Abstract
Curcumin is a primary polyphenol of the rhizomatous perennial plant called Curcuma Longa. Curcumin interferes favorably with the cellular events that take place in the inflammatory and proliferative stages of wound healing, hence its importance in skin regeneration and wound healing. Curcumin is however lipophilic, and this must be considered in the choice of its drug delivery system. Liposomes are spherical vesicles with bi-lipid layers. Liposomes can encapsulate both lipophilic and hydrophilic drugs, hence their suitability as an ideal drug delivery system for curcumin. There is, nevertheless, a tendency for liposomes to be unstable and have low encapsulation efficiency if it is not formulated properly. Formulation optimization of curcumin-loaded liposomes was studied by the application of artificial neural network (ANN) to improve encapsulation efficiency and flux of the liposomes. The input factors selected for optimization of the formulation were sonication time, hydration volume, and lipid/curcumin ratio. The response variables were encapsulation efficiency and flux. The maximum encapsulation efficiency and flux were obtained using lipid/curcumin ratio of 4.35, sonicator time of 15 min, and hydration volume of 25 mL. The maximum encapsulation efficiency and flux predicted were 100% and 51.23 µg/cm2/h, respectively. The experimental values were 99.934% and 51.229 µg/cm2/h, respectively. Curcumin-loaded liposome formulation is a promising drug delivery system in the pharmaceutical industry when formulated using optimized parameters derived from ANN statistically designed models.
Keywords: Response surface methodology, Curcumin, Liposomes, Sonication, Encapsulation
Introduction
Curcumin is a primary polyphenol of the rhizomatous perennial plant called Curcuma longa. Curcumin has several medical uses, its medicinal role as an antifungal, antiviral, antioxidant, and anti-inflammatory have been well established in literature (Sharifi-Rad et al. 2020). Recently curcumin is being investigated for its wound healing properties. Its mechanism of action is that it interferes favorably with pharmacological, biochemical, and cellular events that take place in the inflammatory and proliferative stages of wound healing. Curcumin acts by recruiting macrophages into translucent fatty tissues, it is also involved in cytokine production vital for inflammatory response. Curcumin subsequently reduces inflammation by the activation of the signal pathway linked with associated light chain enhancers of activated B cells. Curcumin acts as an antioxidant scavenger by scavenging reductive–oxidative species. Curcumin is, however, a lipophilic drug, and therefore poorly soluble in water; this factor must be considered in the choice of a delivery system for the drug Curcumin (Rahmani et al. 2018).
Liposomes are biologically degradable and compatible lipid-delivery systems. They are phospholipid bi-layered vesicles that have a lipophilic membrane and an aqueous core. Liposomes can act as biological cargo to both hydrophilic and lipophilic drugs of varying molecular weight. Liposomes deliver drugs to their target sites by housing hydrophilic aqueous soluble drugs in the inner core and lipophilic drugs at its outer bi-lipid layer. Curcumin is a lipophilic drug and can therefore be incorporated into the outer bilayer of liposome for sustained delivery at the wound site. Several parameters and techniques are involved in the formulation of curcumin-loaded liposomes. Parameters or techniques, such as lipid ratio, sonication time, sonication intensity, hydration volume, method of liposome formation, and drug loading, are variable independent parameters that may be manipulated to achieve an optimal formulation. One sure method of achieving it is through a mathematical modeling (Saraf et al. 2020).
Mathematical modeling is a vital interpretation of experimental outcome, hence providing better insight into the optimal formulation variables, parameters, and techniques. A variety of mathematical models for drug encapsulation and release linked with artificial neural networks are useful in building relationships between input and output variables. Artificial neural network modeling (ANN) involves the use of two network architectures, multilayer normal feed-forward (MNFF) and multilayer full feed-forward (MFFF) to determine which input variable is more suitable for the modeling responses. The networks are made of a single input layer two hidden layers and one output layer (Khoo et al. 2019; Ilomuanya et al. 2020a). The use of artificial neural network to optimize liposomal formulations loaded with active pharmaceutical ingredients has been a research spotlight in recent times (Pereira et al. 2020).
Artificial neural network can be described as a useful tool in enhancing decision making though mathematical predictions (Ocampo et al. 2021). Artificial neural network is a mathematical modeling tool based on the architecture of the human neurons (Salehi 2019). According to an investigation by Salehi et al., it was proven that non-linear modeling based on artificial neural network was more accurate with regards to mathematical predictions compared to empirical models (Amini et al. 2021; Salehi 2019). Current research investigations have been published on the utilization of ANN to predict experimental response variables and optimize pharmaceutical formulation processes. In a study by Pereira et al., artificial neural network was used in optimizing the incorporation of tetracaine into liposomes as a drug delivery system (Pereira et al. 2020).
Currently, there is no study that applies artificial neural network as a tool for optimization of input factors, (phospholipid: curcumin ratio, hydration volume and sonication time), to experimentally obtain maximum responses in liposomal encapsulation efficiency and flux (in vitro permeability). Liposomes tend to be unstable with low encapsulation efficiency in case of improper formulation. There is an urgent need in the field of lipid drug delivery to produce stable liposomes that will foster excellent pharmacodynamics and pharmacokinetics of loaded active pharmaceutical ingredients such as curcumin. Encapsulation efficiency and flux directly affect the pharmacological properties of the loaded curcumin drug because they determine how much of the curcumin will be released and absorbed on reaching the target site. The aim of this study is to optimize the process of development of curcumin-loaded liposomes for wound healing using artificial neural network as a mathematical model to determine the best experimental conditions for formulating liposomes with excellent encapsulation efficiency and flux (Ilomuanya et al. 2020a).
Materials and methods
Materials
Curcumin, and phosphatidylcholine (Sigma-Aldrich, MO, USA), methanol (Merck, Darmstadt, Germany), phosphate buffer (Loba Chemie, Mumbai, India), 1% cremophor (RH 40) (Macklin Biochemical, Shangai, China).
