Abstract
Medical application materials must meet multiple requirements, and the designed implant must mimic the bone structure in shape and support the formation of bone tissue (osteogenesis). Magnesium (Mg) alloys, as a “smart” biodegradable material and as “the green engineering material in the twenty-first century”, have become an outstanding bone implant material due to their natural degradability, smart biocompatibility, and desirable mechanical properties. Magnesium is recognised as the next generation of orthopaedic appliances and bioresorbable scaffolds. At the same time, improving the mechanical properties and corrosion resistance of magnesium alloys is an urgent challenge to promote the application of magnesium alloys. Nevertheless, the excessively quick deterioration rate generally results in premature mechanical integrity disintegration and local hydrogen build-up, resulting in restricted clinical bone restoration applicability. The condition of Mg bone implants is thoroughly examined in this study. The relevant approaches to boost the corrosion resistance, including purification, alloying treatment, surface coating, and Mg-based metal matrix composite, are comprehensively revealed. These characteristics are reviewed to assess the progress of contemporary Mg-based biocomposites and alloys for biomedical applications. The fabricating techniques for Mg bone implants also are thoroughly investigated. Notably, laser-based additive manufacturing fabricates customised forms and complicated porous structures based on its distinctive additive manufacturing conception. Because of its high laser energy density and strong controllability, it is capable of fast heating and cooling, allowing it to modify the microstructure and performance. This review paper aims to provide more insight on the present challenges and continued research on Mg bone implants, highlighting some of the most important characteristics, challenges, and strategies for improving Mg bone implants.
Keywords: Biomaterials, Magnesium alloy, Degradability, Mechanical properties, Biocompatibility, Additive manufacturing
Introduction
Several criteria must be met by medical materials, and implants must be morphologically designed to mimic and support bone structure and bone tissue formation (osteogenesis). The fundamental parameters that must be considered are biocompatibility, mechanical properties, and biodegradability (Yuan et al. 2019). Bones have superior regenerative properties and self-healing abilities for the body to recover from physical injury. Patients are experiencing treatments that allow them to maintain their daily activities and quality of life. Orthopaedic implants have been one of the high-demand markets in the last 2 decades. The global biomaterials market was worth US$94.1 billion in 2012 and increased to US$134.3 billion in 2017 (Witte and Eliezer 2012). This rapid increase in the biomaterials market has brought about the development of bone implants. Modern fabrication methods, advanced biomaterials designing, and architecture of medical devices have been greatly developed in the last 20 years. There are some requirements that material must meet to be suitable for applying in the body. There should be a biocompatible chemical composition with no adverse tissue reaction. In other words, materials should be non-toxic, non-inflammatory, non-carcinogenic, and non-allergic. Furthermore, materials must be bio-functional, with sufficient strength and good corrosion resistance to withstand the body’s environment. Depending on the application for which it is used, it may have specific degradation rates and good mechanical properties. Up to date, several types of biomaterials, including ceramics, polymers, composites, and metals, have been investigated for the future application of implants (Table 1).
Table 1.
Biomaterials classification with their advantages, disadvantages and applications
| Type | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|
| Metals and metal alloys | High material strength | Corrosive | Orthopaedic implants, screws, pins, and plates | Zhao et al. (2011) |
| For example, gold, platinum, titanium, steel, chromium, cobalt | Easy to fabricate and sterilise | Aseptic loosening | ||
| Excessive elastic modulus | ||||
| Ceramics and carbon compounds | High material strength | Difficult to mould | Bioactive orthopaedic implants | Zhao et al. (2011) |
| For example, calcium phosphate salts, glass, oxides of aluminium and titanium | Biocompatibility | Excessive elastic modulus | Dental implants | |
| Corrosion resistance | Artificial hearing aids | |||
| Polymers | Biodegradable | Leachable in body fluids | Orthopaedic and dental implants | Tappa and Jammalamadaka (2018) |
| Biocompatible | Hard to sterilise | Prostheses | ||
| Easily mouldable and readily available | Tissue engineering scaffolds | |||
| For example, poly methyl methacrylate (PMMA), polycaprolactone (PCL), PLA, PEEK, polycarbonates, polyurethanes | Suitable mechanical strength | Drug delivery systems | ||
| Composites | Excellent mechanical properties | Expensive | Porous orthopaedic implants | Chandra and Pandey (2020) |
| For example, dental filling composites, carbon fibre-reinforced methyl methacrylate bone cement + ultra-high molecular weight polyethylene | Corrosive resistant | Laborious manufacturing methods | Dental fillings | |
| Rubber catheters and gloves |
Bioceramics are inorganic non-metallic materials used in hard tissue engineering (Navarro et al. 2008). Bioceramics have properties that are ideal for biomedical applications, such as thermochemical stability, (1) abrasion resistance, and (2) ease of moulding. They are also non-immunogenic, biocompatible and non-toxic (Ali and Sen 2017). Compared to magnesium-based alloys, bioceramics such as hydroxyapatite (HAP) are brittle and have lower tensile strength. Commercial ceramics are used in various fields such as implant coating, maxillofacial reconstruction, and drug delivery devices (Festas et al. 2019).
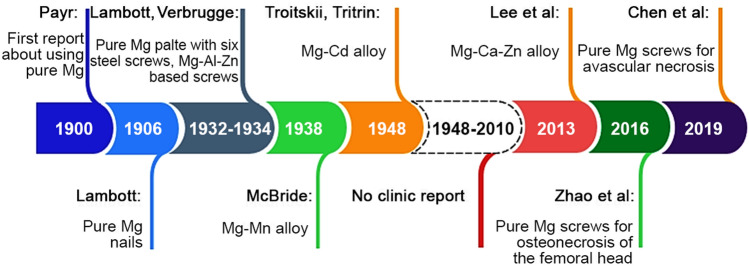
Polymeric materials are used in tissue engineering due to their plasticity, biocompatibility and biodegradability properties. Polymers are made up of small repeating monomers that impart unique properties to the polymer (Sta Agueda et al. 2021). The degree of crosslinking of the monomer determines the physicochemical properties of the polymer (Song et al. 2018). Generally, polymer materials are divided into synthetic polymers and natural polymers. For example, the mechanical strengths of the PEEK-cHAp scaffold for biomedical application were investigated and found that this biocomposite depicts better cell proliferation attachment (Oladapo et al. 2019). Metallic materials are becoming increasingly popular in orthopaedic surgery for the development of orthopaedic devices such as permanent implants (total joint replacement, hip prosthesis, and so on) and temporary implants (pins, bone plates, screws, etc.) (Radha and Sreekanth 2017). Some materials are the preferred options over polymers and ceramics due to their superb mechanical, degradation and biological features. For instance, the composite with the highest proportion of rGO of 5% indicates more biocompatibility and mechanical strength (Oladapo et al. 2019). Practically, metallic biomaterials should benefit from desirable mechanical strength until the affected part of the human body heals. Several biodegradable metals including strontium (Sr), silicon (Si), manganese (Mn), yttrium (Y) magnesium (Mg), calcium (Ca), zirconium (Zr), and zinc (Zn) can meet the requirements of good biocompatibility, biodegradability and adequate mechanical properties when it comes to the healing process for the human body. The features of most currently used metallic materials including 316L Stainless steel, Co–Cr alloys, Ti alloys, Fe-based, Zn-based and Mg-based alloys are briefly introduced in Table 2. Mg is required for the physiological function of many tissues, particularly those of the brain, heart, and musculoskeletal systems, due to its important biological effects. Mg has been used as a biodegradable implant for over a century. Witte (2010) employed magnesium wires as ligatures for blood vessels in 1878, which was the first-time magnesium used as a biomaterial. Following this, different clinicians experimented with pioneering applications of metallic elements in vessel, medical science and abdominal surgery, attracted by the actual fact that this metal corrodes within the body while not venomous effects (Puleo 1999). The historical overview of Mg is summarised in historical order in Table 3.
Table 2.
Advantages, disadvantages and applications of biodegradable and non-biodegradable metallic materials
| Classification | Materials | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|---|
| Biodegradable metallic materials | Mg-based alloys |
Biocompatible Biodegradable Bioresorbable Similar density and Young’s modulus of bone (E = 10–30 GPa) Less stress-shielding effect Light weight |
Hydrogen evolution during degradation Less resistance to corrosion |
Bone screws, bone plates, bone pins, etc | Chandra and Pandey (2020) |
| Fe-based alloys | High tensile strength and formability, fair biocompatibility, MRI compatible (austenitic phase), and no H2 gas production during degradation | Very low degradation rate, high elastic modulus; degradation via 2Fe + 2H2O + O2 → 2Fe(OH)2 | Temporary cardiovascular and orthopaedic implants | Seitz et al. (2015) | |
| Zn-based alloys | Intermediate corrosion rate (falling between corrosion rates of Mg and Fe), fair biocompatibility, no H2 gas evaluation and non-toxic corrosion products, good processability, low melting point, and less reactivity in molten state | Low mechanical strength, age hardening; degradation by 2Zn + 2H2O + O2 | Wound closure devices (biodegradable staples, surgical tacks, plugs, microclips, and rivets), orthopaedic fixation devices (fixative plates, screws, and porous scaffolds), cardiovascular stents, and bone implants | Dambatta et al. (2015) | |
| Non-biodegradable metallic materials | 316L Stainless steel |
Easily available and low cost Excellent fabrication properties Accepted biocompatibility and toughness |
High modulus Poor corrosion resistance Poor wear resistance Allergic reaction in surrounding tissue Stress-shielding effect |
Bone plates, bone screws and pins, wires, etc | Bowen et al. (2016) |
| Co–Cr alloys |
Superior in terms of resistance to corrosion, fatigue and wear High strength Long-term biocompatibility |
Expensive Quite difficult to machine Stress-shielding effect High modulus Biological toxicity due to Co, Cr and Ni ions release |
Shorter term implants—bone plates and wires, total hip replacements (THR)—stem or hard-on-hard bearing system | Bowen et al. (2016) | |
| Ti alloys |
Excellent resistance to corrosion Lower modulus Stronger than stainless steels Light weight Biocompatible |
Poor wear resistance Poor bending ductility Expensive |
Fracture fixation plates, fasteners, nails, rods, screws and wires, femoral hip stems, total joint replacement (TJR) arthroplasty-hips and knees | Venezuela and Dargusch (2019) |
Table 3.
Historical overview of reports on magnesium and its biomedical application in historical order (Witte 2015)
| Year | Author | Magnesium (alloy) | Supplier | Application | Human/animal model |
|---|---|---|---|---|---|
| 1878 | Huse | Pure magnesium | Not reported | Wires as ligature | Humans |
| 1892–1905 | Payr | High-purity Mg | I and CW Rohrbeck, Vienna, Austria, Al and Mg Fabric Hemelingen, Germany | Tubes (intestine, vessel, nerve connector), plates, arrows, wire, sheets, rods | Humans, guinea pigs, rabbits, pigs, dogs |
| 1903 | Höpfner | Pure magnesium | No report | Magnesium cylinders as vessel connectors | Dogs |
| 1900–1905 | Chlumsky´ | High-purity Mg | Friedrich Wosch Company, Germany | Tubes, sheets and cylinder intestine connector, arthroplasty | Humans, rabbits, dogs |
| 1906–1932 | Lambotte | Pure Mg (99.7%) | No report | Rods, plates, screws | Humans, rabbits, dogs |
| 1910 | Lespinasse | Metallic magnesium | No report | Ring-plates for anastomosis | Dogs |
| 1913 | Groves | No report | Intramedullar pegs in bone | Rabbits | |
| 1917 | Andrews | Pure Mg, mix. of eq. part: Mg/Al, Mg/Cd, Mg/Zn | No report | Wires, clips as ligature, anastomosis | Dogs |
| 1924 | Seelig | Pure Mg (99.99%), distilled in vacuum | American Mg Cooperation, Niagara Falls, NY | Wires, strips, bands | Rabbits |
| 1925 | Glass | Pure Mg (99.8–99.9%) | Al and Mg Fabric, Hemelingen, Germany | Magnesium arrows | Humans, rats, cats |
| 1928 | Heinzhoff | Pure magnesium | No report | Magnesium arrows | Rabbits |
| 1933–1937 | Verbrugge | Dow metal: Mg–Al6–Zn3–Mn 0.2%-wt. Electron Mg–Al 8% (wt) | Dow Chemical Corp., USA Griesheim-Electron, Germany | Plate, band, screws, pegs | Humans, dogs, rats, rabbits |
| 1938 | McBride | Mg–Mn 3%-wt, Mg–Al 4–Mn 0.3% (wt) | Not reported | Sheet, plate, band, screw, peg, wire | Humans, dogs |
| 1939 | Nogara | Electron (alloy not specified) | Griesheim-Electron, Germany | Rods | Rabbits |
| 1948 | Tpobwrbq | Mg–Cd | Not reported | Plate, screws, rod-plate | Humans |
| 1940 | Maier | Magnesium | I.G. Farben Industry AG, Bitterfeld, Germany | Band, suture from woven Mg wires, fusiform pins | Humans, rabbits |
| 1951 | Stone | Mg–Al 2% (wt) pure magnesium | Aluminium Company of America, OH, USA | Wires for clotting aneurysms | Dogs |
| 1975 | Fontenier | Ind.-grade purity: Domal Mg (99.9%), TLH Mg not reported Lab-grade purity: ‘‘zone fondue’’ Mg, R69 Mg Mg Mn 1.5% (wt), Mg Al: GAZ8%, GAZ6%, GAZ3% | Not reported | Anodes for implantable batteries to feed pacemaker | Dogs |
| 1980 | Wexler | Mg–Al 2% (wt) | McMaster Univ. Med, Canada | Wires intravascular | Rats |
| 1981 | Hussl | Pure Mg (99.8%) | Goodfellow Metals Ltd, GB | Wires for hemangioma treatment | Rats, rabbits |
| 1981 | Wilflingseder | Pure Mg (99.8%) | Goodfellow Metals Ltd, GB | Wires for hemangioma treatment | Humans |
Mg-based bone implants
The development of biodegradable Mg-based materials is progressing to design orthopaedic implants. Mg and its alloys should be biocompatible if they are to be used as biomaterials. The unknown toxicity of commercial Mg alloying elements poses a potential risk that could exacerbate the use of such alloys in the biomaterial field. In this situation, extreme care must be taken in the selection of biocompatible elements, optimised composition design for new biodegradable Mg alloys with preferred bio-mechanical properties, and viable processing methods to guarantee defect-free products. As for material, Mg and its alloys have revolutionised biomedical implants owing to its superb characteristics such as proper Young’s modulus, good biocompatibility, natural degradability, and high specific strength (Sanchez et al. 2015). As an example, they show the similar Young’s modulus (almost 45 GPa) to that of bone (15–30 GPa) which can be an effective factor for reducing stress shielding in implantation. As for density, the human bone stands for 1.75 g/cm3 while that of for Mg and its alloys is about 1.79 g/cm3. It is important to note that traditional implant metals, including 316L steel (Lavery et al. 2017), titanium (Ti) alloys (Niinomi 2008), Cobalt–Chrome (Co–Cr) alloys (Wang et al. 2016) benefit from the higher Young’s modulus when it comes to that of human bone, despite their superb mechanical strength considering the control of biopolymers and bioceramics exhibited too low mechanical strength. Furthermore, surface treatment, plasticity, and Mg alloys’ stiffness can be easily controlled during the process and sterilisation (Chen et al. 2015; Wang et al. 2018b). Except for the desired mechanical properties, Mg and its alloys exhibit an attractive degradation characteristic and can be completely degraded in vivo (Zhang et al. 2010). Concerning degradability, non-degradable traditional biometals require a second surgery to remove them, resulting in additional pain and increasing the financial burden on patients.
