 |
| Vitamin E | − 0.003 | − 0.008 to 0.002 | 0.215 | − 0.012 | − 0.022 to − 0.003 | 0.012 | 0.003 | − 0.002 to 0.008 | 0.275 |
Bold italics indicates statistical significance (p < 0.05).
CI Confidence interval.
Table 3.
Multivariate linear regression analysis considering FEV1 as dependent variable.
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| Sex | − 0.313 | − 0.406 to − 0.220 | < 0.001 | ||||||
| Age | − 0.031 | − 0.035 to − 0.027 | < 0.001 | − 0.040 | − 0.047 to − 0.033 | < 0.001 | − 0.025 | − 0.029 to − 0.021 | < 0.001 |
| Alcohol Hx | 0.031 | − 0.030 to 0.093 | 0.319 | 0.114 | − 0.048 to 0.276 | 0.169 | 0.031 | − 0.021 to 0.082 | 0.240 |
| Smoking Hx | − 0.057 | − 0.094 to− 0.019 | 0.003 | − 0.061 | − 0.115 to − 0.007 | 0.028 | − 0.035 | − 0.088 to 0.018 | 0.193 |
| Height | 0.026 | 0.021 to 0.031 | < 0.001 | 0.031 | 0.023 to 0.040 | < 0.001 | 0.022 | 0.018 to 0.027 | < 0.001 |
| Weight | 0.005 | 0.002 to 0.008 | 0.003 | 0.006 | 0.001 to 0.012 | 0.025 | 0.000 | − 0.003 to 0.003 | 0.818 |
| Folate | 0.004 | − 0.002 to 0.011 | 0.173 | 0.017 | 0.004 to 0.030 | 0.009 | − 0.003 | − 0.009 to 0.004 | 0.412 |
| Vitamin A | − 0.020 | − 0.108 to 0.147 | 0.763 | − 0.018 | − 0.230 to 0.193 | 0.864 | 0.006 | − 0.133 to 0.144 | 0.934 |
| Vitamin E | − 0.003 | − 0.007 to 0.002 | 0.220 | − 0.010 | − 0.115 to − 0.007 | 0.028 | 0.002 | − 0.002 to 0.006 | 0.361 |
Bold italics indicates statistical significance (p < 0.05).
CI Confidence interval.
Table 4.
Multivariate linear regression analysis considering FEV1/FVC as dependent variable.
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| Sex | 0.019 | 0.002 to 0.036 | 0.029 | ||||||
| Age | − 0.003 | − 0.004 to − 0.003 | < 0.001 | − 0.004 | − 0.006 to − 0.003 | < 0.001 | − 0.002 | − 0.003 to − 0.002 | < 0.001 |
| Alcohol Hx | 0.002 | − 0.009 to 0.013 | 0.735 | 0.010 | − 0.020 to 0.039 | 0.523 | 0.001 | − 0.009 to 0.011 | 0.797 |
| Smoking Hx | − 0.019 | − 0.026 to − 0.012 | < 0.001 | − 0.020 | − 0.030 to − 0.010 | < 0.001 | − 0.017 | − 0.027 to − 0.006 | 0.001 |
| Height | − 0.003 | − 0.004 to − 0.002 | < 0.001 | − 0.003 | − 0.005 to − 0.002 | < 0.001 | − 0.002 | − 0.003 to − 0.001 | < 0.001 |
| Weight | 0.002 | 0.001 to 0.002 | < 0.001 | 0.002 | 0.001 to 0.003 | < 0.001 | 0.001 | 0.001 to 0.002 | < 0.001 |
| Folate | 0.001 | 0.000 to 0.002 | 0.032 | 0.003 | 0.001 to 0.005 | 0.007 | 0.000 | − 0.001 to 0.001 | 0.857 |
| Vitamin A | 0.020 | − 0.003 to 0.043 | 0.090 | 0.025 | − 0.013 to 0.063 | 0.204 | 0.009 | − 0.018 to 0.036 | 0.511 |
| Vitamin E | 0.000 | − 0.001 to 0.001 | 0.673 | − 0.001 | − 0.002 to 0.001 | 0.517 | 0.000 | − 0.001 to 0.001 | 0.940 |
Bold italics indicates statistical significance (p < 0.05).
