Abstract
The objective of this study was to compare the steady-state plasma and intrapulmonary concentrations of orally administered pyrazinamide in normal volunteers and subjects with AIDS. Pyrazinamide was administered at 1 g once daily for 5 days to 40 adult volunteers (10 men with AIDS, 10 normal men, 10 women with AIDS, and 10 normal women). Subjects with AIDS and with more than four stools per day were excluded. Blood was obtained prior to administration of the first dose, 2 h after the last dose, and at the completion of bronchoscopy and bronchoalveolar lavage, which were performed 4 h after the last dose. Standardized bronchoscopy was performed without systemic sedation. The volume of epithelial lining fluid (ELF) recovered was calculated by the urea dilution method. The total number of alveolar cells (AC) was counted in a hemocytometer, and differential cell counting was performed after cytocentrifugation. Pyrazinamide was measured by high-performance liquid chromatography. The presence of AIDS or gender had no significant effect on the concentrations of pyrazinamide in plasma. The concentrations of pyrazinamide in ELF and AC were lower in the subjects with AIDS than in the subjects without AIDS, but the difference was not significant. The concentrations in plasma (mean ± standard deviation) were 25.1 ± 7.6 and 21.1 ± 6.8 μg/ml at 2 and 4 h after the last dose, respectively, and were not significantly different from the concentration (17.4 ± 16.9 μg/ml) in AC. The concentration of pyrazinamide in ELF was high (431 ± 220 μg/ml) and was approximately 4 to 40 times the reported MIC for pyrazinamide-susceptible strains of Mycobacterium tuberculosis. The high concentration of pyrazinamide in ELF may contribute in part to the effectiveness of the drug in treating pulmonary tuberculosis.
Pyrazinamide is an essential drug in the treatment of tuberculosis. Its use in combined antituberculosis drug regimens allows the shortening of the treatment duration from 9 months to 6 months. Elimination half-lives in humans have been reported to be in the range of 7 to 10 h (2, 10, 17, 19) and 23 h (6), providing a pharmacokinetic rationale for once-daily dose administration. Peak concentrations in plasma occur at 2 h postdosing and have been reported to be in the range of 29 to 50 μg/ml following single oral doses of 20 to 35 mg/kg of body weight (2, 6, 17, 19) and 37 to 42 μg/ml after multiple oral doses of 24 to 32 mg of a fixed triple-drug combination of isoniazid, rifampin, and pyrazinamide per kg (2). Several authors in the United States have reported that the absorption of antimycobacterial agents is impaired in patients with AIDS (7, 15, 20, 21, 23), but this effect was not demonstrated in a study of patients with tuberculosis and AIDS in Kenya (10).
Pyrazinamide is active against tubercle bacilli that are growing within macrophages (8, 14, 24). Intracellular drug concentrations within cultured mouse macrophages depend on extracellular drug concentrations and the time of contact (1). Whether pyrazinamide penetrates pulmonary macrophages or epithelial lining fluid (ELF) in vivo in humans has not been previously reported.
We (11–13) and others (3–5) have developed techniques for the measurement in vivo of the concentrations of drugs in pulmonary ELF and alveolar cells (AC). The purpose of this study was to compare the steady-state plasma and intrapulmonary concentrations of pyrazinamide in normal volunteers and men and women with AIDS.
MATERIALS AND METHODS
Study design and subjects.
The investigation was a prospective, nonblind, controlled comparison of the effects of gender and AIDS on the concentrations of pyrazinamide in plasma, AC, and ELF. After giving informed consent, subjects provided a medical history and underwent a physical examination, a purified protein derivative skin test, and baseline laboratory testing, including complete blood count, platelet count, blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and total bilirubin. Normal subjects were required to be at least 18 years old and within 10% of acceptable weight for height according to Metropolitan Life Foundation height and weight tables (27). Women were required to be nonlactating and not pregnant. Subjects were excluded if they had a history of asthma requiring daily therapy, tuberculosis, or a positive skin test (greater than 5 mm induration at 48 to 72 h); had intolerance of pyrazinamide or lidocaine; had clinically significant organ dysfunction; took medications other than self-prescribed vitamins, birth control pills, or thyroid replacement therapy on an ongoing basis; or had abnormal serum creatinine or other screening laboratory values outside the normal range (greater than twice normal for subjects with AIDS). Patients with AIDS were required to meet the revised Centers for Disease Control and Prevention criteria for the diagnosis of AIDS (9) and to have (i) fewer than four soft stools per day without hematochezia, (ii) no abdominal pain or cramping, (iii) no nausea or vomiting, and (iv) a negative chest X-ray within 2 weeks of enrollment. If an x-ray had not been done, it was performed as part of the study. Six of the 20 subjects with AIDS (2 men and 4 women) were receiving a regimen containing zidovudine as part of their medical care. When the preenrollment evaluation was completed, 40 subjects were assigned to one of four comparative groups: normal men, normal women, men with AIDS, and women with AIDS.
