Abstract
Graphene patterning via etching is important for enhancing or controling the properties of devices and supporting their applications in micro- and nano-electronic fields. Herein, we present a simple, low-cost, and scalable wet etching method for graphene patterning. The technique uses hypochlorite solution combined with ultraviolet light irradiation to rapidly remove unwanted graphene areas from the substrate. Raman spectroscopy, atomic force microscopy, scanning electron microscopy, and optical microscopy results showed that well-patterned graphene with micrometer scale regions was successfully prepared. Furthermore, graphene field effect transistor arrays were fabricated, and the obtained devices exhibited good current–voltage characteristics, with maximum mobility of ~ 1600 cm2/Vs, confirming the feasibility of the developed technique.
Subject terms: Chemistry, Materials science
Introduction
Graphene, a one-atom-thick sheet of carbon atoms arranged in a honeycomb-like structure, is the thinnest, strongest, and most conductive material of both electricity and heat1–3. Graphene has attracted widespread attention for its potential use in the next generation of electronic devices and other fields ranging from biosensors to energy storage systems4–8. For these applications to be realized, well-defined architectures at micrometer and nanometer scales will need to be determined.
Various patterning approaches have been proposed, including bottom-up fabrication methods and top-down etching processes involving lithography9. Bottom-up fabrication includes the spatially selective synthesis of graphene arrays by patterning of catalysts10,11, or selective decomposition of silicon carbide (SiC) to form graphene arrays12. Selective synthesis methods suffer from a variety of limitations, including the use of harsh synthesis conditions, complicated steps, demanding large-scale preparation, and structural limitations9. By contrast, top-down patterning by etching of designed areas is expected to be an effective strategy. The techniques of photolithography and electron beam lithography combined with dry etching by oxygen or plasma13–16, reactive ion etching17, helium ion ablation18, and laser ablation19,20 have been used for patterning graphene on substrates. Results indicate high pattern resolution down to 10 nm, and high alignment accuracy18. However, these dry etching processes suffer from a variety of limitations including high cost due to expensive equipment, and complex or inconvenient processing steps that are time-consuming and difficult to scale up. Additionally, the high power of plasma or ion beams may induce defects in patterned graphene as well as the substrate, which may damage the electronic properties.
By contrast, wet etching processes are generally simpler, use low-cost equipment, and are easier to scale up for industry production21. Wet etching also has the advantage of being less damaging to the etched object, which expands the range of its practical applications. However, wet etching methodology has not yet been developed for graphene patterning. It is believed that the stable graphitic structure of graphene makes it difficult to remove or dissolve by chemical agents in solutions.
Recently, we found that carbon nanotubes (CNTs)22–25 and graphene oxide26 could be degraded by hypochlorite, and the degradation rates could be enhanced significantly by combining with ultraviolet (UV) light irradiation. In the present work, we found that sodium hypochlorite (NaClO) could completely degrade monolayer graphene films, which can be used as a wet etchant for graphene patterning. Compared with the dry etching, the wet process is simpler, more affordable, scalable, and easier to control due to the ambient reaction conditions. The patterning process consists of three steps: protection with a gold (Au) cover through convenient electronic beam (EB) evaporation, wet treatment with hypochlorite, and stripping of the Au cover. Using this process, different graphene pattern designs were successfully prepared without damaging the structures, as evidenced by Raman spectra mapping, optical microscopy, scanning electron microscopy (SEM), and atomic force microscopy (AFM). Moreover, as a demonstration, graphene field effect transistor (FET) arrays were fabricated, and the obtained devices exhibited good current–voltage characteristics, confirming the feasibility of the developed technique. The results expand our knowledge about the degradation of graphene in solution, and provide a simple and practical method for electronic processing of graphene, which could expand the application potential of this material for use in micro- and nano-electronic devices.
