Abstract
A combination of atovaquone and proguanil has been found to be quite effective in treating malaria, with little evidence of the emergence of resistance when atovaquone was used as a single agent. We have examined possible mechanisms for the synergy between these two drugs. While proguanil by itself had no effect on electron transport or mitochondrial membrane potential (ΔΨm), it significantly enhanced the ability of atovaquone to collapse ΔΨm when used in combination. This enhancement was observed at pharmacologically achievable doses. Proguanil acted as a biguanide rather than as its metabolite cycloguanil (a parasite dihydrofolate reductase [DHFR] inhibitor) to enhance the atovaquone effect; another DHFR inhibitor, pyrimethamine, also had no enhancing effect. Proguanil-mediated enhancement was specific for atovaquone, since the effects of other mitochondrial electron transport inhibitors, such as myxothiazole and antimycin, were not altered by inclusion of proguanil. Surprisingly, proguanil did not enhance the ability of atovaquone to inhibit mitochondrial electron transport in malaria parasites. These results suggest that proguanil in its prodrug form acts in synergy with atovaquone by lowering the effective concentration at which atovaquone collapses ΔΨm in malaria parasites. This could explain the paradoxical success of the atovaquone-proguanil combination even in regions where proguanil alone is ineffective due to resistance. The results also suggest that the atovaquone-proguanil combination may act as a site-specific uncoupler of parasite mitochondria in a selective manner.
With more than 300 million cases and 2 million deaths estimated to occur annually, malaria continues to be a major problem in the world. The emergence and spread of drug-resistant parasites further exacerbates this serious situation. Quinoline derivatives and parasite dihydrofolate reductase (DHFR) inhibitors have been the drugs most commonly used against malaria during the last 50 years, but resistance to these is now widespread (27, 39). Hence, drugs that target different metabolic features of the malaria parasites are clearly needed, and identification of unique metabolic features of the parasites is a priority in efforts to control malaria. Atovaquone {2-[trans-4-(4′-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone}, a hydroxynaphthoquinone, was developed during the last decade as a potential antimalarial (19–21). Hydroxyquinones were long known to have possible antimitochondrial activity (40) and were explored as antimalarials as early as the 1940s (38). Problems with the toxicity, pharmacokinetics, and metabolic instability of these compounds, however, dampened enthusiasm for them as antimalarial drugs (20). Atovaquone, on the other hand, was found to be well tolerated, metabolically stable, and a very effective agent with a broad-spectrum antiparasite activity (10–21). It is currently used against Pneumocystis carinii pneumonia and toxoplasmosis in patients with AIDS (1, 22, 23). Unfortunately, in clinical trials against Plasmodium falciparum malaria, atovaquone as a single agent met with approximately 30% treatment failure and rapid emergence of resistant parasites (7, 25). A search for drugs with potential synergy with atovaquone identified proguanil [1-(p-chlorophenyl)-5-isopropylbiguanide], an established antimalarial in its own right, as a candidate (4). Clinical trials with an atovaquone-proguanil combination have provided very encouraging results, with cure rates approaching 100% and little evidence of the emergence of resistance (25, 29). This combination (trademark, Malarone) is being registered as an antimalarial, with plans for its controlled distribution in countries where malaria is endemic.
The effectiveness of the atovaquone-proguanil combination, however, is puzzling. Because a major role of the mitochondrial electron transfer chain in malaria parasites is to serve as an electron sink for dihydroorotate dehydrogenase (15, 16), a critical enzyme in the requisite pyrimidine biosynthesis by the parasites, proguanil could act in synergy by inhibiting DHFR, another enzyme important in pyrimidine synthesis. However, proguanil by itself does not have any effect on parasite DHFR; it needs to be metabolized by the host to a cyclic triazine molecule, cycloguanil, the compound that inhibits the parasite DHFR (5, 8). This oxidative conversion is controlled by certain isoforms of cytochrome P450 (2, 17, 41), and about 20% of the Asian and African population are deficient in this metabolic step (18, 37). Furthermore, in Thailand, where the initial atovaquone-proguanil combination clinical trials were conducted, about 90% of the patients with falciparum malaria failed to respond to proguanil alone (25), which was likely due to widespread resistance-imparting point mutations in the parasite DHFR (12, 28, 30). Hence, the synergistic effect of proguanil on atovaquone has been suspected to involve mechanisms other than the inhibition of parasite DHFR.