Pyrazinamide was administered orally in a once-daily dose of 1 g for a total of 5 days. The first and last doses of study medication were administered under direct supervision in the General Clinical Research Center at the University of California, San Francisco (UCSF). Subjects were observed for 30 min after the first dose for adverse effects. Subsequent doses were taken according to verbal and written instructions and documented in writing by the subjects.
Bronchoscopy and BAL.
Standardized bronchoscopy and bronchoalveolar lavage (BAL) (11–13) were performed 4 h after the administration of the last dose. The subjects’ blood pressure, pulse, respiratory rate, and heart rate were recorded prior to, at the completion of, and at 30 and 60 min following the procedure. Fingertip oximetry was monitored throughout the procedure.
Subjects were prepared with a 4% topical lidocaine gargle followed by a 4% topical lidocaine spray. Pledgets soaked with 4% topical lidocaine were then applied to each side of the posterior pharynx, followed by the application of topical 1% lidocaine more distally. No systemic sedation was used. A fiberoptic bronchoscope (Pentax FB-19H) was inserted into the right middle lobe. The instrument was in place for approximately 5 to 7 min.
A total of four 50-ml aliquots of normal saline were instilled into the right middle lobe, and each was immediately aspirated into a trap. The specimens were kept on ice until they were frozen. The first aspirate was discarded. The second, third, and fourth aspirates were pooled (pooled BAL). The volume of the pooled BAL was measured and recorded. Measured aliquots of the pooled BAL were sent to the clinical laboratory at UCSF for cell counts and differential analysis. A known volume of the pooled BAL was immediately spun at 400 × g for 5 min in a refrigerated centrifuge. The supernatant and the cells were separated and frozen at −70°C until assay. A small aliquot of the supernatant was frozen separately for the urea assay.
Specimen processing.
Blood samples were kept on ice until centrifuged. The plasma was separated and then frozen until assay. The cells were resuspended in phosphate-buffered saline (PBS) (pH 7.4) to a 10-fold concentration of the BAL fluid, and the suspension was centrifuged to produce the cell pellet. The cell pellet was sonicated for 2 min with a model 550 Sonic Dismembrator (Fisher Scientific, Santa Clara, Calif.).
Pyrazinamide assay.
Pyrazinamide was measured in plasma, BAL fluid, and AC by high-pressure liquid chromatography. Chromatography was performed at room temperature. Analyses were performed with a Shimadzu (Columbia, Md.) LC-10AD solvent delivery system, a Waters (Milford, Mass.) model 717 Plus Autosampler, and a Perkin-Elmer (Norwalk, Conn.) ABI Spectroflow 783 absorbance detector set at 268 nm and with a sensitivity of 0.02 absorbance unit full scale for plasma and a sensitivity of 0.002 absorbance unit full scale for BAL fluid. The mobile phase consisted of 2.0% acetonitrile in 0.02 M KH2PO4, adjusted to pH 2.6 with phosphoric acid, degassed through a Millipore filter (0.22-μm-pore size), and recycled. It was pumped through a 5-μm Axxiom (Richard Scientific, Novato, Calif.) octadecylsilane column (4.6 mm by 25 cm) at a flow rate of 1.0 ml/min. Chromatograms were integrated on a Shimadzu Chromatopac CR601. The retention times for pyrazinamide and acetazolamide, the internal standard, were 8.4 min and 17.0 min, respectively. PBS was the matrix used for standards and controls against which BAL supernatants and AC were measured. Plasma, BAL supernatant, and AC samples were run for 45 min to avoid late peaks that interfered with the next injection. PBS standards were run for 33 min.
