Abstract
Infants conceived with in vitro fertilization (IVF) are exposed to underlying infertility and the IVF process. High risks of adverse birth outcomes (ABOs) were observed among these infants, including preterm birth, low birth weight, macrosomia, being large/small for gestational age (LGA/SGA). It is unclear whether the specific etiology of the rise of ABOs among IVF infants is IVF technology itself or underlying infertility. A total of 9,480 singletons conceived with IVF and 1,952,419 singletons from the general population were obtained in this study. Multivariable logistic regression model was used to assess variations in risk of ABOs according to causes of infertility. Poisson distributions were applied to calculate standardized risk ratios of IVF infants vs. general population after controlling the causes of infertility. Higher risk of preterm birth and low birth weight were observed among parents with polycystic ovary syndrome, endometriosis, uterine and semen abnormalities. Compared to the general population, after excluding the influence of infertility causes, singletons conceived with IVF were at higher risk of macrosomia (SRR = 1.28, 95% CI 1.14–1.44) and LGA (SRR = 1.25, 95% CI 1.15–1.35). The higher risk of ABOs in IVF was driven by both IVF treatments and infertility, which is important for improving IVF treatments and the managing pregnancies and child development.
Subject terms: Epidemiology, Reproductive disorders, Intrauterine growth, Preterm birth
Introduction
As lifestyles and living environments continue to evolve, infertility is increasing globally, currently affecting more than one-sixth of couples worldwide1–4. Assisted reproductive technology (ART), especially in vitro fertilization (IVF), is widely used for infertility treatment worldwide. Infants conceived by ART have been reported to account for 4–10% of live births in developed countries5,6.
Studies have shown that IVF is associated with an increased risk of adverse birth outcomes (ABOs), such as 1.2–2 times the risk of preterm birth (PTB) and low birth weight (LBW)7, 1.2 times of small for gestational age (SGA)8, and 1.5 times the risk of congenital abnormality9. Infants conceived with IVF are affected by underlying infertility factors in addition to the IVF process. However, it remains unclear whether the association of IVF and ABOs originates from the IVF process or the infertility factors. A systematic review and meta-analysis reported that women with endometriosis had a higher risk of PTB and a similar SGA risk after IVF compared to women without endometriosis10. A cohort study of singletons found a higher risk of PTB and large for gestational age (LGA) after IVF in women with polycystic ovary syndrome (PCOS)11. However, other studies reported that women with unexplained infertility were not at a higher risk of ABOs following IVF12. Infants of subfertile women who conceived naturally had a higher risk of ABOs than infants of fertile women13,14. Taking these studies into consideration, it remains unknown whether IVF increases the risk of ABOs when controlling for the influence of the underlying causes of infertility.
The purpose of this study was to further investigate whether the specific cause of infertility affects the birth outcomes of IVF. In addition, we conducted a large population-based cohort study to assess whether IVF increases the risk of ABOs compared to the general population or whether the increased risks are associated with infertility.
Materials and methods
This population-based retrospective cohort study was approved by the institutional review board of the Shanghai Municipal Centre for Disease Control and Prevention (SCDC), and all methods were performed in accordance with the relevant guidelines and regulations. Following the principles of ethical review in China, it is not necessary to reacquire informed consent from participants for retrospective studies based on population registry information. There, the requirement to obtain written informed consent was waived.
Assisted reproductive technology data
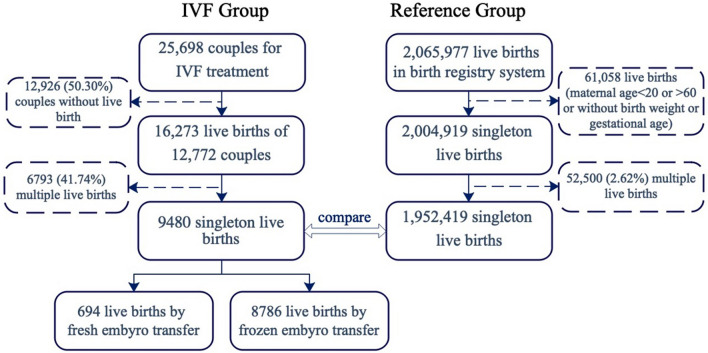
Treatment information and birth outcomes of infants conceived with ART were obtained from the Assisted Reproduction Centre of the Ninth People's Hospital affiliated with Shanghai Jiao Tong University of Medicine, Shanghai, China. The centre provided data for infertility treatments performed from January 2008 through December 2017. Out of 25,698 patients (20–60 years old) who visited the centre for IVF treatment, 12,772 (49.70%) delivered one or more liveborn infants (Fig. 1). After excluding 6793 (41.74%) multiple-birth deliveries, the final sample included 9,480 singleton infants conceived with IVF (694 fresh and 8786 frozen).
Figure 1.
Flow chart of participants included in the study.
As per the technical standard for Human assisted reproduction under the Ministry of Health of China15–17, details of baseline infertility investigations, ART treatments, and resultant births through ART were recorded for each participant. In addition, data on the treatment period, maternal age, pregestational body mass index (BMI), infertility duration, cause of infertility, fresh or frozen embryo transfer, and live birth occurrence were obtained for this study.
Birth registry data
Birth information from the whole population came from the birth registry system of SCDC, which was established in 2003 and provides all hospitals with authorized delivery services in Shanghai. To ensure comparability with ART data, 2,065,977 live births were collected from the birth registry system during 2008–2017 (Fig. 1). Due to the mother’s age (< 20 or > 60) or insufficient data (no birth weight or gestational age), 61,058 (2.96%) live births were excluded. After excluding 52,500(2.62%) multiple-birth deliveries, 1,952,419 singleton births were set up as the reference group for analysis. The birth information includes the date of birth, infant sex, birth weight, gestational age at birth, embryo number, mode of delivery, gravidity, parity, and socio-demographic characteristics of the parents.
Definitions
To compare the gestational age with that of the general population, the gestational age for an IVF infant was calculated as the interval from the date of embryo transfer to the date of birth plus 14 days and the duration of in vitro embryo culture18. The main ABOs concerned in this study included PTB, LBW, macrosomia, LGA, and SGA. According to the World Health Organization, PTB is defined as delivery before 37 completed weeks of gestation (or 259 days). LBW is defined as a birth weight of less than 2500 g and macrosomia is defined as a birth weight of over 4000 g. According to the China national population-based sex-specific reference curve for normal fetal growth, SGA and LGA are defined as having a birth weight < 10th or > 90th percentile for gestational age, respectively19. In the general population, 20–29 years old is the best childbearing age, so the age group of 20–29 years old was set as the reference group20. The remaining ages were divided into groups of 5 years.