In their initial studies to investigate the mechanism of atovaquone action, Fry and Pudney (14) used cholate-lysed mitochondria from P. falciparum and Plasmodium yoelii, supplemented with 100 μM heterologous cytochrome c, to assay electron transport through the cytochrome bc1 complex of the parasite respiratory chain. They found that atovaquone inhibited electron transport in this assay. Because of significant technical difficulties associated with obtaining workable quantities of functional mitochondria from malaria parasites, we previously developed a flow cytometry assay to measure electropotential across the inner mitochondrial membrane (ΔΨm) in live intact parasites and showed that atovaquone collapsed ΔΨm in malaria parasites within minutes (34). The compound was also shown to inhibit electron transport as measured by the respiration rate of the intact parasites (34). We have now used these assays to analyze the effects of the atovaquone-proguanil combination on ΔΨm and electron transport in malaria parasites. Our results suggest that proguanil enhances ΔΨm collapse by atovaquone but has no effect on electron transport inhibition by atovaquone. Parasite DHFR inhibitors, on the other hand, have no effect on atovaquone action.
MATERIALS AND METHODS
Mice.
BALB/cByJ mice, 6 to 8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, Maine) and maintained in our American Association of Laboratory Animal Care-accredited animal facility until they were used for various experiments.
Parasites.
P. yoelii 17XL was maintained in vivo in male or female BALB/cByJ mice by repeated passage. After leukocytes and platelets were removed over a microcrystalline cellulose column (10), infected erythrocytes were enriched for schizonts and trophozoites by centrifugation over a Percoll discontinuous density gradient as described earlier (26). The fractions containing schizonts and trophozoites were pooled, washed twice with RPMI 1640 medium containing 1% fetal bovine serum, and evaluated for leukocyte contamination by Giemsa staining of thin blood smears. These purified schizonts and trophozoites were used in all the experiments described here.
Inhibitors.
The antimalaria compound atovaquone was a gift from Glaxo Wellcome, Research Triangle Park, N.C. Proguanil and cycloguanil were kindly provided by Wilbur Milhous, Experimental Therapeutics, Walter Reed Army Institute of Medical Research, Washington, D.C. Mitochondrial respiratory chain inhibitors, uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP), pyrimethamine, and chloroquine were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Flow cytometric assay for ΔΨm.
Flow cytometric assays for measuring the effects of various compounds either alone or in different combinations were carried out with a FACScan (Becton Dickinson Cellular Imaging) essentially as previously described in detail (34). Briefly, 5 × 106 parasitized erythrocytes were incubated with 2 nM 3,3′ dihexyloxacarbocyanine iodide [DiOC6(3)] (Molecular Probes, Eugene, Oreg.) for 20 min at 37°C. At the end of the incubation period, these cells were aliquoted into different tubes and again incubated for 20 min with the compound to be tested. At the end of the incubation period the parasitized cells were subjected to fluorescence-activated cell sorter (FACS) analysis. In each experiment fluorescence intensity measurements were carried out in the presence and absence of probe as well as in the presence of the protonophore CCCP. The results were expressed as percent inhibition of the fluorescence intensity, using the measurement in the presence of CCCP as a reference for 100% ΔΨm collapse.
Rate of respiration by P. yoelii-infected erythrocytes.
The rate of O2 consumption was measured in a closed system with Clark’s oxygen electrode and K-lC Oxygraph (Gilson Medical Electronics Inc., Middleton, Wis.), following the method of Chance and Williams (6) exactly as described elsewhere in detail (34). The rate of oxygen consumption was measured in a reaction volume of 1.5 ml containing 108 parasitized erythrocytes/ml at 37°C and was calculated as nanoatoms of oxygen (nAO) consumed per 108 infected erythrocytes per minute. The rate of respiration ranged from 15 to 20 nAO/108 infected erythrocytes/min for these experiments. The difference in the rate of O2 consumption by the infected erythrocytes in the presence versus in the absence of the compound was calculated as the measure of respiration inhibition. Various concentrations of each compound were tested individually in separate sets of experiments.
RESULTS
Effect of proguanil on ΔΨm and respiration.