The mean coefficients of variation for all plasma and BAL fluid assays were 5.81% (range, 2.83 to 10.41%) and 2.31% (range, 1.58 to 3.56%), respectively. For both plasma and BAL fluid, there were no significant differences among the means (for each concentration) in intra- and interday precision assessments (P, >0.05; Student’s t test). The mean recoveries of the assay for all determinations with plasma and BAL fluid were 101.3% (range, 95.3 to 108.9%) and 103.1% (range, 100 to 105.6%), respectively. The accuracy ranges for all determinations with plasma and BAL fluid were −4.68 to 8.87% and 0 to 5.6%, respectively. The sensitivities of the assay with plasma and BAL fluid were 0.5 and .005 μg/ml, respectively. The standard curves were prepared with pyrazinamide concentrations of 0.5 to 80 μg/ml in plasma and 0.005 to 0.5 μg/ml in PBS. Weekly determinations of pyrazinamide concentrations in spiked plasma and PBS stored at −70°C revealed no significant degradation of the drug. Spiked PBS controls that were stored frozen were reanalyzed after 1 year and also revealed no significant decrease in drug concentrations.
Quantitation of the volume of ELF and concentrations of antibiotics in ELF and AC.
The amount of ELF recovered was calculated by the urea dilution method (22). The concentration of urea in serum was analyzed by the clinical laboratory at UCSF by use of a coupled urease-glutamate dehydrogenase enzymatic method (28) modified by Boehringer Mannheim Corporation (Indianapolis, Ind.). Measurements were made at a fixed time interval, permitting automated analysis with a BM 747 analyzer (Boehringer). Urea in BAL supernatants was measured by use of a modified enzymatic assay (BUN kit UV-66; Sigma, St. Louis, Mo.) as previously reported (11, 12, 22). Controls were included with every run, and if the results were not within 10% of the known value, the standard curve, control, and specimen assays were repeated.
The volume of ELF in BAL fluid was derived from the following relationship:
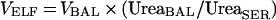 |
where VELF is the volume of ELF sampled by the BAL procedure, VBAL is the volume of aspirated BAL fluid, UreaBAL is the concentration of urea in BAL fluid, and UreaSER is the concentration of urea in serum.
The concentration of antibiotic in ELF (ABXELF) was derived from the following relationship:
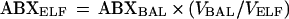 |
where ABXBAL is the measured concentration of antibiotic in BAL fluid.
The volume of AC collected in the pellet suspension was determined from the cell counts determined for the BAL fluid. Cells were counted in a hemocytometer with a lower detection limit of 1.0 × 106/liter. The calculated number of cells in 1.0 ml of pellet suspension was determined to be equal to the number of cells per liter of BAL fluid divided by 100. It has been noted, however, that centrifugation causes an average loss of 21% of cells, so that the actual number of cells recovered may be lower than the number counted and the actual antibiotic concentration may be proportionately lower than that calculated (29). Differential cell counting was performed after the specimen was spun in a cytocentrifuge. The volume of AC collected in the pellet suspension was determined by use of a mean macrophage cell volume of 2.42 μl/106 cells (3).
The concentration of antibiotic in AC (ABXAC) was calculated from the following relationship:
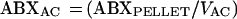 |
where ABXPELLET is the antibiotic concentration in a 1-ml cell suspension and VAC is the volume of AC in a 1-ml cell suspension.
Statistical analysis.
Database management was performed with a Sun 10 Sparcstation (Sun Microsystems, Milpitas, Calif.). PROPHET (version 5.0) (Division of Research Resources, National Institutes of Health, Bethesda, Md., and Bolt, Beranek and Newman, Cambridge, Mass.) (18) was used to compute descriptive statistics, sample sizes, and power curves and to perform the linear regression analyses. Analysis of variance with a two-factor factorial model was used to assess the effects of gender and AIDS status on subject physical characteristics, clinical laboratory values, drug dosage, drug concentrations, AC recovery, ELF recovery, and AC/plasma and ELF/plasma ratios. After data for men and women were grouped, the pyrazinamide concentrations in ELF and AC were compared for subjects with and without AIDS by use of a one-way analysis of variance. Power curves were also calculated for these comparisons. The two-sample equal-variance t test (two sided) was used to compare AC and ELF recoveries and drug concentrations in plasma, AC, and ELF between the groups of women with AIDS who were smokers and nonsmokers. The equality of variances for the smoking and nonsmoking groups was calculated with the F test (Levene’s test). The two-sample Mann-Whitney rank sum test (two sided) was used to compare the CD4 counts in men and women and to compare the serum creatinine determinations between normal subjects and men or women with AIDS. The Shapiro-Wilk test was used to evaluate the normality of the distributions of the data sets prior to comparisons. A P value of <0.05 was regarded as significant.
