Abstract
Diabetic macular edema (DME) remains the major cause of preventable blindness in the working-age population in developed countries, and screening programs are extremely important in the management of this complication of diabetic retinopathy. The introduction of modern imaging modalities and technological advances have facilitated both the early detection and the follow-up of patients with DME, particularly optical coherence tomography angiography and artificial intelligence. Intravitreal therapy is the gold standard treatment for DME, but not all patients respond equally to this therapy, and sometimes it is not easy to apply treatment protocols correctly; for these reasons, clinical practice results may differ from those of clinical trials in terms of vision gain. One approach has been to implement new treatment regimens, such as treat and extend, and new molecules and therapeutic targets are constantly being developed. The main goal of this review paper is to describe the current treatment options and management strategies for DME in Europe and to provide a brief oversight of the novel therapeutic options on the horizon.
Keywords: Diabetic macular edema, Intravitreal therapy, AntiVEGF, Steroids, Diabetic retinopathy
Key Summary Points
| Diabetic macular edema (DME) is the major cause of visual impairment in the working-age population, and with diabetes approaching the status of a pandemic the percentage of patients developing DME is increasing. |
| Intravitreal therapy is the gold standard treatment for DME, but not all patients respond equally to this treatment. |
| There are two main types of intravitreal treatment: anti-vascular endothelial growth factor (VEGF) and corticosteroids, with different therapeutic targets and safety profiles. Here, we review efficacy of these treatments as well as the most relevant new treatments under study. |
| Treatment modalities and regimens are also important, particularly in determining how to obtain the maximum benefit of each treatment according to real-ife practice. |
Background and overview
Diabetes Mellitus and Diabetic Eye Disease in Europe
Diabetes mellitus (DM) affects approximately 60 million people in Europe [1]. In 2019, the International Diabetes Foundation (IDF) estimated that three out four people with DM are of working age and that one in five is older than 65 years, and that these numbers will more than double by 2045 [2]. These estimates emphasize increasing public health and economic challenges that are particularly relevant to aging societies, such as those in the EU [2]. Notably, the age profile of the population of the EU is considerably older than that of the rest of the world. The EU also has the highest incidence of type 1 DM, which affects children and adolescents [2, 3]. Diabetic retinopathy (DR) and diabetic macular edema (DME) are the two elements comprising diabetic eye disease (DED) and constitute the major cause of preventable blindness in the working-age population in Europe [3]. There are currently approximately 6.4 million patients aged over 40 years with DED in the EU. Patients with type 1 DM have a significantly higher prevalence of DED compared to those with type 2 DM (50 vs. 25%) [3]. A recent meta-analysis of 35 studies that evaluated the prevalence of DED in Europe (205,743 individuals) and of four studies on incidence (71,307 persons with type 2 DM) reported a prevalence of 25.7% (95% confidence interval [CI] 22.8–28.8%) for any DR and 3.7% (95% CI 2.2–6.2%) for DME, as well as a pooled mean annual incidence of 4.6% (95% CI 2.3–8.8%) for any DR, 0.5% (95%CI 0.2–0.8%) for sight affected by DR (defined as pre-proliferative or proliferative DR and/or maculopathy requiring referral and/or treatment) and 0.4% (95% CI 0.5–1.4%) for clinically significant DME [3]. It is estimated that the number of patients with DED in Europe will increase from 6.4 million in 2019 to 8.6 million in 2050, with 30% of these patients requiring close monitoring and or treatment [3].
DME can occur at any stage of DR, or as the sole manifestation [4]. In clinical practice, fundus examination or color photography have been the conventional tools used for diagnosing and staging DME based on a classification that takes account the distance of the retinal lesions from the center of the fovea [5]. In 2018 the International Council of Ophthalmology (ICO) updated the DME classification, incorporating optical coherence tomography (OCT) into its toolbox due to its widespread use in clinical practice for the management of DME [4]. The ICO classifies DME into three stages: no DME; non-center-involving DME; and center-involving DME [4]. Specific OCT parameters have recently been proposed for the evaluation of DME which may help to better determine different phenotypes [6], but the ICO classification is currently the most used in clinical practice [7].
The scope of this review is to describe current treatment options and management strategies for DME in Europe, as well as provide a brief oversight of novel therapeutic options on the horizon.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Advances in the Diagnosis of and Screening for Diabetic Maculopathy
The vast majority of cases involving severe vision loss from DR and DME can be prevented by early screening and the timely initiation of treatment. The aim of screening programs is to offer wide coverage to patients and overcome the most common reasons for low patient compliance, such as poor access to medical services, lack of patients’ awareness owing to educational or socioeconomic obstacles and geographical barriers. Ophthalmoscopy has traditionally been the standard tool for DME diagnosis, but in recent years screening methods have be revolutionized with the introduction of modern imaging modalities and technological advances. An annual retinal examination is recommended for patients with diabetes [8]. However, even in the so-called developed countries, such as the USA, only 40–60% of the known diabetic population is screened for DED. The lack of screening is of even greater concern in the developing world as some 80% of people with diabetes live in emerging economies, such as India and China, where medical coverage is poor and there is no access to eye care.
Telemedicine
Telemedicine screening programs not only improve patient compliance in attendance rates for screening but have also been shown to reduce the proportion of those who develop vision-threatening DR over time [9, 10]. DR telescreening is an option that has been found to be cost-effective [11, 12]. With this approach, only those patients who are identified with vision-threatening features are referred to tertiary ophthalmic care. Many countries have implemented telemedicine approaches, including the UK, Singapore, India and China [13–15].