Mg is the fourth most common mineral in the human body, and no one can deny the necessity of this element in bone reconstruction. The restored Mg for maintaining regular functions for a healthy adult is between 21 and 28 g, and the amount is recommended as a daily allowance of Mg for a healthy adult is between 250 and 350 mg (Khayat 2017). Another outstanding feature of the Mg is its unique osteopromotive property (Laires 2004). Natural bone possesses a sophisticated system in terms of the microenvironment. More research regarding the in vivo osteopromotive mechanism on Mg can be referred to as the published literature (Wu and Veillette 2011; de Baaij et al. 2015). The main advantages and drawbacks of Mg are given in Tables 4 and 5, respectively.
Table 4.
The key advantages of Mg (Radha and Sreekanth 2017)
| Benefits | Details |
|---|---|
| Low density | Mg density (1.738 g/cm3) is nearly equivalent to cortical bone density (1.75–2.1 g/cm3) |
| High specific strength | The strength-to-weight ratio of approximately 130 kN m/kg |
| High damping capacity | Mg has the highest energy absorption capacity of any metal and can be used in load-bearing applications |
| High machinability and dimensional accuracy | Mg is the simplest structural metal to machine and it is easy to accomplish steady final dimensions. As a result, advanced models are easily producible, which are critical for the complex shapes often required for medical applications |
| Less stress shielding | Since Mg density is very close to that of bone, stress-shielding issues for many orthopaedic implants can be greatly reduced |
| Good biocompatibility | Mg is a biocompatible mineral that has been found to speed up bone growth |
| Good safe degradation | Mg corrosion in the body would eventually lead to complete breakdown, thus it would be good for patients to be exposed to an implant in the body for a short period |
Table 5.
The key disadvantages of Mg (Radha and Sreekanth 2017)
| Drawbacks | Details |
|---|---|
| Low elastic modulus | Lower Mg elastic modulus may be useful in terms of stress shielding, but any implant must be engineered to withstand its load without distortion |
| Rapid degradation | Mg implants are designed to disintegrate fully, but at a slower rate than bone remodelling. Normal body components (e.g. Zn, Ca and Mn) can, however, be poisonous if the release rate is too great and the amounts are not dealt with effectively (e.g. excess Mg through the kidneys, hydrogen gas through the soft tissues). To prevent the use of harmful alloying elements and provide an adequate release rate for other elements, including those that are naturally occurring, a fully biocompatible Mg alloy is necessary |
| High hydrogen evolution | The emitted H2 gas accumulates at a rapid pace in the surrounding soft tissues. H2 bubbles may cause necrosis of surrounding tissue by delaying healing at the surgical site. Hydrogen evolution rates of 0.01 mL/cm2/day have been documented for several Mg alloys containing Zn, Al and Mn |
Applications of Mg in biomedical implants
Bone fixation device plays a pivotal role when it comes to bone repair. Bone screws, bone plates, bone pins, and so on are considered bone fixation devices. Technically speaking, 316L steel or Ti alloy are known as a traditional biomaterial for bone fixation devices possessing much greater modulus than bone, leading to low-level stress for a bone defect long-term. Therefore, osteoporosis and other symptoms can emerge owing to the lack of mechanical stimulation (Shuai et al. 2018d). Due to the desired mechanical strength of the Mg bone fixation device, sufficient mechanical support, especially at the preliminary phase, is clear, while this particular device undergoes cyclical degradation. In this scenario, load-bearing support decreases gradually. In contrast, the load bearing of bone tissue gradually increases at the fracture site. The introduction of Mg bone devices goes back to the early twentieth century. Several typical bone fixation screws and devices are shown in Figs. 1 and 2, respectively. Payr et al. were the first researchers who reported Mg application for bone pins and plates to fix traumatic bones (Tammann and Schafmeister 1924). According to Lambott (1906), a 17-year-old patient’s fractured leg was mended with an Mg plate and steel nail (Sur divers 1911) but galvanic corrosion of the Mg plate and steel nail was noticed the day following surgery. The Mg plate’s degradation was hastened due to the rapid collection of hydrogen. Another important investigation was implemented by McBride (1938). They concentrated on the application of Mg–Al–Mn alloy for screws, bolts, and plates and experienced 20 fracture treatments. In this scenario, implants were completely absorbed, and no adverse effect was observed. Witte (2010) experienced 34 pseudoarthrosis cases with the Mg–Cd alloy fixation devices. They observed the full degradation of Mg implants, which were replaced by bone tissue regeneration. Despite these accomplishments, in this field, the high degradation rate of magnesium was the main interest of attention, which resulted in a protracted gap in clinical research in the late twentieth century.
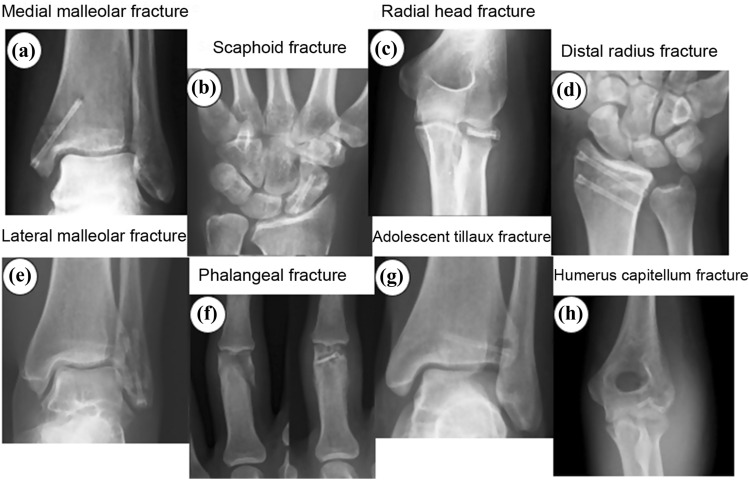
Fig. 1.
Representative of several magnesium screws (Farag and Yun 2014). Copy right from RSNA (Taljanovic et al. 2003)
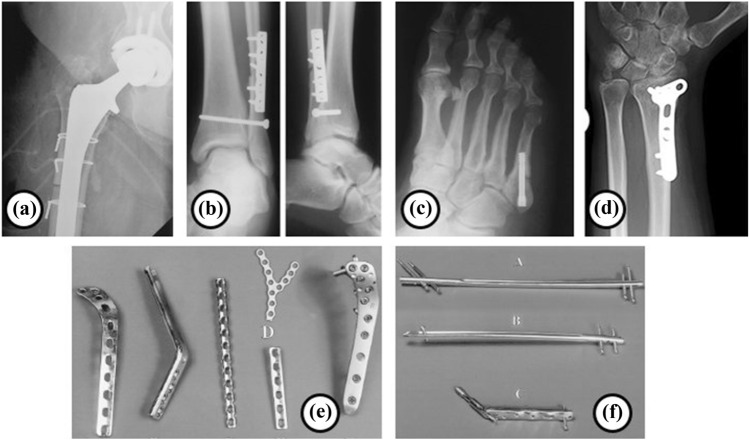
Fig. 2.
Typical bone fixation devices: a cerclage wires used in hip, b interfragmentary screw, c Herbert screw, d wrist fixation, e various types of plates used in internal fixation, f a variety of nails used in internal fixation, under the terms of the Creative Commons CC-BY license, and RSNA (Taljanovic et al. 2003)
During the last decade, tremendous progress has been observed in modifying the Mg degradation rate, thanks to the rapid advancement of research and technology. Lee et al. (2016) used Mg–Ca–Zn screws to repair 53 cases of radius fractures. This type of screw was completely replaced by bone regenerating during the first year of implantation. Zhao et al. (2016) published a study that used Mg screws to secure a vascularised bone graft in a patient's femoral head. In comparison with the conventional methods, this approach exhibited better treatment efficacy. Chen et al. (2018) applied the high pure Mg screws in a 17-year-old patient to treat a vascular necrosis of the femoral head resulted in a 2-year effective follow-up. Another outstanding investigation was implemented by Acar (2018). In 16 cases, Mg screw fixation was inserted in the hallux valgus, and all participants had a full union of the osteotomy after at least a year.
Mg and its alloys also play a pivotal role in bone tissue engineering when it comes to scaffold materials. The transplantation of autologous osteoblasts, bone marrow stromal cells, or chondrocytes onto an artificial scaffold with high biocompatibility and gradual biodegradability in the human body is known as bone tissue engineering (BTE) (Stevens 2008; Gupta et al. 2016). Therefore, scaffold/cell hybrid is implanted into the defect site to provide space for cell growth. In this scenario, since the scaffold is gradually degraded in vivo, cell proliferation occurs, and then new tissue grows directionally into the scaffolds. Considering this implantation, there should be a similarity between the pore size of the scaffold and natural bone, normally about tens of hundreds of microns (Hao et al. 2017). Assuming sufficient mechanical strength, potentially significant porosity, and a pore structure that is well connected are the scaffold requirements for nutrient transfer and metabolites discharge (Yang et al. 2018b). Several prominent cases regarding the application of Mg bone fixation devices are reported in Figs. 3 and 4.
Fig. 3.
Several Mg bone fixation devices in a variety of scenarios
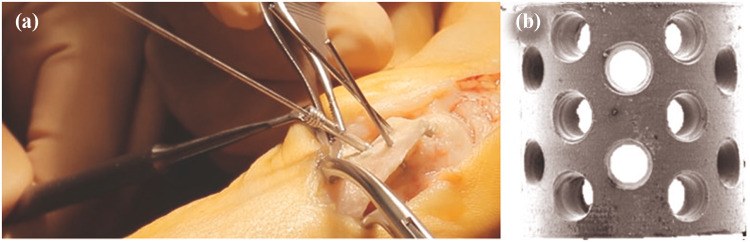
Fig. 4.
Mg-based alloy bioabsorbable screws in orthopaedic surgery (a), low magnification SEM images of a representative porous bone scaffold (b) (Ezechieli et al. 2014)
Mechanical properties of Mg-based bone implants
Considering the fractured bone, the implant requires favourable mechanical properties for internal fixation as a fixture device. The primary role function of any biomedical implant is to fix and hold the fractured bone. From the mechanical properties’ perspective, the elastic moduli of material used as an implant and bone should be as close as possible. For instance, Mg-based alloys have elastic moduli of about 40 GPa which is not dramatically higher than that of bone material which is around 18 GPa (Liu et al. 2018a). This feature results in uniform stress distribution and can reduce the possible stress concentration effects bone and implant joint interface. Regarding the yield strength, the material of implant should benefit from the higher tensile and compressive strengths to refrain from crack development and implant fracture. Notably, yield strength can also be altered depending on the manufacturing process. To increase the functional stability and transfer stress properly from implant to bone, the interfacial shear strength should be increased results in reducing the stress in the implants (Filippi et al. 2020). To avoid brittle fractures caused by cyclic loading circumstances, the implant material should have a high fatigue strength (Saini 2015). Toughness refers to the capacity to retain maximal energy until the implant breaks, whereas ultimate stress refers to the highest stress experienced before the implant fails. Regarding ductility, it indicates the highest strain that an implant without the assembly can tolerate during deformation under the existing loads. Tables 6 and 7 provide the mechanical properties of human bone and several biomaterials for bone implant, and the mechanical properties of some Mg-based alloys, respectively.
Table 6.
Mechanical characteristics of natural bone, Mg alloys and other common bioactive materials (Wu et al. 2014; Li et al. 2016)
| Materials | Density (g/cm3) | Modulus (GPa) | Compressive yield strength (MPa) | Fracture toughness (MPa m1/2) |
|---|---|---|---|---|
| Cortical bone | 1.8–2.1 | 7–20 | 130–180 | 3–6 |
| Cancellous bone | 1–1.4 | 0.01–3 | 2–12 | NA |
| Mg alloys | 1.79–2.0 | 35–45 | 100–200 | 15–35 |
| Ti alloys | 4.2–4.5 | 110–120 | 750–1110 | 55–115 |
| Co alloys | 8.3–9.2 | 230 | 450–1000 | NA |
| 316 L steel | 8.0 | 193 | 190 | 50–200 |
| Tantalum | 16.7 | 186–191 | NA | NA |
| HAp | 3.1 | 80–110 | 0.03–0.3 | 0.6–1.0 |
| TCP | NA | 24–39 | 2–3.5 | 0.3–1.0 |
| PEEK | 1.29 | 3–4 | 95 | NA |
| PLGA | 1.2e1.3 | 1.4–2.8 | 41.4–55.2 | NA |
NA not available
Table 7.
Mechanical properties of some Mg biodegradable alloys, the letters T and C stand for tension and compression, respectively (Chen et al. 2018)
| Elements (alloys) | Yield stress (MPa) | Ultimate stress (MPa) | Elongation (%) |
|---|---|---|---|
| Mg–Zn–Sr | 187.324 ± 4.015(T) | 278.002 ± 5.352(T) | 19.8 ± 3.6(T) |
| Mg–Zn–Zr | 227.509 ± 5.009(T) | 306.052 ± 4.588(T) | 20 ± 2(T) |
| Mg–Zn–Zr–Sr | 322.363 ± 4.547(T) | 376.400 ± 7.526(T) | 16 ± 1(T) |
| Mg–5.5Zn | 115.6 ± 3.9(C) | 303.2 ± 5.6(C) | – |
| Mg–5.5Zn/5HA | 154.7 ± 4.2 (C) | 351.6 ± 5.2(C) | – |
| Mg–5.5Zn/10HA | 165.3 ± 3.7(C) | 379.7 ± 4.1(C) | – |
| Mg–Zn–Mn–Ca | 198(T) | 220(T) | 4.5(T) |
| Mg–1.2Zn–0.5Ca (as-cast) | 64.5 ± 10.5(C), 60.3 ± 3.1(T) | 255.2 ± 7.6 (C), 121.3 ± 5.2(T) | 17.4 ± 0.43(C), 3.2 ± 0.13(T) |
| Mg–1.2Zn–0.5Ca (heat treated) | 124.4 ± 6.9 (C), 84.3 ± 7.1(T) | 309 ± 17.3(C), 150.7 ± 8.5(T) | 18.9 ± 0.3(C), 4.9 ± 0.24(T) |
| Mg–5Zn | 120(T) | 212(T) | 10(T) |
| Mg–5Zn–0.2Sr | 117(T) | 233(T) | 15(T) |
| Mg–5Zn–0.6Sr | 115(T) | 215(T) | 13(T) |
| Mg–5Zn–1.0Sr | 107(T) | 194(T) | 9(T) |
| Zn–1 Mg–0.1Sr (hot rolled) | 196.84 ± 13.20(T) | 300.08 ± 6.09(T) | 22.49 ± 2.52(T) |
Improving the mechanical properties of Mg-based alloys
Biocompatibility and degradation qualities should be also addressed for improving the mechanical properties of biodegradable Mg alloys. The creation of alloys, heat treatment, and plastic deformation are all required to achieve these qualities. When it comes to alloy development, Ca, as an example, is one of the most abundant metal elements in human bone, and it can help in bone repair (Jung et al. 2012; Renkema et al. 2008; Yin et al. 2013). With dispersion along grain boundaries, Mg–Ca plays a critical role in the mechanical characteristics of the Mg–Ca alloy (Seong and Kim 2015). Because of the grain refinement, adding Ca to Mg can boost both strength and elongation rate (Yin et al. 2013). Excessive Ca in magnesium, on the other hand, will reduce corrosion resistance. Ca content in Mg alloys should thus be less than 1 (Cui et al. 2017). Considering Zn, it is one of the important trace elements in human body and a co-factor for optional enzymes in bone and cartilage. Zn has a relatively high solubility in magnesium (6.2% by wt) and can play dual roles in both solid solution and precipitation strengthening. When the content of Zn is over 5%, many MgZn phases would precipitate from Mg matrix along grain boundaries, which could enhance the strength of Mg–Zn alloy due to the dispersion strengthening. Sr is another nutritional element found in biodegradable magnesium alloys that helps osteoblasts development (Atkins et al. 2008). Strontium ranelate (SR) is used in the treatment of osteoporosis to increase bone strength and mineral density. Sr has a grain refining effect, and the refined eutectics result in substantial dispersion strengthening. Only a few publications on Si-containing magnesium alloys exist due to their poor corrosion resistance and mechanical qualities. Ben-Hamu et al. (2008) discovered that Zn is a particularly efficient alloying element for improving the mechanical characteristics of Mg–Si alloys. When 1.6% Zn is added to Mg–0.6Si, the morphology of the Mg2Si phase changes dramatically from a course eutectic structure to a tiny dot or short bar form. Tensile strength, elongation, and bio-corrosion resistance all increased dramatically as a consequence, with elongation improving by 115.7% in particular. The ultimate tensile strength and elongation, respectively, were 182 MPa and 14%. Finally, alloying is an effective method for improving the mechanical characteristics of Mg alloys. For biodegradable Mg alloys, the alloying elements are intended to enhance not only the initial mechanical qualities, but also to maintain mechanical integrity for a longer period of time in vivo by boosting the alloys’ corrosion resistance. Although Ca, Sr, and Si elements have acceptable biocompatibility, their mechanical qualities are undesirable because to their reduced solubility in magnesium alloys when compared to Zn. Their second phases are often thick and dispersed along the grain boundary, which are detrimental to improving the mechanical characteristics of magnesium alloys.