Results
Degradation of monolayer graphene
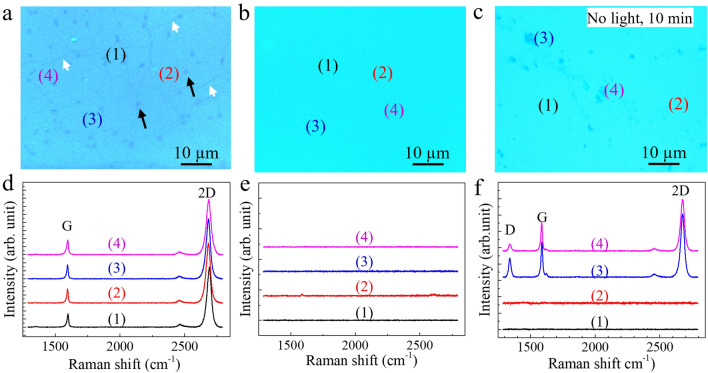
The monolayer graphene film, used as purchased, which was produced by chemical vapor deposition and transferred onto SiO2/Si substrate (labeled graphene/substrate hereafter) via a sacrificial copper film. The graphene film had a smooth surface with some lines and dark spot-like areas (Fig. 1a) that were assumed to be the connected boundaries of graphene domains27 and regions with few layers28, respectively. Raman spectra from four random points on the film-covered substrate were almost the same, indicating uniformity of the graphene film, and all spectra had G-band and 2D-band peaks at ~ 2683 and ~ 1590 cm-1, respectively. The full width at half maximum (FWHM) was ~ 37 cm-1, and the peak intensity ratio (2D/G) was > 5, which indicated monolayer graphene (Fig. 1d)28. When the graphene/substrate was immersed in bleach solution with a NaClO concentration of ~ 6 wt% and then irradiated by UV light for 5 min, the color of the surface of the graphene/substrate appeared brighter and lighter than before treatment. Optical microscopy images revealed no footprints of graphene such as boundaries or spot-like areas (Fig. 1b). The disappearance of graphene was also confirmed by Raman spectra measurements; the absence of peaks indicated that there was no graphene remaining at any points on the treated film (Fig. 1e). On the other hand, when the graphene/substrate was treated with the same bleach solution but without light irradiation for 10 min at room temperature, the graphene film was not completely cleaned; some graphene patches (3 and 4 in Fig. 1c) remained on the surface of the substrate. Raman spectra measurements confirmed that the features were indeed graphene, and the blank areas were cleared spaces (Fig. 1f). This result indicates that UV light irradiation enhanced the graphene degradation rate.
Figure 1.
Degradation of graphene by bleach solution (NaClO, 6 wt%). (a) Optical microscopy images of monolayer graphene film on SiO2/Si substrate. Lines (indicated by white arrows) and dark spot-like areas (indicated by black arrows) represent the domain boundaries and regions with few graphene layers, respectively. Images acquired after treatment with bleach solution assisted by UV light irradiation were captured after 5 min (b), and without light irradiation after 10 min (c). Raman spectra (d, e, and f) for locations (1, 2, 3, and 4) correspond to images (a, b, and c), respectively.
To further prove the complete degradation of graphene by NaClO solution, we treated graphene nanoplatelets, consisting of short stacks of graphene sheets with a few layers, with NaClO (6 wt%) solution (SI, Figure SI-1). Following treatment, the black graphene dispersion became a colorless transparent solution, and the amount of graphene in solution was decreased to almost zero after treatment in an incubator (70 °C) for 6 h, indicating that graphene degraded completely, consistent with our previous results for CNTs23–25.
Patterning graphene by wet etching with hypochlorite
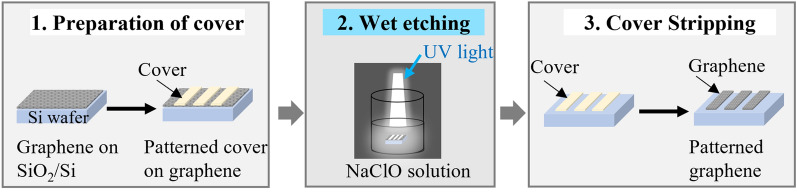
The process for patterning graphene using NaClO as an etchant generally consists of three steps: preparation of the protecting cover, wet etching, and stripping of the cover (Fig. 2). The protective cover with a designed pattern was prepared using metals or photoresistor polymers, as employed for dry processes, for which lithography is a convenient technique. The protective cover used in this study was obtained by EB evaporation of Au with various designed patterns on the surface of the graphene/substrate. After the protective Au cover was prepared, samples of the graphene/substrate were treated with a bleach solution (NaClO = 6 wt%) and irradiated by a UV light for 5 min (Fig. 2). After treatment, the sample was removed immediately, rinsed with water, and immersed in gold etching solution containing KI/I2 to strip the Au cover29.
Figure 2.
Schematic illustration of wet etching by hypochlorite for graphene patterning.