CI Confidence interval.
Table 5.
Multivariate linear regression analysis considering FEF 25–75% as dependent variable.
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| Sex | − 0.194 | − 0.362 to − 0.027 | 0.023 | ||||||
| Age | − 0.048 | − 0.055 to − 0.041 | < 0.001 | − 0.054 | − 0.066 to − 0.041 | < 0.001 | − 0.044 | − 0.053 to − 0.036 | < 0.001 |
| Alcohol Hx | 0.015 | − 0.095 to 0.126 | 0.787 | 0.108 | − 0.169 to 0.386 | 0.443 | − 0.005 | − 0.111 to 0.101 | 0.931 |
| Smoking Hx | − 0.161 | − 0.229 to − 0.093 | < 0.001 | − 0.169 | − 0.262 to 0.076 | < 0.001 | − 0.129 | − 0.238 to 0.020 | 0.021 |
| Height | − 0.003 | − 0.011 to 0.006 | 0.560 | − 0.002 | − 0.016 to 0.013 | 0.836 | − 0.002 | − 0.012 to 0.007 | 0.658 |
| Weight | 0.013 | 0.007 to 0.019 | < 0.001 | 0.013 | 0.004 to 0.023 | 0.006 | 0.010 | 0.004 to 0.017 | 0.002 |
| Folate | 0.008 | − 0.004 to 0.020 | 0.173 | 0.031 | 0.010 to 0.053 | 0.005 | − 0.006 | − 0.019 to 0.006 | 0.340 |
| Vitamin A | 0.141 | − 0.088 to 0.371 | 0.227 | 0.139 | − 0.222 to 0.501 | 0.449 | 0.108 | − 0.179 to 0.395 | 0.461 |
| Vitamin E | − 0.001 | − 0.009 to 0.007 | 0.863 | − 0.008 | − 0.023 to 0.007 | 0.302 | 0.003 | − 0.006 to 0.011 | 0.546 |
Bold italics indicates statistical significance (p < 0.05).
CI Confidence interval.
Figure 1 summarizes the correlation between serum vitamin level and lung function using multivariate linear regression analysis.
Figure 1.
Multivariate linear regression analysis of the effects of serum vitamin levels on pulmonary function in men (A) and women (B) adjusted for age, alcohol history, smoking history, height, weight, and each serum vitamin level. *p < 0.05.
Discussion
In this study, using KNHANES (2016–2018) data and measuring the pulmonary function, we confirmed that there is a positive association between serum folate level and various pulmonary function parameters in older men aged 60–80 years, but not in older women. In particular, serum folate level and FEV1, FEV1/FVC, and FEF 25–75%, which are considered important parameters for evaluating pulmonary function, were positively correlated. To the best of our knowledge, this is the first study to evaluate the relationship between pulmonary function and serum folate levels in the elderly population of Korea. Additionally, no positive association was observed between serum vitamin A and vitamin E levels, and pulmonary function in this study.
Therefore, the results of this study indicated that folate affected the FEV1, FEV1/FVC, and FEF25–75% of pulmonary function associated with obstructive ventilation disorders rather than FVC associated with restricted ventilation disorders in men. In addition, among the subjects included in this study, the proportion of smokers was higher in men than in women, which suggests that folate could help improve obstructive pulmonary function disorders caused by smoking. These results are similar to those of a previous study using KNHANES, which revealed that folate is associated with increased lung functions such as FEV1 in male smokers with COPD14.
Folate is a type of water-soluble vitamin B and is present in many leafy vegetables such as spinach, lettuce, and asparagus15. Folate plays an essential role in DNA methylation, hemoglobin synthesis, normal cell growth, replication, and brain development16. Further, the lack of folate is significantly associated with chronic diseases such as cardiovascular disease, depression, and Alzheimer's disease and causes birth defects such as natural tube defects in fetuses11,17. Several studies have shown the association between serum folate level and pulmonary function in airway diseases such as COPD or asthma. In a pediatric study, folate deficiency had a negative effect on pulmonary function in pediatric asthma patients18. Another study showed that asthma and high serum folate levels have a positive association in adults19.