Methods
Preparation of optimized curcumin-loaded liposomes
Formation of a lipid thin film followed by solvent hydration was used to prepare the liposomes. Exactly 100 mg of phosphatidylcholine was weighed and dissolved with 25 mL of methanol while 435 mg of curcumin was then weighed and added to the mixture. The mixture was dried with the aid of a rotary evaporator to give a thin lipid film and then rehydrated with 25 mL of phosphate buffer (pH 7.4), afterward sonication ensued for 15 min (Umbarkar et al. 2021).
Experimental design
A central composite design (CCD) was used to plan the experiments which involved three variables that were varied over five levels. The independent variables under consideration were phospholipid/curcumin (SPPC/Cur) ratio, sonication time and hydration volume and they were denoted as X1, X2 and X3, respectively. The ranges of these variables including the lower and upper bounds are shown in Table 1 and they were estimated using Eq. 1. The dependent variables or responses investigated were encapsulation efficiency and flux. The experimental design was developed using Design Expert® software (version 7.0.0, Stat-ease, Inc. Minneapolis, USA).
| 1 |
Table 1.
Coded and actual levels of the factors
| Coded and actual levels | ||||||
|---|---|---|---|---|---|---|
| Independent variables | Symbols | − 1.682 | − 1 | 0 | 1 | 1.682 |
| SPPC/CUR ratio | X1 | 1.45 | 2.04 | 2.90 | 3.76 | 4.35 |
| Sonication time (mins) | X2 | 5.00 | 7.03 | 10.00 | 12.97 | 15.00 |
| Hydration volume (mL) | X2 | 25.00 | 40.20 | 62.50 | 84.80 | 100.00 |
In Eq. 1, xi and Xi are the coded and actual values of the independent variables respectively while Xo is the actual value of the independent variable at the center point, and ΔXi is the step change in Xi.
Artificial neural network modeling
Artificial neural networks (ANN) which are computer programs inspired by biological neural networks are particularly useful in building relationships between input and output variables (Ramzi et al., 2015). Thus, a commercially sourced ANN software, Neural Power (version 2.5, C.P.C-X Software USA) was used to map the input variables (SPPC/CUR ratio, sonicator time and hydration volume) onto the output variables (encapsulation efficiency and flux). Two network architectures (multilayer normal feed-forward (MNFF) and multilayer full feed-forward (MFFF) were assessed to determine which was more suitable for modeling the responses. The networks were made up of a single input layer, two hidden layers and one output layer. The networks were then trained using several training algorithms to ascertain the most suitable one. The training algorithms considered were incremental back propagation (IBP), batch back propagation (BBP), quick propagation (QP), generic algorithm (GA), and Levenberg–Marquadt algorithm (LM). The training algorithms employed 70% of the experimental data as training set, 15% as validating set and the remaining 15% as testing set. The best transfer function to map the inputs to the outputs was determined iteratively from among sigmoid, hyperbolic tangent, gaussian, linear, threshold, linear and bipolar linear transfer functions (Amenaghawon and Kazeem 2020). After establishing the best network architecture with its associated training algorithm and transfer function, the optimum number of neurons in both hidden layers was then determined following an iterative process as described by Amenaghawon and Agbedor (Amenaghawon and Agbedor 2021). The number of neurons is important in the sense that very few neurons lead to poor fitting while very many neurons result in over fitting.
Goodness-of-fit assessment of the ANN model
The fit of the ANN model to the experimental observations was assessed using goodness-of-fit statistical indicators, such as correlation coefficient (R), coefficient of determination (R2), + adjusted R2, mean square error (MSE), root mean square error (RMSE), standard error of prediction (SEP), mean absolute error (MAE) and average absolute deviation (AAD), as presented in Eqs. 2, 3, 4, 5, 6, 7, 8 and 9. It is desired that the correlation coefficient and coefficient of determination be as close to unity as possible. Specifically, the R2 value should be a minimum of 0.8 for there to be an acceptable fit between predicted and experimental results. Conversely, the error terms (MSE, RMSE, SEP, MAE and AAD) should be as small as possible (Amenaghawon and Yerimah 2020).
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
In Eqs. 2, 3, 4, 5, 6, 7, 8 and 9, n is the number of experimental runs, is the predicted value, is the experimental values, is the average of the experimental values, is the average of the predicted values and k is the number of input variables.
Optimisation of responses
The optimum values of the responses were obtained by algorithm-based optimization. The optimization process searches for a combination of factor levels that simultaneously satisfy the criteria placed on each of the responses and factors. To include a response in the optimization criteria, it must have a model fit through analysis or supplied by an equation of only simulation. For this work, the optimization was done by maximizing the desired responses. The optimization algorithms considered were genetic algorithm (GA), rotation inherit optimization (RIO) and particle swarm optimization (PSO).
Scanning electron microscopy of curcumin-loaded liposomes
Scanning electron microscopy (SEM) was used to characterize the surface and structure of the liposome (Robson et al. 2018).
Encapsulation efficiency
The ultracentrifugation method was used to ascertain entrapment efficiency, 1 mL of liposomal suspension was transferred into a centrifuge tube and covered with a screw cap. The samples were centrifuged at 30,000 rpm for 10 min at room temperature and the supernatants were used to determine the concentration of non-encapsulated curcumin. The concentration of curcumin was determined using UV–Visible spectrophotometer at a peak wavelength of 425 nm (Corrêa et al. 2019).
The efficiency of encapsulation (EE%) was calculated using the equation:
| 10 |
where EE = Encapsulation efficiency; TCC = Total concentration of curcumin; NCC = Non encapsulated concentration of curcumin.