The effect of proguanil alone on ΔΨm and respiration by live intact parasitized erythrocytes was examined. As shown in Fig. 1A, proguanil concentrations up to 12 μM had no effect on ΔΨm, but a 10-fold-higher concentration (120 μM) reduced DiOC6(3) fluorescence of the parasites. This effect at such high concentration is likely to be irrelevant in pharmacological terms. Hence, we conclude that proguanil by itself has no significant effect on parasite ΔΨm. In comparison, Fig. 1A also shows the effect of atovaquone on ΔΨm. Atovaquone collapsed ca. 70% of ΔΨm at submicromolar concentrations, achieving an EC50 (concentration at which 50% of the maximal effect was observed) of 1.5 nM. The effect of proguanil alone on respiration was also measured (Fig. 1B), and no effect was observed up to a 20 μM concentration of the compound. Atovaquone, as reported earlier (34), inhibited respiration, with an EC50 of 75 nM (Fig. 1B). We conclude that proguanil by itself does not affect the mitochondrial physiology of malaria parasites at pharmacologically relevant concentrations.
FIG. 1.
Concentration-dependent effect of atovaquone and proguanil on ΔΨm and respiration. (A) Concentration-dependent effect of atovaquone and proguanil on ΔΨm. Fluorescence intensity was quantitated by FACS analysis in the presence of various concentrations of atovaquone (■) and proguanil (●). The results are presented as percent inhibition of fluorescence intensity, using CCCP-mediated inhibition as 100%. (B) Concentration-dependent effect of atovaquone and proguanil on parasite respiration. The rate of O2 consumption by P. yoelii-infected erythrocytes was measured in the presence of various concentrations of proguanil (○) and atovaquone (□) independently. The results are plotted as percent inhibition of respiration. The error bars indicate standard deviations.
Because we wished to use the flow cytometric assay for ΔΨm when atovaquone and proguanil are used in combination, it was important to assess the possible effect of this combination on the fluorescence properties of DiOC6(3), the probe used to measure ΔΨm. A wide range of atovaquone concentrations, alone and in combination with various concentrations of proguanil, were tested for their effects on the fluorescence properties of 20 and 250 nM DiOC6(3) by using a fluorospectrophotometer. No quenching or enhancing effects were observed (data not shown), thereby permitting the use of this probe in assessing ΔΨm.
Effect of atovaquone-proguanil combination on ΔΨm.
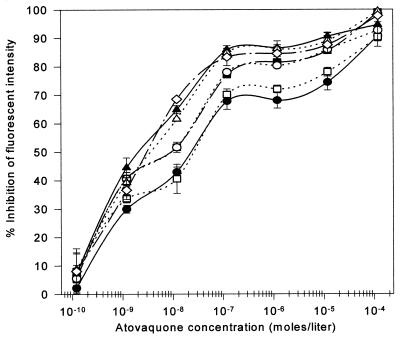
Various concentrations of proguanil, ranging from 1.2 × 10−10 to 1.2 × 10−5 M, were tested for their effects on atovaquone-mediated collapse of ΔΨm in P. yoelii. As shown in Fig. 2, starting with the concentration of 7 × 10−7 M, proguanil enhanced the ability of atovaquone to collapse ΔΨm. The EC50 of atovaquone was reduced approximately sevenfold (from 15 to 2 nM) in the presence of 3.5 × 10−6 M proguanil. More significantly, perhaps, the magnitude of atovaquone-mediated ΔΨm collapse increased from ca. 70 to 85% by inclusion of 3.5 × 10−6 M proguanil. Pharmacokinetic studies have shown that this level of proguanil concentration in plasma is achieved within 3.5 h following therapeutic administration of the drug (18, 24). Hence, the proguanil enhancement seen here appears to approximate therapeutically relevant conditions.
FIG. 2.
Potentiation of atovaquone-mediated collapse of ΔΨm by proguanil. Fluorescence intensity was quantitated by FACS analysis in the presence of various concentrations of atovaquone either alone (●) or in combination with proguanil at various concentrations (3.5 × 10−7 M [□], 7.0 × 10−7 M [■], 1.3 × 10−6 M [○], 3.5 × 10−6 M [▴], 7.0 × 10−6 M [▵], and 1.3 × 10−5 M [◊]). Proguanil concentrations below 3.5 × 10−7 M were also tested but had no effect on ΔΨm collapse by atovaquone. Therefore, the results are not included in the figure for clarity. The error bars indicate standard deviations.
Effect of proguanil on myxothiazole- and antimycin-mediated ΔΨm collapse.
