Abstract
Anti tumour necrosis factor (anti-TNF) drugs increase the risk of serious respiratory infection and impair protective immunity following pneumococcal and influenza vaccination. Here we report SARS-CoV-2 vaccine-induced immune responses and breakthrough infections in patients with inflammatory bowel disease, who are treated either with the anti-TNF antibody, infliximab, or with vedolizumab targeting a gut-specific anti-integrin that does not impair systemic immunity. Geometric mean [SD] anti-S RBD antibody concentrations are lower and half-lives shorter in patients treated with infliximab than vedolizumab, following two doses of BNT162b2 (566.7 U/mL [6.2] vs 4555.3 U/mL [5.4], p <0.0001; 26.8 days [95% CI 26.2 – 27.5] vs 47.6 days [45.5 – 49.8], p <0.0001); similar results are also observed with ChAdOx1 nCoV-19 vaccination (184.7 U/mL [5.0] vs 784.0 U/mL [3.5], p <0.0001; 35.9 days [34.9 – 36.8] vs 58.0 days [55.0 – 61.3], p value < 0.0001). One fifth of patients fail to mount a T cell response in both treatment groups. Breakthrough SARS-CoV-2 infections are more frequent (5.8% (201/3441) vs 3.9% (66/1682), p = 0.0039) in patients treated with infliximab than vedolizumab, and the risk of breakthrough SARS-CoV-2 infection is predicted by peak anti-S RBD antibody concentration after two vaccine doses. Irrespective of the treatments, higher, more sustained antibody levels are observed in patients with a history of SARS-CoV-2 infection prior to vaccination. Our results thus suggest that adapted vaccination schedules may be required to induce immunity in at-risk, anti-TNF-treated patients.
Subject terms: Vaccines, Inflammatory bowel disease, Humoral immunity, SARS-CoV-2
Vaccination is effective in protecting from COVID-19. Here the authors report immune responses and breakthrough infections in twice-vaccinated patients receiving anti-TNF treatments for inflammatory bowel disease, and find dampened vaccine responses that implicate the need of adapted vaccination schedules for these patients.
Introduction
Vaccination programmes have reduced SARS-CoV-2 transmission, hospitalisation and deaths1. Patients treated with immunosuppressive drugs were excluded from the original trials for COVID-19 vaccines2,3. Consequently, data relating to the magnitude and durability of immune responses and subsequent vaccine effectiveness in this population are limited4.
Drugs targeting tumour necrosis factor (TNF), such as infliximab, are the most frequently prescribed biologic therapies used in the treatment of immune-mediated inflammatory disorders (IMIDs). Observational studies indicate that most patients with inflammatory bowel disease (IBD), an archetypal IMID, mount serological responses following SARS-CoV-2 vaccines, although most were underpowered to discern the impact of specific drugs, including immunomodulators (azathioprine, mercaptopurine and methotrexate) and/or biologic therapies5–8. We reported that antibody responses following SARS-CoV-2 infection9,10 or a single dose of either the BNT162b2 or ChAdOx1 nCoV-19SARS-CoV-2 vaccines were impaired in anti-TNF treated patients when compared to vedolizumab-treated patients11. Vedolizumab, is a gut-selective anti-integrin α4β7 monoclonal antibody that, unlike anti-TNF drugs, is not associated with increased susceptibility to systemic infection or attenuated serological responses to vaccination12.
In this work, we show that anti-SARS-CoV-2 spike antibody responses are attenuated and less durable following two doses of the BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in infliximab-treated compared with vedolizumab-treated patients with IBD. Irrespective of biologic drug type, one-fifth of all patients do not mount a T cell response and a minority mount neither antibody nor T cell responses. Breakthrough SARS-CoV-2 infections, which are associated with lower antibody levels after the second dose of vaccine, are more common and occur earlier in infliximab-treated patients. Higher and more sustained antibody levels are observed in patients with a history of SARS-CoV-2 infection. Further work to define immunity after third primary and booster vaccine doses is needed to inform the need for adapted vaccination schedules in at-risk anti-TNF treated patients.
Results
Patient characteristics
Between September 22, 2020 and December 23, 2020, 7226 patients were recruited to the CLARITY study from 92 UK hospitals10. In this analysis we included 2279 infliximab-treated and 1031 vedolizumab-treated participants without a history of prior SARS-CoV-2 infection, who had received uninterrupted biologic therapy since recruitment and had an antibody test between 14 and 70 days after the second dose of either the BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines. Participant characteristics are shown in Table 1.
Table 1.
Baseline characteristics of participants who had anti-S receptor-binding domain antibodies measured 2 to 10 weeks following two doses of SARS-CoV-2 vaccine.
| Variable | Vedolizumab | Infliximab | p |
|---|---|---|---|
| Vaccine | |||
| BNT162b2 | 40.1% (413/1031) | 40.1% (914/2279) | 1.0 |
| ChAdOx1 nCoV-19 | 59.9% (618/1031) | 59.9% (1365/2279) | |
| Age (years) | 48.0 (35.2–61.6) | 40.2 (30.1–53.1) | <0.0001 |
| Sex | |||
| Female | 48.0% (492/1024) | 45.9% (1040/2267) | 0.15 |
| Male | 51.8% (530/1024) | 54.1% (1226/2267) | |
| Intersex | 0.0% (0/1024) | 0.0% (0/2267) | |
| Prefer not to say | 0.2% (2/1024) | 0.0% (1/2267) | |
| Ethnicity | |||
| White | 89.9% (920/1023) | 92.5% (2096/2266) | 0.037 |
| Asian | 6.5% (66/1023) | 4.6% (104/2266) | |
| Mixed | 2.4% (25/1023) | 1.4% (32/2266) | |
| Black | 0.5% (5/1023) | 0.8% (18/2266) | |
| Other | 0.7% (7/1023) | 0.7% (16/2266) | |
| Diagnosis | |||
| Crohn’s disease | 36.9% (380/1031) | 67.2% (1531/2279) | <0.0001 |
| UC/IBDU | 63.1% (651/1031) | 32.8% (748/2279) | |
| Duration of IBD (years) | 9.0 (5.0–17.0) | 8.0 (3.0–16.0) | 0.00017 |
| Age at IBD diagnosis (years) | 33.5 (22.8–47.3) | 27.6 (20.1–39.6) | <0.0001 |
| Immunomodulators at vaccine | 20.4% (210/1031) | 57.2% (1304/2279) | <0.0001 |
| 5-ASA | 33.9% (349/1031) | 20.6% (469/2279) | <0.0001 |
| Steroids | 5.9% (61/1031) | 2.8% (64/2279) | <0.0001 |
| BMI | 26.0 (23.0–29.8) | 25.9 (22.8–30.0) | 0.74 |
| Heart disease | 4.9% (50/1023) | 2.6% (59/2261) | 0.0011 |
| Diabetes | 7.6% (78/1023) | 3.6% (82/2261) | <0.0001 |
| Lung disease | 16.4% (168/1023) | 13.1% (296/2261) | 0.013 |
| Kidney disease | 1.9% (19/1023) | 0.8% (17/2261) | 0.065 |
| Cancer | 1.6% (16/1023) | 0.3% (6/2261) | <0.0001 |
| Smoker | |||
| Yes | 8.2% (84/1023) | 10.8% (245/2261) | 0.0010 |
| Not currently | 37.7% (386/1023) | 30.2% (682/2261) | |
| Never | 54.1% (553/1023) | 59.0% (1334/2261) | |
| Exposure to documented cases of COVID-19 | 7.8% (80/1023) | 8.6% (195/2261) | 0.46 |
| Income deprivation score | 0.089 (0.055 - 0.146) | 0.091 (0.052 - 0.153) | 0.92 |
| Active disease (PRO2) | 10.4% (102/977) | 5.2% (111/2153) | <0.0001 |
| Time between vaccine doses (weeks) | 10.9 (9.7–11.1) | 11.0 (10.0–11.3) | 0.0030 |
| Time from second dose to serum sample (weeks) | 5.7 (3.7– 7.7) | 5.7 (3.7–7.7) | 0.73 |
Values presented are median (interquartile range) or percentage (numerator/denominator).
P values represent the results of a Mann–Whitney U, Kruskal–Wallis or Fisher’s exact test.
UC ulcerative colitis, IBDU IBD unclassified, IBD inflammatory bowel disease, 5-ASA 5-aminosalicylic acid, BMI body mass index, PRO2 IBD disease activity.
Additional analyses are presented for a subset of 211 infliximab-treated and 71 vedolizumab-treated patients included in our T cell experiments (Supplementary Table 1), and a further 530 infliximab-treated and 224 vedolizumab-treated participants who had a history of SARS-CoV-2 infection before vaccination (Supplementary Table 2).