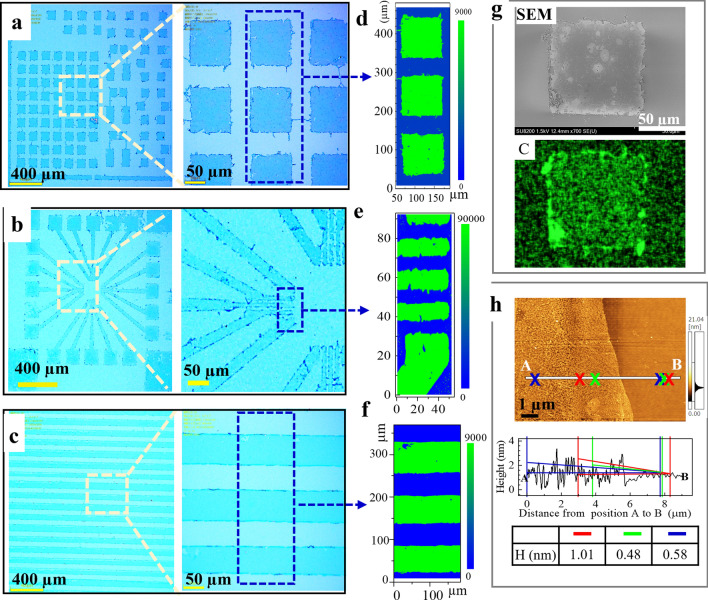
The results showed that well-patterned graphene arrays were successfully prepared using the above process. Three typical graphene, namely squares (Fig. 3a), radiation shapes (Fig. 3b), and ribbons (Fig. 3c), were observed by optical microscopy, with widths of 10–100 µm and no obvious defects. Raman mapping of the peak intensities of 2D-bands at ~ 2683 cm-1 indicated that the patterning of graphene arrays in all three patterns was successful (Fig. 3d, e, and f). SEM images and corresponding energy-dispersive X-ray spectra (EDS) mapping of elemental carbon also confirmed that the patterned area was graphene (Fig. 3g), and the thickness of graphene in prepared patterns was about 0.5–1.0 nm as measured by AFM (Fig. 3h).
Figure 3.
Three typical patterned graphene arrays. Optical microscopy images show close-up views of different areas of the different patterned graphene arrays on Si-wafers obtained by NaClO etching after gold cover removal (a, d, and c). Raman mapping of areas (marked as blue squares in a, b, and c) is shown for 2D peak intensities at ~ 2683 cm-1 (d, e, and f). (g) SEM image from one of the patterned square areas in (a) alongside the elemental carbon (C) mapping image. (h) Atomic force microscopy (AFM) topographic image for one of the patterned graphene ribbon edges in image (c), and the corresponding height profiles for the line from position A to position B (bottom).
To further clarify the structural damage induced by wet treatment, a large area (565 × 660 µm) was evaluated for patterned graphene arrays by Raman mapping of the D-band peak at 1350 cm−1, which corresponds to the amount of disorder or defects in graphene, while the G-band at 1590 cm−1 is indicative of the graphitic structure, as is the typical graphene peak of the 2D-band. The results showed that the intensities of D-bands, as well as G-bands and 2D-bands, were almost uniform for all measured arrays (SI, Figure SI-2). Mapping of the intensity ratio of D and G peaks (D/G) also showed no increase in the domains and edges of graphene arrays. In addition, the intensities of G-band and 2D-band peaks at a few edges were slightly stronger than at other areas, indicating overlap of graphene in these locations, which was confirmed by the results from SEM. SEM images of one patterned graphene square showed that a small part of the patterned graphene edge was rolled up (SI, Figure SI-3). Moreover, images for elemental mapping of carbon (C) and oxygen (O) obtained by EDS measurements showed that O did not increase obviously following treatment at both edges and domains of graphene, but C at the rolled edge was increased slightly compared with the rest of the domain area (SI, Figure SI-3).
Fabrication of the graphene FET
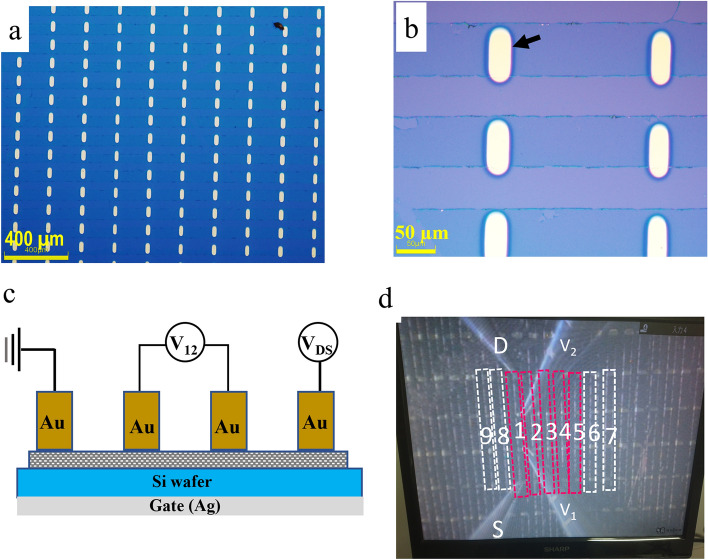
To explore device applications, FET arrays were fabricated as a demonstration. To contact the electronic probes on the patterned graphene arrays obtained using the above process, Au and Cr were deposited on the graphene arrays as electrodes at widths of 60 ± 10 µm; the space between the two electrodes was about 200 ± 10 µm (Fig. 4a and b); and the back gate was prepared by coating Ag paste on the back of the substrate (Fig. 4c). The resulting channel length and width of the patterned graphene FET were 200 ± 10 and 60 ± 10 µm, respectively.