Heat treatment
Heat treatment is used to enhance the mechanical characteristics of Mg alloys when the solubility of specific alloying elements varies with temperature. When compared to other processing procedures, heat treatment typically does not modify the form or chemical content of the materials, but rather modifies their microstructure. The two major methods for increasing the mechanical characteristics of Mg alloys are fine-grain strengthening and second phase strengthening. Solid solution treatment (T4), age treatment (T5), and solid solution + ageing treatment (T6) are the most regularly utilised thermal treatments for Mg alloys (Yu et al. 2017). For example, because of the high solubility of Zn in magnesium, heat treatment is commonly used to enhance the mechanical characteristics of Mg–Zn alloys (Li et al. 2020). Overall, heat treatment is an efficient way for improving the mechanical and biodegradable characteristics of magnesium alloys. After heat treatment, the microstructure and distribution of the second phases will change, which are strongly connected to the mechanical characteristics of magnesium alloys.
Plastic deformation
The dislocation density of Mg alloys rises during plastic deformation, and the grain refining effect is visible (Alaneme and Okotete 2017). As a result of the resistance to dislocation movement, the strength of Mg alloys is increased. Typically, plastic deformation such as extrusion, rolling, drawing, and forging may considerably increase the mechanical characteristics of magnesium and its alloys. Several instances involving magnesium and its alloys have been undertaken in clinic to date. All the findings supported the use of magnesium screws in clinics to treat illnesses. However, in clinic, magnesium and its alloys are mostly utilised as unload-bearing implants, and because of the complicated stress state, plate and screw systems have only been tested in animals (Chaya et al. 2015), the use of a magnesium plate and screw combination has not been implemented. Since the solubility of alloying elements in magnesium is limited, and biocompatibility and biodegradability must be addressed when designing novel biodegradable materials, the ability to increase mechanical qualities is severely constrained. More attention to the combination of new alloy design, heat treatment, and plastic deformation techniques is expected to result in higher mechanical properties of magnesium alloys, and moreover, an approach was recently announced that integrates the strengthening benefits of nanocrystallinity with those of plasticisation to produce a dual-phase material that demonstrates near-ideal strength of 3.3 GPa at room temperature and without sample size effects (Wu and Zhu 2017). Furthermore, with the advancement of magnesium alloys in the medical field, numerous various potential products are being investigated. For several implantable devices, not only the strength, but also the deformability, fatigue resistance, stress corrosion cracking, and so on, should be highlighted, which will widen the research on mechanical properties of magnesium alloys.
Biocompatibility of Mg
The first and most important criteria for every implant material are biocompatibility and nontoxicity. When a foreign substance is implanted in the human body, a series of interactions between the implant material and the host tissues occur, which determine the implant’s acceptance by the body (Shu-Rong et al. 1999). Biocompatibility of permanent implants is determined by how well freshly produced tissue interacts with the implant surface. Temporary implants, on the other hand, are designed to provide support for shattered bone until it heals, after which it is meant to decay at a regulated pace within the body (Yang et al. 2020a). If the degradation product begins to interact negatively with any physiological factor, this is a major problem. As a result, temporary implant materials should be non-toxic and should not produce inflammatory or allergic reactions in the body. Mg is an essential component of bone construction and the fourth most abundant cation in the human body. It has a high biocompatibility, making it one of the best options for temporary implants. Around 20% of the total accessible magnesium is stored in bones, whereas the remaining 35–40% is located in tissues and ligaments. It is critical to understand the effect of Mg-ion concentration on stem cells when evaluating Mg-based alloys for bone implant applications. Stem cells proliferate and differentiate into osteoblasts, which serve as structural components in the formation of new bones. Mg-ions in extracellular matrix can help stimulate the gene expression of melastatin-type transient receptor potential channels in human bone cells, according to a research by Abed and Moreau (2009). They looked at how magnesium ions affected gap junction intercellular communication (GJIC) in human osteoblasts. A 3 mM Mg-ion concentration can significantly boost cell viability. It can also boost alkaline phosphate activity and osteocalcin levels. With the use of computer tomography and fluorescence imaging, the osseous development of Mg implants has been demonstrated in several experimental experiments on various animals. An intramedullary implantation of a pure Mg pin into the distal femur of rats resulted in the development of new bones at the peripheral locations of cortical bones, according to Zhang et al. (2016). They came to the conclusion that Mg-ions can improve the GJIC between bone cells substantially. Several additional investigations have published more extensive overview of the impact of Mg-ions on cell survival (Yang et al. 2010). The effects of various added elements on mechanical and biological properties of Mg are shown in Table 8.
Table 8.
The effects of alloys on the characterisation of Mg
| Alloys | Mechanical properties | Biocompatibility | Potential biomedical application | References |
|---|---|---|---|---|
| Mg–Ca alloys | Increase the strength and the elongation rate; excessive Ca deteriorates the corrosion resistance; Ca concentration should be less than 1%(by wt) | Essential element of the human body; promote bone healing process | Orthopaedic application; screw | Jung et al. (2012) |
| Mg–Zn alloys | Enhance the tensile strength; solid solution strengthening and ageing strengthening; the elongation decreased when Zn concentration was over 5% (by wt) | Important trace elements in human body and a co-factor for optional enzymes in bone and cartilage | Orthopaedic application; suture materials; intestinal tract and bile repairing | Bairagi and Mandal (2021) |
| Mg–Sr alloys | The strength and corrosion resistance improved when Sr concentration below 2% (by wt); excessive Sr decreased mechanical properties | Promotes osteoblast maturation; good for the bone formation | Orthopaedic application; skeletal applications | Atkins et al. (2008) |
| Mg–Si alloys | Mg–Si alloy showed a low ductility due to the presence of coarse Mg2Si; the addition of 1.6% Zn, tensile strength, elongation and bio-corrosion resistance improve significantly | Helps to build the immune system; good for the growth and development of bone and connective tissue | Orthopaedic application; promote the bone healing | Gil-Santos et al. (2017) |
| Mg–Al alloys | Significantly improve ultimate yield strength and ductility at a concentration below 6% | Nerve toxicity and elevated concentration of Al3+ in the brain are related to Alzheimer’s disease | Orthopaedic application; screw | Bakhsheshi-Rad et al. (2015) |
|
Mg–Y alloys Mg–YREZr |
Significantly enhances tensile strength and tensile yield strength with increasing addition of Y; improves elongation with the Y concentration below 3% | Good biocompatibility; promote the bone healing process | Stent and screw | Duan et al. (2012) |
| Mg–Nd alloys | Improves the tensile strength and creep resistance due to the formation of the intermetallics of Mg12Nd; Nd content less than 6% | Good biocompatibility; promoting the bone healing | Stent and screw | Boukhobza et al. 2017) |
Degradability of Mg-based bone implants
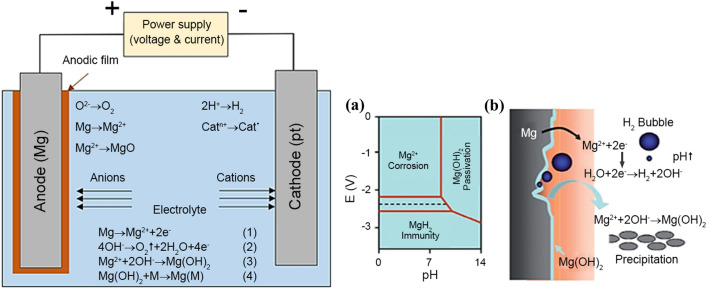
In most cases, repair of bone defects is divided into three phases: inflammation, repair, and reconstruction (Liu et al. 2018b). Within the initial two stages, the fracture location is nearly intolerable. The bone implant is needed to produce comfortable support to safeguard the fracture site from secondary injury. Therefore, bone implants require a slow corrosion rate to take care of sufficient mechanical strength. Within the third stage, the injured bone tissue has to be moderately aroused by a rising load to revive its original supporting operate. Therefore, at the time of reconstruction, the bone implant is expected to degrade completely. The inflammation period is only about a weeklong, whereas the repair period typically lasts 3 to 6 months (Yang et al. 2018a, b, c; Shuai et al. 2019a, b, c). Under the progress of the bone repair, the degradation rate of bone implants should be reasonable, ranging between 0.2 and 0.5 mm/year (Hiromoto et al. 2015). Sadly, previous in vivo studies have reported that Mg alloy indicates a very quick degradation rate to satisfy the necessity of bone repair, although typically, most observations have represented that in vivo degradation is slower than measured in vitro (Bowen et al. 2014; Ren et al. 2019). This rapid degradation characteristic is closely associated with its electrochemical features. Mg is an extremely active metal, which shows a low standard electrode potential of minus 2.37 V (Patil et al. 2019). Taking electrochemical kinetic into account, it benefits from robust electronegatively and is vulnerable to corrosion within the physiological atmosphere made in aggressive chloride ions. This very rapid degradation of Mg bone implants not solely results in the premature loss of mechanical integrity but also leads to aggregation of hydrogen in vivo, causing subcutaneous swelling development and alkaline elevation at the site of implantation (Wu et al. 2019; Song et al. 1999).
| 1 |
| 2 |
| 3 |
Over the last few decades, the corrosion method of Mg in vivo has been thoroughly investigated. Notably, electrochemical corrosion is used to degrade Mg and its alloys, including anodic dissolution and cathodic decrease of Mg metal. It is obvious from the schematic process of Mg corrosion in Fig. 5 that after contact with a liquid body substance, Mg easily oxidises to cations through anodic reactions (Eq. 1), leading to electrons used in a cathodic reduction reaction (Eq. 2) that absorbs water and produces hydrogen (Shuai et al. 2017). Because of the variation between the Mg matrix and the second part of organic molecules adsorbed on the surface, these reactions occur at random on the Mg matrix surface (Yang et al. 2020a, b). Furthermore, a large number of oxygens, protein, and solution ions, such as chloride and hydroxide ions, dissolve within the liquid body substance. Mg with a high electrochemical potential appears to corrode, resulting in the flow of ions from the surface of the metal to the surrounding liquid body substance. After corrosion, accumulated Mg(OH)2 layer generally deposits on the Mg matrix (Eq. 3). The produced corrosion layer on the Mg matrix has a layer with an inner thickness of MgO, together with the heavy outer layer of (Saini 2015). In this scenario, the inner MgO layer fails to provide efficient corrosion protection due to a lack of sufficient dense features. Because of the magnitude relationship between the molar volume of the elementary unit of MgO and that of Mg metal, specifically, the Pilling–Bedworth magnitude relation is 0.8 < 1, whereas the external layer may be dissolved by the chloride ions in physiological circumstances (Song et al. 2017). That is why Mg and its alloys usually degrade speedily in physiological environments.
Fig. 5.
Schematic of Mg corrosion process
Mg alloys’ degradation is considerably suffering from the corrosion type, surface corrosion together with pitting corrosion. Impurity, the cathodic second phase, and corrosive media all play a role in pitting corrosion (such as chloride ions). Pitting corrosion is a type of serious local corrosion caused by the breakdown of the passive film on the Mg matrix (Song and Atrens 1999). The resulting corrosion pit is quite tiny, but it will penetrate deeply into the Mg matrix. Once pitting corrosion occurs, impurities in the Mg matrix and chloride ions in the liquid body substance can stimulate pitting expansion and cause the Mg matrix to break down in a very short time, resulting in reducing the bearing capability. Even so, pitting corrosion generally results in native stress and cracks, which should weaken mechanical behaviour. Surface corrosion, unlike pitting corrosion, is known as consistent chemistry corrosion on the Mg matrix, which can prove the uniform shrinkage of the Mg matrix (Zeng et al. 2006). Surface corrosion is required for two reasons: (1) the Mg matrix’s composition and microstructure are uniforms; (2) corrosion resolution will meet any location on the Mg matrix’s surface equally. It is straightforward to comprehend that surface abrasion results in a standardised loss of bearing capability, which causes far less damage to bone implants than pitting corrosion. To help enhance the biomedical application of magnesium alloys in bone regeneration, numerous authors have concentrated tirelessly to manipulate their biodegradation in physiological environments, and they have made significant progress (Xin et al., 2011; Lee et al. 2016; Liu et al. 2016a, b). Four common procedures, including purifying, alloying, surface coating, and Mg-based composite materials (MMC), have been fully examined and are discussed in this section.
Alloying treatment
One approach to strengthen Mg alloy corrosion resistance by altering the microstructure is alloying treatment (Wang et al. 2018a, b, c; Yang et al. 2016a, b). A collection of Mg alloys for biomedical applications has been conducted by researchers. As shown in Table 2, they concentrate on degradation and physio-chemical characteristics by in vivo testing. One possible methodology is to use alloying components to mitigate the second phase in the magnesium alloy matrix in terms of volume and size, or to create a desirable second phase, which results in lessening chemical reaction caused by the second phase. As an example, Jia et al. (2015) improved AZ91 alloy with Y. It was established that the volume proportion of the Mg17Al12 phase attenuates, and as a result of that, the corrosion rate is reduced. Choi and Kim (2014) found that adding 0.02% (by wt) Ti considerably reduced the volume fraction and size of Mg17Al12 phase, lead to better corrosion resistance. Shuai et al. (2018a, b, c, d) proposed that the CaO-induced (Mg, Al)2Ca phase in AZ61 alloy covered the grains and behaved as an obstacle, potentially blocking corrosion growth in the Mg Matrix phase. Another strategy for improving the degradation rate of Mg alloy is defined as a surface coating. The main application of this method is to apply a protective layer on the Mg matrix to decelerate the degradation. From the medical perspective, the coating material ought to have sensible biocompatibility associated with desirable biodegradability. In other words, the degradation rate of the coating surface ought to be slower than that of the underpinning Mg matrix. As a result, scholars typically focus on perishable bioceramics and biodegradable polymers with sensible biocompatibility and appropriate degradation rates as coating materials (Manoj Kumar et al. 2016; Wang et al. 2018a, b, c).
Bioceramics not only increases the resistance to corrosion of Mg bone implants but also improve the processing of surface bioactivity. The reason is that bioceramics are composed of calcium and phosphorus in chemical compositions similar to natural bone. Moreover, bioceramics play an important role in the interface by stimulating bone tissue growth. Using a calcium stearate protective layer on a magnesium matrix was reported by Zhang et al. (2017) applying the plasma electrolytic oxidation approach. This micro-nano-structured coating indicates superhydrophobicity that avoided Mg corrosion in a simulated bodily fluid. Sankar et al. (2017) used electrophoretic deposition and laser deposition to create HAp protective layer on WE43. The evaluation of degradation behaviour was implemented by soaking the treated samples in Hank’s resolution. According to the results, curves indicate corrosion rates of 0.194 mm/year and electrochemical corrosion polarisation of 0.073 mm/year, which is much less than that of untreated WE43 (0.97 mm/year). Another research done by Chang et al. (2013) was related to the formation of the dicalcium phosphate dehydrate coating on the surface of AZ31. They used a combination of micro-arc oxidation and low-temperature hydrothermal treatment. In this case, after 2 h of soaking in Sodium hydroxide solution, uniform HAp was obtained, resulting in better pitting corrosion resistance. One more investigation implemented by Razavi et al. (2014) discovered that electrophoretic application of nanostructured diopside coating resulted in higher corrosion resistance and physiological activity of Mg alloy (Razavi et al. 2014).