Screening for DR includes the detection of a DME that probably needs treatment. Fundus photography, consisting of a single central field or of two fields centered on the disc and macula, is the most common imaging method used in screening and has been shown to be effective in identifying persons with the signs of DR that are considered to represent features indicating a high risk of progression to sight loss [16, 17]. More recently, as smartphones and tablets have become an indispensable tool in everyday life, several portable, inexpensive and easy-to-operate smartphone-based fundus camera systems have been developed for screening purposes [18]. Nevertheless, robust studies on the diagnostic accuracy of these devices are needed prior to the introduction of these innovative tools into routine screening protocols.
In contrast to biomicroscopy where retinal thickening is identified by direct examination of the fundus, the ability to detect macular thickening on non-stereoscopic fundus photos relies on surrogate markers. Features of referable maculopathy include hard exudates, microaneurysms and hemorrhages within 1–2 disc diameters (DD) from the fovea [19]. The presence of hard exudates within 1 DD from the fovea is one of the strongest predictors of DME (Fig. 1) [20, 21]. The positive predictive value of non-stereoscopic fundus photos for clinical significant macular edema (CSME) detection is relatively low, and DME can be missed if surrogate markers are absent. On the other hand, even eyes with suspected DME due to the presence of surrogate features, such as hard exudates, might not show retinal thickening on optical coherence tomography (OCT) [22–26]. Fewer than 15% of patients who are deemed to require referral on the basis of an image positive for maculopathy on fundus photos are found to have DME upon OCT [20, 21, 27]. In a study with 3170 patients who were identified with diabetic maculopathy based on fundus photographs, only 243 (7.7%) were found to have DME on OCT images [10]. A high rate of false positive tests imposes not only financial burdens on the healthcare system, but might also cause unnecessary psychological stress to patients.
Fig. 1.
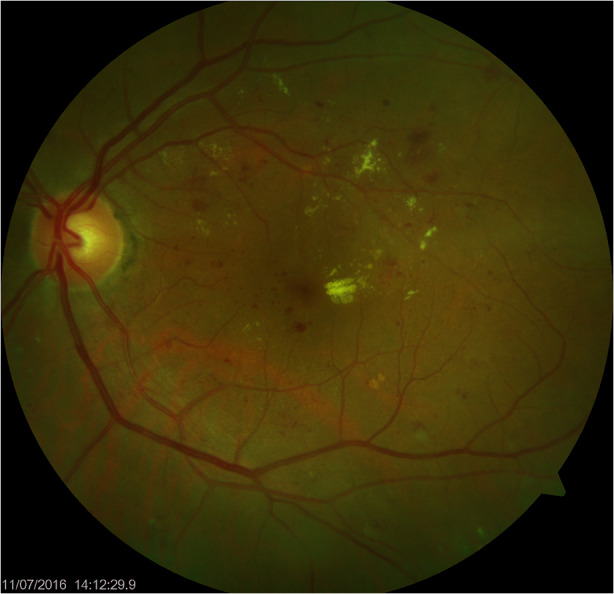
Fundus image of a patient showing hard exudate within 1 disc diameter from the fovea, indicating a high suspicion of macular edema
Owing to the high false positive test rate for referable maculopathy, screening for retinopathy now includes OCT in the UK. This strategy has proved to be effective and generates substantial cost savings [21, 25, 28].
Artificial Intelligence
Decreasing the false positive rate remains an important goal in DME screening programs. The main strength of artificial intelligence (AI) is that it can be applied to analyze details on simple fundus photos that are probably not detectable by a human investigator. Deep learning (DL) models are currently under investigation as potential tools for determining the presence of macular thickening on fundus photographs. In studies using DL models, the authors predicted center-involving DME with a significantly higher specificity than and similar sensitivity as physicians [29, 30]. Another study demonstrated that DL not only has the ability to accurately identify macular thickening of ≥ 250 µm on fundus photos, but also exhibits a capability to predict the value of foveal thickness in micrometers [31]. In addition, new DL models were able to detect the presence of intraretinal and/or subretinal fluid on color images—a task that is impossible for the human eye [12].. Implementing AI to analyze color images acquired during screening for DME can therefore be performed with high diagnostic accuracy using inexpensive hardware, potentially reducing the need for trained manpower that is necessary for manual image grading. However, at present there is a lack of sufficiently large training sets of images from homogenous populations from different regions of the world, nor are there publicly available datasets. Furthermore, there is limited knowledge of how these algorithms will perform under conditions of varying proportions of ungradable images, and how data protection and privacy rules would be overcome.
OCT Angiography
The most important advance with respect to the diagnosis of DME is most likely the use of OCT angiography (OCTA). While spectral-domain OCT offers high-resolution views of retinal structure and enables the acquisition of quantitative metrics, it does not provide information on macular perfusion status. OCTA is a novel tool that provides rapid, non-invasive, high-resolution confocal three-dimensional images of the vasculature of the retina and choroid without the need to inject fluorescent dyes into the systemic circulation. In diabetic patients, OCTA reveals structural abnormalities, such as microaneurysms, telangiectatic vessels, pruning of vessels and areas of capillary non-perfusion in the retinal microcirculation, even before signs of retinopathy are visible clinically [32].