RESULTS
Ten men with AIDS, 10 men without AIDS, 10 women with AIDS, and 10 women without AIDS were enrolled. Because a fixed daily dose of pyrazinamide was used (1 g), the weight-corrected dose (mean ± standard deviation [SD]) was 17% higher for the 20 women (16.1 ± 2.5 mg/kg) than for the 20 men (13.8 ± 2.9 mg/kg) (P, 0.008). The age (mean ± SD) of the 40 volunteers was 36.6 ± 8 years. The subjects with AIDS were older than the subjects without AIDS (41.3 ± 4.5 versus 31.9 ± 8 years) when the four groups were compared (P, ≤0.00001). The CD4 counts (mean ± SD) for the 10 men and 10 women with AIDS were 234 ± 153 and 238 ± 139, respectively, and were not significantly different (P, >0.05). All of the serum creatinine determinations were within normal limits; however, the values (mean ± SD) were significantly higher in men with AIDS (1.09 ± 0.25 mg/dl) than in the normal men (0.87 ± 0.14 mg/dl) (P, <0.05). The serum creatinine determinations were not significantly different when the women with AIDS (0.85 ± 0.2 mg/dl) were compared to the normal women (0.84 ± 0.1 mg/dl) (P, > 0.05). Five of the 10 female subjects with AIDS were cigarette smokers; the remainder of the subjects were nonsmokers.
All 40 subjects recruited for the study successfully underwent bronchoscopy and BAL. There were no major adverse events, and all of the subjects returned to their normal duties. One subject experienced transient chest discomfort after bronchoscopy that subsided spontaneously. The temperature was transiently elevated in eight subjects.
The number (mean ± SD) of AC recovered from BAL fluid in all 40 subjects was 2.1 × 108 ± 1.6 × 108 cells/liter; AC recovery was greater in men and women with AIDS than in the normal subjects (P, 0.0002) (Table 1). Although cell recovery was threefold greater in the smoking than in the nonsmoking women with AIDS, the difference was not significant (P, 0.6). The majority of the cells in both groups (with and without AIDS) were in the monocyte/macrophage class (Table 1). The increased total AC count in AIDS subjects could not be accounted for by any particular cell type. The percentages of neutrophils, lymphocytes, and monocytes/macrophages were the same in both groups for each cell type (P, >0.05). The volume (mean ± SD) of ELF recovered from the 40 subjects was 0.9 ± 0.5 ml and was not significantly different when the four groups were compared (P, >0.05) or when smoking versus nonsmoking women with AIDS were compared (P, 0.48).
TABLE 1.
Recovery of cells from BAL fluid from 40 subjects according to gender and AIDS status
| Measure | Value for:
|
|||
|---|---|---|---|---|
| Men with AIDS (n = 10) | Men without AIDS (n = 10) | Women with AIDS (n = 10) | Women without AIDS (n = 10) | |
| Mean cells/literab | 2.3 × 108 | 1.1 × 108 | 3.5 × 108 | 1.4 × 108 |
| SD cells/liter | 1.6 × 108 | 1.1 × 108 | 3.5 × 108 | 9.9 × 107 |
| Minimum cells/liter | 5.3 × 107 | 5.5 × 107 | 1.7 × 108 | 5.8 × 107 |
| Maximum cells/liter | 5.1 × 108 | 1.7 × 108 | 6.9 × 108 | 3.7 × 108 |
| PMNs (%)bc | 4.1 ± 6.0 | 0.8 ± .8 | 6.0 ± 12.0 | 1.6 ± 1.4 |
| Lymphocytes (%) | 10.9 ± 8.0 | 10.3 ± 20 | 13.6 ± 15.3 | 17.0 ± 16.3 |
| Monocytes/macrophages (%) | 84.4 ± 8.2 | 88.1 ± 20 | 78.5 ± 16.8 | 81.1 ± 16.2 |
| Eosinophils (%) | 0.3 ± 0.7 | 0.1 ± 0.3 | 0.2 ± 0.4 | 0.1 ± 1.0 |
| Degenerated cells (%) | 0.3 ± 0.9 | 0.7 ± 2.2 | 1.5 ± 3.2 | 0.2 ± 0.6 |
Values for men and women with AIDS combined were greater than those for normal subjects (P, 0.0002); there was no effect of gender alone or of gender and the presence of AIDS together on cell recovery (P, >0.05).