Anti-SARS-CoV-2-spike (S) antibody level following two doses of SARS-CoV-2 vaccine
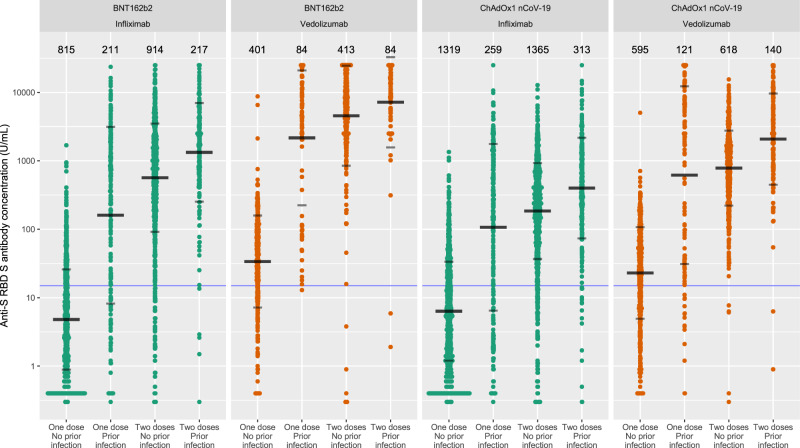
Overall, the geometric mean [geometric SD] of anti-S receptor-binding domain (RBD) antibody concentration was higher in recipients of two doses of the BNT162b2 than ChAdOx1 nCoV-19 vaccines (1084.1 U/mL [7.6] vs 289.9 U/mL[5.2], p < 0.0001). Anti-S RBD antibody concentrations were lower in patients treated with infliximab than in those treated with vedolizumab, following a second dose of BNT162b2 (566.7 U/mL [6.2] vs 4555.3 U/mL [5.4], p < 0.0001) and ChAdOx1 nCoV-19 (184.7 U/mL [5.0] vs 784.0 U/mL [3.5], p < 0.0001) vaccines (Fig. 1).
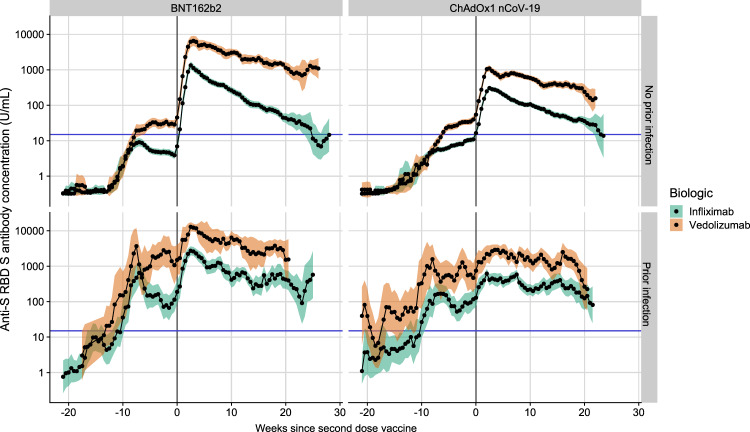
Fig. 1. Anti-S RBD antibody concentration stratified by biologic therapy (infliximab vs vedolizumab), type of vaccine, vaccine dose and history of prior SARS-CoV-2 infection.
The wider bar represents the geometric mean, while the narrower bars are drawn one geometric standard deviation on either side of the geometric mean. Based on published data using neutralisation assays threshold shown of 15 U/mL was used to determine seroconversion11. The biologic treatment infliximab is shown in green and vedolizumab in orange. The number of individuals tested for each group are shown in black at the top of each panel. Source data are provided as a Source Data file.
Crude sensitivity analyses, excluding patients treated with a concomitant immunomodulator, confirmed lower anti-S RBD antibody concentrations in patients treated with infliximab alone versus vedolizumab alone (BNT162b2 809.1 U/mL [4.9] vs 4691.5 U/mL [5.9], p < 0.0001, ChAdOx1 nCoV-19 178.5 U/mL [4.6] vs 778.0 U/mL [3.5], p < 0.0001).
After propensity matching for immunomodulator use and the other factors associated with choice of biologic, we confirmed lower anti-S RBD antibody concentrations in infliximab-treated compared to vedolizumab-treated patients (BNT162b2 600.1 U/mL [6.0] vs 4674.1 U/mL [4.7], p < 0.0001, ChAdOx1 nCoV-19 195.2 U/mL [4.5] vs 779.2 U/mL [3.6], p < 0.0001) (Supplementary Table 3).
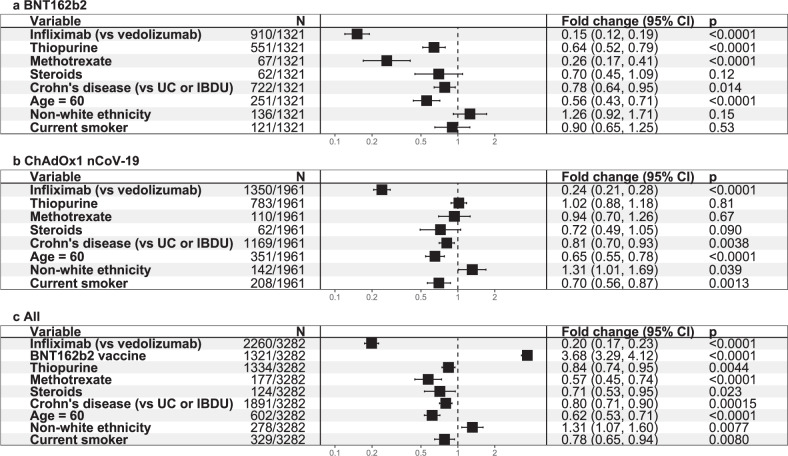
Multivariable linear regression analyses in patients without prior SARS-CoV-2 infection confirmed that antibody concentrations were reduced six and four-fold in infliximab-treated compared with vedolizumab-treated participants who received the BNT162b2 (fold change [FC] 0.15 [95% CI 0.12, 0.19], p < 0.0001) and ChAdOx1 nCoV-19 ([FC] 0.24 [95% CI 0.21, 0.28], p < 0.0001) vaccines (Fig. 2a, b respectively). Age ≥60 years and Crohn’s disease were also independently associated with lower anti-S RBD antibody concentrations in vaccinated participants. Thiopurine or methotrexate use was independently associated with lower anti-S RBD antibody concentrations in participants who received the BNT162b2, but not the ChAdOx1 nCoV-19, vaccine. Current smoking, non-white ethnicity and steroid use were associated with lower anti-S RBD antibody concentrations in participants who received the ChAdOx1 nCoV-19 but not the BNT162b2 vaccine. To assess the effect of vaccine type on antibody responses, we combined our response data in a model that included vaccine type in addition to the significant factors above. Vaccination with the BNT162b2 vaccine compared to the ChAdOx1 nCoV-19 was independently associated with a 3.7 fold [95% CI 3.29–4.12] higher anti-S RBD antibody concentration (p < 0.0001) (Fig. 2c).
Fig. 2. Exponentiated coefficients of linear regression models of log anti-S RBD antibody concentration.
Exponentiated coefficients of the linear regression model of log anti-S RBD antibody concentration in participants who received a BNT162b2 vaccine. b ChAdOx1 nCoV-19 vaccine. c either the BNT162b2 or ChAdOx1 nCoV-19 vaccine. The resultant values represent the fold change of antibody concentration associated with each variable (black square). The horizontal solid line through each square represents the 95% confidence interval. Each vaccine was modelled separately, and then a further model was created using all available data. The vertical dotted line represents a fold change of 1. Tests were two-tailed. p values were derived from linear regression using the t-test statistic and reported without correction for multiple testing. Source data are provided as a Source Data file. UC ulcerative colitis, IBDU IBD unclassified.
Seroconversion rates after the first vaccine dose were lower in infliximab-treated compared to vedolizumab-treated participants (Fig. 1). However, administration of a second vaccine dose resulted in a >100-fold and >25-fold increase in antibody concentrations in recipients of the BNT162b2 and ChAdOx1 nCoV-19 vaccines, respectively (Fig. 1). Overall, more infliximab-treated than vedolizumab-treated patients failed to seroconvert after their second vaccine dose (5.9% vs 1.3%, p < 0.0001). Seroconversion rates stratified by biologic therapy and vaccine type are reported in Supplementary Table 4.