Natural polymers, as well as synthetic polymers, have been employed as Mg matrix surface coating. The controllability of synthetic polymer coating is straightforward, while natural polymer coating provides better cell adhesion and reconstruction. Chen et al. (2011) reported a range of 15–20 µm for coating on pure Mg using polycaprolactone (PCL) and polylactic acid (PLA) led to decreased corrosion rate of the Mg matrix. According to Butt et al. (2017), AZ31 was immersed directly into PLA melt for coating preparation on the surface. It was reported that degradation rapidly began after 8-week immersion. In terms of the effectiveness of coating thickness on Mg corrosion, PCL coating with a thickness of between 2.8 and 13 µm was implemented (Park et al. 2013). The rate of released hydrogen was considered in the Hank solution for 2 weeks. An inverse correlation between degradation rate and coating thickness was observed. In other words, the more the coating thickness increased, the less degradation rate appeared. It should be mentioned that the physical characteristics of polymer or ceramic coating are extremely various from that of underlying Mg metal, which results in a loose interface between the surface coating and Mg matrix. Up to date, much less attention has been paid to the adhesion tests for evaluating the feasibility and stability of the coating. From the electrochemical kinetic point of view, the more impurities and the second phase in the Mg matrix are reduced, the less likely galvanic corrosion will occur (Atrens et al. 2018). That is why researchers proposed the purification methodology to reinforce Mg alloys’ corrosion resistance (Yu et al. 2018). The impurities mostly consist of Fe, Ni, and Cu, since their tolerance thresholds are restricted (Fe, Ni < 0.005% by wt, Cu < 0.05% by wt) in the Mg matrix (Han et al. 2015). Once those impurities’ contents are less than the tolerance thresholds, the corrosion rate will be slow. However, once the impurity content exceeds the tolerance limit, the corrosion rate will be sharply increased. It was discovered that reducing the iron content to 0.5 mm/year minimised the corrosion rate of the Mg matrix from 14.9 to 0.5 mm/year (Qiao et al. 2012). Prasad et al. (2012) combined a suitable ratio of Zr to Mg–X binary alloy (X can be Y, Si, Sn, Ca, Sr, Ce, Gd, Nd, La, Mn, and Zn). It was revealed that when Zr reacted with impurity Fe to shape the Fe2Zr precipitation phase, the rate of corrosion was lessened. Applying sputtering deposition, single-phase Mg–Gd, and Mg–Y alloy was profitably prepared, exhibited a close corrosion rate in a 3.5% NaCl solution compared with that of pure Mg (Schlüter et al. 2014). Despite this, the solid solubility of most alloy components in a-Mg is exceptionally low due to the hexagonal-close-packed atomic arrangement of a-Mg (Tang and El-Awady 2014). As a result, the mechanical strength of purified single-phase a-Mg is insufficient, putting it too far away from desirable clinical bone implants. It has been found that the tensile strength is a less than 50 MPa when it comes to the pure Mg produced by cast approach, associated with 80–90 MPa for pure Mg using the extrusion process, while considerably less than that of compact human bone (100–200 MPa) (Li et al. 2014). Another effective approach to improve the corrosion resistance of Mg alloys by altering the microstructure is alloying treatment (Wang et al. 2018b; Yang et al. 2016b). Scholars have developed a series of Mg alloys for biomedical applications. They focus on degradation and biological characteristics via in vivo assays, as obvious in Table 2. One possible approach is to apply alloying components to decrease the second phase in the Mg matrix in terms of volume and size, or to create a desirable second phase, which results in reducing the galvanic corrosion stem from the second phase. As an example, Jia et al. (2015) improved AZ91 alloy with Y. It was established that the volume fraction of the Mg17Al12 phase attenuates, and as a result of that, the corrosion rate reduced. Choi and Kim (2014) discovered that adding 0.02 wt% Ti lowered the volume fraction and size of the Mg17Al12 phase, resulting in improved resistance to corrosion. According to Shuai et al. (2018a, b, c, d), the CaO-induced (Mg, Al)2 Ca phase in AZ61 alloy encircled the grains and served as a barrier to Mg matrix corrosion progression.
The other practicable way is to use alloying element which results in enhancing the structural integrity of oxide or hydroxide on the surface of Mg, forming a defensive surface film that restrains the enhancing of corrosion. For instance, alloying with Ca and Y, revealed that a more stable layer of hydroxide with less chemical reactivity was placed on the Mg matrix surface, provided a more stable alloy within the simulated bodily fluid (Velikokhatnyi and Kumta 2010). A combination of rare-earth elements (La, Nd, and Ce) to Magnesium matrix was stated by Willbold et al. (2015). According to this research, passive-surface film’s ability was increased thanks to the formation of an oxide layer of rare-earth metal on the surface. Furthermore, Al also boosted the formation of a dense-protecting film on the surface of Mg–Al alloy (Atrens et al. 2013). Not only alloying treatment enhanced the resistance, also, improves the mechanical properties, resulted in a large number of systematic and extensive studies during the past decades (Li et al. 2018a). Presently, the doors of clinical surgeries have been opened up towards many bone fix devices made of Mg alloys, like bone nails and plates, which can be an excellent biomedical-related research approach. Unfortunately, developing an Mg bone tissue engineering scaffold is in the initial stage. There exists no investigation on the clinical analysis of Mg-related bone scaffold to our greatest data, apart from some for only in vivo tests. The reality is that a porous-structured Mg scaffold demands more necessities for degradation rate and mechanical strength. A good combination of corrosion resistance and mechanical properties has been applied to the commercial AZ31 and WE43 to generate porous vascular scaffolds (Heiden and Walker 2015), which have succeeded in achieving CE certification (Zhang et al. 2018). Yet, a slightly faster degradation rate in vivo is obtained as compared to the expectation. Besides, recent studies have established that applying Al element causes cytotoxicity to the particular cells naming neuronal (Wang et al. 2017). From this standpoint, Mg–Al alloy cannot be an appropriate alloy for bone repair application. However, Mg alloy for the bone scaffold is still a requirement to design applicable types. From the biocompatibility point of view, the foremost promising alloying elements are mostly concentrated on several nutrient elements of humans, including Ca, Sn, Si, Sr, and Zn, whereas other alloying elements must meet more biocompatibility requirements for any clinical application.
Reinforcement particles
Alternative approach to adjust the degradation level is preparing reinforcement particles to achieve Mg-based MMC (Kumar et al. 2018; Cui et al. 2019; Kowalski et al. 2018; Dezfuli et al. 2017). In this method, the controllability of the corrosion rate of Mg-oriented MMC can be implemented by applying the category and content reinforcement. At present, some bioactive bioceramics, including HAp, TCP (Shao et al. 2016; Deng et al. 2017; Yin et al. 2019) and bioglass (Wan et al. 2016; Huan et al. 2011) are employed as the reinforcement particles in Mg-based MMC. In fact, these bioactive reinforcing particles can function as apatite nuclei, however, lead to a spontaneous formation of apatite on its own. In this scenario, and other Mg-substituted apatites as the obtained corrosion product layer will be able to fill corrosion pits, form a thicker protective layer, and therefore, slow down the deterioration. In Mg-based MMC, graphene oxide (GO), a low-dimensional nanomaterial, was discovered as an alternative reinforcing phase. In previous research, scholars found that GO improved Mg alloy’s mechanical strength while increased degradation due to the acceleration of galvanic corrosion (Selvam et al. 2016; Turan et al. 2017). According to recent research, GO can also boost the corrosion rate of Mg alloy (Shuai et al. 2019b). Surprisingly, GO may be uniformly distributed across grain boundaries, resulting in a honeycomb nanostructure comprising enclosed Mg grains. This nanostructured was considered a key barrier to prevent corrosion growth because of the superior anti-permeability of GO. GO was also able to reinforce the layer of corrosion by restraining the detachment of corroded Mg grains. In Mg-based MMC, (Yang et al. 2020c) offered mesoporous silica (MS) as a reinforcing particle (Yang et al. 2018a). MS has potential in bone tissue engineering due to its unique pore structure, high specific surface area, and pore volume (Wang et al. 2015). It is worth noting that in Mg matrix, reinforcements are more likely to stick together, especially for nano-size particles with high surface energy (Xiong et al. 2016; Huang et al. 2015; Yan et al. 2017). This aggregation can be evaluated by the physical differences between Mg matrix and reinforcement. The majority of the reinforcement particles will be driven by the growth front of -Mg grains throughout the solidification. In addition, more attention to the interfacial problem between -Mg matrix and reinforcement particles is a requirement. Generally, the coefficients of expansion for Mg metals and reinforcement particles are changeable. Relevant scholars have suggested applying in situ synthesised MgO nanoparticles to improve the interfacial adhesion between the magnesium matrix and the GO (Yuan et al. 2018).
Approaches for producing Mg bone implants
It is crystal clear that the fabrication method directly affects the inherent characteristics of products including degradation rate, biological performance and mechanical properties. So far, several types of fabrication techniques including traditional methods such as casting, wrought, powder metallurgy, and modern technique including laser additive manufacturing have been introduced.
Traditional method
Casting is known as a common approach for the fabrication of Mg bone implants. Low spending, convenient control of the composition of alloy as well as user-friendly operation are the main advantages of casting. The casting process is summarised in heating the metal components more than the melting point, pouring the liquid phase into the designed mould, and natural solidification. Since Mg is sensitive to oxygen and easily oxides, a protective atmosphere such as Ar and should be used during the casting. Currently, biomedical Mg alloys are mostly fabricated by casting as it is convenient to regulate the alloy elements. For example, Ag and Cu can be added into Mg matrix to improve its antibacterial features (Liu et al. 2016a, b; Tie et al. 2013), or Sr can be added to enhance the osteogenic characteristics (Gu et al. 2012). Bone fixation devices mostly demand nail shaped and flat shaped while bone scaffold requires porous structure. Considering this, casting fails to direct fabrication of net shape, however, it is a proper way to produce original material for the processes of forming in which parts can be fabricated with favourable structure and shape (Ali et al. 2016).
Wrought techniques
Another technique for achieving the desired shape parts is wrought techniques which focus on applying a mechanical force to convert the bulk metal into a favourable shape. This method is based on two approaches: cold working and hot working depends on the recrystallisation temperature. When it comes to the manufacturing of Mg bone implants, rolling, extrusion and forging are the commonly used wrought methods. Forging and hot rolling are normally utilised to achieve a profile in a flat shape. Cao et al. (2015) investigated the hot rolling of binary Mg alloy. According to this research, hot rolling homogenises the microstructure with refined grains and reduced the second phase. The Mg–Zn–Zr–Gd alloy was also fabricated by Yao et al. (2018). It was achieved that sample could meet the anticorrosive and mechanical requirements as a bone implant.
Powder metallurgy approach
As for the powder metallurgy technique, it is based on the pressure of the original powder into the desired shape. While sintering, the particles of powder tolerate a series of chemical and physical processes such as fusion welding, dislocation and combination, diffusion and recrystallisation leading to sintering densification (Yang et al. 2020a, b). Mg-based composite can be straight ready by applying the powder metallurgy method. The porous scaffold can also be fabricated utilising a pore-forming agent via powder metallurgy strategy. Obtaining the interconnected porous structure is a challenge in this technique. To achieve a near-net shape and complex implants, metal injection moulding is a typical method. The nearly dense part was obtained by Wolff et al. (2016). They fabricated Mg–Ca alloy by metal injection moulding. Another advanced powder metallurgy approach to produce degradable Mg-based composites is spark plasma sintering (Dutta et al. 2018; Narita et al. 2019; Karasoglu et al. 2018). Self-heating is the nature of this method which affects inside the powder for sintering. The quick rate of heating and consequently short time for sintering results in decreasing the grain growth.
Additive manufacturing
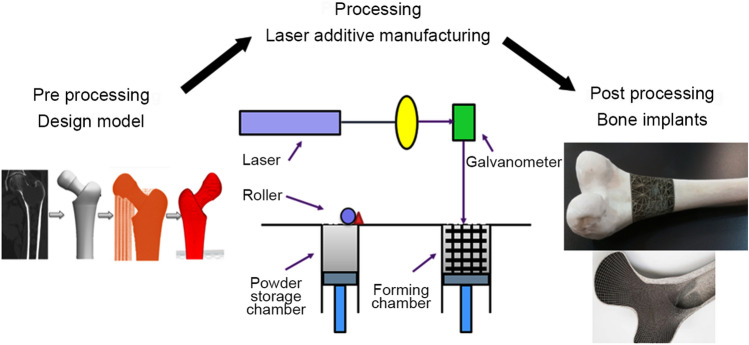
Integrating computer-aided design (CAD), computer numerical control processing, and laser processing lead to the emergence of laser additive manufacturing (LAM). As it is obvious from Fig. 6, a typical LAM consists of a galvanometer for scanning, a laser, a forming cylinder and a computer as a control system. LAM process is divided into few sections as follows: (a) designed a 3D model in the CAD software; (b) obtaining the STL file from the 3D model to gain the slice data of model; (c) placing the layer on the forming cylinder; (d) scanning the powder layer by controlling the laser beam for heating and melting and solidification to achieve a single layer. Laser additive manufacturing (LAM) brings us a quite number of advantages including rapid fabrication, net-shape fabrication, fabrication of complex porous structures, superior process flexibility, freedom of design, using a broad range of materials including ceramics, composites, polymers, and metals and their alloys. In the last 2 years, a few investigations have been done regarding the LAM of Mg scaffolds. Figure 7 indicates several samples fabricated using laser additive manufacturing. For instance, a diamond unit-cell porous Mg scaffold fabricated by Li et al. indicated proper mechanical strength particularly Young’s modulus and favourable degradation rates of 20% in 4 weeks (Li et al. 2018b). Another research in the field of laser additive manufacturing of Mg alloy has been done by Kopp et al. (2019). They concentrated on the WE43 alloy scaffold with various pore sizes. In this case, a small pore size scaffold exhibited a low rate of oxygen and small decreasing of mechanical properties over time. Interestingly, LAM has accurate control of porous structure as compared with other methods. Integrating CAD and computed tomography systems, patient-specific bone implants for various defective sites can be easily fabricated.
Fig. 6.
Schematic LAM process for manufacturing of bone implant
Fig. 7.
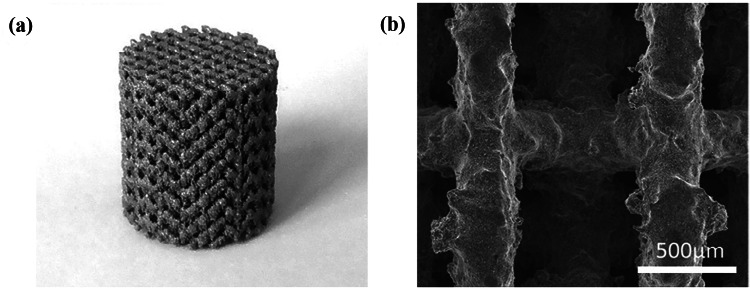
a As-printed WE43 scaffold and b surface morphology of as-polished strut (Li et al. 2018a)
LAM also experiences a quick melting and solidification results in refining the microstructure. The rapid cooling rate can be a hinder to the way of grain growth. According to the research done by Florian Bär et al. (2019), it was found that heat impacts at various places of the molten pool play a vital role. In addition, laser additive manufacturing fabricates a smaller grain size compared to the casting. It is worth mentioning that fine-grain size which is considered as fine-grain strengthening is beneficial to improve the mechanical properties of Mg products. There has been various research on the relationship between grain size and corrosion. Some scholars proposed that grain boundary can act as a corrosion hinder while others concluded that grain boundary can act as crystallographic flaws and resultantly rises Mg corrosion (Zhang et al. 2011b). Considering the investigation on the LAM of Mg bone implants, it can be stated that it is far from mature in comparison with other biometals such as Fe alloy and Ti alloy. The reason goes back to the physical and chemical properties of Mg including a melting point of 650 °C near to the boiling point (1091 °C) which is a huge challenge on the way of processing laser forming. Due to some features of Mg including low boiling point, low surface tension, high vapour pressure, and low density, Mg metal can easily burn out within the laser processing (Wen et al. 2018). More importantly, Mg is more likely to oxides due to its inherent chemical properties leading to low forming quality. Hence, there must be a low-content oxygen protective layer during the laser processing of Mg and its alloys. The fact is LAM of Mg bone implants is still in its infancy while that of Ti and Fe, indicates good formability when it comes to clinical application.