The number of AC and the percentage of polymorphonuclear leukocytes (PMNs) in BAL fluid were not significantly different when smoking women with AIDS were compared to nonsmoking women with AIDS (P for both, 0.63).
Gender, the presence of AIDS, or an interaction between gender and AIDS had no significant effect on the percentage of PMNs in BAL fluid (P, 0.5, 0.07, and 0.8, respectively).
The presence of AIDS had no effect on plasma pyrazinamide concentrations in men or women at 2 or 4 h (Table 2). Plasma pyrazinamide concentrations (mean ± SD) were higher at 2 h (25.1 ± 7.6 μg/ml) than at 4 h (21.1 ± 6.8 μg/ml) following the last dose when the two time periods were compared for all 40 subjects (P, <0.05). Plasma drug concentrations were also higher when all 20 women were compared with all 20 men at 2 h (28.4 ± 7.8 versus 21.8 ± 5.9 μg/ml; P, <0.05) and at 4 h (24.0 ± 6.4 versus 18.2 ± 5.9 μg/ml; P, <0.05). This result was not unexpected, since the fixed dose used in this study, 1 g daily for 5 days, represented a 17% higher weight-adjusted dose for women than for men. There was also an inverse relationship when the weights of all 40 subjects were correlated with the plasma drug concentrations at 2 h (R, −0.53; P, 0.0004) and at 4 h (R, −0.55; P, 0.0002). The weights of the subjects, however, were not correlated with AC (R, −0.20; P, 0.2) or ELF (R, −0.06; P, 0.7) drug concentrations.
TABLE 2.
Pyrazinamide concentrations in plasma, ELF, and AC
| Sample | Valuea for:
|
|||
|---|---|---|---|---|
| Men with AIDS (n = 10) | Men without AIDS (n = 10) | Women with AIDS (n = 10) | Women without AIDS (n = 10) | |
| Plasma | ||||
| 2 h | 20 ± 4.2 | 23.6 ± 7.0 | 28.5 ± 10.3b | 28.2 ± 4.7b |
| 4 h | 16.4 ± 5.42 | 20.1 ± 6.2 | 25.0 ± 7.1c | 23.0 ± 5.8c |
| ELF | ||||
| Mean ± SD | 406 ± 198d | 443 ± 180d | 340 ± 167d | 535 ± 297d |
| Range | 115–674 | 184–752 | 136–577 | 146–1102 |
| AC | ||||
| Mean ± SD | 12.2 ± 10.2d | 21.8 ± 18.6d | 12.2 ± 17.0d | 22.3 ± 21.0d |
| Range | 1.0–32.0 | 3.2–60.0 | 0–53.4 | 3.3–74.6 |
Data are given as the mean ± 1 SD in micrograms per milliliter. Range data are given as minimum to maximum in micrograms per milliliter.
For women versus men, the P value was 0.005; there was no significant effect of the presence of AIDS alone (P, 0.67) or of the presence of AIDS and gender together (P, 0.15).
For women versus men, the P value was 0.005; there was no significant effect of the presence of AIDS alone (P, 0.46) or of the presence of AIDS and gender together (P, 0.39).
There was no significant effect of the presence of AIDS or gender alone or of the presence of AIDS and gender together (P, >0.05) on pyrazinamide concentrations in ELF or AC.
When smoking women with AIDS were compared to nonsmoking women with AIDS, plasma drug concentrations (mean ± SD) at 2 h (30.3 ± 10.6 versus 26.7 ± 10.9 μg/ml) and at 4 h (28.1 ± 5.9 versus 21.9 ± 7.5 μg/ml) were not significantly different (P, > 0.05). When women with AIDS who were receiving zidovudine were compared to women with AIDS who were not receiving zidovudine, the plasma pyrazinamide concentrations at 1 h (35.4 ± 5.3 versus 23.9 ± 10.6 μg/ml) and at 4 h (30.1 ± 4.2 versus 21.6 ± 6.7 μg/ml) were not significantly different. CD4 counts in the 20 human immunodeficiency virus-positive subjects were not correlated with the concentrations of pyrazinamide in plasma at 2 h (R, 0.1; P, 0.5) or 4 h (R, 0.08; P, .7) or in AC (R, 0.3; P, 0.2) or ELF (R, 0.2; P, 0.3).