In vitro drug permeability (Flux)
The experiment was performed in three independent vertical modified Franz®diffusion cells. The apparatus consisted of the donor and receptor compartments. The volume of the receptor compartment was 50 mL and the effective area for dissolution was 3.147 cm2. For the donor compartment, 1 mL of the optimized formulation was applied. Under conditions of stirring at 100 rpm, 37 ± 1.0 °C, 1 mL aliquots were taken from the sampling port of the apparatus and replaced with same volume of media (pH 7.4 phosphate buffer containing Cremophor). This was done at varying time lapse and determined at 425 nm by UV–Visible spectrophotometry (Salamanca et al. 2018).
Results
Artificial neural network architecture and training
Assessments of the multilayer normal feed-forward and multilayer full feed-forward neural networks with the results obtained for the selection of transfer functions and network training are presented in Table 2. It can be clearly seen from Table 2 that the sigmoid transfer function performed best in terms of mapping the input variables onto the output variable for the case of encapsulation efficiency. This was demonstrated in the fact that the sigmoid function yielded the highest R2 value (0.9778) and lowest RMSE value (0.1823) for encapsulation efficiency. However, it was found that the hyperbolic tangent function (Tanh) was more suitable for the case of flux as demonstrated in the high R2 (0.9495) and low RMSE (3.1436) values. Thus, these two transfer functions were chosen for the networks developed for encapsulation efficiency and flux, respectively.
Table 2.
Selection of best transfer function and Selection of best network architecture
| Selection of best transfer function | ||||
|---|---|---|---|---|
| Transfer function | Encapsulation efficiency (%) | Flux (µg/cm2/h) | ||
| R2 | RMSE | R2 | RMSE | |
| Sigmoid | 0.9778 | 0.1823 | 0.9464 | 3.2378 |
| Tanh | 0.9772 | 0.1847 | 0.9495 | 3.1436 |
| Gaussian | 0.4839 | 1.5125 | 0.4275 | 10.581 |
| Linear | 0.3609 | 1.1415 | 0.1099 | 13.193 |
| Threshold linear | 0.3847 | 1.1295 | 0.1217 | 13.106 |
| Bipolar linear | 0.3604 | 1.1415 | 0.1193 | 13.124 |
| Selection of best network architecture | |||||
|---|---|---|---|---|---|
| Network Architecture | Training algorithm | Response | |||
| Encapsulation efficiency | Flux | ||||
| R2 | RMSE | R2 | RMSE | ||
| MNFF | IBP | 0.9770 | 0.1858 | 0.9495 | 3.1436 |
| BBP | 0.9775 | 0.1835 | 0.9454 | 3.2684 | |
| QP | 0.9246 | 0.3360 | 0.9495 | 3.1425 | |
| GA | 0.9456 | 0.2854 | 0.9450 | 3.2792 | |
| LM | 0.9785 | 0.1796 | 0.9495 | 3.1425 | |
| MFFF | IBP | 0.9776 | 0.1832 | 0.9495 | 3.1425 |
| BBP | 0.9782 | 0.1807 | 0.9445 | 3.2956 | |
| QP | 0.9776 | 0.1833 | 0.9495 | 3.1424 | |
| GA | 0.9776 | 0.1832 | 0.9473 | 3.2101 | |
| LM | 0.9785 | 0.1795 | 0.9495 | 3.1424 | |
The results of training the two network architectures are also presented in Table 2. The best network architecture was chosen as the one with the highest R2 values and lowest RMSE value. The best training algorithm was also chosen on the same basis and the results show that for the case of encapsulation efficiency, the multilayer full feed-forward neural network trained with the Levenberg–Marquadt (LM) algorithm performed best with the highest R2 (0.9785) and lowest RMSE (0.1795) values. The same network configuration was also found to adequately model the flux as seen in the highest R2 (0.9495) and lowest RMSE (3.1424) value (Salamanca et al. 2018).
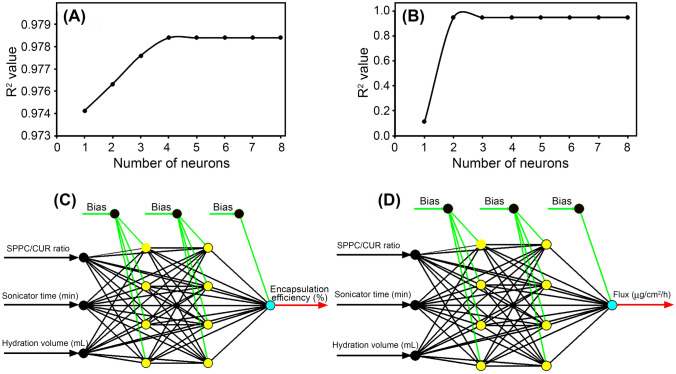
The results obtained after iteratively determining the optimum number of neurons for the network developed for both responses are presented in Fig. 1A and B for encapsulation efficiency and flux, respectively. From the results shown in Fig. 1A, it was observed that the R2 value increased as the number of neurons was increased from 1 to 4. This is an indication of an increase in the predictive capacity of the network (Amenaghawon and Amagbewan, 2017). However, increasing the number of neurons beyond 4 did not improve the R2 value, thus suggesting that the optimum number of neurons is 4. A look at Fig. 1B and following the same analysis as was done for Fig. 1A showed that 4 was also the optimum number of neurons for the network used to model flux. Thus, the two networks used to model encapsulation efficiency and flux were denoted as 3-4-4-1 meaning that the networks contain 3 input variables, 4 neurons each in both hidden layers and one output variable as seen in Fig. 1C and D (Ilomuanya et al. 2020b).
Fig. 1.
Showing A selection of optimum number of neurons for encapsulation efficiency and B selection of optimum number of neurons for flux C architecture of the optimal ANN for predicting encapsulation efficiency D architecture of the optimal ANN for predicting flux
Assessment of ANN model predictions
The values of encapsulation efficiency and flux predicted by the optimum network topologies (Fig. 1C and D respectively) are presented in Table 3 alongside the experimental values for comparison. From the data in Table 3, the model predictions were remarkably close to the experimental observations, indicating minimal deviation between them and thus adequate validity of the ANN models. This position was also corroborated by the goodness-of-fit statistics presented in Table 4.