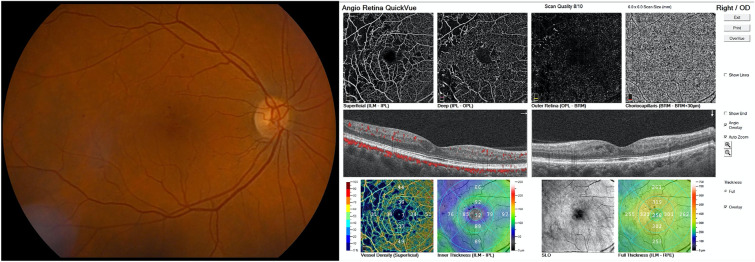
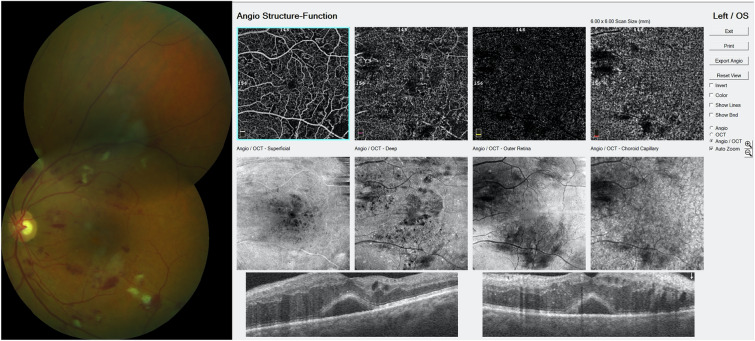
OCTA has several advantages over fluorescein angiography (FA) in terms of the evaluation of diabetic maculopathy. First, it delineates the parafoveal microvasculature and the foveal avascular zone, without any obscuration of the vessels by fluorescein dye leakage [33]. Second, cystoid spaces can be seen clearly as flow-void areas, whereas they might be difficult to identify by FA due to leakage and shadowing [34]. Third, OCTA enables separate visualization and quantification of microvascular changes and vessel density in the multiple vascular layers of the retina (Fig. 2). The segmentation confocally of the inner retina into superficial, mid and deep slabs allows the characterization of DR-related changes in the superficial, intermediate and deep capillary plexi separately [35, 36].
Fig. 2.
Multimodal image. The fundus can be seen to have isolated microhemorrhages and microaneurysms, The optical coherence tomography (OCT) b-scan shows a few cysts within normal ranges, but OCT angiography (OCTA) confirms areas of reduced perfusion/non-perfusion and reduced vascular density in both the superficial and deep plexus. Multimodal imaging and OCTA can provide complementary information on the real-life state of the patient
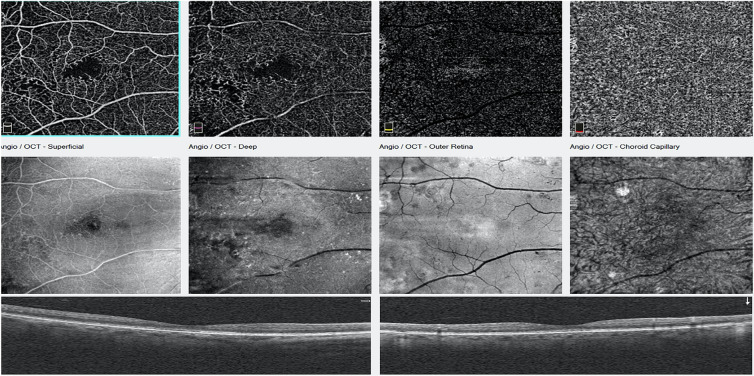
It has been shown that patients with ischemic diabetic maculopathy may be refractive to anti-vascular endothelial growth factor (VEGF) therapy and suffer unexplained visual loss despite resolution of the edema (Fig. 3). In such cases, angiography is needed in the evaluation process in addition to structural OCT. OCTA offers a non-invasive alternative. Still, OCTA can be limited by artifacts from image acquisition and processing [37]. The interpretation of OCTA findings requires an experienced physician. Future studies are needed to define the value and role of OCTA in managing patients with diabetic maculopathy in clinical practice.
Fig. 3.
Top row: OCTA images showing an abnormal avascular zone of a patient referred for blurred vision; the increased foveal avascular zone (FAZ) may be a consequence of macular ischemia. Middle row: En face OCT. Bottom row: Structural or cross-sectional OCT images that are normal
Current Treatment Options
Pathophysiology of Diabetic Macular Edema
Diabetic macular edema is a complex long-term condition with a multifactorial pathogenesis that includes retinal pigment epithelium and Müller cell dysfunction, breakdown of the blood–retinal barrier (BRB), non-perfusion of the retinal capillaries and neuronal damage. All of these are implicated in the complexity of macular edema development [38–40].
VEGF is a potent vasopermeability factor, and as such its role in all manifestations of DR, including DME, is unquestioned. However, biomarkers in addition to VEGF have also been found to be elevated in tissue samples from patients with DME compared to controls [41, 42]. For example, monocyte chemoattractant protein 1 (MCP-1) level is positively correlated to the severity of DME while VEGF level is not [41, 42]. Interestingly, elevated levels of intercellular adhesion molecule-1 (ICAM-1) in aqueous humor samples are correlated with greater macular volume in DME [43]. Recent data suggest that interleukin 6 (IL-6) level is associated with subretinal fluid accumulation but not with intraretinal fluid formation; as such, it may be a potential marker of inflammation and rupture of the retinal pigment epithelium barrier in DME [44].
The ideal treatment for DME should be well tolerated, improve vision and restore macular retinal morphology with durability and with few adverse events, thereby reducing the treatment burden and costs. Anti-VEGF therapy has many of these attributes and has significant clinical benefits, including improving the morphology of the macular retina and providing impressive visual acuity gains in individuals suffering from the disease [45, 46].
Evidence from Clinical Trials
Randomized controlled trials (RCTs) have provided strong evidence supporting intravitreal anti-VEGF as first-line therapy in the management of patients with visual impairment due to DME [47]. Anti-VEGF drugs bevacizumab, ranibizumab and aflibercept are effective and have an impressive safety profile, although the much lower cost of off-label bevacizumab is an important reason for the use of this medication in clinical practice [48].