Statistical analysis
Maternal and neonatal characteristics in the IVF group and reference group were described and the chi-square test was used to compare the difference between the IVF group and the general population. We applied a multivariable logistic regression model to evaluate the influence of parental and treatment-related factors on the risk of PTB, LBW, macrosomia, SGA, and LGA among infants conceived with IVF. The factors evaluated in the model included the period of birth, infant sex, maternal age, pregestational BMI, parity, cause of infertility, years of infertility, number of previous procedures involving ART, fresh or frozen embryo transfer cycles, and whether the sperm was from the husband or a donor. Causes of infertility were identified as semen abnormalities (including oligospermia, azoospermia, and teratozoospermia), endometriosis, PCOS, other ovulation failures, tubal factors, and uterine factors (including malformation or pathological uterine or abnormal cervix). Cases where no cause could be found were defined as unexplained infertility. Each diagnosis was set as a binary variable (Yes/No) in the multivariable logistic regression model.
The number of PTB, LBW, macrosomia, SGA, and LGA infants conceived with IVF were compared with expected numbers following a Poisson distribution, which were calculated based on the Shanghai birth registry data and adjusted according to the birth year, maternal age, infant sex, and parity distributions of women conceived with IVF. Standardized risk ratios (SRR) for PTB, LBW, macrosomia, SGA, and LGA were calculated by dividing the observed numbers by the expected numbers and estimated the 95% confidence intervals (CIs).
Additional analyses were performed on the subsamples to distinguish the effect of IVF procedures from subfertility characteristics and the cause of infertility. First, according to the multivariable logistic regression model mentioned above, the subsample was restricted to infants from couples with normal BMI and without infertility factors, including semen abnormalities, endometriosis, polycystic ovary syndrome and uterine factor, which could have affected the birth outcomes. Second, the subsample was limited to infants born to couples with normal BMI and without any apparent cause of infertility (i.e., the unexplained cause group). Third, the subsample was restricted to infants born to couples with a sole diagnosis of tubal disease and with normal BMI; since these infants were considered to be more likely to be conceived with healthy gametes. The statistical and data analysis software package SAS 9.4 was used for data analysis. A value of p < 0.05 was considered to indicate statistical significance.
Results
Maternal and neonatal characteristics
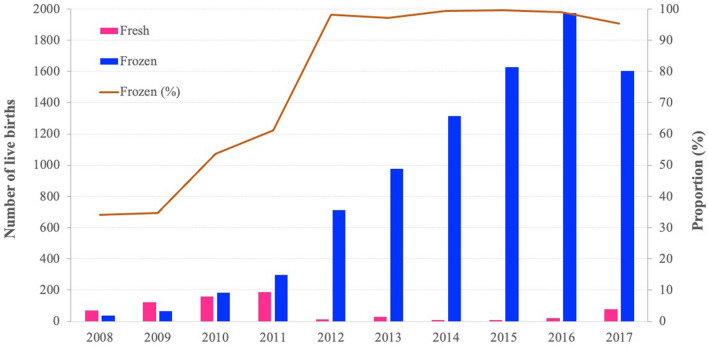
The number of infants conceived with IVF had increased dramatically in recent years, and the utilization rate of frozen embryo transfer had increased from 34% in 2008 to 98% in 2012 and remained above 95% until 2017. (Fig. 2). Maternal and neonatal characteristics showed great differences between the general population and those treated with IVF. The maternal age of women who had conceived with IVF (Mean = 32.80, 95% CI 32.72–32.88) tended to be higher than that in the general population (Mean = 28.15, 95% CI 28.11–28.19) (Table 1). Moreover, women who conceived with IVF were more likely to undergo a caesarean section (76.75% vs. 47.60%, P < 0.0001). The sex ratios at birth of IVF (114.09) and the general population (112.54) were much higher than the United Nations recommendation of 103–107. The average gestational age and birth weight of infants conceived with IVF did not differ significantly from the general population. However, the incidence of PTB (6.59%) and LBW (4.14%) was much higher than that in the general population (4.57 and 2.84%, P < 0.0001). The incidence of macrosomia (8.66%) and LGA (17.82%) was also higher than that in the general population (6.97% and 14.44%, P < 0.0001), while there was no statistically significant difference in the incidence of SGA between IVF (4.98%) and the general population (5.09%, P = 0.6388).
Figure 2.
The trends of live births by fresh- and frozen-embryo transfer. From 2008 to 2017, there was increased in trend in the proportion of frozen-embryo transfer (Cochran−Armitage trend test χ2 = 41.06, P < 0.0001).
Table 1.
Maternal and neonatal characteristics of IVF infants and general population.
| Variable | Variable level | IVF total | Fresh IVF | Frozen IVF | General population | General population VS. IVF | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | P value | ||
| Maternal age | Mean/Std | 32.80 | 4.07 | 32.62 | 3.88 | 32.82 | 4.09 | 28.15 | 4.49 | |
| 20–29 years | 2507 | 26.45 | 187 | 26.95 | 2320 | 26.41 | 1,269,832 | 65.04 | < 0.0001 | |
| 30–34 years | 4232 | 44.64 | 300 | 43.23 | 3932 | 44.75 | 502,893 | 25.76 | ||
| 35–39 years | 2268 | 23.92 | 189 | 27.23 | 2079 | 23.66 | 155,388 | 7.96 | ||
| ≥ 40 years | 473 | 4.99 | 18 | 2.59 | 455 | 5.18 | 24,306 | 1.24 | ||
| Missing | 0 | – | 0 | – | 0 | – | 0 | – | ||
| Maternal education level | Postgraduate | 386 | 5.8 | 7 | 4.64 | 379 | 5.83 | 115,866 | 5.93 | < 0.0001 |
| Graduate | 3963 | 59.59 | 99 | 65.56 | 3864 | 59.45 | 899,125 | 46.05 | ||
| Middle School | 2201 | 33.09 | 43 | 28.48 | 2158 | 33.2 | 383,472 | 19.64 | ||
| Primary School | 101 | 1.52 | 2 | 1.32 | 99 | 1.52 | 553,943 | 28.37 | ||
| Missing | 2829 | – | 543 | – | 2286 | – | 13 | – | ||
| Parity | 0 | 8624 | 90.97 | 630 | 90.78 | 7994 | 90.99 | 1,315,266 | 67.37 | < 0.0001 |
| 1 | 808 | 8.52 | 61 | 8.79 | 747 | 8.5 | 577,600 | 29.58 | ||
| ≥ 2 | 48 | 0.51 | 3 | 0.43 | 45 | 0.51 | 59,553 | 3.05 | ||
| Missing | 0 | – | 0 | – | 0 | – | 0 | – | ||
| Delivery mode | Vaginal | 2202 | 23.25 | 136 | 19.65 | 2066 | 23.54 | 1,022,996 | 52.40 | < 0.0001 |
| Cesarean | 7268 | 76.75 | 556 | 80.35 | 6712 | 76.46 | 929,423 | 47.60 | ||
| Missing | 10 | – | 2 | – | 8 | – | 0 | – | ||
| Sex | Boy | 5052 | 53.29 | 355 | 51.15 | 4697 | 53.46 | 1,033,763 | 52.95 | 0.5041 |
| Girl | 4428 | 46.71 | 339 | 48.85 | 4089 | 46.54 | 918,656 | 47.05 | ||
| Sex ratio | – | 114.09 | – | 104.71 | – | 114.87 | – | 112.54 | ||
| Missing | 0 | – | 0 | – | 0 | – | 0 | – | ||
| Gestational age | Mean/Std | 38.34 | 1.65 | 38.30 | 1.67 | 38.35 | 1.65 | 38.89 | 1.49 | |
| Missing | 0 | – | 0 | – | 0 | – | 0 | – | ||
| PTB | 625 | 6.59 | 37 | 5.33 | 588 | 6.69 | 89,242 | 4.57 | < 0.0001 | |
| Birth weight | Mean/Std | 3347.93 | 502.67 | 3320.15 | 495.44 | 3350.12 | 503.18 | 3347.09 | 453.15 | |
| Missing | 0 | – | 0 | – | 0 | – | 0 | – | ||
| LBW | 392 | 4.14 | 25 | 3.6 | 367 | 4.18 | 55,500 | 2.84 | < 0.0001 | |
| Macrosomia | 821 | 8.66 | 56 | 8.07 | 765 | 8.71 | 136,032 | 6.97 | < 0.0001 | |
| SGA | 472 | 4.98 | 28 | 4.03 | 444 | 5.05 | 99,282 | 5.09 | 0.6388 | |
| LGA | 1689 | 17.82 | 119 | 17.15 | 1570 | 17.87 | 282,006 | 14.44 | < 0.0001 | |
Risk factors of ABOs in IVF
We found a decreased trend of PTB (OR 0.94, 95% CI 0.90–0.98) and LGA (OR 0.97, 95% CI 0.94–1.00) in IVF in recent years (Table 2). In this study, maternal age was not a significant risk factor for ABOs; however, overweight (24 ≤ BMI < 28) and obesity (BMI ≥ 28) was associated with a higher risk of PTB (OR 1.42, 95% CI 1.15–1.77; OR 1.68, 95% CI 1.16–2.44, respectively).