Figure 4.
Illustration of the fabricated graphene field effect transistor (FET). The optical microscopy image (a) and close-up view (b) are shown for patterned graphene arrays with an Au electrode (light yellow rectangle) on the Si-wafer. The schematic diagram shows the back-gate FET (c) and a photograph of the obtained device in the measurement chamber (d).
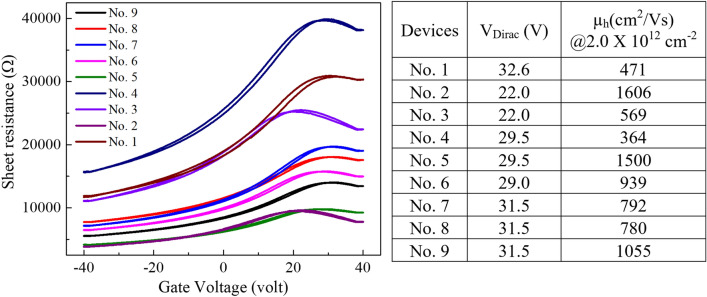
The drain current vs. gate-source voltage (ID-VGS) characteristics of the obtained FET was investigated by measurements made using 4-point probes in a vacuum chamber (2 × 10–2 Pa) at room temperature (Fig. 4d). The results of nine arbitrary devices showed that the electric resistance changed as back-gate bias Vg varied from − 40 to 40 V (Fig. 5, left). The minimum conductivity points in transfer characteristics, also known as Dirac points, were clearly observed from the curves (Fig. 5, right). The positive Dirac points indicated that the devices were p-type in nature.
Figure 5.
Characteristics of the fabricated graphene FET. The transfer characteristics curves of electronic resistance spanning a gate voltage from -40 to 40 V are shown for nine devices (left), along with the Dirac points and electron mobilities (right). The ratio of length to width for graphene channels was presumed to be 3, and n = 2.0 × 1012 cm-2.
Discussion
It is known that graphene and graphite can be oxidized by oxidizing acids such as HNO330, H2SO4/HNO331, and KMnO4/H2SO432 to form graphene oxide or graphene dots, but studies on the complete degradation or elimination of graphene have not been reported, except for combustion. In the present study, we found that a monolayer graphene film could be degraded completely and rapidly using NaClO solution, a household bleach, when assisted by UV light irradiation. The rapid elimination of graphene encouraged us to use the approach for patterning of graphene.
Crucial to the rapid etching of graphene in this study was UV light irradiation. Although the mechanism of graphene degradation by NaClO assisted by UV irradiation is not fully understood, it is known that UV light accelerates the deposition of NaClO to generate photooxidants, such as HO∙, O∙−, and Cl, which possess very strong oxidation ability33. These oxidants react rapidly with graphene, as occurs in water treatment34 and the purification of fuel gas35. The complete oxidation of graphene by NaClO is believed to follow the general reaction formula Cgraphene + 2NaClO → 2NaCl + CO2, similar to CNTs 23. In the case of light irradiation, the super-reactive oxygen species would preferentially react with defective carbon, and oxidation would then spread out, leading to the removal of domains and eventually of the whole graphene film (SI, Figure SI-4). This is supported by the graphene film degradation results showing that some domains of graphene were removed preferentially before complete elimination (SI, Figure SI-5).
Another important factor when using bleach is the inclusion of a surfactant such as an alkylether amine oxide (AO) in addition to NaClO. To explore the effects of the surfactant, we treated graphene films with NaClO solution (~ 6 wt%) with or without addition of AO (1% N,N-D-dimethyldodecyamine-N-oxide) instead of bleach solution. The results showed that etching of graphene for patterning was possible through treatment with light/NaClO solution with or without surfactant, but addition of surfactant made the removal faster and more thorough (Figure SI-6). We believe that the surfactant increased the permeability of NaClO and the affinity of hydrophobic graphene in aqueous solution. However, additional studies using different concentrations of AO surfactant as well as other types of surfactants are clearly needed.