This study confirmed that the serum folate level has a positive association with parameters that measure pulmonary function, such as FEV1, FEV1/FVC, and FEF 25–75%. In particular, FEV1 and FEV1/FVC have a tendency to decrease in patients with obstructive lung disease and are used as a diagnostic criteria for COPD, which is considered important in the pulmonary function test11. Previous studies have shown that in male COPD patients who smoke, high serum folate levels are positively correlated with pulmonary functions such as FEV1, FVC, and PEV14. Another previous study reported that among children, folate deficiency had a negative effect on pulmonary function in girls with asthma18. Similarly, Han et al. reported a positive association between serum folate levels and pulmonary function in children and adults19. Therefore, the results of this study suggests that folate may play a role in improving pulmonary function in elderly Korean men.
Several explanations can be considered for the biological mechanisms by which folate improves pulmonary function or prevents pulmonary function from decreasing. Folate is known to have antioxidant activity, removes free radicals, and is associated with oxidative stress-induced apoptosis20. This antioxidant activity plays a role in preventing the deterioration of pulmonary function by lowering the pathway inflammation associated with pathogenesis in COPD patients21. Furthermore, higher serum folate levels have been reported to play a role in lowering natural killer cell cytotoxicity, which is potentially a biological mechanism22. In addition, folate inhibits the expression of major immunomodulatory genes by promoting DNA methylation and plays several roles related to cell functions associated with the pathogenesis of allergic sensitization23.
Also, we analyzed determinants such as age, alcohol, smoking history, height, and weight known to be associated with pulmonary function12,13. As a result, in FVC, which represents restrictive ventilation disorder, age was negative determinant, whereas height was positive determinant. FEV1, FEV1/FVC, and FEF 25–75% representing obstructive ventilation disorder were negatively correlated with smoking history. Alcohol history did not show any association with pulmonary function in this study.
When we measure the effect size using F-squared (ƒ2). In the multivariate linear regression analysis with each lung function test result as a dependent variable, the ƒ2 in all analysis were 0.35 or more which means a large effect size. This is thought to have resulted from the inclusion of factors related to lung function such as previously known age, height, weight, and smoking history. However, in the unadjusted linear regression analysis, the F value was around 0.002 to 0.02 between each vitamin and pulmonary function test (data not shown), indicating that the effect size was very small between serum vitamins concentration and lung function even though in case with p < 0.05.
There is a positive association between vitamin E and pulmonary function; one study reported that increased vitamin E consumption in COPD patients prevented COPD-related mortality24. Another study reported that vitamin E intake was positively associated with pulmonary functions such as FEV1 and FVC in adults25. However, the results of this study suggest that serum vitamin E value has a negative association with FEV1 and FVC concerning pulmonary function only in men. There may be several reasons for the contrasting results. First, previous studies measured the intake of vitamin E without directly measuring the level of serum vitamin E, whereas this study directly measured the level of serum vitamin E. Second, unlike previous studies targeting adults, this study included the elderly as subjects. Hence, it is difficult to compare the results directly with existing studies, and additional longitudinal research is required.
As far as we know, this is the first large-scale study to examine the relationship between pulmonary function and serum folate, vitamin A, and vitamin E in the elderly population of Korea as per the data available that represents the status of the country population. In addition, in this study, the association between pulmonary function and folate levels in the elderly were compared after excluding subjects with other underlying diseases. Furthermore, the number of subjects was relatively higher than that of other studies, and the serum samples and pulmonary function parameters of the subjects were measured reliably.
Despite these strengths, this study has several limitations. First, we could not include other variables including other vitamins which might be associated with pulmonary function due to the limitations of retrospective studies. Second, due to the nature of the cross-sectional study design, the serum levels of folate, vitamin A, and vitamin E do not represent the long-term status of the subject. Therefore, cohort research and/or analysis at multiple time point are needed for concrete conclusion. Third, the effect size measure showed that the effect of vitamin on lung function was very small as mentioned before. Therefore, further study is needed on the clinical meaning of this result, even though it was statistically significant. Finally, it is essential to investigate the biological mechanisms through which folate improves the pulmonary function.