Anti-spike T cell responses following two doses of BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines
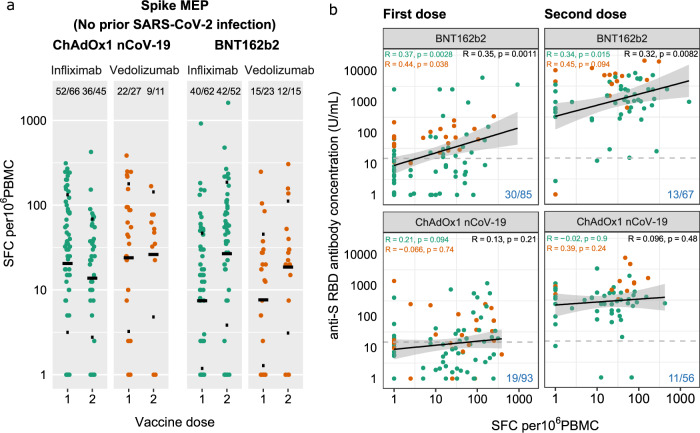
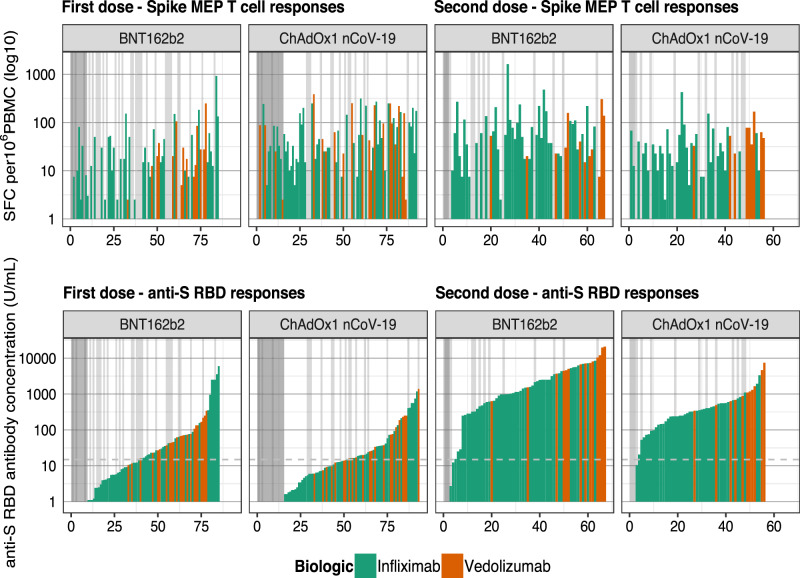
There were no significant differences in the magnitude of anti-spike T cell responses observed in infliximab-treated compared with vedolizumab-treated patients after one or two doses of either vaccine (Fig. 3a). The proportion of patients failing to mount detectable T cell responses were similar in both groups (infliximab 19.6% vs. vedolizumab 19.2%). For recipients of one and two doses of the BNT162b2 vaccine, there was a modest positive correlation between T cell responses and antibody concentration. This association was not observed in recipients following either dose of the ChAdOx1 nCoV-19 vaccine (Fig. 3b). When T cell responses were ranked by magnitude of antibody responses, most patients who did not mount an antibody response after the first vaccine dose (indicated by the dark grey bar) had a detectable T cell response (Fig. 4). In addition to the uncoupling of the T cell and antibody responses demonstrated, this analysis emphasised that about one-fifth of participants made no T cell responses irrespective of vaccine used (indicated by the light grey bars). Moreover, a minority of individuals (3/67) 4.5% for BNT162b2 and (1/56) 1.8% for ChAdOx1 nCoV-19 vaccines carry neither detectable antibody nor T cell responses after two doses of vaccine (Figs. 3b, 4).
Fig. 3. Anti-SARS-CoV-2 spike T cell responses stratified by vaccine platform (BNT162b2 vs ChAdOx1 nCoV-19), biologic therapy (infliximab vs vedolizumab) and vaccine dose (one vs two).
a Spike MEP T cell responses SFC per 106 PBMC stratified by vaccine platform, biologic therapy (infliximab vs vedolizumab) and the number of vaccine doses. The horizontal bar represents the geometric mean and the narrow bars represent one geometric standard deviation on either side of the geometric mean. The number of T cell responders / total number of individuals tested are shown in black at the top of each panel. b Scatterplot demonstrating the correlation between T cell responses against spike MEP pool (SFC per 106 PBMC) and anti-SARS-CoV-2 spike antibody concentration after the first (LHS) and second (RHS) dose of BNT162B2 (top) and ChAdOx1 nCoV-19 (bottom) vaccine. The number of non-T cell responders/total number of individuals tested is shown in blue on the bottom RHS of each panel. The shaded grey band represents the 95% confidence interval. The horizontal dotted line in b represents a threshold of 15 U/mL of anti-S1 SARS-CoV-2 antibody. The tests were two-tailed and p values were reported without correction for multiple testing. The biologic infliximab is shown in green and vedolizumab is shown in orange. Source data are provided as a Source Data file. MEP mapped epitope peptide, SFC spot forming cells, PBMC peripheral blood mononuclear cell, LHS left-hand side, RHS right-hand side, R Spearman’s rank correlation.
Fig. 4. Anti-spike T cell responses ordered by the cumulative magnitude of anti-S RBD following two doses of the BNT162b2 or ChAdOx1 nCoV-19 vaccine show uncoupling of the T cell and antibody responses.
Top panel shows T cell responses to spike, and the bottom panel shows anti-S RBD responses plotted for individual study participants ordered by increasing magnitude of anti-S RBD antibody concentration (U/mL). The vertical dark grey bars at the LHS of the panels indicate individuals with no anti-S RBD response. The vertical light grey bars in the panels indicate individuals with no T cell response. The horizontal dotted line represents a threshold shown of 15 U/mL of anti-S RBD. Source data are provided as a Source Data file. LHS left-hand side, MEP mapped epitope peptide, SFC spot forming cells.
Durability of antibody responses following two doses of BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines
The estimated half-life of anti-S RBD antibodies was shorter in participants receiving the BNT162b2 compared to the ChAdOx1 nCoV-19 vaccines (30.8 days [95% CI 30.3–31.5] vs 40.5 days [95% CI 39.2–41.6], p value <0.0001). When stratified by biologic, half-life estimates were shorter in infliximab-treated than vedolizumab-treated patients following two doses of BNT162b2 (26.8 days [95% CI 26.2–27.5] vs 47.6 [95% CI 45.5–49.8], p value <0.0001) and ChAdOx1 nCoV-19 (35.9 days [95% CI 34.9–36.8] vs 58.0 days [95% CI 55.0–61.3], p value <0.0001) (Supplementary Fig. 1 and Supplementary Table 5).
Overall, following two doses of either vaccine, anti-S RBD antibodies showed minimal decay to the last follow-up in patients treated with vedolizumab (Fig. 5 and Supplementary Fig. 2) and were similar to those observed in participants in the Virus Watch community cohort (Supplementary Fig. 3). However, in infliximab-treated participants, the geometric mean concentrations dropped to the seroconversion threshold by about 25 weeks after the second dose irrespective of the vaccine administered (Fig. 5). Infliximab compared to vedolizumab treatment, current smoking and white ethnicity were associated with a faster fall in anti-S RBD antibody concentration below the seroconversion threshold. (Supplementary Figs. 4, 5).
Fig. 5. Rolling geometric mean antibody concentration over time from the date of the second dose of the SARS-CoV-2 vaccine (week 0) stratified by biologic therapy (infliximab vs vedolizumab), vaccine and history of prior SARS-CoV-2 infection.
Geometric means are calculated using a rolling 15-day window (i.e. 7 days on either side of the day indicated). The shaded areas represent the 95% confidence intervals of the geometric means. The horizontal blue line represents the seroconversion threshold (15 U/mL). The number of participants included at each time point is presented in Supplementary Fig. 2. Overall, data from 4474 participants with no history of prior infection (3029 on infliximab and 1445 on vedolizumab) and 1179 participants with a history of prior infection (833 on infliximab and 346 on vedolizumab) were included in this graph between 22 weeks before and 29 weeks after the second vaccine dose. The biologic treatment infliximab is shown in green and vedolizumab is shown in orange. Source data are provided as a Source Data file.
Breakthrough SARS-CoV-2 infections following two doses of vaccine
Of 5123 participants without polymerase chain reaction (PCR)-positive or serological evidence of prior SARS-CoV-2 infection, 267 had a first positive SARS-CoV-2 PCR test 2 or more weeks after the second vaccine dose. Overall, 89.2% patients were symptomatic: the most commonly reported symptoms were fatigue (73.7%), anosmia/ageusia (71.4%), fever (57.1%), cough (54.9%), myalgia (45.9%), hoarse voice (30.8%), confusion (27.8%) and chest pains (23.3%). Overall, 1.2% (3/253) of participants with PCR-confirmed infection were hospitalised because of COVID-19.
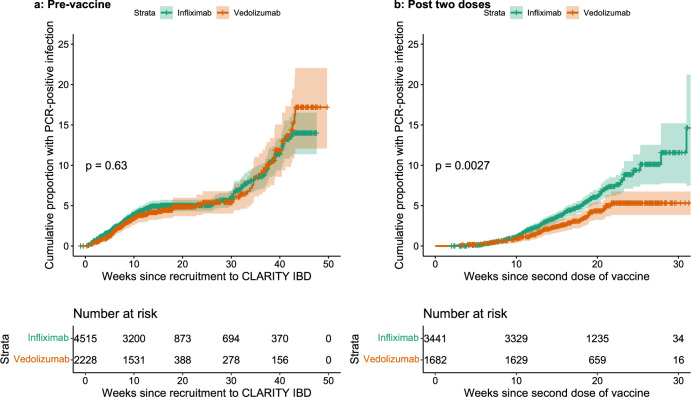
Breakthrough SARS-CoV-2 infections were more frequent (5.8% (201/3441) vs 3.9% (66/1682), p = 0.0039) and the time to breakthrough shorter in patients treated with infliximab than vedolizumab (p = 0.0027) (Fig. 6b). In contrast biologic class did not impact on time to PCR-confirmed infection prior to vaccination (p = 0.63) (Fig. 6a). In a model that included biologic and vaccine type, shorter time to breakthrough infection was associated with infliximab (Hazard Ratio (HR) 1.52 [95% CI 1.15–2.01], p = 0.003) and having received the ChAdOx1 nCoV-19 (HR 1.49 [95% CI 1.15–1.92], p = 0.0023) vaccine. Geometric mean [geometric SD] anti-S RBD antibody concentrations measured 2 to 10 weeks after a second vaccine dose were significantly lower in participants who subsequently had a PCR-confirmed breakthrough SARS-CoV-2 infection: for every tenfold rise in anti-S RBD antibody concentration we observed a 0.8-fold reduction in odds of breakthrough infection ([95% CI 0.70–0.99], p = 0.03).