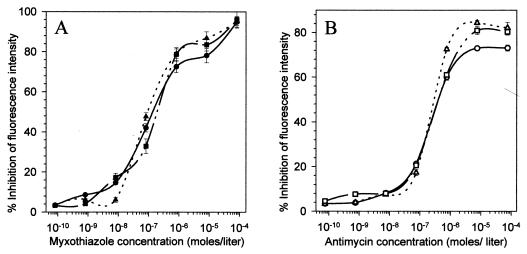
We have previously shown that the well-known cytochrome bc1 complex inhibitors myxothiazole and antimycin collapsed ΔΨm of malaria parasites in a dose-dependent manner (34). Therefore, to test whether proguanil potentiated the effects of all electron transport inhibitors, we examined the effect of proguanil in combination with myxothiazole or antimycin. As shown in Fig. 3, the profiles of ΔΨm collapse by myxothiazole (Fig. 3A) and antimycin (Fig. 3B) were not significantly altered by inclusion of proguanil at 1 μM or higher. A slight increase by inclusion of proguanil in the overall magnitude (70 to 80%) of ΔΨm collapse by the highest concentrations of antimycin (Fig. 3B) is well within the range of magnitude seen at such high inhibitor concentrations and is not likely to be relevant or significant. These results suggest that proguanil enhancement of ΔΨm collapse is specific for its combination with atovaquone and does not occur with other electron transport inhibitors that also work on the cytochrome bc1 complex.
FIG. 3.
Effect of standard mitochondrial inhibitors in combination with proguanil on ΔΨm. (A) Fluorescence intensity was quantitated by FACS analysis in the presence of various concentrations of myxothiazole either alone (●) or in combination with the two highest concentrations of proguanil, i.e., 1.3 × 10−6 (■) and 1.3 × 10−5 M (▴). (B) The effect of antimycin on ΔΨm was determined either alone (○) or in combination with 1.3 × 10−6 (□) and 1.3 × 10−5 (▵) M proguanil. The error bars indicate standard deviations.
Other DHFR inhibitors do not enhance atovaquone-mediated ΔΨm collapse.
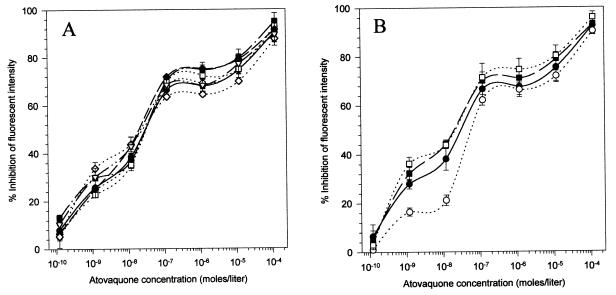
Proguanil is metabolically converted to cycloguanil, which in turn inhibits parasite DHFR, thereby imparting therapeutic value to this antimalarial (5, 8). Although parasites are not believed to possess the cytochrome P450 enzyme needed for cyclization of proguanil, the fact that proguanil was synergistic with atovaquone in P. falciparum cultures (4) raises the possibility that a small amount of cycloguanil could be formed by the parasites themselves, sufficient to inhibit parasite DHFR. Hence, to investigate whether DHFR inhibition was responsible for the potentiation of the atovaquone effect, we used two authentic parasite DHFR inhibitors, cycloguanil and pyrimethamine, to observe their effects on atovaquone-mediated ΔΨm collapse. As shown in Fig. 4, neither of these compounds, over a wide range of concentrations, had any significant effect on atovaquone-mediated ΔΨm collapse. These experiments rule out the possibility that DHFR inhibition was responsible for the enhancement of atovaquone effect.
FIG. 4.
Effect of parasite DHFR inhibitors on atovaquone-mediated collapse of ΔΨm. (A) Concentration-dependent effect of cycloguanil-atovaquone combination on ΔΨm. Fluorescence intensities were quantitated by FACS analysis in the presence of atovaquone, either alone (●) or in combination with a series of cycloguanil concentrations (1.6 × 10−10 M [○], 1.6 × 10−9 M [■] 1.6 × 10−8 M [□], 1.6 × 10−7 M [◊], 1.6 × 10−6 M [◊], and 1.6 × 10−5 M [▵]). (B) Concentration-dependent effect of pyrimethamine-atovaquone combination on ΔΨm. The effect of atovaquone on ΔΨm was determined either alone (●) or in combination with three concentrations of pyrimethamine, i.e., 1.6 × 10−7 M (○), 1.6 × 10−6 M (■), and 1.6 × 10−5 M (□). The error bars indicate standard deviations.