Conclusion
We confirmed a significantly positive correlation between serum folate and FEV1, FEV1/FVC, and FEF 25–75% in elderly Korean men over 60 years of age. Therefore, it can be deduced that serum folate plays an important role in pulmonary function in older men in Korea. Future research is needed on the longitudinal effect of folate and the biological mechanisms of its action on pulmonary function.
Materials and methods
Study population
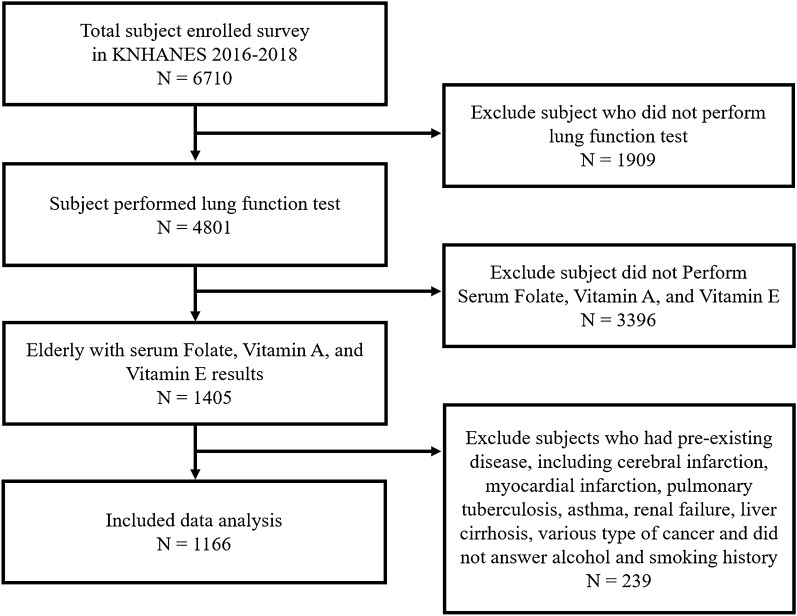
The KNHANES is designed to establish national health policies and conduct nationwide surveys and tests annually to produce national representation and reliability statistics. KNHANES consists of demographic characteristics, chronic disease prevalence, food and nutritional intake, health surveys, and various medical examinations. Detailed information about this study was provided to subjects or legal guardians and informed consent was obtained from all enrolled subjects. We analyzed the association between folate, vitamin A, and vitamin E levels and pulmonary function in the elderly aged 60–80 years from the KNHANES data from 2016 to 2018. Among 6710 subjects, 1,909 patients who were not tested for pulmonary function were first excluded, and 3396 patients who were not tested for vitamin A, vitamin E, and folate were subsequently excluded. The following subjects with one or more underlying diseases were excluded from the study: 50 patients with stroke, 85 with myocardial infarction and angina, 81 with pulmonary tuberculosis, 56 with asthma, 6 with renal failure, 14 with liver cirrhosis, and patients with various cancers (15 stomach cancer, 5 liver cancer, 17 colorectal cancer, 11 breast cancer, 10 cervical cancer, 5 lung cancer, 12 thyroid cancer, and 28 other cancer patients). Further, those who did not disclose their drinking or smoking history were excluded. Finally, 1166 subjects (528 men and 637 women) were enrolled for the analysis. (Fig. 2). All methods and protection of personal information were performed in accordance with the Declaration of Helsinki.
Figure 2.
Flowchart of study participants. Among the 6710 participants in the Korea National Health and Nutrition Examination Survey from 2016 to 2018, 4801 participants who underwent pulmonary function tests were considered. Finally, a total of 1166 subjects, excluding those who met the exclusion criteria, were analyzed in this study.