The intravitreal dexamethasone (DEX) implant (OZURDEX®; Allergan, Dublin, Ireland) and the fluocinolone acetonide (FAc) intravitreal implant (ILUVIEN®; Alimera Sciences, Alpharetta, GA, USA) are commonly used as second-line treatments in DME in eyes recalcitrant to anti-VEGF treatment. However, these latter two therapies are increasingly being used earlier in the treatment algorithms due to their high efficacy and response rates, as well as their proven safety profile in real life [47, 49]. In addition, pharmacological interventions combined with laser photocoagulation (focal or macular grid) may be considered.
Anti-VEGF agents achievesuperior visual outcomes when compared to argon laser, with the benefit clearly evident at both 1 and 2 years after treatment initiation [50]. The Protocol T trial of DRCR.net provided comparative effectiveness data on the efficacy of aflibercept, bevacizumab and ranibizumab, which are the three most commonly used anti-VEGF agents. This trial found that in eyes with better baseline visual acuity (best-corrected visual acuity [BCVA] letter score ≥ 69) all three anti-VEGF agents were similar in their efficacy; however in eyes with relatively worse baseline visual acuity (BCVA letter score < 69), aflibercept was superior to both bevacizumab and ranibizumab at the 1-year follow-up, and only to bevacizumab at 2 years.
Treatment Regimes
The benefit of DME treatment has been demonstrated to be strongly related to the number of injections. For this reason, the current optimal treatment regimen consists of continuous treatment at appropriately fixed retreatment intervals or by the treat-and-extend (TAE) regimen. Also, the treatment-as-needed regimen may be adopted by clinicians. This latter treatment regimen, also named Pro Re Nata (PRN), requires a close monitoring of patients (monthly) and retreatment according to retreatment criteria after a loading dose or after stability according to OCT. The definition of the retreatment criteria is under debate, and more robust OCT features have to be confirmed, but it is considered that changes in central macular thickness (CMT) due to the increase of subretinal and/or intraretinal fluid are a sign of need for treatment (Fig. 4) [51]. The main difference between TAE and PRN is that the former is proactive, with treatment given at every visit and the intervals between visits extended as needed, while the PRN regimen is reactive, with treatment withheld if there is no worsening in vision or OCT thickness representing disease activity. Compared to fixed dosing, the PRN regimen has the advantage of a potential reduction in the number of injections but not visits; in routine practice maintaining monthly visits or injections is difficult, with real-life evidence demonstrating that even a small reduction in number of injections or visits leads to a worse visual outcome compared to that achieved in the rigorous PRN regimens of clinical trials According to the LUMINOUS study, the mean number of injections in a real-world setting at year 1 were 4.5 and the mean visual acuity letter score improved by + 3.5 letters. More recently, Ciulla et al. published the real-world outcomes of anti-VEGF therapy in DME in the USA and showed that visual acuity outcomes were inferior to those of randomized, controlled trials; they also observed no correlation between the initial choice of anti-VEGF agent and visual outcomes. The real-world outcomes were clearly worse than those of the pivotal RIDE and RISE trials in which the mean number of letters gained from baseline were 8.5 and 9.9 letters at 24 months, respectively (adjusted for baseline variables) [52–54].
Fig. 4.
Multimodal image. Fundus shows hemorrhages, microaneurysms and cotton exudates, and the OCT shows an important macular edema where the subretinal fluid is clearly seen
EURETINA Guidelines
According to the EURETINA (European Society of Retina Specialists) DME guidelines, treatment with ranibizumab for DME should be initiated with monthly injections. If the visual acuity improves and/or central retinal thickness (CRT) decreases or other morphological signs of disease activity can be found, monthly injections must be continued until visual acuity and/or OCT stability is reached. Thereafter, monitoring and retreatment intervals should be determined by the physician and based on detection of disease activity, as assessed by changes in visual acuity and/or anatomical parameters. Retreatment can be applied using either a PRN or TAE regimen. As mentioned in the preceding section, the TAE regimen is a a proactive regimen, with the interval between injections varying over time but individualized to the patient’s eye. At each visit, the treating physician decides whether to extend or reduce the subsequent interval on the basis of disease activity criteria; however, an intravitreal injection is always administered. The recommended extension interval is usually 2 weeks.
The same treatment strategy can be considered also for bevacizumab. However, some aspects of the most effective therapeutic strategy in the use of this molecule remain poorly defined, in particular the therapeutic administration plan.
Aflibercept treatment requires a 5-monthly injection loading phase, followed by a bimonthly maintenance phase for the first year [55]. As an alternative to the bimonthly fixed regimen, in the maintenance phase, the treatment schedule may be the TAE regimen.
There is strong evidence highlighting the role of inflammation in the development of DME. Corticosteroids produce an anti-inflammatory effect through multiple pathways and hence are important components in the armamentarium of drugs for the treatment of DME. However, to date, there is limited practical guidance on the use of steroids in routine practice and they remain largely a second-choice therapeutic.
DEX implant is indicated in patients with visual impairment due to DME who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for treatment with non-corticosteroid medications. It has been shown that DEX implants can be used for patients with treatment-naive DME and not just for refractory cases. In cases that do not respond to monthly anti-VEGF injections, the switch to DEX implantation should be considered earlier in the treatment phase as the treatment outcomes are better in the absence of chronicity (EURETINA 2017 [56]).