Table 3.
Comparison of experimental results with ANN predicted results
| Run | Factors | Responses | |||||
|---|---|---|---|---|---|---|---|
| Encapsulation efficiency (%) | Flux (µg/cm2/h) | ||||||
| SPPC/CUR | Sonicator time (min) | Hydration volume (mL) | Experiment | Predicted | Experiment | Predicted | |
| 1 | 3.76 | 7.03 | 40.2 | 99.4 | 99.4 | 28.2 | 28.2 |
| 2 | 2.90 | 10.00 | 100.0 | 97.2 | 97.2 | 8.8 | 8.8 |
| 3 | 2.90 | 10.00 | 62.5 | 97.3 | 97.1 | 5.3 | 7.9 |
| 4 | 2.90 | 10.00 | 62.5 | 97.7 | 97.1 | 5.7 | 7.9 |
| 5 | 2.90 | 10.00 | 62.5 | 96.9 | 97.1 | 5.3 | 7.9 |
| 6 | 3.76 | 7.03 | 84.8 | 97.9 | 97.9 | 15.8 | 15.8 |
| 7 | 2.90 | 10.00 | 25.0 | 99.0 | 99.0 | 12.0 | 12.0 |
| 8 | 1.45 | 10.00 | 62.5 | 99.5 | 99.5 | 44.3 | 44.3 |
| 9 | 2.04 | 12.97 | 84.8 | 98.5 | 98.5 | 33.5 | 33.5 |
| 10 | 2.90 | 10.00 | 62.5 | 96.9 | 97.1 | 5.6 | 7.9 |
| 11 | 2.90 | 5.00 | 62.5 | 97.8 | 97.8 | 0.9 | 0.9 |
| 12 | 3.76 | 12.97 | 40.2 | 99.7 | 99.7 | 35.9 | 35.9 |
| 13 | 2.90 | 10.00 | 62.5 | 96.9 | 97.1 | 5.3 | 7.9 |
| 14 | 2.04 | 12.97 | 40.2 | 99.1 | 99.1 | 24.9 | 24.9 |
| 15 | 2.90 | 10.00 | 62.5 | 96.9 | 97.1 | 20.4 | 7.9 |
| 16 | 4.35 | 10.00 | 62.5 | 99.8 | 99.8 | 49.5 | 49.5 |
| 17 | 2.90 | 15.00 | 62.5 | 97.9 | 97.9 | 18.2 | 18.2 |
| 18 | 2.04 | 7.03 | 40.2 | 99.7 | 99.7 | 8.0 | 7.9 |
| 19 | 3.76 | 12.97 | 84.8 | 98.7 | 98.7 | 19.5 | 19.5 |
| 20 | 2.04 | 7.03 | 84.8 | 98.6 | 98.6 | 20.6 | 20.6 |
Table 4.
Goodness-of-fit statistics for ANN model
| Parameter | Response | |
|---|---|---|
| Encapsulation efficiency | Flux | |
| R | 0.9892 | 0.9744 |
| R2 | 0.9785 | 0.9495 |
| Adjusted R2 | 0.9721 | 0.9470 |
| MSE | 0.0322 | 9.8747 |
| RMSE | 0.1795 | 3.1424 |
| SEP | 0.0381 | 3.7157 |
| MAE | 0.0800 | 1.2450 |
| AAD | 0.08226 | 14.4681 |
Response surface plots
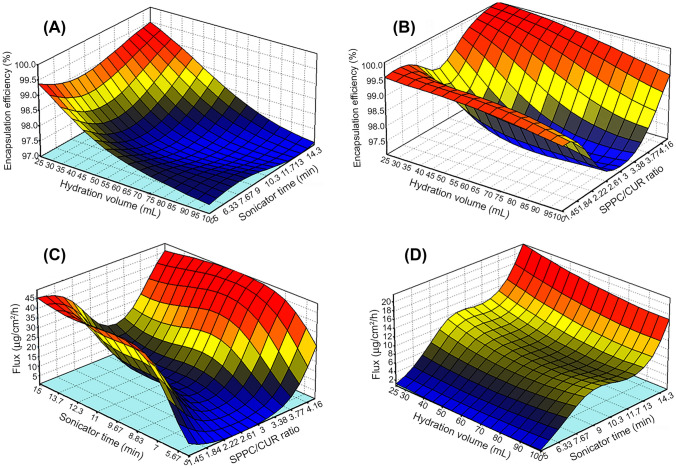
The response surface plots presented in Fig. 2A–D show the relationship between the responses (encapsulation efficiency and flux) and the input factors (SPPC/Cur ratio, sonication time, hydration volume). Response surface plots and contour plots for optimization of curcumin-loaded liposomes was generated using data in Table 3, inputs of 20 experimental runs carried out under conditions established by ANN: Encapsulation efficiency as a function of (A) hydration volume and SPPC/Cur ratio, Encapsulation efficiency as a function of (B) hydration volume and sonicator time, Flux as a function of (C) hydration volume and sonicator time, Flux as a function of (D) sonicator time and SPPC/Cur ratio.
Fig. 2.
Response surface plot showing effect of A hydration volume and sonicator time on encapsulation efficiency B hydration volume and SPPC:Cur ratio on encapsulation efficiency C effect of sonicator time and SPPC: Cur ratio on flux, D hydration volume on flux
Optimization of input factors and responses
The results of optimization of the input factors and the response carried out using genetic algorithm (GA), rotation inherit optimization (RIO) and particle swarm optimization (PSO) are summarized in Table 5. The optimum encapsulation efficiency predicted by all three optimization methods was 100%. GA and RIO both predicted the same optimum values for SPPC/CUR ratio (4.35), sonicator time (15 min) and hydration volume (25 mL) while PSO predicted slightly different values for sonicator time (14.87 min) and hydration volume (25.41 mL). The maximum flux was predicted by GA with RIO and PSO predicting slightly lower values of 51.23 µg/cm2/h and 51.24 µg/cm2/h, respectively. GA and RIO both predicted the same optimum values for SPPC/CUR ratio (4.35), sonicator time (10.49 min) and hydration volume (25 mL) while PSO predicted slightly different values for sonicator time (10.54 min) and hydration volume (25.18 mL).