AM of reactive materials, notably magnesium, has piqued researchers’ interest in recent years, and technology is being developed to reduce the challenges involved with 3D printing. Due to its strong reactivity, magnesium is a challenging metal to 3D print. Magnesium, in its pure state, oxidises uncontrolled and must be kept in a way that precludes oxygen exposure. Powder, liquid resin, and wire types of raw materials are accessible for AM (Qin et al. 2019). The surface energy of the metal increases in this condition, increasing the chance of it interacting with ambient oxygen to permit burning. As a result of these dangers, there has been insufficient investigation into production procedures for magnesium as a possible biodegradable alloy. It will need specialised equipment capable of printing magnesium in an inert atmosphere while also assuring safe material handling. AM of Mg alloys is gaining popularity in the community because to its ability to enable design capabilities not possible with traditional production and its promise for the creation of biodegradable implants. Powder bed fusion (Chung Ng et al. 2011), wire arc AM paste extrusion deposition, friction stir AM, and jetting methods have all been used to show additive manufacturing of magnesium (Guo et al. 2019).
These procedures differ in terms of process mechanics and raw material types. Each technique produces AM components with varying structural characteristics. AM may be utilised to create exceedingly complicated geometries that would be difficult or impossible to create using traditional machining procedures by producing components in this manner. AM allows for more personalised implants that are more precisely aligned with anatomical geometries. Furthermore, AM decreases the production time and cost of implants by eliminating many steps of traditional machining and allowing batch processing (Farag and Yun 2014). The capacity to create complicated internal and exterior geometries utilising AM allows for the creation of geometrical elements that encourage cell growth, proliferation, and bone regeneration. Scaffolds of WE43, a magnesium alloy containing yttrium and rare-earth metals, printed with holes as tiny as 600 µm showed less than 25% toxicity in vitro and retained structural stiffness for 4 weeks. Furthermore, porous depositions may be produced through AM, which may operate as favourable locations for tissue adhesion, hence speeding up the healing process. Porosity may be adjusted across a 3D build by adjusting print process parameters, which have a direct impact on corrosion rates and cell behaviour (Farag and Yun 2014; Palanivel et al. 2015).
Due to the high surface energy of the powder and high electronegativity of the alloy, which drives the quick corrosion rate within the human body, magnesium is a tough material to print biodegradable implants. Multiple techniques in AM, on the other hand, are progressively overcoming these problems. The process parameters of attempts to print Mg utilising PBF, WAAM, paste extrusion deposition, FSAM, and jetting methods have been detailed. Due to the low heat flux and intricate internal and exterior geometries afforded by this technique, powder bed fusion is the most commonly explored approach for printing magnesium alloys. Parts with a density of 96.13% have been attained depending on the type of magnesium alloy utilised. In magnesium AM, creating near-fully dense structures with a density of more than 99% remains a major difficulty. The following table summarises the different elements that impact the AM processes mentioned in this review (Salehi et al. 2019). Table 9 categorises the effects of parameters on the AM process.
Table 9.
Effects of parameters on the AM process
| AM process | Parameters | References | |
|---|---|---|---|
| Powder bed fusion | Laser power and scanning speed | A wide range of laser power and scanning speeds can be deemed as optimal; however, a low laser energy density between 50 and 200 J/mm3 is critical. High energy density leads to element vaporisation while low energy density leads to insufficient melting of Mg powder. Energy density is dependent of alloy composition | Chung Ng et al. (2011) |
| Layer thickness | Layer thickness above 250 μm for pure Mg prevented complete fusion and resulted in higher porosity | ||
| Powder size | Magnesium powder of 50 μm for pure Mg was seen to yield better depositions compared to smaller or larger powders. Particles that are too small result in higher rates of vaporisation and particles that are too big do not achieve a full melt | Chung Ng et al. (2011) | |
| Build envelop | The vaporisation temperature of magnesium increases with higher chamber pressures | ||
| Conditions | This facilitates higher operation temperatures for printing Mg. However, safety risks increase by this approach. In addition, preheating of the work table before printing leads to smoother depositions | ||
| Wire arc additive manufacturing | Deposition speed and feed | Higher speeds and feeds of deposition resulted in more refined, smaller grains | uo et al. (2019) |
| Arc frequency | Small refined grains were observed at arcing frequency of 5–10 Hz for TIG WAAM. Grain size was found to increase above and below this frequency range | ||
| Paste extrusion deposition | Extrusion temperature | Flowability of paste was found to increase at higher temperature during extrusion | Farag and Yun (2014) |
| Paste composition | Higher quantities of gelatin in MgP–gelatin mixture resulted in stronger manufactured samples. However, the strength was still much lesser than other sintering or fusion-based AM processes | ||
| Friction stir additive manufacturing | Tool rotational speed | Higher tool force and speeds resulted in higher cladding temperatures, which led to higher porosity in the components | Palanivel et al. (2015) |
| Tool force | Higher tool force increases temperature due to friction, and thus, residual stresses in components increases | ||
| Jetting technologies for additive manufacturing | Binder jetting |
100% recyclability of powder Binding agent must be chosen carefully by considering its reactivity with powder |
Salehi et al. (2019) |
| Binder-less jetting | Prevents contamination due to absence of binding agent |
Prospect and future trends
Improving functional Mg bone implants
Bacterial infection during orthopaedic treatment results in failed bone repair and intaking of any antibiotics for a long-term period can lead to bacterial resistance in the body of patients (Shuai et al. 2019c). That is why improving the anti-biological Mg implant for bone repair is really important. As Ag and Cu are well known for their strong bactericidal activities (Rtimi et al. 2019). Some scholars applied alloying elements such as Ag and Cu to suppress bacterial infection of Mg alloy. According to the results, Ag and Cu were able to release metal ions that kill bacteria. In contrast, an ongoing investigation has indicated that small amounts of these elements into the Mg matrix cause increased degradation. The reason is that precipitates accelerate the effect of galvanic corrosion (Shuai et al. 2018c). However, more research still needs to be implemented to obtain a balanced level of biodegradability and antimicrobials. Yuan et al. (2019) achieved the rolling and annealing temperature to address the adverse effect of precipitates in Mg–Ag alloy. As stated before, the homogenous distribution of Ag was beneficial for the degradation behaviour. Recent researches indicated the improvement of anti-cancer Mg bone implants (Shuai et al. 2018b). According to this research, Mg–La alloy did not affect typical osteoblasts and controlled the growth of tumour cells. No one can deny the impact of toxicity problems, which resulted from alloying treatment. Hence, more detailed in vivo tests should be done concerning the biocompatibility of functional bone implants. One of the future perspectives of Mg bone implants is a drug-released system. Generally, directly incorporated drugs such as osteoinductive factors into the Magnesium-based implants is a challenge. It can be located into the surface coating, PLLA coating, for instance.
Improving the mechanical properties of Mg alloys
The durability of an implant plays a pivotal role in the injured tissue after implantation to provide structural support. From a mechanical perspective, the mechanical strength of Mg bone implants still requires to be more improvement in various applications. Porous scaffolds can be exemplified for load-bearing applications. Furthermore, this gradual degradation of Mg bone implant in vivo is conducive to rapid mechanical strength loss. Technically speaking, chemical composition, process technology, and grain size are determinant parameters for mechanical strength, including yield strength and ultimate tensile strength (Sezer et al. 2018). Alloying is considered the main approach to improve the mechanical properties of Mg alloy. Alloying with a hexagonal system such as Zn, Sr, and Al can lead to solid solution strengthening of Mg (Ibrahim et al. 2017). Grain refinement is an alternative approach to boost the strength and toughness of Mg alloy, as a boundary with high grain exhibits a remarkable blocking impact on dislocation slip (Jayalakshmi et al. 2018). A deformation process based on external force and rapid solidification technology has been offered for grain refinement of Mg alloys (Drozdenko et al. 2016, 2019; Shalbafi et al. 2017; Lu et al. 2015) while rapid solidification technology is more active than the deformation process. The finer and more homogenous microstructure can be produced by a fast cooling rate regarding cooling rate. As a result, fewer impurities and residues can lead to a less micro-galvanic effect. Recently, melt spinning technology was used for EW62 to obtain its fast solidification, which possessed high resistance to stress corrosion cracking. After that, the consolidated EW62 ribbons were achieved, applying an extrusion process to obtain bars. It is worth mentioning that tubes and plates for repairing biological bone can be produced applying the same extrusion method.
Focus on the dynamic degradation of Mg bone implants
Most of the current researches regarding the Mg-based bone implants are focussed on material design and manufacturing method while much less attention has been paid to the dynamic effect of degradation on mechanical and morphological properties of the bone implant. Apparently, during the degradation of Mg bone implants, any large physical changes may result in different structural features, such as elastic modulus and mechanical strength associated with biological features, such as osteopromotive performance. Due to the difficulty of realistic measurement of Mg implants local features in vivo, simulation of thermochemical mechanical coupling behaviours has drawn scholar’s attention. Grogan et al. (2014) introduced a physio-corrosion model to seize surface changing of a corroding Mg scaffold structure with the aim of predicting device geometry and mechanical efficiency within corrosion. Therefore, dynamics and evolution of the environment, including corrosion products and pH variation, were neglected. A mathematical model for suitable geometry of implants was designed by Bajger et al. (2016). Considering this model, the corrosion products precipitation on the Mg surface and effect on the degradation rate was successfully proposed. Sanz-Herrera et al. (2018) have recently improved a phenomenological approach to simulate Mg corrosion behaviour and explained dynamically physio-chemical interactions. According to the results, the model was able to describe the main variables affecting Mg corrosion. Moreover, a computational method using a dynamic immersion test was revealed by Md Saad et al. (2019) to evaluate the efficacy of morphological changes on the structural features of biodegradable porous Mg. More research should be done in the future; modelling Mg corrosion in more detail to indicate the various physio-chemical characteristics and comprehensive experimental analysis to discover more about Mg corrosion should be investigated. This can result in a better understanding of the design and processes of Mg bone implants.
Modern fabrication of Mg bone implants
Additive manufacturing (AM)g of Mg bone implants should be improved base on the high demand for patient-specific bone implants. Fine and homogenous micro-structured Mg parts can be generated by laser additive manufacturing (LAM), and better mechanical properties and biodegradability is expected. Laser additive manufactured Mg parts possess less formability than other metals, including Fe alloy, Ti alloy and Co alloy. LAM of Mg parts is suffering from several defects, including surface balling, internal pores, and element burning (Liu et al. 2017; Yang et al. 2016a, b). One required approach is a deep understanding of processing factors affecting the mechanism of formability to address this issue. For example, the interaction between the laser beam and magnesium, the processing stability, and the controllability of residual stress will undoubtedly positively impact. Hence, the system for Mg-based material in the LAM process should be developed. The shape of the original Mg alloy powder applied in LAM should be spherical together with a uniform distribution of particle size to ensure its good fluidity (Zhang et al. 2011a). At present, WE43, AZ61 and ZK60 have been employed in LAM. As biological reactions of the current commercial Mg alloys in vivo have not been confirmed, they may not be considered as the best biological bone implants option.
With the advent of hybrid materials, the benefits of some materials can be integrated, which brings great potential for bone implants (Gao et al. 2019a, b). According to a few previous research types, the technology of laser additive manufacturing can process MMC materials (Gao et al. 2019c). The incorporation of high-melting-point ceramics should reduce the formability. However, the high performance of laser additive manufacturing of Mg-based MMC is also considered an inevitable challenge. Focussing on the LAM of bone implant, the structure-oriented design of scaffold should be considered. Appropriate design of porous structure for mimicking human bone is of the essence. Scholars have done a large number of researches on designing a porous structure for non-degradable bone implants. The role of stress distribution, permeability, and biological properties under various pore structures was comprehensively reported (Ali and Sen 2017, 2018; Montazerian et al. 2017) while no researchers have paid attention to the structural design of degradable Mg. Due to the dynamic degradation, mechanical and biological properties of bone implants are various. Hence, the efficacy of different pore structures of Mg scaffold on mechanical properties, biological properties, and degradation rate is a necessity. Various methods, including reverse modelling, mathematical modelling, and CAD design, have been used for designing porous structures (Yousefi et al. 2014). A combination of these methods may meet the requirements of scaffold design for bone healing.
Conclusion
The inherent characteristics of Mg and its alloys for biomedical implants alloys including desirable mechanical properties, biodegradability, biocompatibility and osteopromotive characteristics have been reviewed. Currently, bone tissue engineering scaffold and bone-related fixation devices are the main focus of Mg applications even considering the high corrosion rate rather than our expectation. The existing approaches for reducing the degradation rate of Mg bone implants have been considered as alloying treatment, purification, Mg-based MMC and surface coating. Practically, various approaches can be combined to optimise the corrosion rate of Mg alloy. Various fabrication techniques for Mg bone implants have also been reviewed. Laser additive manufacturing can provide rapid production of complex and shape-customised porous structures in comparison with traditional methods. More importantly, homogenised microstructure which is a favourable feature for improving degradation behaviour and mechanical properties can be achieved due to the fast solidification characteristic of Mg alloys. To achieve the clinical Mg bone implants, more interdisciplinary investigation including designing materials and manufacturing processes are urgently needed in the near future.