The use of steroids as a first-line treatment may also be considered in patients with a history of severe cardiovascular disease, notably those with a prior cardiac infarction as these patients were excluded from all major anti-VEGF trials. Treatment with steroids may also be of value in patients unwilling to attend consultations for monthly injections (and/or monitoring) in the first 6 months of therapy. According to its label the PRN regimen should be maintained for approximately 6 months; however, real-life evidence has shown that a shorter interval may be required as recurrence of disease activity is not uncommon beyond 3 months [57].
Real-world data from Portugal, France, Germany and UK have supported the findings of the FAME trials on FAc, a long-acting steroid, for the treatment of DME. These real-world data support the findings of the FAME trials, showing consistent efficacy and safety profiles of FaC [58–61]. It is important to note that some eyes require additional anti-VEGF intravitreal injections after FAc implantation during the 36-month follow-up period. It is also necessary to monitor the patient’s intraocular pressure (IOP), as is the case for any intravitreal steroid treatment. It has also been recommended that the DEX implant should be used in the first instance to identify possible increases in the IOP to detect potential steroid responders as the FAc implant has an extended durability of 3 years. Intravitreal FAc is contraindicated in the presence of pre-existing glaucoma or active or suspected ocular or periocular infection, including most viral diseases of the cornea and conjunctiva, similar to the DEX implant that is contraindicated in the presence of macular edema secondary to infectious uveitis.
Future Treatment Options
The management of vision impairment in the presence of DME in which the fovea is involved has been transformed by the use of anti-VEGF therapy. Nonetheless, there remain areas of unmet need in the management of DME with anti-VEGF agents. First, a proportion of patients with DME show suboptimal responses to anti-VEGF drugs, which manifest by an unchanging fluid load or presence of residual edema despite regular monthly injections of intravitreal doses of anti-VEGF drugs. Second, withdrawal of treatment or reduction in the dosing frequency after an initial therapeutic response can result in recurrence of DME, or worse still the onset of proliferative DR (PDR). Third, detailed angiographic analyses reveal that capillary non-perfusion does not improve during anti-VEGF therapy even though the overt manifestation of DR consisting of hemorrhages and exudates disappear, giving a misleading impression of a true disease-modifying effect [62]. Therefore, the search is ongoing for new treatments that can address some of these limitations [63].
Enhanced Anti-VEGF Properties and or Durability
To reduce the need for high-frequency retreatment with anti-VEGF agents, newer therapies with properties of greater durability are currently being tested. Two ongoing large phase 3 trials, the KITE and KESTREL trials, are exploring the effectiveness of brolucizumab, a single-chain variable fragment antibody to VEGF, versus aflibercept in patients with DME. Brolucizumab is a much smaller molecule than afilbercept and has already been proven to have increased durability compared to the latter in the treatment of neovascular age-related macular degeneration (AMD) [62]; brolucizumab at the dose of 6.0 mg achieves a concentration in the vitreous that is approximately 12-fold higher than that of aflibercept, which possibly explains the greater effectiveness of brolucizumab in disease control as well as its prolonged durability. The 1-year results in both the KITE and KESTREL studies met the primary endpoint of non-inferiority in change in BCVA from baseline, with more than half of the patients on brolucizumab on a dosing interval of 12 weeks after the loading phase. Even more importantly, these trials showed a well-tolerated safety profile, with an equivalent rate of intraocular inflammation compared to aflibercept. Nevertheless, following the introduction of brolucizumab into routine clinical care, reports of severe intraocular inflammatory reactions with irrecoverable loss of vision in patients who received brolucizumab for wet AMD were confirmed by an independent review committee and also from real-world studies, thus causing concern within the retina community [64–66].
An alternative strategy to increasing both the efficacy and durability of treatment for DME is the simultaneous inhibition of angiotensin 2 and VEGF-A with the bispecific antibody faricimab (Roche AG, Basel, Switzerland). In a large randomized phase 2 trial that enrolled both DME treatment-naïve patients and those previously treated for DME, Sahni et al. showed statistically superior visual acuity gains and a higher proportion of eyes with central subfield reductions to < 325 μM in the faricimab 6.0 mg arm compared to the ranibizumab 0.3 mg arm [67]. This trial examined duration to disease reactivation using predefined criteria by withholding drug administration at week 24; the probability of requiring retreatment was lowest in the 6.0 mg faricimab dosing arm. During the 2021 EURENTINA meeting, the 1-year results of the two identical phase 3 studies, YOSEMITE and RHINE, both of which explored the efficacy, safety and durability of faricimab 6.0 mg delivered at dosing intervals of up to once every 16 weeks (Q16W), were presented. Faricimab Q16W was shown to be non-inferior to aflibercept given once every 8 weeks (Q8W) in terms of BCVA gains. In addition at the 1-year follow-up, faricimab at dosing intervals of up to Q16W had similar vision gains as aflibercept Q8W in about 52% of patients on the Q16W dosing interval and about 72% of those on the ≥ Q12W dosing interval [68].
Several other drugs targeting VEGF are also under investigation These include KSI 301, an antibody biopolymer conjugate (Kodiak Sciences, Palo Alto, CA, USA), OPT 303 (Ophthea, Circadian Technologies Limited, Stoneham, MA, USA), which inhibits VEGF-C and -D, pan 90,806 (PanOptica Inc., Mount Arlington, NJ, USA), a highly selective anti-VEGF agent which is a small molecule delivered topically once daily. The GLEAM study and the GLIMMER study are phase 3 global, multicenter, randomized studies aimed at evaluating the safety, efficacy and durability of KSI-301 in treatment-naïve patients with DME, and recruitment is currently underway.