In addition, we found that NaClO at a concentration of 6 wt% (SI, Figure SI-6) could remove graphene more rapidly than at a concentration of 2.26 wt% (SI, Figure SI-5) and 1.1 wt% (SI, Figure SI-1), indicating that a higher concentration was more effective for degradation of graphene, consistent with the results for CNTs25.
The process for patterning of graphene employed in this study was simple and convenient, and involved the preparation of a protective cover using an established method, and very simple wet etching using a cost-effective etchant (bleach) and inexpensive equipment. Moreover, the wet process was easily controllable and scalable because the reaction was performed by simply immersing the graphene/substrate in bleach solution at room temperature followed by UV light irradiation. For successful patterning, it is important to prepare a suitable protective cover, which can be prepared from a noble metal such as Au (Fig. 3) or by using a negative photoresistor (SI, Figure SI-7). The high precision of the prepared protective cover would yield patterned graphene with similarly high precision. If the protective cover is prepared by Au EB evaporation via a readymade metal mask, the cover will be in the micrometer scale due to current limitations of the technique. In addition, the mild conditions for wet etching and the gentle removal of the protective cover could decrease the roughness of the edges of the pattered graphene.
In addition, the characteristics of the patterned graphene as well as the electrical properties of the FET are also affected by the quality of the original graphene film, especially regarding defects, layers, and the adherence state of graphene to substrates such as SiO2/Si. The FET devices fabricated herein from the commercially available graphene samples exhibited ambipolar current–voltage characteristics. Although it is necessary to take electrode configuration into account when estimating hole mobility (µh), the highest value obtained herein was ~ 1600 cm2/Vs, confirming the feasibility of our wet etching technique. Notably, the gate voltage at Dirac points (VDirac) and the mobilities for different devices varied from 29 to 31 V and from 400 to 1600 cm2/Vs, respectively. This might be due to the different properties of local original graphene structures and/or interactions with substrates. Although the value of the charge mobility in the graphene FET obtained in this study was not outstanding compared with previous reports36, the results indicate that our wet etching process is applicable to the patterning of graphene for fabrication of electronic devices.
The p-type properties of these FET devices were consistent with previous reports for monolayer chemical vapor deposition (CVD) graphene on SiO237–40. It is probable that the degree of graphene coupling to the SiO2 substrate might induce hole doping37. We believe that p-type conduction was not due to the etching process using aqueous NaClO solution. The obtained hole mobilities of monolayer CVD graphene were in the range of reported data, which were calculated from channels of almost the same size38–40. To ensure good reproducibility, a high-quality and homogeneous graphene structure as well as appropriate adherence to the substrate are extremely important, alongside the patterning process.
In conclusion, we found that monolayer graphene could be rapidly and completely eliminated by etching with a household bleach assisted by UV light irradiation. The etching process was simple, cost-efficient, and scalable, which enabled the successful patterning of graphene with various fine-tuned patterns. The FET arrays fabricated as a demonstration exhibited good performance in terms of ambipolar current–voltage characteristics and high hole mobility, indicating the feasibility of the developed wet etching technique. Further improvement in the graphene patterning process may be possible by preparing a high-precision protective cover at sub-micrometer scale, and by developing more appropriate conditions for etching graphene on different substrates. We believe that the wet etching technique proposed herein could be useful for various processes using graphene in electronic applications.
Methods
Degradation of graphene by sodium hypochlorite
Monolayer graphene films with a thickness of ~ 0.345 nm were used as purchased (Catalog no. 773700, Sigma-Aldrich, Tokyo, Japan). Films were deposited on SiO2/Si by direct CVD via a sacrificial copper film. Graphene on the SiO2/Si substrate was 1 × 1 cm in size, with a coverage > 95%. The thickness of substrate was ~ 525 µm with a coating of thermal oxide (SiO2) of ~ 300 nm on both wafer sides. NaClO solution used in this study was a household bleach solution (Kitchen Power Bleach, Lion Hygiene, Tokyo, Japan) with a NaClO concentration of ~ 6 wt% and AO surfactant (1–5 wt%). This bleach solution was used directly without dilution within 3 months after purchase. Besides the standard bleach solution, NaClO solutions with 1.1 wt%, 2.26 wt%, or 6 wt% were also tested, and were prepared by dissolving NaClO·5H2O powder (Wako, Tokyo, Japan) in deionized water.