Table 5.
Optimized conditions for encapsulation efficiency and flux
| Variables | Optimisation algorithms | |||||
|---|---|---|---|---|---|---|
| GA | RIO | PSO | ||||
| Encapsulation efficiency (%) | Flux (µg/cm2/h) | Encapsulation efficiency (%) | Flux (µg/cm2/h) | Encapsulation efficiency (%) | Flux (µg/cm2/h) | |
| SPPC/CUR | 4.35 | 4.35 | 4.35 | 4.35 | 4.35 | 4.35 |
| Sonicator time (min) | 15.00 | 10.49 | 15.00 | 10.49 | 14.87 | 10.54 |
| Hydration volume (mL) | 25.00 | 25.01 | 25.00 | 25.00 | 25.41 | 25.18 |
| Maximum value | 100.00 | 51.26 | 100.00 | 51.23 | 100.00 | 51.24 |
Scanning electron microscopy of liposomes and in vitro permeability
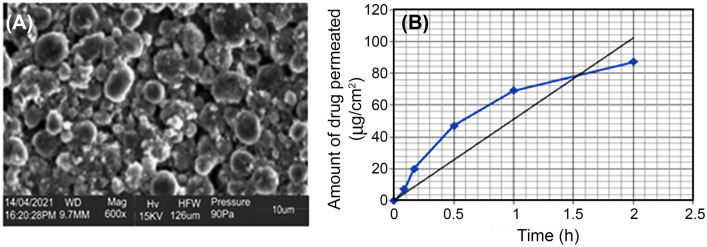
Considering the results of the scanning electron microscopy in Fig. 3A, the size liposomes were spherical with uniformed size distribution. Liposomes prepared through thin-film hydration method were observed under scanning electron microscope to characterize the morphologies and shapes of the liposomes. All samples were observed under magnification to confirm the occurrence of liposomes. Scanning electron microscope images show that liposomes were formed in the formulations. Liposomes produced were large well-defined spherical liposomes 10 µm in size.
Fig. 3.
A SEM of curcumin-loaded liposomes, showing the morphology and size, B shows the graph of flux (in vitro permeability) over time
To obtain quantitative and qualitative information, the release properties of liposomal formulations loaded with curcumin were determined using a modified Franz diffusion cell and plain buffer saline (pH 7.4) as the diffusion fluid. In this study, release kinetics showed an initial burst release of liposomal curcumin within the first 30 min, which is considered important for attaining higher levels of curcumin for cutaneous penetration (Fig. 3B). There is a continuous increase in the cumulative release of curcumin over the next hour, which levels out in the next 30 min. The optimal in vitro was obtained after preparing the optimized liposomes formulation with the parameters derived from the artificial neural network mathematical modeling. Sonicator time was 15 min, phospholipid–curcumin ratio was 4.35 and hydration volume was 25 mL.
Discussion
Artificial neural network architecture and training
Artificial neural network architecture is based on the structure and function of the biological neural network of the human system. Artificial neural network is composed of neurons which are arranged in several layers like neurons in the brain. It is made of three layers, namely input layer, output layer, and hidden intermediate layers. Artificial neural network is used to predict result based on scientific experiments with the aid of a transfer function which translates the input signals to output signals. For the selection of best transfer function, the best transfer function for the percentage encapsulation efficiency was the sigmoid transfer function because it had the highest R2 value and the lowest (root mean squared value) RMSE value. While that of the flux was Tanh with the highest R2 value and the lowest RMSE value. Hence, the sigmoid and Tanh transfer functions were selected for artificial neural architecture. Artificial neural architecture can be explained as a group of complex structures made of artificial neurons that can take in multiple inputs and translate to meaningful output. For the selection of best network architecture, the Levenberg–Marquadt algorithm had the lowest RMSE value and the highest R2 value for encapsulation efficiency and flux. The Levenberg–Marquardt algorithm was independently designed by Kenneth Levenberg and Donald Marquardt, presents a mathematical solution to the pickle of minimizing a non-linear function (Cavuslu and Sahin 2018). In the field of artificial neural networks, this algorithm is suitable for training minor and medium-sized problems. The two architectural networks used for the encapsulation efficiency and flux had three input variables and four hidden layers of neurons and one output variable (Amenaghawon and Amagbewan 2017; Ilomuanya et al. 2020a).
Assessment of ANN model predictions
Generally, the ANN models for encapsulation efficiency and flux displayed high predictive capacity as seen in the high R (0.9892 and 0.9744, respectively), R2 (0.9785 and 0.9495, respectively) and adjusted R2 values (0.9721 and 0.9470, respectively) as well as low MSE, RMSE, SEP, MAE and AAD values. The higher R, R2 and adjusted R2 values as well as the lower MSE, RMSE, SEP, MAE and AAD values for the case of ANN model for encapsulation efficiency compared with those of the model for flux indicates that the ANN model for encapsulation efficiency performed better than that for flux. Nevertheless, all the R2 values were greater than 0.8 indicating adequate fit between experimental observations and model predictions.