Funding
The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Human/animal rights
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet-derived growth factor. Am J Physiol Cell Physiol. 2009;297(2):C360–C368. doi: 10.1152/ajpcell.00614.2008. [DOI] [PubMed] [Google Scholar]
- Acar B, Unal M, Turan A, Kose O. Isolated lateral malleolar fracture treated with a bioabsorbable magnesium compression screw. Cureus. 2018 doi: 10.7759/cureus.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaneme KK, Okotete EA. Enhancing plastic deformability of Mg and its alloys—a review of traditional and nascent developments. J Magnes Alloys. 2017;5(4):460–475. doi: 10.1016/j.jma.2017.11.001. [DOI] [Google Scholar]
- Ali D, Sen S. Finite element analysis of mechanical behavior, permeability and fluid induced wall shear stress of high porosity scaffolds with gyroid and lattice-based architectures. J Mech Behav Biomed Mater. 2017;75:262–270. doi: 10.1016/j.jmbbm.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Ali D, Sen S. Computational fluid dynamics study of the effects of surface roughness on permeability and fluid flow-induced wall shear stress in scaffolds. Ann Biomed Eng. 2018;46(12):2023–2035. doi: 10.1007/s10439-018-2101-z. [DOI] [PubMed] [Google Scholar]
- Ali Y, Qiu D, Jiang B, Pan F, Zhang MX. The influence of CaO addition on grain refinement of cast magnesium alloys. Scr Mater. 2016;114:103–107. doi: 10.1016/j.scriptamat.2015.12.015. [DOI] [Google Scholar]
- Atkins GJ, Welldon KJ, Halbout P, Findlay DM. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos Int. 2008;20(4):653–664. doi: 10.1007/s00198-008-0728-6. [DOI] [PubMed] [Google Scholar]
- Atrens A, Song GL, Cao F, Shi Z, Bowen PK. Advances in Mg corrosion and research suggestions. J Magnes Alloys. 2013;1(3):177–200. doi: 10.1016/j.jma.2013.09.003. [DOI] [Google Scholar]
- Atrens A, Johnston S, Shi Z, Dargusch MS. Viewpoint—understanding Mg corrosion in the body for biodegradable medical implants. Scrip Mat. 2018;154:92–100. doi: 10.1016/j.scriptamat.2018.05.021. [DOI] [Google Scholar]
- Bairagi D, Mandal S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: current status, challenges, and future prospects. J Magnes Alloys. 2021 doi: 10.1016/j.jma.2021.09.005. [DOI] [Google Scholar]
- Bajger P, Ashbourn JMA, Manhas V, Guyot Y, Lietaert K, Geris L. Mathematical modelling of the degradation behaviour of biodegradable metals. Biomech Model Mechanobiol. 2016;16(1):227–238. doi: 10.1007/s10237-016-0812-3. [DOI] [PubMed] [Google Scholar]
- Bakhsheshi-Rad H, Hamzah E, Abdul-Kadir M, Daroonparvar M, Medraj M. Corrosion and mechanical performance of double-layered nano-Al/PCL coating on Mg–Ca–Bi alloy. Vacuum. 2015;119:95–98. doi: 10.1016/j.vacuum.2015.04.039. [DOI] [Google Scholar]
- Bär F, Berger L, Jauer L, Kurtuldu G, Schäublin R, Schleifenbaum JH, Löffler JF. Laser additive manufacturing of biodegradable magnesium alloy WE43: a detailed microstructure analysis. Act Biomater. 2019;98:36–49. doi: 10.1016/j.actbio.2019.05.056. [DOI] [PubMed] [Google Scholar]
- Ben-Hamu G, Eliezer D, Shin K. The role of Mg2Si on the corrosion behavior of wrought Mg–Zn–Mn alloy. Intermetallics. 2008;16(7):860–867. doi: 10.1016/j.intermet.2008.03.003. [DOI] [Google Scholar]
- Boukhobza A, Brioua M, Benaicha S, Fedaoui K. Biomechanical characterization of failure the 316L stainless steel for femoral compression plates. J Biomim Biomater Biomed Eng. 2017;34:68–74. doi: 10.4028/www.scientific.net/jbbbe.34.68. [DOI] [Google Scholar]
- Bowen PK, Drelich A, Drelich J, Goldman J. Rates of in vivo(arterial) and in vitro biocorrosion for pure magnesium. J Biomed Mater Res Part A. 2014;103(1):341–349. doi: 10.1002/jbm.a.35179. [DOI] [PubMed] [Google Scholar]
- Bowen PK, Shearier ER, Zhao S, Guillory RJ, Zhao F, Goldman J, Drelich JW. Biodegradable metals for cardiovascular stents: from clinical concerns to recent Zn-Alloys. Adv Healthc Mater. 2016;5(10):1121–1140. doi: 10.1002/adhm.201501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt MS, Bai J, Wan X, Chu C, Xue F, Ding H, Zhou G. Mechanical and degradation properties of biodegradable Mg strengthened poly-lactic acid composite through plastic injection molding. Mater Sci Eng C. 2017;70:141–147. doi: 10.1016/j.msec.2016.08.051. [DOI] [PubMed] [Google Scholar]
- Cao F, Shi Z, Song GL, Liu M, Dargusch MS, Atrens A. Influence of hot rolling on the corrosion behavior of several Mg–X alloys. Corr Sci. 2015;90:176–191. doi: 10.1016/j.corsci.2014.10.012. [DOI] [Google Scholar]
- Chandra G, Pandey A. Preparation strategies for mg-alloys for biodegradable orthopaedic implants and other biomedical applications: a review. IRBM. 2020 doi: 10.1016/j.irbm.2020.06.003. [DOI] [Google Scholar]
- Chang L, Tian L, Liu W, Duan X. Formation of dicalcium phosphate dihydrate on magnesium alloy by micro-arc oxidation coupled with hydrothermal treatment. Corr Sci. 2013;72:118–124. doi: 10.1016/j.corsci.2013.03.017. [DOI] [Google Scholar]
- Chaya A, Yoshizawa S, Verdelis K, Myers N, Costello BJ, Chou DT, Pal S, Maiti S, Kumta PN, Sfeir C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Act Biomater. 2015;18:262–269. doi: 10.1016/j.actbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Chen Y, Song Y, Zhang S, Li J, Zhao C, Zhang X. Interaction between a high purity magnesium surface and PCL and PLA coatings during dynamic degradation. Biomed Mater. 2011;6(2):025005. doi: 10.1088/1748-6041/6/2/025005. [DOI] [PubMed] [Google Scholar]
- Chen LY, Xu JQ, Choi H, Pozuelo M, Ma X, Bhowmick S, Yang JM, Mathaudhu S, Li XC. Processing and properties of magnesium containing a dense uniform dispersion of nanoparticles. Nature. 2015;528(7583):539–543. doi: 10.1038/nature16445. [DOI] [PubMed] [Google Scholar]
- Chen J, Tan L, Yu X, Etim IP, Ibrahim M, Yang K. Mechanical properties of magnesium alloys for medical application: a review. J Mech Behav Biomed Mater. 2018;87:68–79. doi: 10.1016/j.jmbbm.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Choi J, Kim W. Significant effects of adding trace amounts of Ti on the microstructure and corrosion properties of Mg–6Al–1Zn magnesium alloy. J Alloys Compd. 2014;614:49–55. doi: 10.1016/j.jallcom.2014.06.052. [DOI] [Google Scholar]
- Chung Ng C, Savalani M, Chung Man H. Fabrication of magnesium using selective laser melting technique. Rap Prototyp J. 2011;17(6):479–490. doi: 10.1108/13552541111184206. [DOI] [Google Scholar]
- Cui LY, Li XT, Zeng RC, Li SQ, Han EH, Song L. In vitro corrosion of Mg–Ca alloy—the influence of glucose content. Front Mater Sci. 2017;11(3):284–295. doi: 10.1007/s11706-017-0391-y. [DOI] [Google Scholar]
- Cui Z, Li W, Cheng L, Gong D, Cheng W, Wang W. Effect of nano-HA content on the mechanical properties, degradation and biocompatible behavior of Mg–Zn/HA composite prepared by spark plasma sintering. Mater Charact. 2019;151:620–631. doi: 10.1016/j.matchar.2019.03.048. [DOI] [Google Scholar]
- Dambatta M, Kurniawan D, Izman S, Yahaya B, Hermawan H. Review on Zn-Based alloys as potential biodegradable medical devices materials. Appl Mech Mater. 2015;776:277–281. doi: 10.4028/www.scientific.net/amm.776.277. [DOI] [Google Scholar]
- de Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: Implications for health and disease. Physiol Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- Deng Y, Yang Y, Gao C, Feng P, Guo W, He C, Chen J, Shuai C. Mechanism for corrosion protection of β-TCP reinforced ZK60 via laser rapid solidification. Int J Bioprint. 2017 doi: 10.18063/ijb.v4i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfuli SN, Leeflang S, Huan Z, Chang J, Zhou J. Fabrication of novel magnesium-matrix composites and their mechanical properties prior to and during in vitro degradation. J Mech Behav Biomed. 2017;67:74–86. doi: 10.1016/j.jmbbm.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Drozdenko D, Bohlen J, Yi S, Minárik P, Chmelík F, Dobroň P. Investigating a twinning–detwinning process in wrought Mg alloys by the acoustic emission technique. Act Mater. 2016;110:103–113. doi: 10.1016/j.actamat.2016.03.013. [DOI] [Google Scholar]
- Drozdenko D, Yamasaki M, Máthis K, Dobroň P, Lukáč P, Kizu N, Inoue SI, Kawamura Y. Optimization of mechanical properties of dilute Mg–Zn–Y alloys prepared by rapid solidification. Mater Des. 2019;181:107984. doi: 10.1016/j.matdes.2019.107984. [DOI] [Google Scholar]
- Duan Y, Sun Y, He J, Guo Z, Fang D. Corrosion behavior of As-Cast Pb–Mg–Al alloys in 3.5% NaCl solution. Corrosion. 2012;68(9):822–826. doi: 10.5006/0570. [DOI] [Google Scholar]
- Dutta S, Devi KB, Gupta S, Kundu B, Balla VK, Roy M. Mechanical and in vitro degradation behavior of magnesium-bioactive glass composites prepared by SPS for biomedical applications. J Biomed Mater Res Part B Appl Biomater. 2018;107(2):352–365. doi: 10.1002/jbm.b.34127. [DOI] [PubMed] [Google Scholar]
- Ezechieli M, Ettinger M, König C, Weizbauer A, Helmecke P, Schavan R, Lucas A, Windhagen H, Becher C. Biomechanical characteristics of bioabsorbable magnesium-based (MgYREZr-alloy) interference screws with different threads. Knee Surg Sports Traumatol Arthrosc. 2014;24(12):3976–3981. doi: 10.1007/s00167-014-3325-6. [DOI] [PubMed] [Google Scholar]
- Farag M, Yun HS. Effect of gelatin addition on fabrication of magnesium phosphate-based scaffolds prepared by additive manufacturing system. Mater Lett. 2014;132:111–115. doi: 10.1016/j.matlet.2014.06.055. [DOI] [Google Scholar]
- Festas A, Ramos A, Davim J. Medical devices biomaterials—a review. Proc Inst Mech Eng Part L J Mater Des Appl. 2019;234(1):218–228. doi: 10.1177/1464420719882458. [DOI] [Google Scholar]
- Filippi M, Born G, Chaaban M, Scherberich A. Natural polymeric scaffolds in bone regeneration. Front Bioeng Biotechnol. 2020 doi: 10.3389/fbioe.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Yao M, Li S, Feng P, Peng S, Shuai C. Highly biodegradable and bioactive Fe–Pd-bredigite biocomposites prepared by selective laser melting. J Adv Res. 2019;20:91–104. doi: 10.1016/j.jare.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Yao M, Shuai C, Peng S, Deng Y. Nano-SiC reinforced Zn biocomposites prepared via laser melting: microstructure, mechanical properties and biodegradability. J Mater Sci Technol. 2019;35(11):2608–2617. doi: 10.1016/j.jmst.2019.06.010. [DOI] [Google Scholar]
- Gao X, Lin X, Yu J, Li Y, Hu Y, Fan W, Shi S, Huang W. Selective laser melting (SLM) of in-situ beta phase reinforced Ti/Zr-based bulk metallic glass matrix composite. Scr Mater. 2019;171:21–25. doi: 10.1016/j.scriptamat.2019.06.007. [DOI] [Google Scholar]
- Gil-Santos A, Marco I, Moelans N, Hort N, van der Biest O. Microstructure and degradation performance of biodegradable Mg–Si–Sr implant alloys. Mater Sci Eng C. 2017;71:25–34. doi: 10.1016/j.msec.2016.09.056. [DOI] [PubMed] [Google Scholar]
- Grogan J, Leen S, McHugh P. A physical corrosion model for bioabsorbable metal stents. Act Biomater. 2014;10(5):2313–2322. doi: 10.1016/j.actbio.2013.12.059. [DOI] [PubMed] [Google Scholar]
- Gu X, Xie X, Li N, Zheng Y, Qin L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Act Biomater. 2012;8(6):2360–2374. doi: 10.1016/j.actbio.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Guo Y, Pan H, Ren L, Quan G. Microstructure and mechanical properties of wire arc additively manufactured AZ80M magnesium alloy. Mater Lett. 2019;247:4–6. doi: 10.1016/j.matlet.2019.03.063. [DOI] [Google Scholar]
- Gupta P, Adhikary M, Kumar M, Bhardwaj N, Mandal BB. Biomimetic, osteoconductive non-mulberry silk fiber reinforced tricomposite scaffolds for bone tissue engineering. ACS Appl Mater Interfaces. 2016;8(45):30797–30810. doi: 10.1021/acsami.6b11366. [DOI] [PubMed] [Google Scholar]
- Han P, Cheng P, Zhang S, Zhao C, Ni J, Zhang Y, Zhong W, Hou P, Zhang X, Zheng Y, Chai Y. In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt%) screw with femoral intracondylar fractured rabbit model. Biomaterials. 2015;64:57–69. doi: 10.1016/j.biomaterials.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Hao Z, Song Z, Huang J, Huang K, Panetta A, Gu Z, Wu J. The scaffold microenvironment for stem cell based bone tissue engineering. Biomater Sci. 2017;5(8):1382–1392. doi: 10.1039/c7bm00146k. [DOI] [PubMed] [Google Scholar]
- Heiden M, Walker E. Magnesium, iron and zinc alloys, the trifecta of bioresorbable orthopaedic and vascular implantation—a review. J Biotechnol Biomater. 2015 doi: 10.4172/2155-952x.1000178. [DOI] [Google Scholar]
- Hiromoto S, Inoue M, Taguchi T, Yamane M, Ohtsu N. In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Act Biomater. 2015;11:520–530. doi: 10.1016/j.actbio.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Huan Z, Leeflang S, Zhou J, Zhai W, Chang J, Duszczyk J. In vitro degradation behavior and bioactivity of magnesium-Bioglass® composites for orthopedic applications. J Biomed Mater Res Part B Appl Biomater. 2011;100B(2):437–446. doi: 10.1002/jbm.b.31968. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu D, Anguilano L, You C, Chen M. Fabrication and characterization of a biodegradable Mg–2Zn–0.5Ca/1β-TCP composite. Mater Sci Eng C. 2015;54:120–132. doi: 10.1016/j.msec.2015.05.035. [DOI] [PubMed] [Google Scholar]
- Ibrahim H, Esfahani SN, Poorganji B, Dean D, Elahinia M. Resorbable bone fixation alloys, forming, and post-fabrication treatments. Mater Sci Eng C. 2017;70:870–888. doi: 10.1016/j.msec.2016.09.069. [DOI] [PubMed] [Google Scholar]
- Jayalakshmi S, Arvind Singh R, Sankaranarayanan S, Shabadi R, Konovalov S, Chen X, Gupta M. Structure-property correlation in magnesium nanocomposites synthesized by disintegrated melt deposition technique. Mater Today Proc. 2018;5(8):16280–16285. doi: 10.1016/j.matpr.2018.05.120. [DOI] [Google Scholar]
- Jia R, Zhang M, Zhang L, Zhang W, Guo F. Correlative change of corrosion behavior with the microstructure of AZ91 mg alloy modified with y additions. J Alloys Compd. 2015;634:263–271. doi: 10.1016/j.jallcom.2015.02.019. [DOI] [Google Scholar]
- Jung JY, Kwon SJ, Han HS, Lee JY, Ahn JP, Yang SJ, Cho SY, Cha PR, Kim YC, Seok HK. In vivo corrosion mechanism by elemental interdiffusion of biodegradable Mg–Ca alloy. J Biomed Mater Res Part B Appl Biomater. 2012;100B(8):2251–2260. doi: 10.1002/jbm.b.32795. [DOI] [PubMed] [Google Scholar]