Fig. 6. Kaplan–Meier graphs comparing the time to PCR-confirmed SARS-CoV-2 infection stratified by biologic therapy (infliximab vs vedolizumab) in participants before vaccination and after receiving two doses of vaccine.
a The time to PCR-confirmed SARS-CoV-2 infection in participants who have not received any dose of either vaccine stratified by biologic therapy (infliximab vs vedolizumab). b The time to a PCR-confirmed SARS-CoV-2 breakthrough infection in participants following two doses of either vaccine stratified by biologic therapy. The biologic treatment infliximab is shown in green and vedolizumab in orange. The number of participants at each time point are displayed in black at the bottom of each figure. P values are calculated using the log-rank test. Source data are provided as a Source Data file.
Antibody responses in patients with prior SARS-CoV-2 infection
Amongst patients with a history of SARS-CoV-2 infection before vaccination, geometric mean [SD] anti-S RBD antibody concentrations were lower in infliximab-treated compared with vedolizumab-treated patients after a second dose of BNT162b2 (1330.0 U/mL [5.3] vs 7169.5 U/mL [4.6], p < 0.0001) and ChAdOx1 nCoV-19 (399.7 U/mL [5.4] vs 2077.3 [4.6] p < 0.0001) vaccines. In all patients, antibody concentrations following vaccination were higher in patients without a history of SARS-CoV-2 infection (Fig. 1). Irrespective of vaccine or biologic type, minimal decay of anti-S RBD antibodies were observed up to a follow-up of 21 weeks.
Discussion
We have shown that in infliximab-treated patients, anti-SARS-CoV-2 spike antibody responses are attenuated following two doses of the BNT162b2 and ChAdOx1 nCoV-19SARS-CoV-2 vaccines. One-fifth of both infliximab-treated and vedolizumab-treated patients did not mount a T cell response and a small subset of patients had neither antibody nor T cell responses. Antibody half-lives were shorter in infliximab-treated patients. Breakthrough SARS-CoV-2 infections were more common and occurred earlier in infliximab-treated patients who received the ChAdOx1 nCoV-19 vaccine. The risk of breakthrough infection was predicted by lower antibody levels after the second dose of the vaccine. Irrespective of biologic treatment, higher and more sustained antibody levels were observed in patients with a history of SARS-CoV-2 infection.
Sustained antibody responses observed in vaccinated patients with a history of prior SARS-CoV-2 infection indicate that third antigen exposure enhances the serological response. This supports the rationale for prioritising a third dose of vaccine to clinically vulnerable patient populations13–16, who otherwise may face further periods of social distancing or hospitalisation following infection. Whilst drawing direct comparisons between IBD patients and patients treated with more potent chemotherapies is limited by the degree to which patients are immunosuppressed, data from solid organ transplant recipients shows that a third dose of vaccine also leads to sustained immune responses17.
Irrespective of biologic or immunosuppressant use, and in keeping with the original trials2,18, the highest antibody responses were seen in recipients of the BNT162b2 vaccine. Like in the general population, these responses waned more quickly than in the recipients of the ChAdOx1 nCoV-19 vaccine19. Unlike the general population20, but similar to renal transplant recipients4, we did not observe differences in T cell ELISpot responses between recipients of the BNT162b2 and ChAdOx1 nCoV-19 vaccines. The differences observed in breakthrough infection by vaccine type reported here are consistent with the differences in efficacy reported in the respective clinical trials2,3,21. The higher peak antibody levels and the lower rate of SARS-CoV-2 breakthrough infections suggest that the BNT162b2 rather than the ChAdOx1 nCoV-19 vaccine should be used for primary vaccination in infliximab-treated patients and, although untested, supports the use of BNT162b2 for third doses in all patients treated with an anti-TNF regardless of the primary vaccine type.
All patients treated with anti-TNF therapy should receive a third primary dose of the SARS-CoV-2 vaccine and our data support recent recommendations that this should occur about 4–8 weeks after the second dose13,14,16 during periods of high transmission in the population. Our data demonstrate that patients treated with vedolizumab and infliximab-treated patients with prior SARS-CoV-2 infection have sustained antibody levels beyond 6 months.
When starting a biologic, it would be reasonable to consider differences in SARS-CoV-2 vaccine response as one of the factors when determining which drug to use. For patients who need to start anti-TNF therapy, the benefits of combination immunomodulator therapy should be weighed against the risk of attenuated vaccine response, and whenever feasible, patients should first receive a SARS-CoV-2 vaccine dose. Further research to determine whether timing third vaccine doses towards the end of anti-TNF treatment cycles when drug levels are lowest leads to greater immunogenicity9 is needed. Other strategies including the temporary discontinuation of immunomodulators22, the use of heterologous vaccines23 and adjuvants including the influenza vaccines (ComFluCOV)24 need to be studied in immunosuppressed patient groups.
The biology underpinning loss of durable antibody responses and uncoupling of the B cell and T cell responses merit further research. TNF is a pleiotropic cytokine and its activities include maturation of antigen-presenting cells, modulation of T cell responses and stimulation of immunoglobulin synthesis25–27. TNF neutralisation, or genetic ablation, results in substantial loss of B-cells in primary follicles in germinal centres, reduced numbers of memory B-cells in the periphery but preserved numbers of T cells25. Uncoupling of humoral and T cell immunity to SARS-CoV-2 has been observed in healthy individuals28, and although the relative contributions of memory B cell and T cell responses have yet to be fully defined in SARS-CoV-2 immunity, the preservation of T cell immunity reported here should provide some reassurance for anti-TNF treated patients. However, it is noteworthy that one-fifth made no anti-spike T cell response following two doses of either vaccine. Chronic TNF exposure, a feature of many IMIDs, can render T cells anergic and can be reversed by anti-TNF treatment29. This may in part explain why the magnitude of T cell responses observed in anti-TNF-treated patients in this study did not differ significantly from patients treated with vedolizumab.
Although our data show major differences in the magnitude and durability of antibody responses, we have not assessed the impact of biologic therapy on specific immunoglobulin classes, antibody neutralisation or mucosal immune responses, which may be impaired, in particular, with anti-a4b7 therapy30,31. However, previous studies have demonstrated that anti-RBD antibody levels such as the ones measured in this study, strongly correlate with Wuhan Hu-1 live virus and variant S RBD neutralisation assays32,33, and we have demonstrated here that early antibody responses to vaccination correlates with the subsequent risk of breakthrough infection in immunosuppressed patients.
Infliximab was associated with attenuated, less durable vaccine-induced anti-SARS-CoV-2 spike antibody responses and a 50% increase in subsequent breakthrough SARS-CoV-2 infection. Further work to define immunity after third primary and booster vaccine doses is needed to inform the need for adapted vaccination schedules in at-risk anti-TNF treated patients.
Methods
Patient and settings
impaCt of bioLogic therApy on saRs-cov-2 Infection and immuniTY (CLARITY) IBD is a UK-wide, multicentre, prospective observational cohort study investigating the impact of infliximab and vedolizumab and/or concomitant immunomodulators (azathioprine, mercaptopurine and methotrexate) on SARS-CoV-2 acquisition, illness and immunity in patients with IBD.
Study methods have been previously described10,11. Consecutive patients were recruited at the time of attendance at infusion units between 22 September 2020 and 23 December 2020 (Supplementary Table 1). Patients aged 5 years and over, with a diagnosis of IBD, treated with infliximab or vedolizumab were eligible for inclusion. Follow-up visits coincided with biologic infusions and occurred eight-weekly. Here, we report vaccine-induced antibody responses after the second dose of either the BNT162b2 or ChAdOx1 nCoV-19 vaccines. Participants were eligible for our primary immunogenicity analysis, if they had had an anti-S RBD antibody test between 14 and 70 days after a second-dose vaccine, defined as the second dose of any of the licenced COVID-19 vaccines, 10-14 weeks after the first dose. Anti-S RBD antibody levels were compared with samples from 605 fully vaccinated adult participants from the Virus Watch study, a household community cohort of 10,000 individuals representative of the UK population of England and Wales recruited between 1 June 2020 to 31 August 202119. Peripheral blood mononuclear cells (PBMC) for T cell experiments were collected from patients 4 to 6 weeks after the first and second dose of vaccine at the time of biologic infusions, at selected sites which could facilitate PBMC extraction within 12 h of venepuncture.
Outcome measures
Our primary outcome was anti-S RBD antibodies 2 to 10 weeks after the second dose of the BNT162b2 or ChAdOx1 nCoV-19 vaccines.
Secondary outcomes were:
-
(i)
the proportion of participants who seroconverted
-
(ii)
anti-spike T cell responses in patients following the first and second dose of vaccines
-
(iii)
the durability of vaccine responses
-
(iv)
risk of breakthrough infections two or more weeks after two doses of vaccine
-
(v)
antibody concentrations and seroconversion rates in patients with PCR or serological evidence of past SARS-CoV-2 infection at, or prior, to the post-vaccination serum sample
Variables
Variables recorded by participants were demographics (age, sex, ethnicity, comorbidities, height and weight, smoking status, and postcode), IBD disease activity (PRO2), SARS-CoV-2 symptoms aligned to the COVID-19 symptoms study (symptoms, previous testing, and hospital admissions for COVID-19) and vaccine uptake (type and date of primary vaccination). Study sites completed data relating to IBD history (age at diagnosis, disease duration, and phenotype according to the Montreal classifications, previous surgeries, and duration of current biologic and immunomodulator therapy)10. We linked our data by NHS number or Community Health Index to Public Health England, Scotland, and Wales archive dates and results of all SARS-CoV-2 PCR tests undertaken and vaccines administered. Data were entered electronically into a purpose-designed REDCap database hosted at the Royal Devon and Exeter NHS Foundation Trust34. Participants without access to the internet or electronic device completed their questionnaires on paper case record forms that were subsequently entered by local research teams.