To investigate the degradation of graphene, the graphene/substrate was immersed in bleach or NaClO solution (~ 5 mL) at room temperature, and then irradiated by a UV light spot (1 cm diameter) using a Lightningcure LC5 light source unit (Hamamatsu Photonics, Hamamatsu, Japan) composed of a Hg-Xe lamp (200 W) and fiber cables. The UV light intensity at 365 nm was ~ 3.5 W/cm2, and the wavelength had a range of 240–550 nm.
Graphene patterning
Preparation of the protective cover on graphene film
The protective cover was made by decomposition of Au (thickness = 100 nm) on graphene film through a patterned mask using an EW-100S vacuum EB evaporation instrument (Eiko Corporation, Tokyo, Japan). Two types of patterned mask were tested: one was a photoresistor polymer assisted by lithography (SI, Figure SI-8), which was used for preparation of pattern A (Fig. 3a) and pattern B (Fig. 3b); the other was a custom-made metal (Ni) mask used for designed pattern C (Fig. 3c), which was prepared by the manufacturer (Fuji Semitu Industries, Osaka, Japan) using a traditional metal etching method.
Wet etching
After covering with the patterned protective Au film, the graphene/substrate was immersed in bleach solution or NaClO solution and then irradiated by UV light for ~ 5 min. The graphene/substrate was removed immediately, rinsed three times with deionized water, and dried naturally at room temperature blown gently with N2 gas.
Stripping the protective cover
After wet etching, the graphene/substrate samples were immersed in KI/I2 solution29 for 10–60 s to remove the Au cover. KI/I2 solution was prepared by dissolving 1.6 g KI powder (Wako) and 0.4 g I2 (Wako) in 50 mL water. When the yellow pieces of Au cover were floating on the surface, the graphene/substrate was taken out, rinsed three times with deionized water, and dried naturally at room temperature or blown gently using N2 gas.
Evaluation of graphene after wet etching
After treatment or patterning, graphene was first evaluated by optical microscopy using a DSX 510 instrument (Olympus, Tokyo, Japan) with 10× and 50× lenses. Images were captured using bright field (BF) mode, and samples were checked by micro Raman spectroscopy (inVia, Renishaw, UK). Raman measurements were taken at an excitation wavelength of 532 nm. Raman mapping of patterned graphene was performed by measuring the peak intensities for 2D-bands at ~ 2683 cm-1, G-bands at ~ 1590, and D-bands at 1350 cm-1. SEM using an SU8220 instrument (Hitachi High Tech Corporation, Tokyo, Japan) combined with EDS using a 5060FlatQUAD instrument (Bruker Corporation, Yokohama, Japan) were employed to assess the structure of patterned graphene, and to investigate the elemental distributions by mapping of carbon and oxygen in the graphene arrays. SEM and EDS measurements were performed at an acceleration voltage of 5 kV and an emission current of 10 µA. AFM images were obtained by a Nanosearch scanning probe microscope combined with an optical/laser microscope (Shimadzu, Tokyo, Japan).
Fabrication and evaluation of the graphene FET array
Patterned graphene ribbon arrays were selected for preparation of FETs. The drain and source electronic probes were prepared using Cr/Au EB evaporation by an EW-100S vacuum EB evaporation instrument (Eiko Corporation) and a custom-made metal (Ni) mask. Cr and Au were successively deposed at a thickness of ~ 20 and ~ 100 nm, respectively.
To assess the performance of fabricated graphene FETs, the patterned graphene ribbons with Au/Cr electrodes were thermally treated at 623 K under an H2 atmosphere (40 Pa) for 2 h to remove residues on the graphene surfaces that may otherwise cause resistance. Samples were then transferred to another vacuum system (Thermal Block Company, Kawaguchi, Saitama, Japan) for electrical measurements. The devices were heated to 473 K in the chamber before measuring electrical properties to remove any water molecules adhered to the graphene. ID as a function of VGS was measured at room temperature below 2.0 × 10–2 Pa using a standard four-probe technique with a 4200A-SCS semiconductor parameter analyzer (Tektronix Inc., Tokyo, Japan). Under a constant drain-source voltage (VDS) of 0.1 V, VGS was swept from -40 to 40 V. The hole mobility was calculated using the following equation:
where Rs is the sheet resistance of the patterned graphene, e is the elementary charge (1.6 × 1019 C), Cox is the gate insulator capacitance (tSiO2 = 300 nm), and VDirac is the gate voltage at the Dirac point. The mobility at a hole concentration (p) of 2.0 × 1012 cm-2 is discussed in this study.