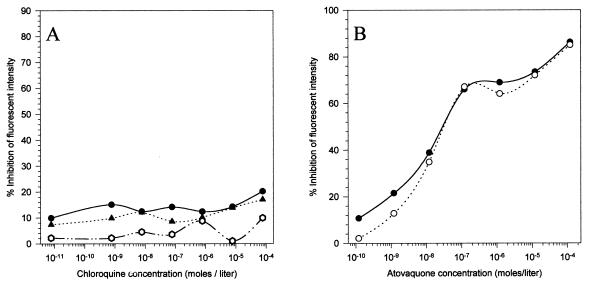
Chloroquine-proguanil combination does not affect ΔΨm. To assess the possibility that proguanil augments ΔΨm collapse in a dying parasite, we tested the chloroquine-proguanil combination for its effect on ΔΨm.
Similarly, the atovaquone-chloroquine combination was also tested for the possible effect of chloroquine on atovaquone-mediated collapse of ΔΨm. As shown in Fig. 5, the proguanil-chloroquine combination had no effect on the ΔΨm of P. yoelii and chloroquine did not affect the profile of atovaquone-mediated ΔΨm collapse.
FIG. 5.
Effect of chloroquine-proguanil and chloroquine-atovaquone combinations on ΔΨm. (A) Fluorescence intensity was quantitated by FACS analysis in the presence of various concentrations of chloroquine either alone (●) or in combination with the two highest concentrations of proguanil, i.e., 1.3 × 10−6 M (○) and 1.3 × 10−5 M (▴). (B) Effects of various concentrations of atovaquone on ΔΨm either alone (●) or in combination with the highest concentration of chloroquine (1.25 × 10−5 M [○]).
Proguanil has no significant effect on respiration inhibition by atovaquone.
We tested the effect of the atovaquone-proguanil combination on mitochondrial electron transport as judged by the respiration rate of the intact parasitized erythrocytes. As shown in Fig. 6, inclusion of proguanil did not alter the profile of atovaquone-mediated respiration inhibition. Concentrations of proguanil (>10−6 M) that significantly enhanced ΔΨm collapse by atovaquone (Fig. 2) had no effect on respiration inhibition by atovaquone. This suggests that inhibition of electron transfer by atovaquone is unaffected by the presence of proguanil.
FIG. 6.
Concentration-dependent effect of atovaquone-proguanil combination on parasite respiration. The rate of O2 consumption was measured in the presence of various concentrations of atovaquone either alone (○) or in combination with the two highest concentrations of proguanil (2.3 × 10−6 M [■] and 2.3 × 10−5 M [□]). The error bars indicate standard deviations.
DISCUSSION
At first glance, the collapse of ΔΨm by atovaquone in malaria parasites could be interpreted as a consequence of electron transport inhibition, especially since a lack of ATP synthase (13) may prevent restoration of ΔΨm. However, the results of the experiments described in this paper raise the possibility that atovaquone-mediated ΔΨm collapse is not entirely a consequence of electron transport inhibition. Proguanil by itself had minimal effects on electron transport and ΔΨm of P. yoelii, but it enhanced the ability of atovaquone to collapse membrane potential without affecting electron transport inhibition. This suggests that the respiration-inhibitory property of atovaquone can be dissociated from its uncoupling ability. A large number of metabolic processes relegated to mitochondria (35) are dependent upon metabolic transport across the inner membrane, which in turn is dependent upon maintenance of ΔΨm. Hence, collapsed ΔΨm may have more far-reaching consequences than electron transport inhibition. In this regard, the site-specific protonophoric activity of the atovaquone-proguanil combination suggested by our results may prove to be a novel mechanism of action.