Targeting Alternative Pathogenetic Pathways
There has been a resurgence of interest in the inflammatory mechanisms and cytokine profiles underpinning the pathogenesis of DME because approximately one half of all patients receiving anti-VEGF therapy are either poor or suboptimal responders [69]. Steroid inhibitors of inflammation are currently used as second-line treatments for DME in patients who respond suboptimally to anti-VEGF therapies, including DEX and FAc implants (MEAD and FAME trials) [49, 70]. In eyes with DME resistant to anti-VEGF treatment, inflammatory cytokine overexpression and redistribution of Claudin 5, which mediates leakage from blood vessels, has been observed and represents a potential new target for drug development. A range of new biologicals are currently being tested in phase 1 and 2 safety and efficacy and dose ranging studies. These include Risuteganib, an integrin (Allegro Pharmaceuticals, LLC, San Juan Capistrano, CA, USA) which mitigates oxidative stress reducing inflammation, apoptosis and neural cell degeneration. In the phase IIb DEL MAR trial, risuteganib was non-inferior to bevacizumab (Avastin; Roche AG/Genentech, Basel, Switzerland/South San Francisco, CA, USA), in patients with persistent DME who had suboptimal treatment response to anti-VEGF drugs [71]. Another potential pathogenetic mechanism that is dysregulated in diabetes is the Kallikrein–Kinin pathway, and the biopharmaceutical company Oxurion (Leuven, Belgium) is testing its novel drug THR-149, which targets plasma kallikrein, in a phase II trial of patients with DME [72]. Because neuroglia have been implicated in the pathogenesis of DME, minocycline, which belongs to the tetracycline group of drugs and part of our therapeutic armamentarium against common diseases such as acne, is also being tested in DME. One study found that an oral dose of 100 mg twice daily, which is sufficient to inhibit microglial activity when administered to patients with DME, resulted in visual acuity improvements along with concomitant reductions in central subfield thickness and angiographic leakage 6 months after commencement of the drug. This drug may be repurposed for the management of DME but requires testing in large phase 3 trials [73].
In summary, we have a number of effective treatments for DME but owing to the complex pathophysiology of the disease, insufficient disease control or the relatively short durability of currently available therapies, new targets and molecules are needed to improve the management and control of DME.
Acknowledgements
During the peer review process, Alimera Sciences was offered the opportunity to comment on the article. Changes resulting from comments received were made by the author based on their scientific and editorial merit.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Patricia Udaondo, Mariacristina Parravano, Stela Vujosevic, Dinah Zur and Usha Chakravarthy have nothing to disclose.
Compliance with Ethics Guideliens
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.World Health Organization Europe. Diabetes. Data and statistics. 2010. http://www.euro.who.int/en/health-topics/noncommunicable-diseases/diabetes/data-and-statistics. Accessed 14 Apr 2020.
- 2.International Diabetes Federation . IDF diabetes atlas. 9. Brussels: International Diabetes Federation; 2019. [PubMed] [Google Scholar]
- 3.Li JQ, Welchowski T, Schmid M, et al. Prevalence, incidence and future projection of diabetic eye disease in Europe: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(1):11–23. doi: 10.1007/s10654-019-00560-z. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608–1622. doi: 10.1016/j.ophtha.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scale. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 6.Panozzo G, Cicinelli MV, Augustin AJ, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: the European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol. 2020;30(1):8–18. doi: 10.1177/1120672119880394. [DOI] [PubMed] [Google Scholar]
- 7.Vujosevic S, Aldington SJ, Silva P. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8(4):337–347. doi: 10.1016/S2213-8587(19)30411-5. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1–15. [DOI] [PubMed]
- 9.Mansberger SL, Sheppler C, Barker G, et al. Long-term comparative effectiveness of telemedicine in providing diabetic retinopathy screening examinations: a randomized clinical trial. JAMA Ophthalmol. 2015;133(5):518–525. doi: 10.1001/jamaophthalmol.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pareja-Rios A, Bonaque-Gonzalez S, Serrano-Garcia M, Cabrera-Lopez F, Abreu-Reyes P, Marrero-Saavedra MD. Tele-ophthalmology for diabetic retinopathy screening: 8 years of experience. Arch Soc Esp Oftalmol. 2017;92(2):63–70. doi: 10.1016/j.oftal.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HV, Tan GSW, Tapp RJ, et al. Cost-effectiveness of a National Telemedicine Diabetic Retinopathy Screening Program in Singapore. Ophthalmology. 2016;123(12):2571–2580. doi: 10.1016/j.ophtha.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Tozer K, Woodward MA N-CP. Telemedicine and diabetic retinopathy: review of published screening programs. J Endocrinol Diabetes. 2015;2(4):10.15226/2374-6890/2/4/00131. [DOI] [PMC free article] [PubMed]
- 13.Peng J, Zou H, Wang W, et al. Implementation and first-year screening results of an ocular telehealth system for diabetic retinopathy in China. BMC Health Serv Res. 2011;4(11):250. doi: 10.1186/1472-6963-11-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sim DA, Mitry D, Alexander P, et al. The evolution of teleophthalmology programs in the united kingdom: beyond diabetic retinopathy screening. J Diabetes Sci Technol. 2016;10(2):308–317. doi: 10.1177/1932296816629983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachapelle S, Legood R, Alavi Y, et al. The cost-utility of telemedicine to screen for diabetic retinopathy in India. Ophthalmology. 2013;120(3):566–573. doi: 10.1016/j.ophtha.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Li HK, Horton M, Bursell S-E, et al. Telehealth practice recommendations for diabetic retinopathy, second edition. Telemed J E Health. 2011;17(10):814–837. doi: 10.1089/tmj.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pieczynski JGA. Review of diabetic retinopathy screening methods and programmes adopted in different parts of the world. Eur Ophthalmic Rev. 2015;9(1):49–55. [Google Scholar]