Table 2.
Risk factors of adverse birth outcomes in singletons conceived with IVF.
| Variable | Variable level | PTB | LBW | Macrosomia | SGA | LGA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Rate (%) | OR (95%CI) | N | Rate (%) | OR (95%CI) | N | Rate (%) | OR (95%CI) | N | Rate (%) | OR (95%CI) | N | Rate (%) | OR (95%CI) | ||
| Year of birth | Change per year | – | – | 0.94(0.90,0.98) | – | – | 0.98(0.93,1.04) | – | – | 0.99(0.95,1.03) | – | – | 1.01(0.96,1.06) | – | – | 0.97(0.94,1.00) |
| Maternal age | 20–29 years | 166 | 6.76 | Ref | 101 | 4.11 | Ref | 226 | 9.20 | Ref | 127 | 5.17 | Ref | 451 | 18.36 | Ref |
| 30–34 years | 263 | 6.35 | 0.87(0.71,1.07) | 172 | 4.15 | 0.95(0.74,1.23) | 361 | 8.72 | 0.88(0.73,1.05) | 192 | 4.64 | 0.93(0.73,1.17) | 730 | 17.63 | 0.90(0.78,1.02) | |
| 35–39 years | 142 | 6.42 | 0.86(0.67,1.11) | 87 | 3.93 | 0.89(0.65,1.22) | 187 | 8.46 | 0.80(0.64,0.99) | 116 | 5.25 | 1.11(0.84,1.47) | 395 | 17.87 | 0.86(0.73,1.01) | |
| ≥ 40 years | 45 | 9.78 | 1.29(0.88,1.88) | 21 | 4.57 | 1.03(0.61,1.72) | 34 | 7.39 | 0.62(0.41,0.92) | 24 | 5.22 | 1.17(0.72,1.87) | 81 | 17.61 | 0.77(0.58,1.02) | |
| Pregestational BMI | < 18.5 | 65 | 6.01 | 1.02(0.77,1.34) | 39 | 3.60 | 0.97(0.69,1.37) | 45 | 4.16 | 0.48(0.35,0.65) | 86 | 7.95 | 1.84(1.43,2.37) | 103 | 9.52 | 0.50(0.40,0.62) |
| 18.5–23.9 | 395 | 6.08 | Ref | 247 | 3.80 | Ref | 518 | 7.98 | Ref | 292 | 4.50 | Ref | 1114 | 17.16 | Ref | |
| 24–28 | 120 | 8.82 | 1.42(1.15,1.77) | 72 | 5.29 | 1.41(1.07,1.85) | 200 | 14.70 | 2.03(1.70,2.43) | 68 | 5.00 | 1.15(0.88,1.51) | 360 | 26.45 | 1.75(1.52,2.01) | |
| ≥ 28 | 36 | 10.84 | 1.68(1.16,2.44) | 23 | 6.93 | 1.84(1.17,2.90) | 45 | 13.55 | 1.89(1.35,2.64) | 13 | 3.92 | 0.92(0.52,1.63) | 80 | 24.10 | 1.55(1.19,2.03) | |
| Gender | Boy | 347 | 7.03 | Ref | 184 | 3.73 | Ref | 516 | 10.45 | Ref | 228 | 4.62 | Ref | 882 | 17.86 | Ref |
| Girl | 269 | 6.22 | 0.89(0.75,1.05) | 197 | 4.55 | 1.23(1.00,1.52) | 292 | 6.75 | 0.62(0.53,0.72) | 231 | 5.34 | 1.17(0.97,1.42) | 775 | 17.91 | 1.01(0.91,1.12) | |
| Parity | 0 | 542 | 6.43 | Ref | 350 | 4.15 | Ref | 721 | 8.55 | Ref | 427 | 5.06 | Ref | 1479 | 17.54 | Ref |
| 1 | 70 | 8.89 | 1.41(1.07,1.85) | 29 | 3.68 | 0.94(0.63,1.40) | 82 | 10.42 | 1.18(0.92,1.52) | 31 | 3.94 | 0.77(0.52,1.13) | 166 | 21.09 | 1.22(1.01,1.47) | |
| ≥ 2 | 4 | 8.51 | 1.31(0.46,3.70) | 2 | 4.26 | 1.09(0.26,4.59) | 5 | 10.64 | 1.19(0.46,3.06) | 1 | 2.13 | 0.42(0.06,3.06) | 12 | 25.53 | 1.58(0.81,3.08) | |
| Year of infertility | < 5 years | 424 | 6.32 | Ref | 265 | 3.95 | Ref | 583 | 8.69 | Ref | 339 | 5.05 | Ref | 1187 | 17.69 | Ref |
| 5–10 years | 162 | 7.59 | 1.15(0.95,1.40) | 98 | 4.59 | 1.11(0.87,1.42) | 189 | 8.86 | 0.99(0.82,1.18) | 101 | 4.74 | 0.96(0.76,1.22) | 389 | 18.24 | 0.99(0.87,1.13) | |
| ≥ 10 years | 30 | 7.08 | 1.01(0.68,1.52) | 18 | 4.25 | 1.03(0.62,1.72) | 36 | 8.49 | 0.97(0.67,1.40) | 19 | 4.48 | 0.88(0.54,1.45) | 81 | 19.10 | 1.03(0.79,1.34) | |
| Number of failure cycle | 0 | 368 | 6.37 | Ref | 228 | 3.95 | Ref | 485 | 8.40 | Ref | 299 | 5.18 | Ref | 1005 | 17.40 | Ref |
| 1 | 99 | 6.10 | 0.87(0.69,1.11) | 57 | 3.51 | 0.86(0.63,1.17) | 153 | 9.43 | 1.17(0.96,1.43) | 69 | 4.25 | 0.80(0.60,1.05) | 300 | 18.48 | 1.07(0.92,1.24) | |