Our results clearly show that proguanil acts to enhance the atovaquone effect as a biguanide rather than as its metabolite cycloguanil. Biguanides have a long history of use as agents that affect cellular physiology (32). Drugs such as metformin are currently used as hypoglycemic agents for the treatment of insulin-independent diabetes (9). It was initially suggested that these compounds are uncouplers of oxidative phosphorylation (31); however, the uncoupling effect is seen at millimolar concentrations, which are not pharmacologically relevant. The puzzle of atovaquone-proguanil efficacy against malaria in areas where a large number of individuals do not metabolize proguanil, and where proguanil-resistant parasites are widespread, can now possibly be explained by the results described here. The slow uptake of atovaquone and its high lipophilicity (21) may result in a relatively prolonged period of parasite exposure to suboptimal concentrations of the drug when it is used as a single agent. Under these conditions, atovaquone-resistant parasites appear to emerge frequently (7, 25). The inclusion of proguanil with atovaquone will effectively lower the in vivo 50% inhibitory concentration of atovaquone, thereby resulting in parasite demise at atovaquone concentrations which otherwise would have been suboptimal. The net result will be a much lower incidence of treatment failure and resistance emergence, which is what has been observed in clinical trials (25, 29). Pharmacokinetic studies have shown that proguanil concentrations required for the enhancement of atovaquone effect shown here are achieved in an adult within 3.5 h after an oral dose of 200 mg (18, 24).
The molecular basis for proguanil enhancement is unclear. Fidock and Wellems (11) have suggested that proguanil may have intrinsic activity independent of its metabolic activation and DHFR inhibition. Indeed, at concentrations higher than 12 μM, proguanil does seem to have an effect on ΔΨm in malaria parasites, although the specificity of this effect is unclear, since a number of compounds can affect mitochondrial physiology at high concentrations. The concentrations of proguanil used by Fidock and Wellems (11) are significantly higher (50% inhibitory concentration, 50-75 μM) than those achieved in vivo (18, 24). Thus, the relevance of the intrinsic in vitro activity of proguanil to the clinical situation remains unclear. The synergistic activity of proguanil in our experiments was observed at pharmacologically achievable drug concentrations.
Atovaquone appears to block electron transfer from ubiquinol to cytochrome c (14). Atovaquone binds the cytochrome bc1 complex at or around the ubiquinol oxidation (Qo) site, a site believed also to be occupied by standard Qo site inhibitors, such as myxothiazole (3). The crystal structure of the bovine cytochrome bc1 complex revealed that myxothiazole binds in a pocket within the cytochrome b that lies between the low-potential heme and the iron-sulfur cluster of the Rieske protein (42). Indeed, characterization of atovaquone-resistant malaria parasites has identified a discrete cavity within the parasite cytochrome b where atovaquone is likely to bind (33). The specificity of this binding appears to be dictated by the unique structural features of the parasite cytochrome b compared to its mammalian homologue. This binding could explain the electron transport inhibition by atovaquone. The rapidity of the ΔΨm collapse, however, suggests an additional direct effect of atovaquone acting as a site-specific protonophore. This suggestion is consistent with the observation that proguanil is able to enhance ΔΨm collapse without affecting electron transport inhibition. We would like to speculate that, in addition to electron transport inhibition, atovaquone acts to destabilize the cytochrome bc1 complex, causing proton leakage to occur through this site, and that proguanil, through as yet unclear means, enhances this destabilization. This would then result in proton leakage at lower concentrations of atovaquone. Examination of other compounds, especially other biguanides, for their ability to act in a similar synergistic manner could provide additional leads in devising drug combinations that target mitochondrial physiology in malaria parasites.
ACKNOWLEDGMENTS
We thank Joanne Morrisey for expert technical assistance, Hagai Rottenberg for discussion and advice, Glaxo Wellcome for providing atovaquone, and Wilbur Milhous and Dennis Kyle for providing proguanil and cycloguanil.
This work was supported by a grant (AI28398) from the National Institutes of Health.