Response surface plots
Figure 2A–D shows the 3D plots (for maximum encapsulation efficiency and flux) in a way that visualizes the interactions of the independent variables. The response surface plots show the relationship between the dependent variables (encapsulation efficiency and flux), and the input factors (lipid/curcumin ratio, sonication time, and hydration volume). Figure 2A shows the response surface plot of the effect of hydration volume and SSPC/Cur ratio (lipid/drug ratio) on encapsulation efficiency. Figure 2A shows an increase in encapsulation efficiency with a decrease in hydration volume and an increase in SPPC/Cur ratio. The values of hydration volume and SPPC/Cur ratio correspond to 25 mL and above 4.16, respectively, produced liposomes with a maximum encapsulation efficiency of 99.6%. Hence, an SSPC/ Cur ratio of above 4.16 and hydration volume of 25 mL is highly recommended.
Curcumin is a hydrophobic drug molecule and is trapped within the bilayer of the liposomes. An increase in lipid ratio will increase the sites for which curcumin can be trapped thus leading to a higher amount of entrapped curcumin and higher encapsulation efficiency. In a study by Torres-Flores et al., it was also observed that the increased lipid/drug ratio led to an increase in encapsulation efficiency of the hydrophobic drug Everolimus. This occurred because of the increase in the number of sites that Everolimus can be entrapped (Torres-Flores et al., 2020).
Figure 2B shows that there is an increase in encapsulation efficiency with a decrease in hydration volume. The values of sonication time and hydration volume corresponding to 14.3 min at 25 mL, respectively, produced liposomes with 99.3% encapsulation efficiency. The liposomes obtained with 99.3% encapsulation efficiency were highly stable and had the ability to store large quantities of curcumin in their vesicle space with minimum leakage. Parameters such as hydration volume of over 25 mL during the process of production of liposomes led to lower encapsulation efficiency due to the dilution of the medium (Torres-Flores et al. 2020). Encapsulation efficiency decreased as the hydration volume was increased suggesting an antagonistic effect as shown in Fig. 2B. This trend was observed for all levels of sonication time and SPPC/Cur ratio. Concerning the sonication time, there was no observable effect on the encapsulation efficiency by the sonication time, although there was a slight upward trend at low hydration volumes. The encapsulation efficiency had an overall positive trend for SPPC/Cur ratio as shown in Fig. 2A. Maximum encapsulation efficiency was recorded at high SPPC/Cur ratios and low hydration volumes. Flux which is the in vitro drug permeability was positively influenced by both sonication time and SPPC/Cur ratio. An increase in sonication time and SPPC/Cur ratio led to increasing flux. At a maximum sonication time of 15 min and an SPPC/Cur ratio of above 4.16, a flux of 45 µg/cm2/h was observed in Fig. 2D. The same observation was also revealed in Fig. 2C for sonication time. Although the effect of hydration volume was not significant at low sonication, there was however a slight antagonistic effect on flux at higher sonication times.
Sonication reduces the size of the liposomes, thus enhancing liposomal stability. The size of a liposome is determined during the formulation process, and it reduces because of the inclusion of external energy. The energy input during sonication breaks down the lipid layer into smaller bits and then these bits enclose to form vesicles (de Matos et al.2019). In this study, energy input was in the form of sonication. Longer periods of sonication meant more input of energy therefore smaller and more stable liposomes. Stable liposomes lead to minimal content leakage; hence, more amount of drug remain encapsulated for release at the point of in vitro membrane permeation. Higher SPPC/Cur also leads to increased flux as a high lipid ratio will lead to higher encapsulation of the drug, hence more drug-loaded content for release. In a study by Nam et al., the liposomes size developed was smaller on subjection to an ultra-sonicator probe thereby improving liposome stability (Nam et al. 2018).
Size and morphology of optimized curcumin-loaded liposomes
The shape and size of developed curcumin-loaded liposomal formulation were observed by scanning electron microscope. The liposomes were spherical with a smooth surface and homogenous size distribution with a mixed population of uni-lamellar small- and medium-sized vesicles. The liposome size distribution is vital to the performance of the liposomal delivery drug system as they influence the drug pharmacokinetics and bioavailability (Peretz et al. 2018). It was observed that with increasing curcumin concentrations, there is a proportional increase in the size of liposomes. This may be as a result to the incorporation of the curcumin in the outer bi-lipid layer around liposomes. The results are comparable with previous studies which investigated the effect of liposome formulation techniques and variables on their physical characters. In a study by Salem et al., the liposome size and morphology were dependent on the concentration of starch used in liposome preparation (Salem et al. 2018).
Encapsulation efficiency of optimized formulation
The percentage encapsulation efficiency of the developed curcumin-loaded liposome was found to be 99.934 ± 1.0% (mean ± SD, n = 3). The percentage of loaded drug content indicated that the liposomes were stable, and the curcumin was well loaded in vesicular dispersions. A percentage encapsulation efficiency near 100% (predicted value) indicated minimal leakage from liposomes and good stability of liposomes. In a study by Vakili-Gharvatol, the liposomal loading conditions of the drug Docetaxel were optimized, the transmission electron microscope of the loaded liposomes was also spherical; however, the Docetaxel which is also a hydrophobic drug showed low drug-to-lipid ratios and comparatively low encapsulation efficiency of 34.3–67.18% (Vakili-Ghartavol 2020). This may be due to unstable liposomes. In another study by Gim Ming Ong, the effect of liposome encapsulation efficiency on the bioavailability of the hydrophobic drug griseofulvin was investigated (Ong et al. 2016). Two griseofulvin-loaded liposomes were formulated and labeled F1 and F2 with encapsulation efficiencies of 32% and 98%, respectively. It was observed that the griseofulvin-loaded liposomes (F2) with 98% encapsulation efficiency, showed a higher bioavailability in adult male, Sprague–Dawley rats. The size and high lipid ratio in the curcumin-loaded liposomes encouraged good encapsulation efficiency (Sadarani et al. 2019).