| 2 | 69 | 8.03 | 1.20(0.91,1.58) | 43 | 5.01 | 1.24(0.88,1.75) | 78 | 9.08 | 1.16(0.90,1.51) | 40 | 4.66 | 0.88(0.62,1.24) | 159 | 18.51 | 1.10(0.91,1.34) | |
| ≥ 3 | 80 | 7.94 | 1.16(0.88,1.51) | 53 | 5.26 | 1.29(0.93,1.79) | 92 | 9.13 | 1.17(0.91,1.50) | 51 | 5.06 | 0.97(0.70,1.33) | 193 | 19.15 | 1.16(0.96,1.38) | |
| Sperm | Husband sperm | 608 | 6.66 | Ref | 372 | 4.08 | Ref | 799 | 8.76 | Ref | 449 | 4.92 | Ref | 1635 | 17.92 | Ref |
| Donor sperm | 8 | 5.63 | 0.84(0.40,1.78) | 9 | 6.34 | 1.42(0.68,2.93) | 9 | 6.34 | 0.73(0.36,1.49) | 10 | 7.04 | 1.45(0.72,2.90) | 22 | 15.49 | 0.80(0.49,1.29) | |
| Embryo transfer | Fresh | 37 | 5.51 | Ref | 25 | 3.72 | Ref | 56 | 8.33 | Ref | 28 | 4.17 | Ref | 118 | 17.56 | Ref |
| Frozen | 579 | 6.74 | 1.51(1.03,2.21) | 356 | 4.14 | 1.14(0.72,1.81) | 752 | 8.75 | 1.07(0.77,1.47) | 431 | 5.01 | 1.20(0.78,1.84) | 1539 | 17.91 | 1.13(0.90,1.43) | |
| Unexplained | No | 603 | 6.63 | Ref | 373 | 4.10 | Ref | 795 | 8.74 | Ref | 447 | 4.91 | Ref | 1626 | 17.87 | Ref |
| Yes | 13 | 7.83 | 1.29(0.69,2.41) | 8 | 4.82 | 1.51(0.69,3.29) | 13 | 7.83 | 0.74(0.40,1.36) | 12 | 7.23 | 1.45(0.75,2.81) | 31 | 18.67 | 0.92(0.60,1.42) | |
| Semen abnormalities | No | 515 | 6.59 | Ref | 309 | 3.95 | Ref | 688 | 8.81 | Ref | 385 | 4.93 | Ref | 1397 | 17.88 | Ref |
| Yes | 101 | 6.95 | 1.08(0.86,1.35) | 72 | 4.95 | 1.31(1.00,1.71) | 120 | 8.25 | 0.90(0.74,1.11) | 74 | 5.09 | 1.04(0.80,1.35) | 260 | 17.88 | 0.99(0.85,1.15) | |
| Endometriosis | No | 532 | 6.52 | Ref | 325 | 3.98 | Ref | 726 | 8.89 | Ref | 400 | 4.90 | Ref | 1484 | 18.18 | Ref |
| Yes | 84 | 7.62 | 1.29(1.01,1.65) | 56 | 5.08 | 1.37(1.01,1.85) | 82 | 7.43 | 0.85(0.66,1.08) | 59 | 5.35 | 1.05(0.78,1.40) | 173 | 15.68 | 0.88(0.73,1.05) | |
| PCOS | No | 542 | 6.35 | Ref | 342 | 4.01 | Ref | 746 | 8.74 | Ref | 432 | 5.06 | Ref | 1517 | 17.77 | Ref |
| Yes | 74 | 10.11 | 1.65(1.25,2.18) | 39 | 5.33 | 1.30(0.90,1.87) | 62 | 8.47 | 0.74(0.55,0.99) | 27 | 3.69 | 0.74(0.49,1.12) | 140 | 19.13 | 0.90(0.73,1.10) | |
| Other ovulation failure | No | 588 | 6.60 | Ref | 363 | 4.08 | Ref | 779 | 8.75 | Ref | 436 | 4.90 | Ref | 1605 | 18.02 | Ref |
| Yes | 28 | 7.76 | 1.28(0.85,1.92) | 18 | 4.99 | 1.36(0.83,2.24) | 29 | 8.03 | 0.88(0.59,1.32) | 23 | 6.37 | 1.33(0.85,2.08) | 52 | 14.40 | 0.75(0.55,1.02) | |
| Tubal factor | No | 112 | 7.87 | Ref | 67 | 4.71 | Ref | 128 | 9.00 | Ref | 79 | 5.55 | Ref | 265 | 18.62 | Ref |
| Yes | 504 | 6.43 | 0.95(0.74,1.21) | 314 | 4.00 | 1.03(0.76,1.40) | 680 | 8.67 | 0.88(0.70,1.11) | 380 | 4.84 | 0.92(0.69,1.23) | 1392 | 17.75 | 0.89(0.76,1.06) | |
| Uterine factor | No | 503 | 6.30 | Ref | 302 | 3.78 | Ref | 702 | 8.80 | Ref | 391 | 4.90 | Ref | 1438 | 18.02 | Ref |
| Yes | 113 | 8.78 | 1.42(1.14,1.77) | 79 | 6.14 | 1.60(1.23,2.08) | 106 | 8.24 | 0.89(0.72,1.11) | 68 | 5.28 | 1.08(0.83,1.42) | 219 | 17.02 | 0.90(0.77,1.06) | |
| Others* | No | 599 | 6.64 | Ref | 372 | 4.13 | Ref | 783 | 8.69 | Ref | 449 | 4.98 | Ref | 1609 | 17.85 | Ref |
| Yes | 17 | 6.75 | 1.05(0.63,1.75) | 9 | 3.57 | 0.80(0.40,1.59) | 25 | 9.92 | 1.21(0.78,1.87) | 10 | 3.97 | 0.68(0.35,1.30) | 48 | 19.05 | 1.18(0.85,1.65) | |
*Others: including chromosomal abnormality, immune factors, pituitary lesions and sexual dysfunction.
Statistical differences are shown in bold.