Supplementary Information
Acknowledgements
This work was partially supported by the Research Institute of Materials and Chemistry of AIST for the projects in elucidation of the mechanism of materials and process research (R3-005). The authors thank Dr. Naoko Tajima for help with electronic beam evaporation experiments.
Author contributions
M.Z., T.O., and T.Y. conceived and designed the project. M.Z. and M.Y. conducted wet etching experiments. Y.O. conducted fabrication and evaluation of graphene FETs. M.Y., H.N., and Y.I. conducted material characterization. M.Z. wrote the manuscript and analyzed the experimental results. All authors discussed the results and commented on the manuscript.
Data availability
All experimental data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Minfang Zhang, Email: m-zhang@aist.go.jp.
Takatoshi Yamada, Email: takatoshi-yamada@aist.go.jp.
Toshiya Okazaki, Email: toshi-okazaki@aist.go.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08674-3.
References
- 1.Novoselov KS, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 2.Novoselov KS, et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature. 2005;438:197–200. doi: 10.1038/nature04233. [DOI] [PubMed] [Google Scholar]
- 3.Castro Neto AH, et al. The electronic properties of graphene. Rev. Mod. Phys. 2009;81:109. doi: 10.1103/RevModPhys.81.109. [DOI] [Google Scholar]
- 4.Bae S, et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nature Nanotech. 2010;5:574–578. doi: 10.1038/nnano.2010.132. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, et al. Graphene electrostatic microphone and ultrasonic radio. PNAS. 2015;112(29):8942–8946. doi: 10.1073/pnas.1505800112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sordan R, Traversi F, Russo V. Logic gates with a single graphene transistor. Appl. Phys. Lett. 2009;94:073305. doi: 10.1063/1.3079663. [DOI] [Google Scholar]
- 7.Mohanty N, Berry V. Graphene-based single-bacterium resolution biodevice and DNA transistor: Interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Lett. 2008;8(12):4469–5447. doi: 10.1021/nl802412n. [DOI] [PubMed] [Google Scholar]
- 8.Wang JTW, et al. Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 2014;14:724–730. doi: 10.1021/nl403997a. [DOI] [PubMed] [Google Scholar]
- 9.Wei T, Bao L, Hauke F, Hirsch A. Recent advances in graphene patterning. Chem. Plus Chem. 2020;85:1655–1668. doi: 10.1002/cplu.202000419. [DOI] [PubMed] [Google Scholar]
- 10.Kim KS, et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457:706–710. doi: 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- 11.Lee E, et al. Direct growth of highly stable patterned graphene on dielectric insulators using a surface-adhered solid carbon source. Adv. Mater. 2018;30:1706569. doi: 10.1002/adma.201706569. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, et al. Pattern induced ordering of semiconducting graphene ribbons grown from nitrogen seeded SiC. Carbon. 2015;82:360–367. doi: 10.1016/j.carbon.2014.10.081. [DOI] [Google Scholar]
- 13.Wang X, Dai H. Etching and narrowing of graphene from the edges. Nature Chem. 2010;2:661–665. doi: 10.1038/nchem.719. [DOI] [PubMed] [Google Scholar]
- 14.Bai J, Duan X, Huang Y. Rational fabrication of graphene nanoribbons using a nanowire etch mask. Nano Lett. 2009;9(5):2083–2087. doi: 10.1021/nl900531n. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Loh KP. Making patterns on graphene. Adv. Mater. 2010;22:3615–3620. doi: 10.1002/adma.201000436. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, et al. Patterning graphene film by magnetic-assisted UV Ozonation. Sci. Rep. 2017;7:46583. doi: 10.1038/srep46583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraswat V, Jacobberger RM, Arnold MS. Materials science challenges to graphene nanoribbon electronics. ACS Nano. 2021;15(3):3674–3708. doi: 10.1021/acsnano.0c07835. [DOI] [PubMed] [Google Scholar]
- 18.Liu LZ, et al. Tunable periodic graphene antidot lattices fabricated by e-beam lithography and oxygen ion etching. Vacuum. 2014;105:21–25. doi: 10.1016/j.vacuum.2014.01.015. [DOI] [Google Scholar]