- Karasoglu M, Karaoglu S, Arslan G. Mechanical properties of Mg-based materials fabricated by mechanical milling and spark plasma sintering. Proc Inst Mech Eng Part L J Mater Des Appl. 2018;233(10):1972–1984. doi: 10.1177/1464420718805119. [DOI] [Google Scholar]
- Khayat S. Minerals in pregnancy and lactation: a review article. J Clin Diagn Res. 2017 doi: 10.7860/jcdr/2017/28485.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A, Derra T, Müther M, Jauer L, Schleifenbaum JH, Voshage M, Jung O, Smeets R, Kröger N. Influence of design and postprocessing parameters on the degradation behavior and mechanical properties of additively manufactured magnesium scaffolds. Act Biomater. 2019;98:23–35. doi: 10.1016/j.actbio.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Kowalski K, Jurczyk MU, Wirstlein PK, Jakubowicz J, Jurczyk M. Properties of ultrafine-grained Mg-based composites modified by addition of silver and hydroxyapatite. Mater Sci Technol. 2018;34(9):1096–1103. doi: 10.1080/02670836.2018.1424381. [DOI] [Google Scholar]
- Kumar AM, Hassan SF, Sorour AA, Paramsothy M, Gupta M. Investigation on the controlled degradation and in vitro mineralization of carbon nanotube reinforced AZ31 nanocomposite in simulated body fluid. Met Mater Int. 2018;25(1):105–116. doi: 10.1007/s12540-018-0161-0. [DOI] [Google Scholar]
- Laires MJ. Role of cellular magnesium in health and human disease. Front Biosci. 2004;9(1–3):262. doi: 10.2741/1223. [DOI] [PubMed] [Google Scholar]
- Lavery N, Cherry J, Mehmood S, Davies H, Girling B, Sackett E, Brown S, Sienz J. Effects of hot isostatic pressing on the elastic modulus and tensile properties of 316L parts made by powder bed laser fusion. Mater Sci Eng A. 2017;693:186–213. doi: 10.1016/j.msea.2017.03.100. [DOI] [Google Scholar]
- Lee JW, Han HS, Han KJ, Park J, Jeon H, Ok MR, Seok HK, Ahn JP, Lee KE, Lee DH, Yang SJ, Cho SY, Cha PR, Kwon H, Nam TH, Han JHL, Rho HJ, Lee KS, Kim YC, Mantovani D. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of mg alloy. Proc Nat Acad Sci. 2016;113(3):716–721. doi: 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zheng Y, Qin L. Progress of biodegradable metals. Prog Nat Sci Mater Int. 2014;24(5):414–422. doi: 10.1016/j.pnsc.2014.08.014. [DOI] [Google Scholar]
- Li X, Liu X, Wu S, Yeung K, Zheng Y, Chu PK. Design of magnesium alloys with controllable degradation for biomedical implants: from bulk to surface. Act Biomater. 2016;45:2–30. doi: 10.1016/j.actbio.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Li T, He Y, Zhou J, Tang S, Yang Y, Wang X. Influence of albumin on in vitro degradation behavior of biodegradable Mg-1.5Zn-0.6Zr-0.2Sc alloy. Mater Lett. 2018;217:227–230. doi: 10.1016/j.matlet.2018.01.082. [DOI] [Google Scholar]
- Li Y, Zhou J, Pavanram P, Leeflang M, Fockaert L, Pouran B, Tümer N, Schröder KU, Mol J, Weinans H, Jahr H, Zadpoor A. Additively manufactured biodegradable porous magnesium. Act Biomater. 2018;67:378–392. doi: 10.1016/j.actbio.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Li S, Yang X, Hou J, Du W. A review on thermal conductivity of magnesium and its alloys. J Magnes Alloys. 2020;8(1):78–90. doi: 10.1016/j.jma.2019.08.002. [DOI] [Google Scholar]
- Liu C, Fu X, Pan H, Wan P, Wang L, Tan L, Wang K, Zhao Y, Yang K, Chu PK. Biodegradable Mg–Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci Rep. 2016 doi: 10.1038/srep27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sun J, Qiu K, Yang Y, Pu Z, Li L, Zheng Y. Effects of alloying elements (ca and sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5Mg alloy. J Alloys Compd. 2016;664:444–452. doi: 10.1016/j.jallcom.2015.10.116. [DOI] [Google Scholar]
- Liu C, Zhang M, Chen C. Effect of laser processing parameters on porosity, microstructure and mechanical properties of porous Mg–Ca alloys produced by laser additive manufacturing. Mater Sci Eng A. 2017;703:359–371. doi: 10.1016/j.msea.2017.07.031. [DOI] [Google Scholar]
- Liu C, Ren Z, Xu Y, Pang S, Zhao X, Zhao Y. Biodegradable magnesium alloys developed as bone repair materials: A review. Scanning. 2018;2018:1–15. doi: 10.1155/2018/9216314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li D, Zhang Y, Li M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem Cell Biol. 2018;149(4):393–404. doi: 10.1007/s00418-018-1643-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu C, Zhao J, Zeng W, Wang Z. Modification of grain refinement and texture in AZ31 Mg alloy by a new plastic deformation method. J Alloys Compd. 2015;628:130–134. doi: 10.1016/j.jallcom.2014.12.196. [DOI] [Google Scholar]
- Manoj Kumar R, Kuntal KK, Singh S, Gupta P, Bhushan B, Gopinath P, Lahiri D. Electrophoretic deposition of hydroxyapatite coating on Mg–3Zn alloy for orthopaedic application. Surf Coat Technol. 2016;287:82–92. doi: 10.1016/j.surfcoat.2015.12.086. [DOI] [Google Scholar]
- McBride E. Magnesium screw and nail transfixion in fractures. South Med J. 1938;31(5):508–514. doi: 10.1097/00007611-193805000-00010. [DOI] [Google Scholar]
- Md Saad APM, Prakoso AT, Sulong MA, Basri H, Wahjuningrum DA, Syahrom A. Impacts of dynamic degradation on the morphological and mechanical characterisation of porous magnesium scaffold. Biomech Model Mechanobiol. 2019;18(3):797–811. doi: 10.1007/s10237-018-01115-z. [DOI] [PubMed] [Google Scholar]
- Montazerian H, Davoodi E, Asadi-Eydivand M, Kadkhodapour J, Solati-Hashjin M. Porous scaffold internal architecture design based on minimal surfaces: a compromise between permeability and elastic properties. Mater Des. 2017;126:98–114. doi: 10.1016/j.matdes.2017.04.009. [DOI] [Google Scholar]
- Narita K, Tian Q, Johnson I, Zhang C, Kobayashi E, Liu H. Degradation behaviors and cytocompatibility of Mg/β-tricalcium phosphate composites produced by spark plasma sintering. J Biomed Mater Res Part B Appl Biomater. 2019;107(7):2238–2253. doi: 10.1002/jbm.b.34316. [DOI] [PubMed] [Google Scholar]
- Navarro M, Michiardi A, Castaño O, Planell J. Biomaterials in orthopaedics. J R Soc Interface. 2008;5(27):1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinomi M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J Mech Behav Biomed. 2008;1(1):30–42. doi: 10.1016/j.jmbbm.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Oladapo BI, Zahedi S, Adeoye A. 3D printing of bone scaffolds with hybrid biomaterials. Compos Part B Eng. 2019;158:428–436. doi: 10.1016/j.compositesb.2018.09.065. [DOI] [Google Scholar]
- Palanivel S, Nelaturu P, Glass B, Mishra R. Friction stir additive manufacturing for high structural performance through microstructural control in an Mg based WE43 alloy. Mater Des. 2015;1980–2015(65):934–952. doi: 10.1016/j.matdes.2014.09.082. [DOI] [Google Scholar]
- Park M, Lee JE, Park CG, Lee SH, Seok HK, Choy YB. Polycaprolactone coating with varying thicknesses for controlled corrosion of magnesium. J Coat Technol Res. 2013;10(5):695–706. doi: 10.1007/s11998-013-9474-6. [DOI] [Google Scholar]
- Patil SS, Misra RDK, Gao M, Ma Z, Tan L, Yang K. Bioactive coating on a new Mg–2Zn–0.5Nd alloy: modulation of degradation rate and cellular response. Mater Technol. 2019;34(7):394–402. doi: 10.1080/10667857.2019.1574956. [DOI] [Google Scholar]
- Prasad A, Uggowitzer PJ, Shi Z, Atrens A. Production of high purity magnesium alloys by melt purification with Zr. Adv Eng Mater. 2012;14(7):477–490. doi: 10.1002/adem.201200054. [DOI] [Google Scholar]
- Puleo D. Understanding and controlling the bone–implant interface. Biomaterials. 1999;20(23–24):2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- Qiao Z, Shi Z, Hort N, Zainal Abidin NI, Atrens A. Corrosion behaviour of a nominally high purity Mg ingot produced by permanent mould direct chill casting. Corros Sci. 2012;61:185–207. doi: 10.1016/j.corsci.2012.04.030. [DOI] [Google Scholar]
- Qin Y, Wen P, Guo H, Xia D, Zheng Y, Jauer L, Poprawe R, Voshage M, Schleifenbaum JH. Additive manufacturing of biodegradable metals: current research status and future perspectives. Act Biomater. 2019;98:3–22. doi: 10.1016/j.actbio.2019.04.046. [DOI] [PubMed] [Google Scholar]
- Radha R, Sreekanth D. Insight of magnesium alloys and composites for orthopedic implant applications—a review. J Magnes Alloys. 2017;5(3):286–312. doi: 10.1016/j.jma.2017.08.003. [DOI] [Google Scholar]
- Razavi M, Fathi M, Savabi O, Mohammad Razavi S, Hashemi Beni B, Vashaee D, Tayebi L. Controlling the degradation rate of bioactive magnesium implants by electrophoretic deposition of akermanite coating. Ceram Int. 2014;40(3):3865–3872. doi: 10.1016/j.ceramint.2013.08.027. [DOI] [Google Scholar]
- Ren T, Gao X, Xu C, Yang L, Guo P, Liu H, Chen Y, Sun W, Song Z. Evaluation of as-extruded ternary Zn–Mg–Zr alloys for biomedical implantation material: in vitro and in vivo behavior. Mater Corros. 2019;70(6):1056–1070. doi: 10.1002/maco.201810648. [DOI] [Google Scholar]
- Renkema K, Alexander R, Bindels R, Hoenderop J. Calcium and phosphate homeostasis: Concertedinterplay of new regulators. Annals Of Medicine. 2008;40(2):82–91. doi: 10.1080/07853890701689645. [DOI] [PubMed] [Google Scholar]
- Rtimi S, Dionysiou DD, Pillai SC, Kiwi J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl Catal B Environ. 2019;240:291–318. doi: 10.1016/j.apcatb.2018.07.025. [DOI] [Google Scholar]
- Saini M. Implant biomaterials: a comprehensive review. World J Clin Cases. 2015;3(1):52. doi: 10.12998/wjcc.v3.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Maleksaeedi S, Sapari MAB, Nai MLS, Meenashisundaram GK, Gupta M. Additive manufacturing of magnesium–zinc–zirconium (ZK) alloys via capillary-mediated binderless three-dimensional printing. Mater Des. 2019;169:107683. doi: 10.1016/j.matdes.2019.107683. [DOI] [Google Scholar]
- Sanchez AHM, Luthringer BJ, Feyerabend F, Willumeit R. Mg and mg alloys: how comparable are in vitro and in vivo corrosion rates? A review. Act Biomater. 2015;13:16–31. doi: 10.1016/j.actbio.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Sankar M, Suwas S, Balasubramanian S, Manivasagam G. Comparison of electrochemical behavior of hydroxyapatite coated onto WE43 Mg alloy by electrophoretic and pulsed laser deposition. Surf Coat Technol. 2017;309:840–848. doi: 10.1016/j.surfcoat.2016.10.077. [DOI] [Google Scholar]
- Sanz-Herrera J, Reina-Romo E, Boccaccini A. In silico design of magnesium implants: macroscopic modeling. J Mech Behav Biomed. 2018;79:181–188. doi: 10.1016/j.jmbbm.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Schlüter K, Shi Z, Zamponi C, Cao F, Quandt E, Atrens A. Corrosion performance and mechanical properties of sputter-deposited MgY and MgGd alloys. Corros Sci. 2014;78:43–54. doi: 10.1016/j.corsci.2013.08.027. [DOI] [Google Scholar]
- Seitz JM, Durisin M, Goldman J, Drelich JW. Recent advances in biodegradable metals for medical sutures: a critical review. Adv Healthc Mater. 2015;4(13):1915–1936. doi: 10.1002/adhm.201500189. [DOI] [PubMed] [Google Scholar]
- Selvam M, Saminathan K, Siva P, Saha P, Rajendran V. Corrosion behavior of Mg/graphene composite in aqueous electrolyte. Mater Chem Phys. 2016;172:129–136. doi: 10.1016/j.matchemphys.2016.01.051. [DOI] [Google Scholar]
- Seong J, Kim W. Development of biodegradable Mg–Ca alloy sheets with enhanced strength and corrosion properties through the refinement and uniform dispersion of the Mg2Ca phase by high-ratio differential speed rolling. Act Biomater. 2015;11:531–542. doi: 10.1016/j.actbio.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Sezer N, Evis Z, Kayhan SM, Tahmasebifar A, Koç M. Review of magnesium-based biomaterials and their applications. J Magnes Alloys. 2018;6(1):23–43. doi: 10.1016/j.jma.2018.02.003. [DOI] [Google Scholar]
- Shalbafi M, Roumina R, Mahmudi R. Hot deformation of the extruded Mg–10Li–1Zn alloy: constitutive analysis and processing maps. J Alloys Compd. 2017;696:1269–1277. doi: 10.1016/j.jallcom.2016.12.087. [DOI] [Google Scholar]
- Shao H, He Y, Fu J, He D, Yang X, Xie J, Yao C, Ye J, Xu S, Gou Z. 3D printing magnesium-doped wollastonite/β-TCP bioceramics scaffolds with high strength and adjustable degradation. J Eur Ceram Soc. 2016;36(6):1495–1503. doi: 10.1016/j.jeurceramsoc.2016.01.010. [DOI] [Google Scholar]
- Shuai C, Yang Y, Wu P, Lin X, Liu Y, Zhou Y, Feng P, Liu X, Peng S. Laser rapid solidification improves corrosion behavior of Mg–Zn–Zr alloy. J Alloys Compd. 2017;691:961–969. doi: 10.1016/j.jallcom.2016.09.019. [DOI] [Google Scholar]
- Shuai C, He C, Xu L, Li Q, Chen T, Yang Y, Peng S. Wrapping effect of secondary phases on the grains: increased corrosion resistance of Mg–Al alloys. Virtual Phys Prototyp. 2018;13(4):292–300. doi: 10.1080/17452759.2018.1479969. [DOI] [Google Scholar]
- Shuai C, Liu L, Yang Y, Gao C, Zhao M, Yi L, Peng S. Lanthanum-containing magnesium alloy with antitumor function based on increased reactive oxygen species. Appl Sci. 2018;8(11):2109. doi: 10.3390/app8112109. [DOI] [Google Scholar]
- Shuai C, Liu L, Zhao M, Feng P, Yang Y, Guo W, Gao C, Yuan F. Microstructure, biodegradation, antibacterial and mechanical properties of ZK60-Cu alloys prepared by selective laser melting technique. J Mater Sci Technol. 2018;34(10):1944–1952. doi: 10.1016/j.jmst.2018.02.006. [DOI] [Google Scholar]
- Shuai C, Yang W, Peng S, Gao C, Guo W, Lai Y, Feng P. Physical stimulations and their osteogenesis-inducing mechanisms. Int J Bioprint. 2018 doi: 10.18063/ijb.v4i2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai C, Li S, Peng S, Feng P, Lai Y, Gao C. Biodegradable metallic bone implants. Mater Chem Front. 2019;3(4):544–562. doi: 10.1039/c8qm00507a. [DOI] [Google Scholar]
- Shuai C, Wang B, Yang Y, Peng S, Gao C. 3D honeycomb nanostructure-encapsulated magnesium alloys with superior corrosion resistance and mechanical properties. Compos Part B Eng. 2019;162:611–620. doi: 10.1016/j.compositesb.2019.01.031. [DOI] [Google Scholar]
- Shuai C, Xu Y, Feng P, Wang G, Xiong S, Peng S. Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver. Chem Eng J. 2019;374:304–315. doi: 10.1016/j.cej.2019.03.273. [DOI] [Google Scholar]