Measurements of variables
Subjects were assessed on their alcohol consumption and smoking history through a self-survey, and height and weight measurements were performed. Vitamin A and vitamin E serum levels were measured using the high-performance liquid chromatography-fluorescence detection (HPLC-FID) test method, with Agilent 1200 (Agilent, Santa Clara, CA, USA) test equipment, and Chromsystems (Chromsystems Instruments & Chemicals, Gräfelfing, Germany) reagents. The adult reference ranges for serum vitamin A and vitamin E were considered to be 0.30–0.70 (mg/L) and 5.00–20.00 (mg/L), respectively. Serum folate levels were measured using ARCHITECT i4000Sr (Abbott, Abbott Park, IL, USA) test equipment and ARCHITECT Folate reagent (Abbott, Abbott Park, IL, USA) using the Chemiluminescent Microparticle Immunoassay (CMIA method). The reference range of serum folate was 3.1–20.5 (ng/mL). Pulmonary function tests (PFTs) were performed using a pulmonary function test device with disposable filters (Vyntus™ Spiro; CareFusion, San Diego, CA, USA) by experienced examiners. All pulmonary function tests were performed without bronchodilator use. We measured the following parameters by pulmonary function tests: forced vital capacity (FVC); refers to the total expiratory volume exhaled with one breath during the maximum effort expiration, forced expiratory volume in one second (FEV1); refers to the expiratory volume expelled in the first second of the maximum effort expiration and is associated with the severity of obstructive ventilation disorder, forced expiratory volume in one second/forced vital capacity (FEV1/FVC), forced expiratory flow at 25% and 75% of the pulmonary volume (FEF25–75%); the mean forced expiratory flow during the middle half of the FVC, which helps diagnose peripheral small airway obstruction and is the first abnormal finding in smokers, forced expiratory volume in 6 s (FEV6), and peak expiratory velocity (PEV).
Statistical analysis
We used the chi-square test for comparative analysis based on the alcohol consumption status, while the Student’s t-test analysis was performed to compare each variable between men and women. A linear-by-linear association test was performed for comparative analysis based on the amount of smoking. Regression analysis was performed by considering FEV1, FVC, FEF25–75%, FEV1/FVC, PEV, and FEV6 as dependent variables to evaluate the effect of each item on each pulmonary function value. In addition, Cohen's D in t-test result and F-squared values in linear regression analysis were used to measure the effect size. And, the method used is described in the supplementary file. (Supplemental method) We used SPSS statistical software package version 17 (SPSS Inc., Chicago, USA) for all analyses and a p value < 0.05 was considered statistically significant.
Ethics statement
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the Institutional Review Board of Jeju National University Hospital who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the Institutional Review Board of Jeju National University Hospital.
Supplementary Information
Acknowledgements
The authors are very grateful to Myong Hee Kim, Suan Kang, and Iaan Kang for their enormous support in preparing this manuscript. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing.
Author contributions
Conceptualization, M.B.K. and J.W.K.; Funding acquisition, S.W.C; Data curation, S.W.C., M.B.K., and J.W.K.; Formal analysis, S.W.C., M.B.K., and J.W.K; Validation, S.W.C., M.B.K. and J.W.K.; Writing—original draft and review, S.W.C., M.B.K., and J.W.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was also supported by the Basic Science Research Program through the NRF funded by the Ministry of Education (NRF-2018R1C1B5046919). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All available data generated or analyzed during this study are included in this published article. Other raw data are not available because of regulation of data sharing in the Republic of Korea.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Suk Won Chang and Min Bum Kim.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08234-9.
References
- 1.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: Data from the National Health and Nutrition Examination Survey, 1999–2000. Am. J. Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern. Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 3.Packer L. Protective role of vitamin E in biological systems. Am. J. Clin. Nutr. 1991;53(4 Suppl):1050s–s1055. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 4.Harding AH, Wareham NJ, Bingham SA, et al. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer–Norfolk prospective study. Arch. Intern. Med. 2008;168(14):1493–1499. doi: 10.1001/archinte.168.14.1493. [DOI] [PubMed] [Google Scholar]