In men and women with AIDS, the AC pyrazinamide concentrations were approximately 50% those observed in the subjects without AIDS, but the difference was statistically significant (P, 0.09) (Table 2). When the male and female data were grouped to create a larger sample size (n = 20 in each group), the difference between subjects with and without AIDS was still not significant (P, 0.08). The power curve indicated that the two-sided t test would only be able to detect a difference in the observed mean ± SD (12.8 ± 12.9 μg/ml in subjects with AIDS versus 22.0 ± 19.3 μg/ml in subjects without AIDS) as significant (P, < 0.05) with a power of 40%. Therefore, the variability of the AC pyrazinamide concentrations would require larger sample sizes (approximately 50 subjects in each combined male-female group) to detect the observed difference as significant (P, < 0.05) with a greater certainty (power of 80%). The AC pyrazinamide concentration/plasma pyrazinamide concentration ratios (mean ± SD) were 0.68 ± 0.6 and 0.83 ± 0.7 when the 2- and 4-h plasma drug concentrations were used for the calculations, respectively. AC pyrazinamide concentrations (mean ± SD) in smoking (19.2 ± 21.2 μg/ml) and nonsmoking (7.4 ± 3.8 μg/ml) women with AIDS were not significantly different (P, >0.05).
Pyrazinamide was highly concentrated in ELF (Table 2). As with the AC pyrazinamide concentrations, the ELF pyrazinamide concentrations were lower in the subjects with AIDS than in the subjects without AIDS, but the difference was not statistically significant (P, 0.10). When the male and female data were grouped to create a larger sample size (n = 20 in each group), the difference between subjects with and without AIDS was still not significant (P, 0.10). The power curve indicated that the two-sided t test would only be able to detect a difference in the observed mean ± SD (373.2 ± 181.5 μg/ml in subjects with AIDS versus 489.2 ± 243.9 μg/ml in subjects without AIDS) as significant (P, <0.05) with a power of 40%. As with the AC pyrazinamide concentration data, the variability of the data would require larger sample sizes (approximately 55 subjects in each combined male-female group) to detect the observed difference as significant (P, < 0.05) with a greater certainly (power of 80%). For all 40 subjects, the ELF pyrazinamide concentration/plasma pyrazinamide concentration ratios (mean ± SD) at 2 and 4 h were 17.8 ± 8.4 and 22.0 ± 11.8, respectively. ELF pyrazinamide concentrations (mean ± SD) in smoking (311 ± 203 μg/ml) and nonsmoking (379 ± 138 μg/ml) women with AIDS were not significantly different (P, >0.05).
DISCUSSION
MICs for sensitive strains of Mycobacterium tuberculosis tested with the BACTEC system have been reported to be in the range of <6.2 to 50 μg/ml or 25 to 400 μg/ml when tested at pH 5.5 or pH 5.95, respectively (25). A bimodal distribution of sensitive and resistant strains tested with the BACTEC system at pH 6.0 has suggested a breakpoint of 100 μg/ml to indicate susceptibility (26). For clinical purposes, the recommended breakpoints for susceptible, moderately susceptible, moderately resistant, and resistant strains are <100, 300, 900, and >900 μg/ml, respectively (16).
We have demonstrated that the absorption of pyrazinamide is not affected by gender or by the presence of AIDS, as defined in our subjects. Plasma drug concentrations were higher in women than in men in this study, but the difference is explainable by the higher weight-adjusted dose received by the women. In all subjects, plasma drug concentrations at 2 h were significantly higher than those at 4 h, consistent with previous reports that have described the kinetics of pyrazinamide in plasma (17, 19). It is of some concern that the plasma and intracellular drug concentrations achieved in this study, even allowing for the low dose used, would be at or below the MICs that have been reported for many pyrazinamide-susceptible strains of M. tuberculosis. While the clinical significance of this finding is unknown, the observation suggests the possibility that marginal increases in the MIC for the organism may translate to in vivo resistance to pyrazinamide therapy. The optimum dose of pyrazinamide in relation to the MIC for the organism has not been determined. The relationship between the MIC of pyrazinamide for M. tuberculosis and drug effectiveness requires further investigation.
We have also shown that pyrazinamide penetrates AC at concentrations that are not significantly different from concentrations in plasma. It is of interest that the pyrazinamide concentrations in AC and ELF were not higher in the women, as were the concentrations in plasma, and were not negatively correlated with the weight of the subjects. The mechanism for this finding is unknown, but the observation suggests that the AC and ELF drug concentrations were determined by factors other than the size of the patient. The dose of pyrazinamide used in this pharmacokinetic study (1 g daily) was intentionally lower than that recommended for clinical purposes (20 to 25 mg/kg/day) in order to avoid toxicity in volunteer subjects.