- Shu-Rong Z, Huai-Mei Z, Snao-Zhen Q, Guo-Wei S, Kaper RF. A long-term study of the efficacy and acceptability of a single-rod hormonal contraceptive implant (implanon®) in healthy women in China. Eur J Contracept Reprod Health Care. 1999;4(2):85–93. doi: 10.3109/13625189909064009. [DOI] [PubMed] [Google Scholar]
- Song G, Atrens A (1999) Corrosion mechanisms of magnesium alloys. Adv Eng Mater 1(1):11–33. 10.1002/(sici)1527-2648(199909)1:1
- Song Y, Shan D, Han EH. Pitting corrosion of a rare earth mg alloy GW93. J Mater Sci Technol. 2017;33(9):954–960. doi: 10.1016/j.jmst.2017.01.014. [DOI] [Google Scholar]
- Song R, Murphy M, Li C, Ting K, Soo C, Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des Dev Ther. 2018;12:3117–3145. doi: 10.2147/dddt.s165440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sta Agueda JRH, Chen Q, Maalihan RD, Ren J, da Silva TGM, Dugos NP, Caldona EB, Advincula RC. 3D printing of biomedically relevant polymer materials and biocompatibility. MRS Commun. 2021;11(2):197–212. doi: 10.1557/s43579-021-00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11(5):18–25. doi: 10.1016/s1369-7021(08)70086-5. [DOI] [Google Scholar]
- Sur divers altisides du nord de l’Afrique avec description de plusieursformesnouvelles (1911) Bulletin de La Société Entomologique de France. pp 9–11. 10.5962/bhl.part.2956
- Taljanovic MS, Jones MD, Ruth JT, Benjamin JB, Sheppard JE, Hunter TB. Fracture fixation. Radiographics. 2003;23(6):1569–1590. doi: 10.1148/rg.236035159. [DOI] [PubMed] [Google Scholar]
- Tammann G, Schafmeister P. MetallographischeMitteilungen. CXVIII. Über die verteilungeinesmetalleszwischenzweiflüssigenmetallischenphasen. Z Anorg Allg Chem. 1924;138(1):219–232. doi: 10.1002/zaac.19241380121. [DOI] [Google Scholar]
- Tang Y, El-Awady JA. Formation and slip of pyramidal dislocations in hexagonal close-packed magnesium single crystals. Act Mater. 2014;71:319–332. doi: 10.1016/j.actamat.2014.03.022. [DOI] [Google Scholar]
- Tappa K, Jammalamadaka U. Novel biomaterials used in medical 3D printing techniques. J Funct Biomater. 2018;9(1):17. doi: 10.3390/jfb9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie D, Feyerabend F, Müller WD, Schade R, Liefeith K, Kainer K, Willumeit R. Antibacterial biodegradable Mg–Ag alloys. Eur Cells Mater. 2013;25:284–298. doi: 10.22203/ecm.v025a20. [DOI] [PubMed] [Google Scholar]
- Turan ME, Sun Y, Akgul Y, Turen Y, Ahlatci H. The effect of GNPs on wear and corrosion behaviors of pure magnesium. J Alloys Compd. 2017;724:14–23. doi: 10.1016/j.jallcom.2017.07.022. [DOI] [Google Scholar]
- Velikokhatnyi OI, Kumta PN. First-principles studies on alloying and simplified thermodynamic aqueous chemical stability of calcium-, zinc-, aluminum-, yttrium- and iron-doped magnesium alloys☆. Act Biomater. 2010;6(5):1698–1704. doi: 10.1016/j.actbio.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Venezuela J, Dargusch M. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: a comprehensive review. Act Biomater. 2019;87:1–40. doi: 10.1016/j.actbio.2019.01.035. [DOI] [PubMed] [Google Scholar]
- Wan Y, Cui T, Li W, Li C, Xiao J, Zhu Y, Ji D, Xiong G, Luo H. Mechanical and biological properties of bioglass/magnesium composites prepared via microwave sintering route. Mater Des. 2016;99:521–527. doi: 10.1016/j.matdes.2016.03.096. [DOI] [Google Scholar]
- Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed Nanotechnol Biol Med. 2015;11(2):313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Wang H, Feng Q, Li N, Xu S. Evaluation of metal-ceramic bond characteristics of three dental Co–Cr alloys prepared with different fabrication techniques. J Prosthet Dent. 2016;116(6):916–923. doi: 10.1016/j.prosdent.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Fan X, Yuan S, Jiao W, Liu B, Cao J, Jiang W. Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food Funct. 2017;8(8):2924–2934. doi: 10.1039/c7fo00659d. [DOI] [PubMed] [Google Scholar]
- Wang P, Xiong P, Liu J, Gao S, Xi T, Cheng Y. A silk-based coating containing GREDVY peptide and heparin on Mg–Zn–Y–Nd alloy: improved corrosion resistance, hemocompatibility and endothelialization. J Mater Chem B. 2018;6(6):966–978. doi: 10.1039/c7tb02784b. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu D, Wu R, Chen X, Peng Q, Jin L, Xin Y, Zhang Z, Liu Y, Chen X, Chen G, Deng K, Wang H. What is going on in magnesium alloys? J Mater Sci Technol. 2018;34(2):245–247. doi: 10.1016/j.jmst.2017.07.019. [DOI] [Google Scholar]
- Wang Y, Tie D, Guan R, Wang N, Shang Y, Cui T, Li J. Microstructures, mechanical properties, and degradation behaviors of heat-treated Mg-Sr alloys as potential biodegradable implant materials. J Mech Behav Biomed. 2018;77:47–57. doi: 10.1016/j.jmbbm.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Wen P, Voshage M, Jauer L, Chen Y, Qin Y, Poprawe R, Schleifenbaum JH. Laser additive manufacturing of Zn metal parts for biodegradable applications: processing, formation quality and mechanical properties. Mater Des. 2018;155:36–45. doi: 10.1016/j.matdes.2018.05.057. [DOI] [Google Scholar]
- Willbold E, Gu X, Albert D, Kalla K, Bobe K, Brauneis M, Janning C, Nellesen J, Czayka W, Tillmann W, Zheng Y, Witte F. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Act Biomater. 2015;11:554–562. doi: 10.1016/j.actbio.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Witte F. The history of biodegradable magnesium implants: a review☆. Act Biomater. 2010;6(5):1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Witte F, Eliezer A. Biodegradable Metals. Berlin Heidelberg: Springer; 2012. [Google Scholar]
- Witte F. Reprint of: the history of biodegradable magnesium implants: a review. Act Biomater. 2015;23:S28–S40. doi: 10.1016/j.actbio.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Wolff M, Schaper JG, Suckert MR, Dahms M, Ebel T, Willumeit-Römer R, Klassen T. Magnesium powder injection molding (MIM) of orthopedic implants for biomedical applications. JOM. 2016;68(4):1191–1197. doi: 10.1007/s11837-016-1837-x. [DOI] [Google Scholar]
- Wu N, Veillette A. Magnesium in a signalling role. Nature. 2011;475(7357):462–463. doi: 10.1038/475462a. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhu Y. Heterogeneous materials: a new class of materials with unprecedented mechanical properties. Mater Res Lett. 2017;5(8):527–532. doi: 10.1080/21663831.2017.1343208. [DOI] [Google Scholar]
- Wu S, Liu X, Yeung KW, Liu C, Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater Sci Eng Rep. 2014;80:1–36. doi: 10.1016/j.mser.2014.04.001. [DOI] [Google Scholar]
- Wu Y, Wang Y, Zhao D, Zhang N, Li H, Li J, Wang Y, Zhao Y, Yan J, Zhou Y. In vivo study of microarc oxidation coated Mg alloy as a substitute for bone defect repairing: degradation behavior, mechanical properties, and bone response. Colloid Surf B Biointerfaces. 2019;181:349–359. doi: 10.1016/j.colsurfb.2019.05.052. [DOI] [PubMed] [Google Scholar]
- Xin Y, Hu T, Chu P. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: a review. Act Biomater. 2011;7(4):1452–1459. doi: 10.1016/j.actbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Xiong G, Nie Y, Ji D, Li J, Li C, Li W, Zhu Y, Luo H, Wan Y. Characterization of biomedical hydroxyapatite/magnesium composites prepared by powder metallurgy assisted with microwave sintering. Curr Appl Phys. 2016;16(8):830–836. doi: 10.1016/j.cap.2016.05.004. [DOI] [Google Scholar]
- Yan Y, Kang Y, Li D, Yu K, Xiao T, Deng Y, Dai H, Dai Y, Xiong H, Fang H. Improvement of the mechanical properties and corrosion resistance of biodegradable β-Ca3 (PO4) 2/Mg-Zn composites prepared by powder metallurgy: the adding β-Ca3 (PO4) 2, hot extrusion and aging treatment. Mater Sci Eng C. 2017;74:582–596. doi: 10.1016/j.msec.2016.12.132. [DOI] [PubMed] [Google Scholar]
- Yang C, Yuan G, Zhang J, Tang Z, Zhang X, Dai K. Effects of magnesium alloys extracts on adult human bone marrow-derived stromal cell viability and osteogenic differentiation. Biomed Mater. 2010;5(4):045005. doi: 10.1088/1748-6041/5/4/045005. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu P, Lin X, Liu Y, Bian H, Zhou Y, Gao C, Shuai C. System development, formability quality and microstructure evolution of selective laser-melted magnesium. Virtual Phys Prototyp. 2016;11(3):173–181. doi: 10.1080/17452759.2016.1210522. [DOI] [Google Scholar]
- Yang Y, Wu P, Wang Q, Wu H, Liu Y, Deng Y, Zhou Y, Shuai C. The enhancement of mg corrosion resistance by alloying mn and laser-melting. Materials. 2016;9(4):216. doi: 10.3390/ma9040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Guo X, He C, Gao C, Shuai C. Regulating degradation behavior by incorporating mesoporous silica for Mg bone implants. ACS Biomater Sci Eng. 2018;4(3):1046–1054. doi: 10.1021/acsbiomaterials.8b00020. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang G, Liang H, Gao C, Peng S, Shen L, Shuai C. Additive manufacturing of bone scaffolds. Int J Bioprint. 2018;11:12. doi: 10.18063/IJB.v5i1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yuan F, Gao C, Feng P, Xue L, He S, Shuai C. A combined strategy to enhance the properties of zn by laser rapid solidification and laser alloying. J Mech Behav Biomed. 2018;82:51–60. doi: 10.1016/j.jmbbm.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zan J, Yang W, Qi F, He C, Huang S, Peng S, Shuai C. Metal organic frameworks as a compatible reinforcement in a biopolymer bone scaffold. Mater Chem Front. 2020;4(3):973–984. doi: 10.1039/c9qm00772e. [DOI] [Google Scholar]
- Yang Y, He C, Dianyu E, Yang W, Qi F, Xie D, Shen L, Peng S, Shuai C. Mg bone implant: features, developments and perspectives. Mater Des. 2020;185:108259. doi: 10.1016/j.matdes.2019.108259. [DOI] [Google Scholar]
- Yang Y, Lu C, Peng S, Shen L, Wang D, Qi F, Shuai C. Laser additive manufacturing of Mg-based composite with improved degradation behaviour. Virtual Phys Prototyping. 2020;15(3):278–293. doi: 10.1080/17452759.2020.1748381. [DOI] [Google Scholar]
- Yao H, Wen J, Xiong Y, Lu Y, Ren F, Cao W. Extrusion temperature impacts on biometallic Mg–2.0Zn–0.5Zr–3.0Gd (wt%) solid-solution alloy. J Alloys Compd. 2018;739:468–480. doi: 10.1016/j.jallcom.2017.12.225. [DOI] [Google Scholar]
- Yin P, Li NF, Lei T, Liu L, Ouyang C. Effects of Ca on microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Ca alloys. J Mater Sci Mater Med. 2013;24(6):1365–1373. doi: 10.1007/s10856-013-4856-y. [DOI] [PubMed] [Google Scholar]
- Yin Y, Huang Q, Liang L, Hu X, Liu T, Weng Y, Long T, Liu Y, Li Q, Zhou S, Wu H. In vitro degradation behavior and cytocompatibility of ZK30/bioactive glass composites fabricated by selective laser melting for biomedical applications. J Alloys Compd. 2019;785:38–45. doi: 10.1016/j.jallcom.2019.01.165. [DOI] [Google Scholar]
- Yousefi AM, Hoque ME, Prasad RGSV, Uth N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res A. 2014;103(7):2460–2481. doi: 10.1002/jbm.a.35356. [DOI] [PubMed] [Google Scholar]
- Yu D, Zhang D, Sun J, Luo Y, Xu J, Zhang H, Pan F. Improving mechanical properties of ZM61 magnesium alloy by aging before extrusion. J Alloys Compd. 2017;690:553–560. doi: 10.1016/j.jallcom.2016.08.128. [DOI] [Google Scholar]
- Yu Y, Lu H, Sun J. Long-term in vivo evolution of high-purity Mg screw degradation—local and systemic effects of Mg degradation products. Act Biomater. 2018;71:215–224. doi: 10.1016/j.actbio.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Yuan QH, Zhou GH, Liao L, Liu Y, Luo L. Interfacial structure in AZ91 alloy composites reinforced by graphene nanosheets. Carbon. 2018;127:177–186. doi: 10.1016/j.carbon.2017.10.090. [DOI] [Google Scholar]
- Yuan L, Ding S, Wen C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: a review. Bioact Mater. 2019;4:56–70. doi: 10.1016/j.bioactmat.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng RC, Zhang J, Huang WJ, Dietzel W, Kainer K, Blawert C, Ke W. Review of studies on corrosion of magnesium alloys. Trans Nonferrous Met Soc. 2006;16:s763–s771. doi: 10.1016/s1003-6326(06)60297-5. [DOI] [Google Scholar]
- Zhang S, Zhang X, Zhao C, Li J, Song Y, Xie C, Tao H, Zhang Y, He Y, Jiang Y. Research on an Mg–Zn alloy as a degradable biomaterial. Act Biomater. 2010;6(2):626–640. doi: 10.1016/j.actbio.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wei QS, Lin GK, Zhao X, Shi YS. Effects of powder characteristics on selective laser melting of 316L stainless steel powder. Adv Mater Res. 2011;189–193:3664–3667. doi: 10.4028/www.scientific.net/amr.189-193.3664. [DOI] [Google Scholar]
- Zhang T, Shao Y, Meng G, Cui Z, Wang F. Corrosion of hot extrusion AZ91 magnesium alloy: I-relation between the microstructure and corrosion behavior. Corros Sci. 2011;53(5):1960–1968. doi: 10.1016/j.corsci.2011.02.015. [DOI] [Google Scholar]
- Zhang Y, Xu J, Ruan YC, Yu MK, O’Laughlin M, Wise H, Chen D, Tian L, Shi D, Wang J, Chen S, Feng JQ, Chow DHK, Xie X, Zheng L, Huang L, Huang S, Leung K, Lu N, Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feyerabend F, Tang S, Hu J, Lu X, Blawert C, Lin T. A study of degradation resistance and cytocompatibility of super-hydrophobic coating on magnesium. Mater Sci Eng C. 2017;78:405–412. doi: 10.1016/j.msec.2017.04.057. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Wang W, Huang H, Pei J, Qu H, Yuan G, Li Y. The degradation and transport mechanism of a Mg–Nd–Zn–Zr stent in rabbit common carotid artery: a 20-month study. Act Biomater. 2018;69:372–384. doi: 10.1016/j.actbio.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Zhao XF, Niinomi M, Nakai M, Hieda J (2011) OS07-2-2 development of new Ti-Mo alloys with changeable Young’s modulus for spinal fixture devices. The Abstracts of ATEM: International Conference on Advanced Technology in Experimental Mechanics: Asian Conference on Experimental Mechanics, 2011.10(0), _OS07-2. 10.1299/jsmeatem.2011.10._os07-2-2
- Zhao D, Huang S, Lu F, Wang B, Yang L, Qin L, Yang K, Li Y, Li W, Wang W, Tian S, Zhang X, Gao W, Wang Z, Zhang Y, Xie X, Wang J, Li J. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016;81:84–92. doi: 10.1016/j.biomaterials.2015.11.038. [DOI] [PubMed] [Google Scholar]