LBW (OR 1.41, 95% CI 1.07–1.85; OR 1.84, 95% CI 1.17–2.90, respectively), macrosomia (OR 2.03, 95% CI 1.70–2.43; OR 1.89, 95% CI 1.35–2.64, respectively), and LGA (OR 1.75, 95% CI 1.52–2.01; OR 1.55, 95% CI 1.19–2.03, respectively), while lower pregestational BMI (< 18.5) was associated with SGA (OR 1.84, 95% CI 1.43–2.37). In addition, female infants had a higher risk of LBW (OR 1.23, 95% CI 1.00–1.52) and a lower risk of macrosomia (OR 0.62, 95% CI0.53–0.72). Frozen embryo transfer was associated with a higher risk of PTB (OR 1.51, 95% CI 1.03–2.21), but there was no statistically significant difference in LBW, macrosomia, SGA and LGA.
Regarding the causes of infertility, semen abnormalities (OR 1.31, 95% CI 1.00–1.71) were related to a higher risk of LBW; endometriosis was related to a higher risk of PTB (OR 1.29, 95% CI 1.01–1.65) and LBW (OR 1.37, 95% CI 1.01–1.85); PCOS was linked to a higher risk of PTB (OR 1.65, 95% CI 1.25–2.18) and lower risk of macrosomia (OR 0.74, 95% CI 0.55–0.99); and uterine factor infertility was related to a higher risk of PTB (OR 1.42, 95% CI 1.14–1.77) and LBW (OR 1.60, 95% CI 1.23–2.08). There was no statistically significant difference in the incidence of ABOs between women with or without tubal infertility.
Comparison of ABOs risk between IVF and the general population
Compared with the general population, singletons conceived with IVF had a higher risk of PTB (SRR = 1.15, 95% CI 1.06–1.24), LBW (SRR = 1.12, 95% CI 1.02–1.24), macrosomia (SRR = 1.34, 95% CI 1.26–1.44), and LGA (SRR = 1.26, 95% CI 1.20–1.32) (Table 3). We stratified IVF procedures based on fresh and frozen embryo transfer cycles and found that the risks of all ABOs categories were still significantly higher in frozen cycles, whereas only the risks of LGA (SRR = 1.21, 95% CI 1.01–1.45) remained higher in fresh cycles.
Table 3.
Observed and expected cases of adverse birth outcomes among infants conceived with IVF in mothers with normal BMI.
| ABOs | Group | IVF | IVF-Fresh | IVF-Frozen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total no | No. of events | SRR* (95%CI) | Total no | No. of events | SRR* (95%CI) | Total no | No. of events | SRR* (95%CI) | |||||
| Observed | Expected# | Observed | Expected# | Observed | Expected# | ||||||||
| PTB | IVF Total | 9480 | 625 | 543.2 | 1.15(1.06,1.24) | 694 | 37 | 39.8 | 0.93(0.67,1.28) | 8786 | 588 | 503.4 | 1.17(1.08,1.27) |
| Excluding infertility factors affecting ABOs $ | 3787 | 229 | 217.0 | 1.06(0.93,1.20) | 336 | 16 | 19.3 | 0.83(0.51,1.36) | 3451 | 213 | 197.7 | 1.08(0.94,1.23) | |
| Unexplained infertility cause | 111 | 8 | 6.4 | 1.26(0.63,2.52) | – | – | – | – | 99 | 8 | 5.7 | 1.41(0.71,2.82) | |
| Tubal factor infertility | 3333 | 199 | 191.0 | 1.04(0.91,1.20) | 286 | 13 | 16.4 | 0.79(0.46,1.37) | 3047 | 186 | 174.6 | 1.07(0.92,1.23) | |
| LBW | IVF Total | 9480 | 392 | 348.9 | 1.12(1.02,1.24) | 694 | 25 | 25.5 | 0.98(0.66,1.45) | 8786 | 367 | 323.3 | 1.14(1.02,1.26) |
| Excluding infertility factors affecting ABOs $ | 3787 | 133 | 139.4 | 0.95(0.81,1.13) | 336 | 12 | 12.4 | 0.97(0.55,1.71) | 3451 | 121 | 127.0 | 0.95(0.80,1.14) | |
| Unexplained infertility cause | 111 | 3 | 4.1 | 0.73(0.24,2.28) | – | – | – | – | 99 | 3 | 3.6 | 0.82(0.27,2.55) | |
| Tubal factor infertility | 3333 | 115 | 122.7 | 0.94(0.78,1.13) | 286 | 10 | 10.5 | 0.95(0.51,1.77) | 3047 | 105 | 112.1 | 0.94(0.77,1.13) | |
| Macrosomia | IVF total | 9480 | 821 | 610.5 | 1.34(1.26,1.44) | 694 | 56 | 44.7 | 1.25(0.96,1.63) | 8786 | 765 | 565.8 | 1.35(1.26,1.45) |
| Excluding infertility factors affecting ABOs $ | 3787 | 308 | 243.9 | 1.26(1.13,1.41) | 336 | 23 | 21.6 | 1.06(0.71,1.60) | 3451 | 285 | 222.2 | 1.28(1.14,1.44) | |
| Unexplained Infertility cause | 111 | 10 | 7.1 | 1.40(0.75,2.60) | 12 | 1 | 0.8 | 1.29(0.18,9.19) | 99 | 9 | 6.4 | 1.41(0.73,2.71) | |
| Tubal factor infertility | 3333 | 271 | 214.6 | 1.26(1.12,1.42) | 286 | 22 | 18.4 | 1.19(0.79,1.81) | 3047 | 249 | 196.2 | 1.27(1.12,1.44) | |
| SGA | IVF total | 9480 | 472 | 486.3 | 0.97(0.89,1.06) | 694 | 28 | 35.6 | 0.79(0.54,1.14) | 8786 | 444 | 450.7 | 0.99(0.90,1.08) |
| Excluding infertility factors affecting ABOs $ | 3787 | 174 | 194.3 | 0.90(0.77,1.04) | 336 | 16 | 17.2 | 0.93(0.57,1.52) | 3451 | 158 | 177.0 | 0.89(0.76,1.04) | |
| Unexplained infertility cause | 111 | 7 | 5.7 | 1.23(0.59,2.58) | 12 | 1 | 0.6 | 1.62(0.23,11.53) | 99 | 6 | 5.1 | 1.18(0.53,2.63) | |
| Tubal factor infertility | 3333 | 149 | 171.0 | 0.87(0.74,1.02) | 286 | 12 | 14.7 | 0.82(0.46,1.44) | 3047 | 137 | 156.3 | 0.88(0.74,1.04) | |
| LGA | IVF total | 9480 | 1689 | 1342.4 | 1.26(1.20,1.32) | 694 | 119 | 98.3 | 1.21(1.01,1.45) | 8786 | 1570 | 1244.1 | 1.26(1.20,1.33) |
| Excluding infertility factors affecting ABOs $ | 3787 | 662 | 536.2 | 1.23(1.14,1.33) | 336 | 53 | 47.6 | 1.11(0.85,1.46) | 3451 | 609 | 488.7 | 1.25(1.15,1.35) | |
| Unexplained infertility cause | 111 | 22 | 15.7 | 1.40(0.92,2.13) | 12 | 1 | 1.7 | 0.59(0.08,4.18) | 99 | 21 | 14.0 | 1.50(0.98,2.30) | |
| Tubal factor infertility | 3333 | 589 | 472.0 | 1.25(1.15,1.35) | 286 | 47 | 40.5 | 1.16(0.87,1.54) | 3047 | 542 | 431.5 | 1.26(1.15,1.37) | |
#The number of expected cases was calculated by applying the rates of PTB, LBW, macrosomia, SGA and LGA from the birth registry data to the population of infants conceived with assisted reproductive technology. The values were adjusted to account for differences in the distributions of year of birth, maternal age, infant sex and parity between the two populations.