The in vitro drug permeability of the optimized formulation (flux)
The flux which is the in vitro drug permeability release value and also the slope of the graph of the amount of curcumin permeated in vitro (µg/cm2) as a function of time (h) was found to be 51.229 µg/cm2/h (mean ± SD, n = 3). The predicted value was 51.23 µg/cm2/h. The closeness of the predicted value to the actual experimental value shows the reliability of the mathematical model. In Fig. 3B above the graph shows a gradual increase in the permeation of curcumin with a peak after 2 h. Flux describes an effect that appears to sip through a membrane from one side to the other. Flux is also dependent on the size, morphology, and encapsulation efficiency of the liposome formulation. A higher lipid content leads to the formation of more liposomes with a greater amount of bi-lipid area to accommodate the hydrophobic drug curcumin. This leads to a more efficient encapsulation. Increased encapsulation efficiency of curcumin is associated with high levels flux as more drug is available in liposome to permeate the membrane over time (Sadarani et al. 2019).
In a study by Sadarani et al., methotrexate was encapsulated in deformable liposomes and embedded in a hydroxyethyl cellulose gel for topical use. The effect of liposome size and entrapment efficiency on the flux of the drug were investigated. The flux of the liposomal formulation was determined using a modified Franz cell apparatus (Sadarani et al. 2019). The drug entrapment was about 42%, while the in vitro drug permeability was about 17.37 μg/cm2/h. The low drug entrapment brought about low in vitro drug permeability. In another study by Maione-Silva, ascorbic acid was encapsulated into negatively charged liposomes to evaluate skin permeation and retention (Maione-Silva et al. 2019). Drug flux was also affected by the liposome composition, presence of cholesterol, and the liposomes' negative surface charge (Katuwavila et al. 2016).
In an investigation by Fathalla et al. (2020) liposomal gels were prepared for the dermal delivery of Anthralin as therapy for psoriasis. To assess the effect of drug/lipid ratio on encapsulation efficiency, the liposomes were formulated at varying drug lipid ratios and results showed that higher encapsulation and flux were observed at a higher drug/lipid ratio. Finally, in a study by Katuwavila et al. (2016) caffeic acid was encapsulated by liposomes to enhance its delivery. The encapsulation efficiency was observed as 70% with a flux of about 80% of liposomal drug permeability over 8 h, the high encapsulation efficiency translated to a high liposomal drug release and permeability (Fathalla et al. 2020).
Conclusion
Artificial neural networking was successfully employed for the optimization of the development of curcumin-loaded liposome formulations. The results of the above optimization study displayed that the tuning of the phospholipid/curcumin ratio, sonication time, and hydration volume led to an enhancement in rate and extent of in vitro release (flux) and encapsulation efficiency. The maximum encapsulation efficiency and flux predicted through ANN were 100% and 51.23 µg/cm2/h, respectively. The values obtained experimentally were 99.934% and 51.229 µg/cm2/h, respectively. The close fit between the two consecutive values and the high values obtained encapsulation efficiency and flux lead to a conclusion that the proposed artificial neural networking is a very useful tool for the preparation and optimization of curcumin-loaded liposomes for use in varying applications.
Author contributions
IC carried out the experiments. NAA and MI analyzed the data. MI, IC, AND NAA designed the project. IC, MI, NAA, CA drafted the manuscript. IC and MI performed the manuscript editing and final improvement. All authors approved the manuscript for submission.
Funding
This research received no funding.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Ethical approval is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amenaghawon N, Agbedor M. Multilayer full feed forward neural network for modelling and optimizing nutrient medium composition for oxalic acid production from sweet potato peels. ATBU J Sci Tech Edu. 2021;9(1):44–53. [Google Scholar]
- Amenaghawon N, Amagbewan E. Evaluating the effect of acid mixtures and solids loading on furfural production from sugarcane bagasse: optimization using response surface methodology and artificial neural network. Niger Res J Eng Environ Sci. 2017;2(2):578–587. [Google Scholar]
- Amenaghawon N, Kazeem J. Evaluation of the comparative performance of response surface methodology, artificial neural network and adaptive neuro fuzzy inference system for modelling and optimising oxalic acid production from pineapple waste. FUW Tre Sci Tech J. 2020;5(1):255–263. [Google Scholar]
- Amenaghawon N, Yerimah E. Application of plant oils and surfactants as stimulating agents for optimum citric acid production from cassava bagasse. FUW Tre Sci Tech J. 2020;5(2):407–413. [Google Scholar]
- Amini G, Salehi F, Rasouli M. Drying kinetics of basil seed mucilage in an infrared dryer: application of GA-ANN and ANFIS for the prediction of drying time and moisture ratio. J Food Process Preserve. 2021 doi: 10.1111/jfpp.15258. [DOI] [Google Scholar]
- Çavuşlu MA, Şahin S. FPGA implementation of ANN training using Levenberg and Marquardt algorithms. Neural Net World. 2018;28(2):161–178. doi: 10.14311/NNW.2018.28.010. [DOI] [Google Scholar]
- Corrêa ACNTF, Pereira PR, Paschoalin VMF. Preparation and characterization of nanoliposomes for the entrapment of bioactive hydrophilic globular proteins. J Visual Exp. 2019 doi: 10.3791/59900. [DOI] [PubMed] [Google Scholar]