REFERENCES
- 1.Araujo F G, Huskinson J, Remington J S. Remarkable in vitro and in vivo activities of 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkett D J, Rees D, Andersson T, Gonzalez F J, Miners J O, Veronese M E. In vitro activation of proguanil to cycloguanil by human liver microsomes is mediated by CYP3A isoforms as well as by S-mephenytoin hydrokylase. Br J Clin Pharmacol. 1994;37:413–420. doi: 10.1111/j.1365-2125.1994.tb05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasseur G, Saribas A S, Daldal F. A composition of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc1 complex. Biochim Biophys Acta. 1996;1275:61–69. doi: 10.1016/0005-2728(96)00051-5. [DOI] [PubMed] [Google Scholar]
- 4.Canfield C J, Pudney M, Gutteridge W E. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol. 1995;80:373–381. doi: 10.1006/expr.1995.1049. [DOI] [PubMed] [Google Scholar]
- 5.Carrington H C, Crowther A F, Davey D G, Levi A A, Rose F L. A metabolite of “Paludrine” with high antimalarial activity. Nature. 1951;168:1080. doi: 10.1038/1681080a0. [DOI] [PubMed] [Google Scholar]
- 6.Chance B, Williams G R. A simple and rapid assay of oxidative phosphorylation. Nature. 1955;175:1120–1121. doi: 10.1038/1751120a0. [DOI] [PubMed] [Google Scholar]
- 7.Chiodini P L, Conlon C P, Hutchinson D B, Farquhar J A, Hall A P, Peto T E, Birley H, Warrell D A. Evaluation of atovaquone in the treatment of patients with uncomplicated Plasmodium falciparum malaria. J Antimicrob Chemother. 1995;36:1073–1078. doi: 10.1093/jac/36.6.1073. [DOI] [PubMed] [Google Scholar]
- 8.Crowther A F, Levi A A. Proguanil—the isolation of a metabolite with high antimalarial activity. Br J Pharmacol. 1953;8:93–97. doi: 10.1111/j.1476-5381.1953.tb00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn C J, Peters D H. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs. 1995;49:721–749. doi: 10.2165/00003495-199549050-00007. [DOI] [PubMed] [Google Scholar]
- 10.Eling W. Ficoll fractionation for the separation of parasitized erythrocytes from malaria infected blood. Bull W H O. 1977;55:105–114. [PMC free article] [PubMed] [Google Scholar]
- 11.Fidock D A, Wellems T E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foote S J, Galatis D, Cowman A F. Amino acids in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance. Proc Natl Acad Sci USA. 1990;87:3014–3017. doi: 10.1073/pnas.87.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry M, Webb E, Pudney M. Effect of mitochondrial inhibitors on adenosinetriphosphate levels in Plasmodium falciparum. Comp Biochem Physiol. 1990;96B:775–782. doi: 10.1016/0305-0491(90)90230-q. [DOI] [PubMed] [Google Scholar]
- 14.Fry M, Pudney M. Site of action of the antimalarial hydroxynapthoquinone, 2-[-4-(4′-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-napthoquinone (566C80) Biochem Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 15.Gutteridge W E, Dave D, Richards W H G. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979;582:390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- 16.Hammond D J, Burchell J R, Pudney M. Inhibition of pyrimidine biosynthesis de novo by 2-(4-t-butylcyclohexyl)-3-hydroxy-1,4,napthoquinone in vitro. Mol Biochem Parasitol. 1985;14:97–109. doi: 10.1016/0166-6851(85)90109-4. [DOI] [PubMed] [Google Scholar]
- 17.Helsby N A, Ward S A, Howell R E, Breckenridge A M. The pharmacokinetics and activation of proguanil in man: consequences of variability in drug metabolism. Br J Clin Pharmacol. 1990;30:287–291. doi: 10.1111/j.1365-2125.1990.tb03818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helsby N A, Edwards G, Breckenridge A M, Ward S A. The multiple dose pharmacokinetics of proguanil. Br J Clin Pharmacol. 1993;35:653–656. doi: 10.1111/j.1365-2125.1993.tb04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson A T, Randall A W, Ginger C D, Hill B, Latter V S, McHardy N, Williams R B. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoan activity. Parasitology. 1985;90:45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- 20.Hudson A T, Dickins M, Ginger C D, Gutteridge W E, Holdrich T, Hutchinson D B A, Pudney M, Randall A W, Latter V S. 566C80: a broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drugs Exp Clin Res. 1991;17:427–435. [PubMed] [Google Scholar]
- 21.Hudson A T. Lapinone, menoctone, hydroxyquinolinequinones and similar structures. In: Peters W, Richards W H G, editors. Handbook of experimental pharmacology. 68. Antimalarial drugs. New York, N.Y: Springer-Verlag; 1984. pp. 343–361. [Google Scholar]