*SRR standardized risk ratio.
$Infertility factors affecting ABOs meant infertility factors that influenced the birth outcomes, including semen abnormalities, endometriosis, polycystic ovary syndrome and uterine factor infertility.
Statistical differences are shown in bold.
In the subgroup analyses, we found that the risks of PTB and LBW no longer increased. However, the risks of macrosomia and LGA remained elevated in analyses restricted to subgroups of the infants conceived by women with a sole diagnosis of tubal factor infertility (macrosomia, SRR = 1.26, 95% CI 1.12–1.42; LGA, SRR = 1.25, 95% CI 1.15–1.35) or by women without infertility factors (semen abnormalities, endometriosis, PCOS, and uterine factor infertility) (macrosomia, SRR = 1.26, 95% CI 1.13–1.41; LGA, SRR = 1.23, 95% CI 1.14–1.33). The risk of LGA (SRR = 1.50, 95% CI 0.98–2.30) was not statistically significant in the group of women diagnosed with unexplained infertility, but the sample size (n = 99) was relatively small.
Discussion
With the wide application of IVF to treat infertility, a large number of ABOs have been observed among infants conceived with IVF8,9,18,21. This large sample study demonstrated that singleton infants conceived with IVF were at a higher risk of ABOs relative to singletons in the general population of Shanghai. These risks could not be explained by the known differences between the two populations in the distribution of sex of infants, maternal age, maternal parity, or maternal BMI. Factors causing infertility, such as semen abnormalities, endometriosis, PCOS, and uterine factors were associated with PTB and abnormal birth weight. However, the increased risks of macrosomia and LGA remained significant when the sample was restricted to (1) parents without infertility factors affecting ABOs wherein the mother has a normal BMI, (2) mothers who have infertility caused by a fallopian tube abnormality. Therefore, this study suggests that the increased risk of birth weight among singletons conceived with IVF may be associated with treatments for infertility. Although the risk of macrosomia and LGA was higher than that in the general population, the incidence of LGA showed a decreasing trend across the entire cohort of IVF cycles, which was also observed in Israel and Sweden22,23.
Consistent with previous studies, our study showed that low pregestational BMI (< 18.5) increased the risk of SGA, and being overweight or obese (BMI > 24) before pregnancy increased the risk of PTB, LBW, macrosomia, and LGA24,25. BMI abnormalities are more common in the infertile population and may be related to ovulation failure, irregular menses, poor oocyte quality, and hormonal imbalances26–28. Additionally, abnormal BMI may be a risk factor for abnormal birth weight in infants conceived with IVF. Therefore, it is necessary to eliminate the influence of abnormal BMI to reveal the real impact of IVF on ABOs.
This study found that PCOS, endometriosis and uterine factors were associated with a higher risk of PTB. Semen abnormalities, endometriosis, and uterine factors were associated with a higher risk of LBW. The association of PCOS with increased risk of PTB but not LBW is similar to previous studies where women with PCOS were found to have a higher risk of gestational diabetes, hypertensive disorders, PTB, and LGA11,29. Moreover, the literature showed that after the correction of hypertensive disorders, the increased risk of PTB was eliminated, and even after the correction of gestational diabetes, the increased risk of LGA remained significant11. Endometriosis, which affects 10–15% of reproductive age women, is associated with inflammation, fibrosis, and aberrant angiogenesis30. Literature shows that endometriosis is associated with a higher risk of PTB and LBW10,31, however, this association is influenced by BMI32. In this study, we also found that the association between endometriosis and risk of PTB and LBW increased after adjusting for maternal BMI. This finding suggests that the higher risk is associated with mechanisms specific to endometriosis33. Additionally, uterine factors such as uterine defects, uterine inflammation, and cervical insufficiency were also associated with a higher risk of PTB and LBW in this study. This finding is supported by literature showing a relationship between uterine factors and many obstetric complications34,35. Some studies have reported that tubal factors increase the risk of PTB and LBW for singletons; the etiological reasons were mainly attributed to inflammation and infections31,36,37. Conversely, other studies showed that a unilateral tubal block did not increase the risk of ABOs38,39. In this study, we did not find any association between tubal factor infertility and PTB or LBW. This difference may be explained by the epidemiological differences in the cause and severity of tubal infertility; hence, further study of the underlying mechanism is warranted.
Another novel finding of this study was that semen abnormality was associated with a higher incidence of LBW but not PTB. This finding contrasts with that of most other studies, which found no significant association between male infertility factors and increased risk of PTB and LBW compared to unexplained infertility31. Moreover, semen parameters did not influence embryo quality or live birth outcomes40,41. Another study showed that male-factor infertility has been associated with LBW and LBW at full term in singletons conceived with ART18; however, it could not be distinguished whether the effect was from the ART or male-factor infertility. This study found that sperm abnormality, oligospermia, and asthenozoospermia were associated with a higher risk of LBW. The increased risk may be due to sperm DNA damage42, but the mechanisms underlying the association remain unclear and warrants further research.
Several additional analyses were performed on the subsamples to distinguish the effects of IVF procedures from underlying characteristics of the patients and the cause of infertility. We found a significant association of macrosomia and LGA with IVF, even when abnormal BMI and infertile causes, such as semen abnormalities, endometriosis, polycystic ovary syndrome and uterine factor infertility for abnormal birth weight were eliminated from the study group. Although increased risk was not observed for fresh embryo transfers, this analysis had greatly reduced sample sizes and should therefore be interpreted with caution. Previous studies found that frozen embryo transfer was associated with increased birth weight and a higher risk of macrosomia and LGA, compared with spontaneous conception and fresh embryo transfer43,44; however, potential confounding factors, such as maternal BMI or cause of infertility had not been adjusted in those studies. In this study, after adjusting for confounding factors, singletons born after frozen embryo transfer had a higher rate of LGA and macrosomia compared with the general population. This finding may partly be explained by aspects related to ART procedure, such as improved endometrial reception, higher quality embryos surviving the freezing–thawing process45, and the effect of cryoprotectants46. The embryos were cultured in cryoprotectants and had undergone freezing and thawing procedures at a critical and vulnerable development stage, which could cause epigenetic changes resulting in a larger gestational size47. Currently, the mechanism remains unclear and epigenetic effects in infants conceived with ART require further investigation. While LGA and macrosomia may seem less threatening to infant survival, they are associated with an increased risk of cardiovascular diseases in adulthood48,49. Thus, more attention should be paid to the association between IVF and increased risk of LGA and macrosomia, and longer-term follow-up of children’s development is also important.