Laboratory methods
To determine antibody responses specific to vaccination we used the Roche Elecsys Anti-SARS-CoV-2 spike (S) immunoassay35 alongside the nucleocapsid (N) immunoassay36. This double sandwich electrochemiluminescence immunoassay uses a recombinant protein of the receptor-binding domain on the spike protein as an antigen for the determination of antibodies against SARS-CoV-2. Sample electrochemiluminescence signals are compared to an internal calibration curve and quantitative values are reported as units (U)/mL. In-house assay validation experiments on the Roche Elecsys Anti-SARS-CoV-2 spike (S) immunoassay were performed on 20 samples from healthy individuals who have been vaccinated. This demonstrated:
-
i.
The intra-assay and inter-assay coefficient of variation were 1.3% and 5.6%, respectively
-
ii.
Anti-SARS-CoV-2 (S) antibodies were stable in uncentrifuged blood and serum at ambient temperature for up to seven days permitting postal transport
-
iii.
No effect was observed on recovery of anti-SARS-CoV-2 (S) antibodies following four freeze/thaw cycles
-
iv.
No analytical interference was observed for the detection of anti-SARS-CoV-2 (S) with infliximab or vedolizumab up to 10,000 and 60,000 mg/L, respectively, or with anti-drug antibodies to infliximab or vedolizumab up to 400 and 38 AU/mL, respectively (data not shown).
Seroconversion was defined at a threshold of 15 U/mL. ElecSys Anti-SARS-CoV-2 spike (S) RBD concentrations of greater than or equal to 15 U/ml are associated with neutralisation of ≥20% with a positive predictive value of 99.10% (95% CI: 97.74–99.64)11.
At the entry to CLARITY IBD and at follow-up visits, all patients were tested for previous SARS-CoV-2 infection using the Roche Elecsys anti-SARS-CoV-2 (N) immunoassay. We have previously reported that anti-N antibody responses following SARS-CoV-2 natural infection are impaired in patients treated with infliximab or vedolizumab11. As such, a threshold 0.12 times above the cut-off index was set, using receiver operator characteristic curve and area under the curve analysis of anti-N antibody results from participants two weeks following a PCR-confirmed infection to maximise specificity, beyond which patients were deemed to have had prior SARS-CoV-2 infection (Supplementary Fig. 6). Patients with a PCR test confirming SARS-CoV-2 infection at any time prior to vaccination were deemed to have evidence of past infection irrespective of any antibody test result. Breakthrough infections were defined by a positive SARS-CoV-2 PCR test 2 or more weeks after the second vaccine dose.
Peripheral blood mononuclear cell isolation
Whole blood was collected in lithium heparin tubes and PBMCs were isolated by density-gradient centrifugation using LymphoprepTM (Stem Cell Technologies) layered onto SepMateTM (Stem Cell Technologies) tubes. PBMC isolation was performed within 12 h of venepuncture. Purified PBMCs were cryopreserved in 10% DMSO/50% FBS and stored in liquid nitrogen pending batch analysis.
Spike-peptide specific T cell responses
IFN-γ T cell ELISpot assays were performed using pre-coated plates (Mabtech 3420-2APT) and using the protocol described previously28,32. Two-hundred thousand cells were seeded per well and cells were stimulated with a peptide pool, containing 18 peptides derived from SARS-CoV-2 spike protein37 at a concentration of 10 μg/ml/peptide; the peptide pool utilises a mapped epitope pool (MEP) or 12–20mer peptides, mapped as eliciting high-prevalence CD4 responses covering diverse HLA-II haplotypes28,32. Use of this spike MEP in otherwise healthy SARS-CoV-2 seropositive individuals elicits a T cell response in 83% of individuals at 16–18 weeks after natural SARS-CoV-2 infection and 91% of healthy individuals 2–3 weeks after two-dose vaccination with seronegative individuals showing a level of response indistinguishable from pre-pandemic controls28,32. Plates were cultured for 18–20 h before development and data were collected using an AID classic ELISpot plate reader (Autoimmun Diagnostika GMBH). Results are expressed as differences in (delta) spot forming cells (SFC) per 106 PBMC between peptide stimulation and a media-only control. A response below 2 standard deviations of the media-only control wells was deemed to be a null response. Data were excluded if the response to the positive control anti-CD3 stimulation was <200 SFC per 106 PBMCs.
Sample size
The sample size for CLARITY IBD was based on the number of participants required to demonstrate a difference in the impact of infliximab and vedolizumab on seroprevalence and seroconversion following SARS-CoV-2 infection, with an estimated background seroprevalence of 0.05. We calculated that a sample of 6970 patients would provide 80% power to detect differences in the seroprevalence of SARS-CoV-2 antibodies in infliximab-treated compared with vedolizumab-treated patients, whilst controlling for immunomodulator status at the 0.05 significance level.
Statistical analyses
Analyses were undertaken using R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-tailed and p values were reported without correction for multiple testing. P values <0.05 were considered significant. We included patients with missing clinical data in analyses for which they had data and have specified the denominator for each variable. Anti-S RBD antibody concentrations are reported as geometric means and standard deviations. Other continuous data are reported as a median and interquartile range, and discrete data as numbers and percentages, unless otherwise stated.
Univariable analyses, using Spearman’s rank correlation coefficients, and t-tests of log-transformed anti-S RBD antibody concentration were used to identify demographic, disease, vaccine and treatment-related factors associated with the concentration of anti-S RBD antibodies across the cohort. Crude sensitivity analyses excluding patients treated without a concomitant immunomodulator were undertaken to control for the effect of immunomodulator use on anti-S RBD antibody concentrations. Propensity matching was used to account for the other significant differences in baseline variables between infliximab-treated and vedolizumab-treated patients using the MatchIt package38. A priori, patients were matched exactly on diagnosis, immunomodulator use, and then using optimal matching, on age, the number of comorbidities, ethnicity, and presence of active disease. Multivariable linear regression models were used to identify factors independently associated with log anti-S RBD concentration. A priori, we included age, ethnicity, biologic medication and immunomodulator use. Results are presented after exponentiation so that the coefficients of the model correspond to the fold change (FC) associated with each binary covariate. For age, a cut-off was chosen based on a graphical inspection of the relationship between age and anti-S RBD antibody concentrations.
Mann–Whitney U-test was used to compare the magnitude of T cell response (SFC/106 PBMCs) stratified by treatment and vaccine received, and Spearman’s rank correlation coefficient was calculated to determine the correlation between antibody and T cell responses.
Anti-S RBD antibody half-lives were estimated using an exponential model of decay. Linear mixed models were fit using the lme4 and lmerTest package, with biologic treatment and vaccine type as fixed effects and each subject as a random effect. Each of these effects were estimated independently for gradient and intercept. 95% confidence intervals of fixed effects were calculated using likelihood ratios. P values for comparison of half-lives were estimated from the full linear mixed-effects model that incorporated vaccine, biologic drug and prior SARS-CoV-2 infection status.
We visualised the durability of antibody responses by calculating 15-day rolling geometric mean anti-S RBD antibody concentrations. For this analysis we included participants who had an antibody test carried out between 1 and 70 days after the second vaccine dose. Cox proportional hazard regression models were used to identify the demographic, disease and treatment-related factors associated with the time to fall in anti-S RBD antibody concentration below the seroconversion threshold.
Kaplan–Meier curves and Cox proportional hazard regression model was used to identify treatment-related factors associated with time to breakthrough infection. A linear regression model of log-transformed geometric mean anti-S RBD antibody concentration was used to determine the risk of breakthrough infections.
Where appropriate the same analyses were used to compare antibody responses in participants with PCR evidence of SARS-CoV-2 infection at any time prior to vaccination.