- 22.Hughes W T, Gray V L, Gutteridge W E, Latter V S, Pudney M. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental Pneumocystis carinii. Antimicrob Agents Chemother. 1990;34:225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs J A. Efficacy of atovaquone in treatment of toxoplasmosis in patients with AIDS. Lancet. 1992;340:637–688. doi: 10.1016/0140-6736(92)92172-c. [DOI] [PubMed] [Google Scholar]
- 24.Kusaka M, Setiabudy R, Chiba K, Ishizaki T. Simultaneous measurements of proguanil and its metabolites in human plasma and urine by reversed-phase high performance liquid chromatography, and its preliminary application in relation to genetically determined S-mephenytoin 4′-hydroxylation status. Am J Trop Med Hyg. 1996;54:189–196. doi: 10.4269/ajtmh.1996.54.189. [DOI] [PubMed] [Google Scholar]
- 25.Looareesuwan S, Viravan C, Webester H K, Kyle D E, Hutchinson D B A, Canfield C J. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 26.McNally J, O’Donovan S M, Dalton J P. Plasmodium berghei and Plasmodium chabaudi chabaudi: development of simple in vitro erythrocyte invasion assays. Parasitology. 1992;105:355–362. doi: 10.1017/s0031182000074527. [DOI] [PubMed] [Google Scholar]
- 27.Peters W. Plasmodium: resistance to antimalarial drugs. Ann Parasitol Hum Comp. 1990;65(Suppl. 1):103–106. doi: 10.1051/parasite/1990651103. [DOI] [PubMed] [Google Scholar]
- 28.Peterson D S, Milhous W K, Wellems T E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radloff P D, Philipps J, Nkeyi M, Hutchinson D B A, Kremsner P G. Atovaquone and proguanil for Plasmodium falciparum malaria. Lancet. 1996;340:1511–1514. doi: 10.1016/s0140-6736(96)90671-6. [DOI] [PubMed] [Google Scholar]
- 30.Reeder J C, Rieckmann K H, Genton B, Lorry K, Wines B, Cowman A F. Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes and in vitro susceptibility to pyrimethamine and cycloguanil of Plasmodium falciparum isolates from Papua New Guinea. Am J Trop Med Hyg. 1997;55:209–213. doi: 10.4269/ajtmh.1996.55.209. [DOI] [PubMed] [Google Scholar]
- 31.Schafer G. Site specific uncoupling and inhibition of oxidative phosphorylation by biguanides. Biochim Biophys Acta. 1969;172:334–337. doi: 10.1016/0005-2728(69)90077-2. [DOI] [PubMed] [Google Scholar]
- 32.Schafer G. Biguanides. a review of history, pharmacodynamics and therapy. Diabetes Metab. 1983;9:148–163. [PubMed] [Google Scholar]
- 33.Srivastava, I. K., J. M. Morrisey, E. Darrouzet, F. Daldal, and A. B. Vaidya. Submitted for publication.
- 34.Srivastava I K, Rottenberg H, Vaidya A B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in malaria parasites. J Biol Chem. 1997;272:3961–3966. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya A B. Mitochondrial physiology as a target for atovaquone and other antimalarials. In: Sherman I W, editor. Malaria: parasite biology, pathogenesis, and protection. Washington, D.C: ASM Press; 1998. pp. 355–368. [Google Scholar]
- 36.Vaidya A B, Lashgari M S, Pologe L G, Morrisey J. Structural features of Plasmodium cytochrome b that may underlie susceptibility to 8-aminoquinolines and hydroxynaphthoquinones. Mol Biochem Parasitol. 1993;58:33–42. doi: 10.1016/0166-6851(93)90088-f. [DOI] [PubMed] [Google Scholar]
- 37.Ward S A, Helsby N A, Skjelbo E, Brosen K, Gram L F, Breckenridge A M. The activation of the biguanide antimalarial proguanil co-segregates with mephenytoin oxidation polymorphism—a panel study. Br J Clin Pharmacol. 1991;31:689–692. doi: 10.1111/j.1365-2125.1991.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendel W B. The influence of naphthoquinones upon the respiratory and carbohydrate metabolism of malaria parasites. Fed Proc. 1946;5:406–407. [PubMed] [Google Scholar]
- 39.Wernsdorfer W H, Payne D. The dynamics of drug resistance in Plasmodium falciparum. Pharmacol Ther. 1991;50:95–121. doi: 10.1016/0163-7258(91)90074-v. [DOI] [PubMed] [Google Scholar]
- 40.Wiselogle F Y, editor. A survey of antimalarial drugs 1941–1945. Vol. 1. Ann Arbor, Mich: Edwards; 1946. [Google Scholar]
- 41.Wright J D, Helsby N A, Ward S A. The role of S-mephenytoin hydroxylase (CYP2C19) in the metabolism of the antimalarial biguanides. Br J Clin Pharmacol. 1995;39:441–444. doi: 10.1111/j.1365-2125.1995.tb04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia D, Yu C A, Kim H, Xia J H, Kachurin A M, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]