Strengths and limitations
One of the strengths of this study is its large sample size and detailed information available on the treatments from the infertility service centre. Additionally, the availability of outcomes from many frozen embryo transfers is higher compared with many previously reported studies. Confounding factors that affect embryonal development and underlying infertility have prevented researchers from evaluating the association between IVF and ABOs for a long time. This study adjusted for the confounding effects of underlying infertility to compare the fetal growth between IVF infants and the general population. Our research results indicated that the risk of macrosomia and LGA in IVF was increased, especially when frozen embryo transfer was used.
This study has some limitations. The general population was used as the reference group; however, the general population may include 1.7–4.0% of infants conceived with ART as it is not possible to accurately identify and exclude these births. Therefore, the association between ABOs and IVF was likely underestimated. This retrospective cohort study covers a relatively long period and may be affected by inevitable changes to routines for data collection, approach to diagnosis, and improved procedures for IVF. For example, such changes mean that there may be effects from changes to ovarian stimulation and oocyte collection methods that are hard to distinguish. We also did not collect data on pregnancy complications that may have affected the incidence of ABOs; however, we did include most risk factors for pregnancy complications, such as maternal age, pregestational BMI, endometriosis, and PCOS.
Conclusions
This cohort study found that causes of infertility, including endometriosis, PCOS, uterine factors, and semen abnormalities increased the risk of ABOs. After adjusting for these factors, compared with the general population, the risk of LBW and PTB did not increase, but the risk of macrosomia and LGA was still increased. Although we could not exclude the potential effect of pregnancy complications, the findings in this study may nonetheless provide insight for patients seeking ART treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation for Young Scientists of China (82003486), the Fifth Round of the Three-Year Public Health Action Plan of Shanghai (GWV-10.1-XK08), and Cross project of medical engineering in Shanghai Jiaotong University (YG2019QNA14).
Author contributions
Study concept and design: Y.K., H.Y. and Z.L.; Methodology: H.Y., Z.L. and T.X.; Formal Analysis: H.Y. and Z.L.; Data Curation: Z.L., R.C. and S.J.; Writing—Original Draft: H.Y.; Writing—Review & Editing: H.Y., Z.L., T.X. and Y.K.; Project administration: Y.K. and C.W.; Funding acquisition: H.Y. and Z.L.; Critical revision of the manuscript for important intellectual content: All authors.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: In the original version of this Article Huiting Yu and Zhou Liang were omitted as equally contributing authors.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Huiting Yu and Zhou Liang.
Change history
5/19/2022
A Correction to this paper has been published: 10.1038/s41598-022-12512-x
Contributor Information
Tian Xia, Email: xiatian@scdc.sh.cn.
Chunfang Wang, Email: 13761019425@163.com.
Yanping Kuang, Email: htychihealth@126.com.
References
- 1.Zhou Z, Zheng D, Wu H, et al. Epidemiology of infertility in China: A population-based study. BJOG. 2018;125(4):432–441. doi: 10.1111/1471-0528.14966. [DOI] [PubMed] [Google Scholar]
- 2.Population GBD, Fertility C. Population and fertility by age and sex for 195 countries and territories, 1950–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1995–2051. doi: 10.1016/S0140-6736(18)32278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Y, He X, Qi K, et al. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. (China) 2019;77:210–217. doi: 10.1016/j.jes.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Mouzon J, Goossens V, Bhattacharya S, et al. Assisted reproductive technology in Europe, 2006: Results generated from European registers by ESHRE. Hum. Reprod. 2010;25(8):1851–1862. doi: 10.1093/humrep/deq124. [DOI] [PubMed] [Google Scholar]
- 6.Hargreave M, Jensen A, Hansen MK, et al. Association between fertility treatment and cancer risk in children. JAMA. 2019;322(22):2203–2210. doi: 10.1001/jama.2019.18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mcdonald SD, Han Z, Mulla S, et al. Preterm birth and low birth weight among in vitro fertilization singletons: A systematic review and meta-analyses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;146(2):138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Dunietz GL, Holzman C, Zhang Y, et al. Assisted reproductive technology and newborn size in singletons resulting from fresh and cryopreserved embryos transfer. PLoS ONE. 2017;12(1):e0169869. doi: 10.1371/journal.pone.0169869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu HT, Yang Q, Sun XX, et al. Association of birth defects with the mode of assisted reproductive technology in a Chinese data-linkage cohort. Fertil. Steril. 2018;109(5):849–856. doi: 10.1016/j.fertnstert.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Horton J, Sterrenburg M, Lane S, et al. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update. 2019;25(5):592–632. doi: 10.1093/humupd/dmz012. [DOI] [PubMed] [Google Scholar]
- 11.Sterling L, Liu J, Okun N, et al. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil. Steril. 2016;105(3):791–797. doi: 10.1016/j.fertnstert.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Isaksson R, Gissler M, Tiitinen A. Obstetric outcome among women with unexplained infertility after IVF: A matched case-control study. Hum. Reprod. 2002;17(7):1755–1761. doi: 10.1093/humrep/17.7.1755. [DOI] [PubMed] [Google Scholar]
- 13.Luke B, Gopal D, Cabral H, et al. Pregnancy, birth, and infant outcomes by maternal fertility status: The Massachusetts Outcomes Study of Assisted Reproductive Technology. Am. J. Obstet. Gynecol. 2017;217(3):327 e1–327 e14. doi: 10.1016/j.ajog.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. NEJM. 2012;366(19):1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y. Big data analysis and quality control of Assisted reproductive Technology in China. China Med. News. 2018;33(16):1. [Google Scholar]
- 16.Peng A, Qing M, Zhu L, et al. Analysis on development trend of assisted reproductive technology in Shanghai from 2006 to 2015. Matern. Child Health Care China. 2018;33(15):3361–3365. [Google Scholar]
- 17.China N H C O T P S R O Specification for human assisted reproductive technology. Chin. J. Reprod. Health. 2004;15(1):4–9. [Google Scholar]
- 18.Schieve LA, Meikle SF, Ferre C, et al. Low and very low birth weight in infants conceived with use of assisted reproductive technology. NEJM. 2002;346(10):731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 19.Dai L, Deng C, Li Y, et al. Birth weight reference percentiles for Chinese. PLoS ONE. 2014;9(8):e104779. doi: 10.1371/journal.pone.0104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jingyi N, Linli C, Lijing C, et al. Relationship between maternal age and adverse birth outcomes and inspiration for appropriate age of childbearing. Mod. Prev. Med. 2021;48(20):3720–3724. [Google Scholar]