Ethical consideration
Patients were included after providing informed, written consent and compensation for participation was not provided. The sponsor was the Royal Devon and Exeter NHS Foundation Trust. The Surrey Borders Research Ethics committee approved the study (REC reference: REC 20/HRA/3114) in September 2020. The protocol is available online at https://www.clarityibd.org. The study was registered with the ISRCTN registry (10.1186/ISRCTN45176516).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
CLARITY IBD is an investigator-led, UK National Institute for Health Research COVID-19 urgent public health study, funded by the Royal Devon and Exeter NHS Foundation Trust, NIHR Imperial Biomedical Research Centre, Hull University Teaching Hospital NHS Trust, UKRI (MR/V036939/1) and by unrestricted educational grants from F. Hoffmann-La Roche AG (Switzerland), Biogen GmbH (Switzerland), Celltrion Healthcare (South Korea), Takeda (UK) and Galapagos NV (Belgium). None of our funding bodies had any role in study design, data collection or analysis, writing, or decision to submit for publication. The NIHR Clinical Research Network supported study set-up, site identification and delivery of this study. This was facilitated by Professor Mark Hull, the National Speciality Lead for Gastroenterology. We acknowledge the contribution of our Patient Advisory Group who helped shape the trial design around patient priorities. Our partners, Crohn’s and Colitis UK (CCUK), continue to support this group and participate in Study Management Team meetings. We thank Professor Graham Cooke and Dr Katrina Pollock for their helpful discussions and review of the data. Laboratory tests were undertaken by the Exeter Blood Sciences Laboratory at the Royal Devon and Exeter NHS Foundation Trust. The Exeter NIHR Clinical Research Facility coordinated sample storage and management. This research used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (research which commenced between 1 October 2020–31 March 2021 grant ref MC_PC_20029; 1 April 2021–30 September 2022 grant ref MC_PC_20058). Tariq Malik and James Thomas from Public Health England, Guy Stevens, Katie Donelon, Elen de Lacy from Public Health Wales and Johanna Bruce from Public Health Scotland supported linkage of central SARS-CoV-2 PCR test results with study data. Roche Diagnostics Limited provided the Elecsys Anti-SARS-CoV-2 immunoassay for the study. Faculty of Medicine at Imperial College London, Exeter NIHR Clinical Research Facility, Jeffrey Cheah Biomedical Centre at the University of Cambridge, Newcastle University Medical School and The Queen’s Medical Research Institute at the University of Edinburgh facilitated PBMC extractions for the T cell experiments. We thank Professor Robert Aldridge for access to data from the Virus Watch Collaborative. S.L. is supported by a Wellcome GW4-CAT fellowship (222850/Z/21/Z). N.C. acknowledges support from CCUK. J.C.L. is a Lister Prize Fellow and acknowledges support from the Cambridge NIHR Biomedical Research Centre and the Francis Crick Institute which receives core funding from Cancer Research UK (FC001169), the UK Medical Research Council (FC001169) and the Wellcome Trust (FC001169). G.-R.J. is supported by a Wellcome Trust Clinical Research Career Development Fellowship (220725/Z/20/Z). C.A.L. acknowledges support from the NIHR Newcastle Biomedical Research Centre and the support of the Programmed Investigation Unit at Royal Victoria Infirmary, Newcastle upon Tyne. C.W.L. is funded by a UKRI Future Leaders Fellowship. R.J.B. and D.M.A. are supported by MRC (MR/W020610/1, MR/S019553/1, MR/R02622X/1 and MR/V036939/1), NCSi4P, NIHR EME NIHR134607 and NIHR COV-LT2-0027, Innovate UK SBRI894, NIHR Imperial Biomedical Research Centre (BRC):ITMAT, Cystic Fibrosis Trust SRC (2019SRC015) and Horizon 2020 Marie Skłodowska-Curie Innovative Training Network (ITN) European Training Network (No 860325). N.P. is supported by the NIHR Imperial Biomedical Research Center (BRC). We acknowledge the study co-ordinators of the Exeter Inflammatory Bowel Disease Research Group: Marian Parkinson and Helen Gardner-Thorpe for their ongoing administrative support to the study. The sponsor of the study was the Royal Devon and Exeter NHS Foundation Trust.
Source data
Author contributions
N.A.K., J.R.G., C.B., S.S., N.P. and T.A. participated in the conception and design of this study. C.B. was the project manager and coordinated patient recruitment. R.N. and T.J.M. coordinated all biochemical analyses and central laboratory aspects of the project. S.L., N.A.K., A.S., D.M.S., C.J.R., R.C.S., S.H.K., F.P.P., K.-M.L., D.K.B., N.C., D.C., C.B., M.J., S.S., J.L.A., L.C., J.C.L., C.D.M., A.L.H., P.M.I., G.-R.J., K.B.K., C.A.L., C.W.L., D.M.A., R.J.B., J.R.G., N.P. and T.A. were involved in the acquisition, analysis or interpretation of data. D.M.S., C.J.R., K.-M.L., D.K.B. and F.F.P. performed, analysed and interpreted T cell experiments. T cell experiments were supervised, designed, analysed and interpreted by R.J.B. and D.M.A. Data analysis was done by N.A.K., D.M.S. and R.J.B. Drafting of the manuscript was done by S.L., N.A.K., N.C., S.S., C.W.L., D.M.A., R.J.B., J.R.G., N.P. and T.A. R.J.B., N.P. and T.A. obtained the funding for the study. All the authors contributed to the critical review and final approval of the manuscript. N.A.K., N.P. and T.A. have verified the underlying data.
Peer review
Peer review information
Nature Communications thanks Eloisa Bonfa, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The study protocol including the statistical analysis plan is available at https://www.clarityibd.org/. Individual participant de-identified data that underlie the results reported in this article will be available immediately after publication for a period of 5 years. Due to the sensitive nature of the data, this will be made available to investigators whose proposed use of the data has been approved by an independent review committee. Analyses will be restricted to the aims in the approved proposal. Proposals should be directed to tariq.ahmad1@nhs.net. To gain access data requestors will need to sign a data access agreement. Data from the Virus Watch study is currently being archived on the Office of National Statistics Secure Research Service and will be available shortly. Source data are provided with this paper in the Source Data file. Source data are provided with this paper.
Code availability
Statistical analyses were undertaken in R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria. Code has been made available at: https://github.com/exeteribd/clarityibd-public.
Competing interests
Dr. S Lin reports non-financial support from Pfizer, non-financial support from Ferring, outside the submitted work. Dr. Kennedy reports grants from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, non-financial support from Immundiagnostik, during the conduct of the study; grants and non-financial support from AbbVie, grants and personal fees from Celltrion, personal fees and non-financial support from Janssen, personal fees from Takeda, personal fees and non-financial support from Dr. Falk, outside the submitted work. Dr. Saifuddin has received travel expense support from Dr. Falk Pharma. Dr. Chee reports non-financial support from Ferring, personal fees and non-financial support from Pfizer, outside the submitted work. Prof. Sebastian reports grants from Takeda, Abbvie, AMGEN, Tillots Pharma, personal fees from Jaansen, Takeda, Galapagos, Celltrion, Falk Pharma, Tillots pharma, Cellgene, Pfizer, Pharmacocosmos, outside the submitted work. Dr. Alexander reports sponsorship from Vifor Pharma for accommodation/travel to BSG 2019, outside the submitted work. Dr. Lee reports personal fees from Abbvie, personal fees from C4X Discovery, personal fees from PredictImmune and personal fees from AG pus diagnostics. Dr. Hart reports personal fees from Abbvie, personal fees from Allergan, personal fees from BMS, personal fees from Celltrion, personal fees from Falk, personal fees from GSK, personal fees from Takeda, personal fees from Pfizer, personal fees from Janssen, personal fees from Galapogos, personal fees from AstraZeneca, outside the submitted work. Dr. Irving reports grants and personal fees from Takeda, grants from MSD, grants and personal fees from Pfizer, personal fees from Galapagos, personal fees from Gilead, personal fees from Abbvie, personal fees from Janssen, personal fees from Boehringer Ingelheim, personal fees from Topivert, personal fees from VH2, personal fees from Celgene, personal fees from Arena, personal fees from Samsung Bioepis, personal fees from Sandoz, personal fees from Procise, personal fees from Prometheus, outside the submitted work. Dr. Jones has received speaker fees from Takeda, Ferring and Janssen. Dr. Kok reports personal fees from Janssen, personal fees from Takeda, personal fees from PredictImmune, personal fees from Amgen, outside the submitted work. Dr. Lamb reports grants from Genentech, grants and personal fees from Janssen, grants and personal fees from Takeda, grants from AbbVie, personal fees from Ferring, grants from Eli Lilly, grants from Pfizer, grants from Roche, grants from UCB Biopharma, grants from Sanofi Aventis, grants from Biogen IDEC, grants from Orion OYJ, personal fees from Dr. Falk Pharma, grants from Astra Zeneca, outside the submitted work. Prof. Lees reports personal fees from Abbvie, personal fees from Janssen, personal fees from Pfizer, personal fees from Takeda, grants from Gilead, personal fees from Gilead, personal fees from Galapagos, personal fees from Iterative Scopes, personal fees from Trellus Health, personal fees from Celltion, personal fees from Ferring, personal fees from BMS, during the conduct of the study. Prof Boyton and Prof Altmann are members of the Global T cell Expert Consortium and have consulted for Oxford Immunotec outside the submitted work. Dr. Goodhand reports grants from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, non-financial support from Immundiagnostik, during the conduct of the study. Dr. Powell reports personal fees from Takeda, personal fees from Janssen, personal fees from Pfizer, personal fees from Bristol-Myers Squibb, personal fees from Abbvie, personal fees from Roche, personal fees from Lilly, personal fees from Allergan, personal fees from Celgene, outside the submitted work; and Dr. Powell has served as a speaker/advisory board member for Abbvie, Allergan, Bristol-Myers Squibb, Celgene, Falk, Ferring, Janssen, Pfizer, Tillotts, Takeda and Vifor Pharma. Prof. Ahmad reports grants and non-financial support from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, non-financial support from Immundiagnostik, during the conduct of the study; personal fees from Biogen inc, grants and personal fees from Celltrion Healthcare, personal fees and non-financial support from Immundiagnostik, personal fees from Takeda, personal fees from ARENA, personal fees from Gilead, personal fees from Adcock Ingram Healthcare, personal fees from Pfizer, personal fees from Genentech, non-financial support from Tillotts, outside the submitted work. The following authors have nothing to declare: Diana Muñoz Sandoval, Catherine Reynolds, Rocio Castro Seoane, Sherine H Kottoor, Franziska Pieper, Kai-Min Lin, David Butler, Neil Chanchlani, Claire Bewshea, Rachel Nice, Laura Constable, Charles D Murray, Timothy J McDonald.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Simeng Lin, Nicholas A Kennedy, Aamir Saifuddin, Diana Muñoz Sandoval, Rosemary J Boyton, James R Goodhand, Nick Powell and Tariq Ahmad.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Tariq Ahmad, Email: tariq.ahmad1@nhs.net.