- 18.Debuc DC. The role of retinal imaging and portable screening devices in tele-ophthalmology applications for diabetic retinopathy management. Curr Diab Rep. 2016;16(12):132. [DOI] [PubMed]
- 19.Peto T, Tadros C. Screening for diabetic retinopathy and diabetic macular edema in the United Kingdom. Curr Diab Rep. 2012;12(4):338–345. doi: 10.1007/s11892-012-0285-4. [DOI] [PubMed] [Google Scholar]
- 20.Prescott G, Sharp P, Goatman K, et al. Improving the cost-effectiveness of photographic screening for diabetic macular oedema: a prospective, multi-centre, UK study. Br J Ophthalmol. 2014;98(8):1042–1049. doi: 10.1136/bjophthalmol-2013-304338. [DOI] [PubMed] [Google Scholar]
- 21.Litvin TV, Ozawa GY, Bresnick GH, et al. Utility of hard exudates for the screening of macular edema. Optom Vis Sci. 2014;91(4):370–375. doi: 10.1097/OPX.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Date RC, Shen KL, Shah BM, Sigalos-Rivera MA, Chu YI, Weng CY. Accuracy of detection and grading of diabetic retinopathy and diabetic macular edema using teleretinal screening. Ophthalmol Retin. 2019;3(4):343–349. doi: 10.1016/j.oret.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Litvin TV, Bresnick GH, Cuadros JA, Selvin S, Kanai K, Ozawa GY. A revised approach for the detection of sight-threatening diabetic macular edema. JAMA Ophthalmol. 2017;135(1):62–68. doi: 10.1001/jamaophthalmol.2016.4772. [DOI] [PubMed] [Google Scholar]
- 24.Wang YT, Tadarati M, Wolfson Y, Bressler SB, Bressler NM. Comparison of prevalence of diabetic macular edema based on monocular fundus photography vs optical coherence tomography. JAMA Ophthalmol. 2016;134(2):222–228. doi: 10.1001/jamaophthalmol.2015.5332. [DOI] [PubMed] [Google Scholar]
- 25.Leal J, Luengo-Fernandez R, Stratton IM, Dale A, Ivanova K, Scanlon PH. Cost-effectiveness of digital surveillance clinics with optical coherence tomography versus hospital eye service follow-up for patients with screen-positive maculopathy. Eye. 2019;33(4):640–647. doi: 10.1038/s41433-018-0297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackenzie S, Schmermer C, Charnley A, et al. SDOCT imaging to identify macular pathology in patients diagnosed with diabetic maculopathy by a digital photographic retinal screening programme. PLoS ONE. 2011;6(5):1–6. doi: 10.1371/journal.pone.0014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong RLM, Tsang CW, Wong DSH, et al. Are we making good use of our public resources? The false-positive rate of screening by fundus photography for diabetic macular oedema. Hong Kong Med J. 2017;23(4):356–364. doi: 10.12809/hkmj166078. [DOI] [PubMed] [Google Scholar]
- 28.Olson J, Sharp P, Goatman K, et al. Improving the economic value of photographic screening for optical coherence tomography-detectable macular oedema: a prospective, multicenter, UK study. Health Technol Assess (Rockv) 2013;17(51):1–141. doi: 10.3310/hta17510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varadarajan AV, Bavishi P, Ruamviboonsuk P, et al. Predicting optical coherence tomography-derived diabetic macular edema grades from fundus photographs using deep learning. Nat Commun. 2020;11:30. 10.1038/s41467-019-13922-8. [DOI] [PMC free article] [PubMed]
- 30.Dupas B, Walter T, Erginay A, et al. Evaluation of automated fundus photograph analysis algorithms for detecting microaneurysms, haemorrhages and exudates, and of a computer-assisted diagnostic system for grading diabetic retinopathy. Diabetes Metab. 2010;36(3):213–220. doi: 10.1016/j.diabet.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Arcadu F, Benmansour F, Maunz A, et al. Deep learning predicts OCT measures of diabetic macular thickening from color fundus photographs. Invest Ophthal Vis Sci 2019;60:852–7. [DOI] [PubMed]
- 32.de Carlo TE, Chin AT, Bonini Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35(11):2364–2370. doi: 10.1097/IAE.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 33.Soares M, Neves C, Marques IP, et al. Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br J Ophthalmol. 2017;101(1):62–68. doi: 10.1136/bjophthalmol-2016-309424. [DOI] [PubMed] [Google Scholar]
- 34.de Carlo TE, Chin AT, Joseph T, et al. Distinguishing diabetic macular edema from capillary nonperfusion using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47(2):108–114. doi: 10.3928/23258160-20160126-02. [DOI] [PubMed] [Google Scholar]
- 35.Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):362–370. doi: 10.1167/iovs.15-18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onishi AC, Nesper PL, Roberts PK, et al. Importance of considering the middle capillary plexus on OCT angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:2167–2176. doi: 10.1167/iovs.17-23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new question. Eye. 2002;16:242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- 39.Das A, McGuire P, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Opthalmology. 2015;122:1375–1394. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, et al. Parainflammation in aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 42.Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 43.Hillier RJ, Ojaimi E, Wong DT, et al. Aqueous humor cytokine levels as biomarkers of disease severity in diabetic macular edema. Retina. 2017;37(4):761–769. doi: 10.1097/IAE.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 44.Sonoda S, Sakamoto S, Yamashita T, et al. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina. 2014;34:741–774. doi: 10.1097/IAE.0b013e3182a48917. [DOI] [PubMed] [Google Scholar]