The high concentration of pyrazinamide in ELF was an unexpected finding. ELF drug concentration/plasma drug concentration ratios were approximately 20:1. Based upon the reported range of MICs of pyrazinamide for M. tuberculosis (25, 26), this finding would indicate that considerable antituberculosis activity resides in ELF. For example, for an organism for which the MIC is 20 μg/ml, inhibitory ratios of 15:1 to 25:1 would be present in ELF.
The physiological basis for the differential penetration of pyrazinamide into ELF is unknown. Further, the in vivo effect of high ELF concentrations on antimicrobial activity and the clinical significance of plasma, AC, or ELF pyrazinamide concentrations are not known. In general, high inhibitory or killing ratios are viewed as favorable in the treatment of infectious diseases. It is likely that the high intrapulmonary drug concentrations observed in this study are in part responsible for the effectiveness of pyrazinamide in the treatment of pulmonary tuberculosis.
We were unable to demonstrate a difference in plasma, AC, or ELF drug concentrations in smoking versus nonsmoking women with AIDS or an effect of gender or AIDS on drug concentrations in ELF or AC. However, the concentrations of pyrazinamide in AC and ELF were lower in subjects with AIDS than in those without AIDS. Because of the interpatient variability of the data, the power of this study to detect a significant difference was low, and investigation of a larger group of subjects might detect such a difference. The clinical significance of lower AC and ELF pyrazinamide concentrations in subjects with AIDS is unknown and merits further investigation.
We were also unable to confirm the potential drug interaction between zidovudine and pyrazinamide described in an earlier study (21). Plasma pyrazinamide concentrations in women receiving zidovudine were not significantly different from those in women who were not receiving zidovudine. The study was not designed to detect interactions among the many other drugs taken by our AIDS patients and pyrazinamide.
ACKNOWLEDGMENTS
This work was carried out with funds provided by NIH grant AI36054 and with funds provided by NIH grant MO1RR00079 (to the General Clinical Research Center at UCSF).
We thank Charles L. Daley for his assistance, Margareta Andersson for performing the assays, and Belinda Biggs and Eve Benton for manuscript preparation.
REFERENCES
- 1.Acocella G, Carlone N A, Cuffini A M, Cavallo G. The penetration of rifampicin, pyrazinamide, and pyrazinoic acid into mouse macrophages. Am Rev Respir Dis. 1985;132:1268–1273. doi: 10.1164/arrd.1985.132.6.1268. [DOI] [PubMed] [Google Scholar]
- 2.Acocella G, Nonis A, Perna G, Patane E, Gialdroni-Grassi G, Grassi C. Comparative bioavailability of isoniazid, rifampin, and pyrazinamide. Am Rev Respir Dis. 1988;138:886–890. doi: 10.1164/ajrccm/138.4.886. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin D R, Wise R, Andrews J M, Ashby J P, Honeybourne D. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J. 1990;3:886–890. [PubMed] [Google Scholar]
- 4.Baldwin D R, Wise R, Honeybourne D. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother. 1992;36:1171–1175. doi: 10.1128/aac.36.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin D R, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agents Chemother. 1992;36:1176–1180. doi: 10.1128/aac.36.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bareggi S R, Cerutti R, Pirola R, Riva R, Cisternino M. Clinical pharmacokinetics and metabolism of pyrazinamide in healthy volunteers. Arzneimittelforschung. 1987;37:849–854. [PubMed] [Google Scholar]
- 7.Berning S E, Huitt G A, Iseman M D, Peloquin C A. Malabsorption of antituberculosis medications by a patient with AIDS. N Engl J Med. 1992;327:1817–1818. doi: 10.1056/NEJM199212173272514. [DOI] [PubMed] [Google Scholar]
- 8.Carlone N A, Acocella G, Cuffini A M, Forno-Pizzoglio M. Killing of macrophage-ingested mycobacteria by rifampicin, pyrazinamide, and pyrazinoic acid alone and in combination. Am Rev Respir Dis. 1995;132:1274–1277. doi: 10.1164/arrd.1985.132.6.1274. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269:729–730. [PubMed] [Google Scholar]
- 10.Choudhri S H, Hawken M, Gathua S, Minyiri G O, Watkins W, Sahai J, Sitar D S, Aoki F Y, Long R. Pharmacokinetics of antimycobacterial drugs in patients with tuberculosis, AIDS, and diarrhea. Clin Infect Dis. 1997;25:104–111. doi: 10.1086/514513. [DOI] [PubMed] [Google Scholar]