CLARITY IBD study:
Klaartje B. Kok, Farjhana Bokth, Bessie Cipriano, Caroline Francia, Nosheen Khalid, Hafiza Khatun, Ashley Kingston, Irish Lee, Anouk Lehmann, Kinnari Naik, Elise Pabriaga, Nicolene Plaatjies, Kevin Samuels, Rebecca Saich, Hayley Cousins, Wendy Fraser, Rachel Thomas, Matthew Brown, Benjamin White, Nikolaos Kirkineziadis, Bernadette Tilley, Rafeeq Muhammed, Rehana Bi, Catherine Cotter, Jayne Grove, Kate Hong, Ruth Howman, Monica Mitchell, Sophie Clayton, Sugrah Sultan, Melanie Rooney, Charlotte Cottrill, Salil Singh, Chris Dawe, Robert Hull, Natalie Silva, Jonathan Manning, Lauren Finlayson, Allison Roebuck, Joy Dawson, Sunil Sonwalkar, Naomi Chambers, Matthew Robinson, Andrew Haigh, Lear Matapure, Tim Raine, Varun George, Christina Kapizioni, Konstantina Strongili, Tina Thompson, Mohamed Ahmed, Christos Kontos, Christophe Bourges, Isabella Barbutti, Megan E. Gozzard, Philip Hendy, Rhian Bull, Patricia Costa, Lisa Davey, Hayley Hannington, Kribashnie Nundlall, Catarina Martins, Laura Avanzi, Jaime Carungcong, Sabrina Barr, Richard Appleby, Emma Johnson, Kath Phillis, Rachel Gascoyne, Amanda Crowder, Amanda Whileman, Ian London, Jenny Grounds, Emmeline Martin, Susie Pajak, Jude Price, Kathryn Cawley, Anjan Dhar, Ellen Brown, Amanda Cowton, Kimberley Stamp, Ben Warner, Carmel Stuart, Louise Lacey, Shanika de Silva, Clare Allcock, Philip Harvey, Lesley Jones, Elise Cooke, Johanne Brooks, Pearl Baker, Hannah Beadle, Carina Cruz, Debbie Potter, Joe Collum, Farzana Masters, Aashish Kumar, Samantha Coetzee, Mihaela Peiu, Becky Icke, Meena Raj, Edward Gaynor, Sibongile Chadokufa, Bonita Huggett, Hamza Meghari, Sara El-Khouly, Fevronia Kiparissi, Waffa Girshab, Andrew Claridge, Emily Fowler, Laura McCafferty, Karolina Christodoulides, Angela Clifford, Patrick Dawson, Sailish Honap, Samuel Lim, Raphael Luber, Karina Mahiouz, Susanna Meade, Parizade Raymode, Rebecca Reynolds, Anna Stanton, Sherill Tripoli, Naomi Hare, Senthuran Balachandran, Emma North, Jessica North, Bria Browne, Ella Jameson, Yih Harn Siaw, Lane Manzano, Jonathan Segal, Ibrahim Al-Bakir, Imran Khakoo, Nora Thoua, Katherine Davidson, Jagrul Miah, Lisa Canclini, Alex Hall, Melony Hayes, Sally Myers, Alison Talbot, Jack Turnbull, Emma Whitehead, Katie Stamp, Alison Pattinson, Verghese Mathew, Leanne Sherris, Angela Harvey, Lucy Hicks, Tara-Marie Byrne, Leilani Cabreros, Hannah Downing-Wood, Sophie Hunter, Hemanth Prabhudev, Sharmili Balarajah, Hajir Ibraheim, Melissa Torkizadeh, Jonathan W. Lo, Zhigang Liu, Helen Sutherland, Elva Wilhelmsen, Katherine Mackintosh, Ajay M. Verma, Juliemol Sebastian, Mohammad Farhad Peerally, Parizade Raymode, Anne-marie Guerdette, Alexandra Kent, Lee Meng Choong, Benedetta Pantaloni, Pantelis Ravdas, Babu Vadamalayan, Stephen Foley, Becky Arnold, Cheryl Heeley, Wayne Lovegrove, Donna Sowton, Lynne Allsop, Heidi Gregory, Philip J. Smith, Giovanna Bretland, Sarah King, Martina Lofthouse, Lindsey Rigby, Sreedhar Subramanian, David Tyrer, Kate Martin, Christopher Probert, Nikolaos Kamperidis, Temi Adedoyin, Manisha Baden, Jeannette Brown, Feba Chacko, Michela Cicchetti, Mohammad Aamir Saifuddin, Priya Yesupatham, Rohit Gowda, Maureen Williams, Karen Kemp, Rima Akhand, Glaxy Gray, Anu John, Maya John, Tasnim Mohammed, Diamond Sathe, Natasha Jones, Jennifer Soren, Michael Sprakes, Julie Burton, Patricia Kane, Stephanie Lupton, Jacqueline Bartholomew, George MacFaul, Diane Scaletta, Loria Siamia, Felicity Williams, Chloe Green, Zeljka Ver, Christopher A. Lamb, Mary Doona, Ashleigh Hogg, Lesley Jeffrey, Andrew King, R. Alexander Speight, Jennifer Doyle, Ruth Owen, Craig Mowat, Debbie Rice, Susan MacFarlane, Anne MacLeod, Samera Mohammed, Shona Murray, Anne Elliott, Mary Anne Morris, Louise Coke, Grace Hindle, Eirini Kolokouri, Catherine Wright, Claire Lee, Nicola Ward, Adele Dann, Melanie Lockett, Charlotte Cranfield, Louise Jennings, Ankur Srivastava, Lana Ward, Nouf Jeynes, Poonam Ranga, Praveen Rajasekhar, Lisa Gallagher, Linda Patterson, Jill Ward, Rae Basnett, Judy Murphy, Lauren Parking, Emma Lawson, Stacey Short, David Devadason, Gordon Moran, Neelam Khan, Lauren Tarr, Charmaine Olivia, Jimmy Limdi, Kay Goulden, Asad Javed, Lauren McKenzie, Pradeep Bhandari, Michelle Baker-Moffatt, Joanne Dash, Alison Le Poidevin, Hayley Downe, Lucille Bombeo, Helen Blackman, Alan Wiles, Hannah Bloxham, Jose Dias, Evelyn Nadar, Hollie Curgenven, Jonathan Macdonald, Shona Finan, Faye McMeeken, Misbah Mahmood, Stephanie Shields, John Paul Seenan, Des DeSilva, Susanna Malkakorpi, Rachel Carson, Simon Whiteoak, Kelli Edger-Earley, Luke Vamplew, Sarah Ingram, Sharon Botfield, Fiona Hammonds, Clare James, Tariq Ahmad, Gemma Aspinall, Sarah Hawkins, Suzie Marriott, Clare Redstone, Halina Windak, Ana-Marie Adam, Hannah Mabb, Charles Murray, Cynthia Diaba, Fexy Joseph, Glykeria Pakou, Yvonne Gleeson, James Berrill, Natalie Stroud, Carla Pothecary, Lisa Roche, Keri Turner, Lisa Deering, Lynda Israel, Evelyn Baker, Sean Cutler, Rina Mardania Evans, Maxine Nash, Georgina Mallison, Anna Roynon, John Gordon, Emma Levell, Silvia Zagalo, Wendy Fraser, Ina Hoad, Nikolaos Kirkineziadis, Richard Russell, Paul Henderson, Margaret Millar, Andrew Fagbemi, Felicia Jennings, Imelda Mayor, Jill Wilson, Christopher Alexakis, Natalia Michalak, John Saunders, Helen Burton, Vanessa Cambridge, Tonia Clark, Charlotte Ekblad, Sarah Hierons, Joyce Katebe, Emma Saunsbury, Rachel Perry, Matthew Brookes, Kathryn Davies, Marie Green, Ann Plumbe, Clare Ormerod, Helen Christensen, Anne Keen, Jonathan Ogor, Alpha Anthony, Emily Newitt, Fiona Trim, Ruth Casey, Katherine Seymour, Edward Fogden, Kalisha Russell, Anne Phillips, Muaad Abdulla, Jeff Butterworth, Colene Adams, Elizabeth Buckingham, Danielle Childs, Alison Magness, Jo Stickley, Nichola Motherwell, Louise Tonks, Hannah Gibson, S. Pajak, Caradog Thomas, Elaine Brinkworth, Lynda Connor, Amanda Cook, Tabitha Rees, Rachel Harford, Emma Wesley, Alison Moss, Jacob Lucas, Claire Lorimer, Maria Oleary, Maxine Dixon, Arvind Ramadas, Julie Tregonning, Olaku Okeke, Wendy Jackson, Ioannis Koumoutsos, Viji George, Swapna Kunhunny, Sophie Laverick, Isla Anderson, Sophie Smith, Kamal Patel, Mariam Ali, Hilda Mhandu, Aleem Rana, Katherine Spears, Joana Teixeira, Richard Pollok, Mark Mencias, Abigail Seaward, Jessica Sousa, Nooria Said, Mark Soomaroo, Valentina Raspa, Asha Tacouri, Nicholas Reps, Rebecca Martin, Christian Selinger, Jenelyn Carbonell, Felicia Onovira, Doris Quartey, Alice L’Anson, Andrew Ashworth, Jessica Bailey, Angie Dunn, Zahid Mahmood, Racheal Campbell, Liane Marsh, Monira Rahman, Sarah Davies, Ruth Habibi, Ellen Jessup-Dunton, Teishel Joefield, Reina Layug, Vinod Patel, Joanne Vere, Victoria Turner, Susan Kilroy, Gareth Walker, Stacey Atkins, Jasmine Growdon, Charlotte McNeill, Rachel Cooney, Lillie Bennett, Louise Bowlas, Sharafaath Shariff, Aileen Fraser, Dwayne Punnette, Charlotte Bishop-Hurman, Elizabeth Undrell, Katherine Belfield, Said Din, Catherine Addleton, Marie Appleby, Johanna Brown, Kathleen Holding, Patricia Hooper, John deCaestecker, Olivia Watchorn, Chris Hayward, Susan Inniss, Lucy Pritchard, Karen Rudge, Amanda Carney, Jervoise Andreyev, Caroline Hayhurst, Carol Lockwood, Lynn Osborne, Amanda Roper, Karen Warner, Julia Hindle, Caroline Watt, Kinga Szymiczek, Shameer Mehta, James Bell, William Blad, Lisa Whitley, Durai Dhamaraj, Mark Baker, Elizabeth John Sivamurugan, Mim Evans, Fraser Cummings, Clare Harris, Amy Jones, Liga Krauze, Sohail Rahmany, Michelle Earl, Jenny Vowles, Audrey Torokwa, Mirela Petrova, Andrew Procter, Jo Stanley, Claudia Silvamoniz, Marion Bettey, Amar Wahid, Zoe Morrison, Rhian Thomas-Turner, Louise Yendle, Jennifer Muller, Marcus Mitchell, John Kirkwood, Anna Barnes, Rakesh Chaudhary, Melanie Claridge, Chiara Ellis, Cheryl Kemp, Ogwa Tobi, Jentus Milton, Emma Johnston, Metod Oblak, Jo Godden, Charlie Lees, Debbie Alexander, Kate Covil, Lauranne Derikx, Sryros Siakavellas, Helen Baxter, Scott Robertson, Linda Smith, Beena Poulose, Anne Colemam, Margareta Balint, Gareth Rhys-Jones, Kerrie Johns, Rachel Hughes, Janet Phipps, Abigail Taylor, Catherine MacPhee, Suzanne Brooks, Katie Smith, Linda Howard, Dianne Wood, Ajay Muddu, Laura Barman, Janine Mallinson, Tania Neale, Diana Ionita, Kerry Elliot, Alison Turnball, Iola Thomas, Kelly Andrews, Jonathon Sutton, Caroline Mulvaney Jones, Julia Roberts, and Jeannie Bishop