- de Matos MBC, Deckers R, van Elburg B, Lajoinie G, de Miranda BS, Versluis M, Schiffelers R, Kok RJ. Ultrasound-sensitive liposomes for triggered macromolecular drug delivery: formulation and in vitro characterization. Front Pharmacol. 2019 doi: 10.3389/fphar.2019.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathalla D, Youssef EMK, Soliman GM. Liposomal and ethosomal gels for the topical delivery of anthralin: preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics. 2020;12(5):446. doi: 10.3390/pharmaceutics12050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilomuanya MO, Elesho R, Amenaghawon A, Adetuyi A, Velusamy V, Akanmu S. Development of trigger sensitive hyaluronic acid/palm oil-based organogel for in vitro release of HIV/AIDS microbicides using artificial neural networks. Future J Pharm Sci. 2020;6:1. doi: 10.1186/s43094-019-0015-8. [DOI] [Google Scholar]
- Ilomuanya MO, Onwubuya CP, Amenaghawon AN. Development and optimization of antioxidant polyherbal cream using artificial neural network aided response surface methodology. J Pharm Technol. 2020;1(2):46–53. [Google Scholar]
- Katuwavila NP, Perera ADLC, Samarakoon SR, Soysa P, Karunaratne V, Amaratunga GAJ, Karunaratne DN. Chitosan-alginate nanoparticle system efficiently delivers doxorubicin to MCF-7 Cells. J Nanomater. 2016;2016:1–12. [Google Scholar]
- Khoo G, Lam K, Lee T. Advances in feedstock conversion technologies for alternative fuels and bioproducts. Elsevier; 2019. Microscale and Macroscale modeling of microalgae cultivation in photobioreactor: a review and perspective; pp. 1–19. [Google Scholar]
- Maione-Silva L, de Castro EG, Nascimento TL, Cintra ER, Moreira LC, Cintra BAS, Valadares MC, Lima EM. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci Rep. 2019 doi: 10.1038/s41598-018-36682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JH, Kim S-Y, Seong H. Investigation on physicochemical characteristics of a nanoliposome-based system for dual drug delivery. Nanoscale Res Lett. 2018 doi: 10.1186/s11671-018-2519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo I, López RR, Camacho-León S, Nerguizian V, Stiharu I. Comparative evaluation of artificial neural networks and data analysis in predicting liposome size in a periodic disturbance micromixer. Micromachines. 2021;12(10):1164. doi: 10.3390/mi12101164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S, Ming L, Lee K, Yuen K. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics. 2016;8(3):25. doi: 10.3390/pharmaceutics8030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AKV, de Barbosa RM, Fernandes MAC, Finkler L, Finkler CLL. Comparative analyses of response surface methodology and artificial neural networks on incorporating tetracaine into liposomes. Brazilian J Pharm Sci. 2020 doi: 10.1590/s2175-97902019000317808. [DOI] [Google Scholar]
- Peretz Damari S, Shamrakov D, Varenik M, Koren E, Nativ-Roth E, Barenholz Y, Regev O. Practical aspects in size and morphology characterization of drug-loaded nano-liposomes. Int J Pharm. 2018;547(1–2):648–655. doi: 10.1016/j.ijpharm.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Rahmani A, Alsahli M, Aly S, Khan M, Aldebasi Y. Role of curcumin in disease prevention and treatment. Adv Biomed Res. 2018;7(1):38. doi: 10.4103/abr.abr_147_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzi M, Kashaninejad M, Salehi F, Sadeghi Mahoonak AR, Ali Razavi SM. Modeling of rheological behavior of honey using genetic algorithm–artificial neural network and adaptive neuro-fuzzy inference system. Food Biosci. 2015;9:60–67. doi: 10.1016/j.fbio.2014.12.001. [DOI] [Google Scholar]
- Robson A-L, Dastoor PC, Flynn J, Palmer W, Martin A, Smith DW, Woldu A, Hua S. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front Pharmacol. 2018 doi: 10.3389/fphar.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarani B, Majumdar A, Paradkar S, Mathur A, Sachdev S, Mohanty B, Chaudhari P. Enhanced skin permeation of Methotrexate from penetration enhancer containing vesicles: in vitro optimization and in vivo evaluation. Biomed Pharmacother. 2019;114:108770. doi: 10.1016/j.biopha.2019.108770. [DOI] [PubMed] [Google Scholar]
- Salamanca CH, Barrera-Ocampo A, Lasso JC, Camacho N, Yarce CJ. Franz diffusion cell approach for pre-formulation characterisation of Ketoprofen semi-solid dosage forms. Pharmaceutics. 2018;10(3):148. doi: 10.3390/pharmaceutics10030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi F. Recent advances in the modeling and predicting quality parameters of fruits and vegetables during postharvest storage: a review. In J Fru Sci. 2019;20(3):506–520. doi: 10.1080/15538362.2019.1653810. [DOI] [Google Scholar]
- Salem H, Kharshoum R, Mahmoud M, Saleh A, Ebeid E. Development, and characterisation of a novel nano-liposomal formulation of Alendronate sodium loaded with biodegradable polymer. Ars Pharmaceutica. 2018;59(1):9–20. [Google Scholar]
- Saraf S, Jain A, Tiwari A, Verma A, Panda PK, Jain SK. Advances in liposomal drug delivery to cancer: an overview. J Drug Delivery Sci Technol. 2020;56:101549. doi: 10.1016/j.jddst.2020.101549. [DOI] [Google Scholar]
- Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K, Zielińska D, Salehi B, Setzer WN, Dosoky NS, Taheri Y, El Beyrouthy M, Martorell M, Ostrander EA, Suleria HAR, Cho WC, Maroyi A. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharma. 2020 doi: 10.3389/fphar.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Flores G, Gonzalez-Horta A, Vega-Cantu YI, Rodriguez C, Rodriguez-Garcia A. Preparation and characterization of liposomal everolimus by thin-film hydration technique. Adv Polym Tech. 2020;2020:1–9. doi: 10.1155/2020/5462949. [DOI] [Google Scholar]
- Umbarkar M, Thakare S, Surushe T, Giri A, Chopade V. Formulation and evaluation of liposome by thin film hydration method. J Drug DeliveRy Ther. 2021;11(1):72–76. doi: 10.22270/jddt.v11i1.4677. [DOI] [Google Scholar]
- Vakili-Ghartavol R, Rezayat SM, Faridi-Majidi R, Sadri K, Jaafari MR. Optimization of docetaxel loading conditions in liposomes: proposing potential products for metastatic breast carcinoma chemotherapy. Sci Rep. 2020 doi: 10.1038/s41598-020-62501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]