- 5.Rafighi Z, Shiva A, Arab S, Mohd YR. Association of dietary vitamin C and E intake and antioxidant enzymes in type 2 diabetes mellitus patients. Global J. Health Sci. 2013;5(3):183–187. doi: 10.5539/gjhs.v5n3p183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keranis E, Makris D, Rodopoulou P, et al. Impact of dietary shift to higher-antioxidant foods in COPD: A randomised trial. Eur. Respir. J. 2010;36(4):774–780. doi: 10.1183/09031936.00113809. [DOI] [PubMed] [Google Scholar]
- 7.Britton JR, Pavord ID, Richards KA, et al. Dietary antioxidant vitamin intake and lung function in the general population. Am. J. Respir. Crit. Care Med. 1995;151(5):1383–1387. doi: 10.1164/ajrccm.151.5.7735589. [DOI] [PubMed] [Google Scholar]
- 8.Hong JY, Lee CY, Lee MG, Kim YS. Effects of dietary antioxidant vitamins on lung functions according to gender and smoking status in Korea: A population-based cross-sectional study. BMJ Open. 2018;8(4):e020656. doi: 10.1136/bmjopen-2017-020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung YJ, Lee SH, Chang JH, Lee HS, Kang EH, Lee SW. The impact of changes in the intake of fiber and antioxidants on the development of chronic obstructive pulmonary disease. Nutrients. 2021;13(2):580. doi: 10.3390/nu13020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama F, Lee AH, Terasawa K, Kagawa Y. Folate intake associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease. Asia Pac. J. Clin. Nutr. 2010;19(1):103–109. [PubMed] [Google Scholar]
- 11.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Lee E, Hyun T. Dietary folate intake and food sources of children and adolescents in Chungcheong area-using nutrient database revised by measured folate in selected foods. J. Nutr. Health. 2015;48(1):94–104. doi: 10.4163/jnh.2015.48.1.94. [DOI] [Google Scholar]
- 13.Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of nutrition for development-folate review. J. Nutr. 2015;145(7):1636s–s1680. doi: 10.3945/jn.114.206599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naderi N, House JD. Recent developments in folate nutrition. Adv. Food Nutr. Res. 2018;83:195–213. doi: 10.1016/bs.afnr.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Kruman II, Kumaravel TS, Lohani A, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2002;22(5):1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papamichael MM, Katsardis C, Tsoukalas D, Lambert K, Erbas B, Itsiopoulos C. Potential role of folate status on pulmonary function in pediatric asthma. Nutrition. 2021;90:111267. doi: 10.1016/j.nut.2021.111267. [DOI] [PubMed] [Google Scholar]
- 17.Han YY, Forno E, Rosser F, Celedón JC. Serum folate metabolites, asthma, and lung function in a nationwide US study. J. Allergy Clin. Immunol. 2020;146(1):220–2.e8. doi: 10.1016/j.jaci.2020.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozyürek M, Bektaşoğlu B, Güçlü K, Güngör N, Apak R. Simultaneous total antioxidant capacity assay of lipophilic and hydrophilic antioxidants in the same acetone-water solution containing 2% methyl-beta-cyclodextrin using the cupric reducing antioxidant capacity (CUPRAC) method. Anal. Chim. Acta. 2008;630(1):28–39. doi: 10.1016/j.aca.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 19.Angelis N, Porpodis K, Zarogoulidis P, et al. Airway inflammation in chronic obstructive pulmonary disease. J. Thorac. Dis. 2014;6(Suppl 1):S167–S172. doi: 10.3978/j.issn.2072-1439.2014.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paniz C, Bertinato JF, Lucena MR, et al. A daily dose of 5 mg folic acid for 90 days is associated with increased serum unmetabolized folic acid and reduced natural killer cell cytotoxicity in healthy Brazilian adults. J. Nutr. 2017;147(9):1677–1685. doi: 10.3945/jn.117.247445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson A, Pollard SL, Lima JJ, et al. Serum folate concentrations, asthma, atopy, and asthma control in Peruvian children. Respir. Med. 2017;133:29–35. doi: 10.1016/j.rmed.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talaminos Barroso A, Márquez Martín E, Roa Romero LM, Ortega RF. Factors affecting lung function: A review of the literature. Arch. Bronconeumol. (Engl. Ed.) 2018;54(6):327–332. doi: 10.1016/j.arbr.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Mottram C. Ruppel’s Manual of Pulmonary Function Testing. 10. Mosby; 2012. [Google Scholar]
- 24.Walda IC, Tabak C, Smit HA, et al. Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countries. Eur. J. Clin. Nutr. 2002;56(7):638–643. doi: 10.1038/sj.ejcn.1601370. [DOI] [PubMed] [Google Scholar]
- 25.Hanson C, Lyden E, Furtado J, et al. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin. Nutr. 2016;35(1):169–174. doi: 10.1016/j.clnu.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data generated or analyzed during this study are included in this published article. Other raw data are not available because of regulation of data sharing in the Republic of Korea.