- 11.Conte J E, Jr, Golden J, Duncan S, McKenna E, Zurlinden E. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob Agents Chemother. 1995;39:334–338. doi: 10.1128/aac.39.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte J E, Jr, Golden J, Duncan S, McKenna E, Zurlinden E. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cerfuroxime in volunteer subjects. Antimicrob Agents Chemother. 1996;40:1617–1622. doi: 10.1128/aac.40.7.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte J E, Jr, Golden J. Intrapulmonary and systemic pharmacokinetics of aerosolized pentamidine used for prophylaxis of Pneumocystis carinii pneumonia in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1995;35:1166–1173. doi: 10.1002/j.1552-4604.1995.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 14.Crowle A J, Sbarbaro J A, May M H. Inhibition by pyrazinamide of tubercle bacilli within cultured human macrophages. Am Rev Respir Dis. 1986;134:1052–1055. doi: 10.1164/arrd.1986.134.5.1052. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S M, Horsburgh C R, Peloquin C A. Low serum levels of oral antimycobacterial agents in patients with disseminated Mycobacterium avium complex disease. J Infect Dis. 1993;168:1559–1562. doi: 10.1093/infdis/168.6.1559. [DOI] [PubMed] [Google Scholar]
- 16.Heifits L. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am Rev Respir Dis. 1988;137:1217–1222. doi: 10.1164/ajrccm/137.5.1217. [DOI] [PubMed] [Google Scholar]
- 17.Lacroix C, Hoang T P, Nouveau J, Guyonnaud C, Laine G, Duwoos H, Lafont O. Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur J Clin Pharmacol. 1989;36:395–400. doi: 10.1007/BF00558302. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Research Resources. PROPHET 5.0. Bethesda, Md: National Center for Research Resources, National Institutes of Health; 1997. ., and BBN Systems and Technologies, Cambridge, Mass. [Google Scholar]
- 19.Peloquin C, Jaresko G, Yong C L, Keung A, Bulpitt A, Jelliffe R. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother. 1997;41:2670–2679. doi: 10.1128/aac.41.12.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peloquin C A. Malabsorption of antimycobacterial medications. N Engl J Med. 1993;329:1122–1123. doi: 10.1056/NEJM199310073291513. [DOI] [PubMed] [Google Scholar]
- 21.Peloquin C A, Nitta A T, Burman W J, Brudney K F, Miranda-Massari J R, McGuinness M E, Berning S E, Gerena G T. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother. 1996;30:919–925. doi: 10.1177/106002809603000901. [DOI] [PubMed] [Google Scholar]
- 22.Rennard S I, Basset G, Lecossier D, O’Donnell K M, Pinkston P, Martin P G, Crystal R G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as a marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 23.Sahai J, Gallicano K, Swick L, Tailor S, Garber G, Seguin I, Oliveras L, Walker S, Rachlis A, Cameron D W. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann Intern Med. 1997;127:289–293. doi: 10.7326/0003-4819-127-4-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Salfinger M, Crowle A J, Reller L B. Pyrazinamide and pyrazinoic acid activity against tubercle bacilli in cultured human macrophages and in the BACTEC system. J Infect Dis. 1990;162:201–207. doi: 10.1093/infdis/162.1.201. [DOI] [PubMed] [Google Scholar]
- 25.Salfinger M, Heifets L B. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob Agents Chemother. 1988;32:1002–1004. doi: 10.1128/aac.32.7.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salfinger M, Reller L B, Demchuk B, Johnson Z T. Rapid radiometric method for pyrazinamide susceptibility testing of Mycobacterium tuberculosis. Res Microbiol. 1989;140:301–309. doi: 10.1016/0923-2508(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 27.Statistical Bulletin of the Metropolitan Life Foundation. 1983 Metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 28.Talke H, Schubert G E. Enzymatische Harnstoffbestimmung im Blut und Serum im optischem Test nach Warburg. Klin Wochenschr. 1965;43:174. doi: 10.1007/BF01484513. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox M, Kervitsky L, Watters C, King T E. Quantification of cells recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1988;138:74–80. doi: 10.1164/ajrccm/138.1.74. [DOI] [PubMed] [Google Scholar]