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28517-z.
References
- 1.Bernal, J. L. et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ373, n1088 (2021). [DOI] [PMC free article] [PubMed]
- 2.Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. 397, 99–111 (2021). [DOI] [PMC free article] [PubMed]
- 3.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prendecki M, et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398:1482–1484. doi: 10.1016/S0140-6736(21)02096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong, S.-Y. et al. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel bisease patients receiving biologic therapies. Gastroenterology161, 715–718 (2021). [DOI] [PMC free article] [PubMed]
- 6.Kappelman, M. D. et al. Humoral immune response to mRNA COVID-19 vaccines among patients with IBD. Gastroenterology161, 1340–1343 (2021). [DOI] [PMC free article] [PubMed]
- 7.Kearns, P. et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – The OCTAVE Trial. SSRN Electron. J. 10.2139/SSRN.3910058 (2021).
- 8.Sakuraba, A., Luna, A. & Micic, D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology162, 88–108.e9 (2021). [DOI] [PMC free article] [PubMed]
- 9.Chanchlani, N. et al. Adalimumab and infliximab impair SARS-CoV-2 antibody responses: results from a therapeutic drug monitoring study in 11422 biologic-treated patients. J. Crohn’s Colitis10.1093/ECCO-JCC/JJAB153 (2021). [DOI] [PMC free article] [PubMed]
- 10.Kennedy NA, et al. Inflammatory bowel disease anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy, N. A. et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19SARS-CoV-2 vaccines in patients with IBD. Gut70, 1884–1893 (2021). [DOI] [PubMed]
- 12.Harrington, J. E., Hamilton, R. E., Ganley-Leal, L., Farraye, F. A. & Wasan, S. K. The immunogenicity of the influenza, pneumococcal, and hepatitis B vaccines in patients with inflammatory bowel disease treated With vedolizumab. Crohn’s Colitis 360, 2 (2020). [DOI] [PMC free article] [PubMed]
- 13.Joint Committee on Vaccination and Immunisation. Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination. https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination (2021).
- 14.Centre for Disease Control and Prevention. COVID-19 vaccines for moderately to severely immunocompromised people. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (2021).
- 15.European Medicines Agency. ECDC and EMA highlight considerations for additional and booster doses of COVID-19 vaccines. https://www.ema.europa.eu/en/news/ecdc-ema-highlight-considerations-additional-booster-doses-covid-19-vaccines (2021).
- 16.National Advisory Committee on Immunization (NACI). Summary of National Advisory Committee on Immunization (NACI) rapid response: additional dose of COVID-19 vaccine in immunocompromised individuals following a 1- or 2-dose primary series. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/summary-september-10-2021-additional-dose-covid-19-vaccine-immunocompromised-following-1-2-dose-series.html?utm_campaign=hc-sc-covidvaccine-21-22 (2021).
- 17.Hall, V. G. et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N. Engl. J. Med. 385, 1244–1246 (2021). [DOI] [PMC free article] [PubMed]
- 18.EE W, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrotri, M. et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet398, 385–387 (2021). [DOI] [PMC free article] [PubMed]
- 20.McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. npj Vaccines. 2021;6:1–14. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 Months. N. Engl. J. Med.385, 1761–1773 (2021). [DOI] [PMC free article] [PubMed]
- 22.Park JK, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann. Rheum. Dis. 2017;76:1559–1565. doi: 10.1136/annrheumdis-2017-211128. [DOI] [PubMed] [Google Scholar]
- 23.Barros-Martins, J. et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med.27, 1525–1529 10.1038/s41591-021-01449-9 (2021). [DOI] [PMC free article] [PubMed]
- 24.Lazarus R, et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. Lancet. 2021;398:2277–2287. doi: 10.1016/S0140-6736(21)02329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritter U, Meissner A, Ott J, Körner H. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-α reveals an essential role for TNF. J. Leukoc. Biol. 2003;74:216–222. doi: 10.1189/jlb.1202587. [DOI] [PubMed] [Google Scholar]
- 27.Salinas GF, et al. Anti-TNF treatment blocks the induction of T cell-dependent humoral responses. Ann. Rheum. Dis. 2013;72:1037–1043. doi: 10.1136/annrheumdis-2011-201270. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds, C. J. et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci. Immunol. 5, eabf3698 (2020). [DOI] [PMC free article] [PubMed]
- 29.Cope AP, et al. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J. Clin. Invest. 1994;94:749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 31.Bourges D, et al. Differential expression of adhesion molecules and chemokines between nasal and small intestinal mucosae: implications for T- and sIgA+ B-lymphocyte recruitment. Immunology. 2007;122:551–561. doi: 10.1111/j.1365-2567.2007.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds CJ, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418–1423. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dailey, J. et al. Antibody responses to SARS-CoV-2 after infection or vaccination in children and young adults with inflammatory bowel disease. Inflamm. Bowel Dis. 10.1093/IBD/IZAB207 (2021) [DOI] [PMC free article] [PubMed]
- 34.Harris, P. A. et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform.95, 103208 (2019). [DOI] [PMC free article] [PubMed]
- 35.Roche Diagnostics GmbH. Elecsys® Anti-SARS-CoV-2 S assay method sheet. https://diagnostics.roche.com/gb/en/products/params/elecsys-anti-sars-cov-2-s.html (2020).
- 36.Muench P, et al. Development and validation of the elecsys anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2. J. Clin. Microbiol. 2020;58:1694–1714. doi: 10.1128/JCM.01694-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol including the statistical analysis plan is available at https://www.clarityibd.org/. Individual participant de-identified data that underlie the results reported in this article will be available immediately after publication for a period of 5 years. Due to the sensitive nature of the data, this will be made available to investigators whose proposed use of the data has been approved by an independent review committee. Analyses will be restricted to the aims in the approved proposal. Proposals should be directed to tariq.ahmad1@nhs.net. To gain access data requestors will need to sign a data access agreement. Data from the Virus Watch study is currently being archived on the Office of National Statistics Secure Research Service and will be available shortly. Source data are provided with this paper in the Source Data file. Source data are provided with this paper.
Statistical analyses were undertaken in R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria. Code has been made available at: https://github.com/exeteribd/clarityibd-public.