- 45.Miller K, Fortun JA. Diabetic macular edema: current understanding, pharmacologic treatment options, and developing therapies. Asian Pac J Ophthalmol (Paris) 2018;7:28–35. doi: 10.22608/APO.2017529. [DOI] [PubMed] [Google Scholar]
- 46.Bahrami B, Hong T, Gilles MC, Chang A. Anti-VEGF therapy for diabetic eye diseases. Asia Pac J Ophthalmol (Phila) 2017;6:535–545. doi: 10.22608/APO.2017350. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237:185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 48.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab or ranibizumab for diabetic macular edema. Two-year results form a comparative effectiveness randomized clinical trial. Opthalmology. 2016;123(6):1351–1359. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi: 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 50.Virgili G, Parravano M, Menchini F, et al. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014;10:CD007419. doi: 10.1002/14651858.CD007419.pub4. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell P, Sheidow TG, Farah ME, et al. Effectiveness and safety of Ranibizumab 0.5 mg in treatment-naïve patients with diabetic macular edema: results from the real-world global LUMINOUS Study. PLoS ONE. 2020;15(6):e023359. doi: 10.1371/journal.pone.0233595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciulla T, Amador AG, Zinman B. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophtalmol Retina. 2018;2:1179–1187. doi: 10.1016/j.oret.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RIDE and RISE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 54.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-months results from two phase III trials:RIDE and RISE. Ophthalmology. 2013;120(10):2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 55.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Hernández Martínez A, Pereira Delgado E, Silva Silva G, et al. Early versus late switch: How long should we extend the anti-vascular endothelial growth factor therapy in unresponsive diabetic macular edema patients? Eur J Ophthalmol. 2020;30(5):1091–8. [DOI] [PubMed]
- 57.Bellocq D, Akesi J, Matonti, et al. The pattern of recurrence in diabetic macular edema treated by dexamethasone implant: the PREDIAMEX Study. Ophthalmol Retina 2018;2(6):567–73. [DOI] [PubMed]
- 58.Figueira J, Henriques J, Amaro M, et al. A nonrandomized, open-label, multicenter, phase 4 pilot study on the effect and safety of Iluvien in chronic diabetic macular edema patients considered insufficiently responsive to available therapies (RESPOND) Ophthalmic Res. 2017;57(3):166–172. doi: 10.1159/000455235. [DOI] [PubMed] [Google Scholar]
- 59.Massin P, Erginay A, Dupas B, et al. Efficacy and safety of sustained-delivery fluocinolone acetonide intravitreal implant in patients with chronic diabetic macular edema insufficiently responsive to available therapies: a real-life study. Clin Ophthalmol. 2016;10:1257. doi: 10.2147/OPTH.S105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Augustin A, Bopp S, Fechner M, et al. Three-year results from the Retro-IDEAL study: real-world data from diabetic macular edema (DME) patients treated with ILUVIEN® (0.19 mg fluocinolone acetonide implant) Eur J Ophthalmol. 2020;30:382–391. doi: 10.1177/1120672119834474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fusi-Rubiano W, Mukherjee C, Lane M, et al. Treating diabetic macular oedema (DMO): real world UK clinical outcomes for 0.19 mg fluocinolone acetonide intravitreal implant (Iluvien) at 2 years. BMC Ophthalmol. 2018;18(1):62. doi: 10.1186/s12886-018-0726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnin S, Dupas B, Lavia C, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. 2019;39:426–434. doi: 10.1097/IAE.0000000000002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wubben TJ, Johnson MW. Anti-vascular endothelial growth factor therapy for diabetic retinopathy: consequences of inadvertent treatment interruptions. Am J Ophthalmol. 2019;204:13–18. doi: 10.1016/j.ajo.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Mones J, Srivastava S, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–1059. doi: 10.1016/j.ophtha.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi: 10.1016/j.ophtha.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 66.Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519–527. doi: 10.1016/j.oret.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Sahni J, Patel SS, Dugel PU, et al. Simultaneous inhibition of angiogensin 2 and VEGF with faricimab in diabetic macular edema: Boulevard phase two randomized controlled clinical trial. Ophthalmology. 2019;126:1155–1170. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 68.Tadayoni R. Efficacy, safety, and durability of faricimab in diabetic macular edema: one-year results from the phase 3 YOSEMITE and RHINE trials. EURETINA. 2021. https://euretina.org/resource/abstract_2021_efficacy-safety-and-durability-of-faricimab-in-diabetic-macular-edema-one-year-results-from-the-phase-3-yosemite-and-rhine-trials/.
- 69.Arema M, Nakao S, Yamaguchi M, et al. Claudin-5 Redistribution induced by inflammation leads to antiVEGF-resistant diabetic macular edema. Diabetes. 2020;69:981–999. doi: 10.2337/db19-1121. [DOI] [PubMed] [Google Scholar]
- 70.Boyer D, Yoon YH, Belfort R, Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 71.Kaiser PK. The latest data on anti-integrin therapy for patients with DME. 2019. http://retinatoday.com/2017/12/the-latest-data-on-anti-integrin-therapy-for-patients-with-dme.
- 72.Abdulaal M, Haddad NM, Sun JK, Silva PS. The role of plasma kallikrein-kinin pathway in the development of diabetic retinopathy. Pathophysiology and therapeutic approaches. Semin Ophthalmol. 2016;31:19–24. doi: 10.3109/08820538.2015.1114829. [DOI] [PubMed] [Google Scholar]
- 73.Cukras CA, Petrou P, Chew EY, et al. Oral minocycline for the treatment of diabetic macular edema (DME): results of a phase I/II clinical study. IOVS. 2012;53:3865–3874. doi: 10.1167/iovs.11-9413. [DOI] [PMC free article] [PubMed] [Google Scholar]