- 19.Kalhor N, Boden SA, Mizuta H. Sub-10 nm patterning by focused He-ion beam milling for fabrication of downscaled graphene nano devices. Microelectron. Eng. 2014;114:70–77. doi: 10.1016/j.mee.2013.09.018. [DOI] [Google Scholar]
- 20.Sahin R, Simsek E, Akturk S. Nanoscale patterning of graphene through femtosecond laser ablation. Appl. Phys. Lett. 2014;104:053118. doi: 10.1063/1.4864616. [DOI] [Google Scholar]
- 21.Yoo JH, Park JB, Ahn S, Grigoropoulos CP. Laser-induced direct graphene patterning and simultaneous transferring method for graphene sensor platform. Small. 2013;9:4269–4275. doi: 10.1002/smll.201300990. [DOI] [PubMed] [Google Scholar]
- 22.Nayak AP, Islam MS, Logeeswaran VJ. Wet Etching. In: Bhushan B, editor. Encyclopedia of Nanotechnology. Dordrecht: Springer; 2012. [Google Scholar]
- 23.Zhang M, et al. A simple method for removal of carbon nanotubes from wastewater using hypochlorite. Sci. Rep. 2019;9:1284. doi: 10.1038/s41598-018-38307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, et al. Diameter-dependent degradation of 11 types of carbon nanotubes: safety implications. ACS Appl. Nano Mater. 2019;2:4293–4301. doi: 10.1021/acsanm.9b00757. [DOI] [Google Scholar]
- 25.Yang M, Okazaki T, Zhang M. Removal of carbon nanotubes from aqueous solutions by sodium hypochlorite: effects of treatment conditions. Toxics. 2021;9:223. doi: 10.3390/toxics9090223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman L, et al. Hypochlorite degrades 2D graphene oxide sheets faster than 1D oxidised carbon nanotubes and nanohorns. npj 2D Mater Appl. 2017;39:774–776. [Google Scholar]
- 27.Park KS, et al. Wafer-scale single-domain-like graphene by defect-selective atomic layer deposition of hexagonal ZnO. Nanoscale. 2015;7:17702. doi: 10.1039/C5NR05392G. [DOI] [PubMed] [Google Scholar]
- 28.Reina A, et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009;9(1):30–35. doi: 10.1021/nl801827v. [DOI] [PubMed] [Google Scholar]
- 29.Hokazono A, et al. Enhancement/depletion MESFETs of diamond and their logic circuits. Diam. Relat. Mater. 1997;6:339–343. doi: 10.1016/S0925-9635(96)00726-1. [DOI] [Google Scholar]
- 30.Dong Y, et al. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012;22:8764. doi: 10.1039/c2jm30658a. [DOI] [Google Scholar]
- 31.Peng J, et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012;12:844–849. doi: 10.1021/nl2038979. [DOI] [PubMed] [Google Scholar]
- 32.Hummers WS, Jr, Offeman RE. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958;80:1339. doi: 10.1021/ja01539a017. [DOI] [Google Scholar]
- 33.Fang J, Fu Y, Shang C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014;48:1859–1868. doi: 10.1021/es4036094. [DOI] [PubMed] [Google Scholar]
- 34.Zyara AM, Torvinen E, Veijalainen AM, Heinonen-Tanski H. The effect of UV and combined chlorine/UV treatment on coliphages in drinking water disinfection. Water. 2016;8(4):130. doi: 10.3390/w8040130. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, et al. UV-enhanced NaClO oxidation of nitric oxide from simulated flue gas. J. Chem. 2016;2016:6065019. [Google Scholar]
- 36.Rahimi S, et al. Toward 300 mm wafer-scalable high-performance polycrystalline chemical vapor-deposited graphene transistors. ACS Nano. 2014;8:10471–10479. doi: 10.1021/nn5038493. [DOI] [PubMed] [Google Scholar]
- 37.Ryu S, et al. Atmospheric oxygen binding and hole doping in deformed graphene on a SiO2 Substrate. Nano Lett. 2010;10:4944–4951. doi: 10.1021/nl1029607. [DOI] [PubMed] [Google Scholar]
- 38.Ago H, et al. Epitaxial growth and electronic properties of large hexagonal graphene domains on Cu (111) thin film. Appl. Phys. Express. 2013;6:075101. doi: 10.7567/APEX.6.075101. [DOI] [Google Scholar]
- 39.Okigawa Y, et al. Effects of outgassing on graphene synthesis by plasma treatment. Carbon. 2016;108:351. doi: 10.1016/j.carbon.2016.07.030. [DOI] [Google Scholar]
- 40.Yamada T, Okigawa Y, Hasegawa M. Potassium-doped n-type bilayer graphene. Appl. Phys. Lett. 2018;112:043105. doi: 10.1063/1.5012808. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All experimental data that support the findings of this study are available from the corresponding author upon reasonable request.