- 21.Kawwass JF, Badell ML. Maternal and fetal risk associated with assisted reproductive technology. Obstet. Gynecol. 2018;132(3):763–772. doi: 10.1097/AOG.0000000000002786. [DOI] [PubMed] [Google Scholar]
- 22.Shah JS, Vaughan DA, Leung A, et al. Perinatal outcomes in singleton pregnancies after in vitro fertilization cycles over 24 years. Fertil. Steril. 2021;116(1):27–35. doi: 10.1016/j.fertnstert.2021.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Finnstrom O, Kallen B, Lindam A, et al. Maternal and child outcome after in vitro fertilization: A review of 25 years of population-based data from Sweden. Acta Obstet. Gynecol. Scand. 2011;90(5):494–500. doi: 10.1111/j.1600-0412.2011.01088.x. [DOI] [PubMed] [Google Scholar]
- 24.Shaw GM, Wise PH, Mayo J, et al. Maternal prepregnancy body mass index and risk of spontaneous preterm birth. Paediatr. Perinat. Epidemiol. 2014;28(4):302–311. doi: 10.1111/ppe.12125. [DOI] [PubMed] [Google Scholar]
- 25.Vinturache AE, Chaput KH, Tough SC. Pre-pregnancy body mass index (BMI) and macrosomia in a Canadian birth cohort. J. Matern. Fetal. Neonatal Med. 2017;30(1):109–116. doi: 10.3109/14767058.2016.1163679. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen RH, Wilcox AJ, Skjaerven R, et al. Men's body mass index and infertility. Hum. Reprod. 2007;22(9):2488–2493. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- 27.Imterat M, Agarwal A, Esteves SC, et al. Impact of Body Mass Index on female fertility and ART outcomes. Panminerva Med. 2019;61(1):58–67. doi: 10.23736/S0031-0808.18.03490-0. [DOI] [PubMed] [Google Scholar]
- 28.Mushtaq R, Pundir J, Achilli C, et al. Effect of male body mass index on assisted reproduction treatment outcome: An updated systematic review and meta-analysis. Reprod. Biomed. Online. 2018;36(4):459–471. doi: 10.1016/j.rbmo.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Lonnebotn M, Natvig GK, Benediktsdottir B, et al. Polycystic ovary syndrome, body mass index and hypertensive disorders in pregnancy. Pregnancy Hypertens. 2018;11:32–37. doi: 10.1016/j.preghy.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012;98(3):511–514. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunkara SK, Antonisamy B, Redla AC, et al. Female causes of infertility are associated with higher risk of preterm birth and low birth weight: Analysis of 117,401 singleton live births following IVF. Hum. Reprod. 2021;36(3):676–682. doi: 10.1093/humrep/deaa283. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Yang X, Huang J, et al. Effect of maternal body mass index on neonatal outcomes in women with endometriosis undergoing IVF. Reprod. Biomed. Online. 2020;40(4):559–567. doi: 10.1016/j.rbmo.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Lopez FR, Villagrasa-Boli P, Munoz-Olarte M, et al. Association between endometriosis and preterm birth in women with spontaneous conception or using assisted reproductive technology: A systematic review and meta-analysis of cohort studies. Reprod. Sci. 2018;25(3):311–319. doi: 10.1177/1933719117749760. [DOI] [PubMed] [Google Scholar]
- 34.Zyla MM, Wilczynski J, Nowakowska-Glab A, et al. Pregnancy and delivery in women with uterine malformations. Adv. Clin. Exp. Med. 2015;24(5):873–879. doi: 10.17219/acem/23171. [DOI] [PubMed] [Google Scholar]
- 35.Boyle AK, Rinaldi SF, Norman JE, et al. Preterm birth: Inflammation, fetal injury and treatment strategies. J. Reprod. Immunol. 2017;119:62–66. doi: 10.1016/j.jri.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Kawwass JF, Crawford S, Kissin DM, et al. Tubal factor infertility and perinatal risk after assisted reproductive technology. Obstet. Gynecol. 2013;121(6):1263–1271. doi: 10.1097/AOG.0b013e31829006d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunietz GL, Holzman C, Zhang Y, et al. Assisted reproduction and risk of preterm birth in singletons by infertility diagnoses and treatment modalities: A population-based study. J. Assist. Reprod. Genet. 2017;34(11):1529–1535. doi: 10.1007/s10815-017-1003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: A prospective study of 144,018 treatment cycles. PLoS Med. 2011;8(1):e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan J, Tannus S, Taskin O, et al. The effect of unilateral tubal block diagnosed by hysterosalpingogram on clinical pregnancy rate in intrauterine insemination cycles: Systematic review and meta-analysis. BJOG. 2019;126(2):227–235. doi: 10.1111/1471-0528.15457. [DOI] [PubMed] [Google Scholar]
- 40.Mariappen U, Keane KN, Hinchliffe PM, et al. Neither male age nor semen parameters influence clinical pregnancy or live birth outcomes from IVF. Reprod. Biol. 2018;18(4):324–329. doi: 10.1016/j.repbio.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Anifandis G, Bounartzi T, Messini CI, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia. 2015;47(3):295–302. doi: 10.1111/and.12259. [DOI] [PubMed] [Google Scholar]
- 42.Simon L, Zini A, Dyachenko A, et al. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017;19(1):80–90. doi: 10.4103/1008-682X.182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litzky JF, Boulet SL, Esfandiari N, et al. Effect of frozen/thawed embryo transfer on birthweight, macrosomia, and low birthweight rates in US singleton infants. Am. J. Obstet. Gynecol. 2018;218(4):433 e1–433 e10. doi: 10.1016/j.ajog.2017.12.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wennerholm UB, Henningsen AK, Romundstad LB, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: A Nordic cohort study from the CoNARTaS group. Hum. Reprod. 2013;28(9):2545–2553. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 45.Pinborg A, Loft A, Aaris Henningsen AK, et al. Infant outcome of 957 singletons born after frozen embryo replacement: The Danish National Cohort Study 1995–2006. Fertil. Steril. 2010;94(4):1320–1327. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 46.Young L, Fernandes K, Mcevoy TG, et al. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 2001;27(2):153–154. doi: 10.1038/84769. [DOI] [PubMed] [Google Scholar]
- 47.Pinborg A. Short- and long-term outcomes in children born after assisted reproductive technology. BJOG. 2019;126(2):145–148. doi: 10.1111/1471-0528.15437. [DOI] [PubMed] [Google Scholar]
- 48.Mohseni R, Mohammed SH, Safabakhsh M, et al. Birth weight and risk of cardiovascular disease incidence in adulthood: A dose-response meta-analysis. Curr. Atheroscler. Rep. 2020;22(3):1–12. doi: 10.1007/s11883-020-0829-z. [DOI] [PubMed] [Google Scholar]
- 49.Skilton MR, Siitonen N, Würtz P, et al. High birth weight is associated with obesity and increased carotid wall thickness in young adults: The cardiovascular risk in young Finns study. Arterioscler. Thromb. Vasc. Biol. 2014;34(5):1064–1068. doi: 10.1161/ATVBAHA.113.302934. [DOI] [PubMed] [Google Scholar]