Abstract
Preclinical safety requirements and test methods have been standardized over time to guide medical device developers in the path needed to manufacture safe devices and achieve regulatory approval. Today, femtosecond lasers are commonly used in cataract and refractive surgeries. Currently, an industry standard to guide developers in preclinical testing of ophthalmic lasers does not exist. Consequently, the data presented in regulatory submissions may vary between manufacturers, making the regulatory review process more ambiguous. Here, the authors present a comprehensive discussion of preclinical test methods applied to the evaluation of an ophthalmic laser. We include in vitro and ex vivo models, as well as an in vivo rabbit model subject to corneal refractive treatments, for consideration in a preclinical safety evaluation plan. Scientific rationale to support the ocular endpoints of evaluation in the rabbit model to demonstrate safety is also presented and discussed.
Keywords: Femtosecond laser, In vivo model, Ophthalmic laser safety, Preclinical, Refractive surgery, Regulatory
Key Summary Points
| Why carry out this study? |
| Femtosecond lasers are commonly used in cataract and refractive surgeries. Preclinical animal studies are performed prior to use in human patients to understand the effect of laser procedures on the cornea, internal eye structures, and ocular function |
| Standardized preclinical test methods to guide manufacturers in the assessment of safety and performance of ophthalmic lasers do not exist. Consequently, the data presented in regulatory submissions may vary between manufacturers making the regulatory review process more ambiguous |
| Preclinical test methods are needed to show safety and effectiveness of refractive laser treatments on ocular tissue prior to human clinical trials |
| What was learned from the study? |
| Various in vitro, ex vivo, and in vivo models were shown to be effective in evaluation of safety and effectiveness of the refractive laser treatments on ocular tissue |
| An in vivo rabbit model subject to corneal refractive treatments was shown to be a useful tool for the evaluation of the laser energy on ocular tissue and ocular function |
Introduction
Refractive lasers, including the femtosecond laser, have become a standard tool in the field of ophthalmology, replacing surgical procedures traditionally performed manually, with faster and more precise treatments that have widely improved patient outcomes. The versatility and unique properties of lasers have allowed for multiple applications in ophthalmology including the correction of corneal refractive errors [1, 2], non-invasive treatment of glaucoma and retinal conditions [3, 4], and, most recently, improvements of several steps in cataract surgery [5–8].
Prior to femtosecond (FS) laser introduction into the corneal refractive treatment arena, excimer lasers were utilized in a technique called photo-refractive keratectomy (PRK), which involved removing a large area of epithelium (approximately 9 mm) manually with a spatula or brush or a pretreatment with the laser to expose the lamellar bed subsequently photoablated to the desired refractive outcome [9]. The next evolution was use of a microkeratome, a bladed micro-plane, to slice a flap of tissue to expose the lamella for photoablation and then replacement of the flap over the treated bed. This reduced the healing time and improved visual acuity more rapidly [2]. The popularity of corneal refractive surgery, the process of changing the curvature of the cornea to improve visual acuity, was heightened with the development of laser-assisted in situ keratomileusis, commonly referred to as LASIK.
The LASIK procedure was significantly changed with the commercial introduction of Intralase® FS Laser (Johnson & Johnson Surgical Vision) for corneal resection surgery in 2001 [10, 11]. In this bladeless method, the FS laser creates a corneal flap, replacing the keratome and eliminating most of the mechanical flap-related complications [2, 12, 13]. The success of IntraLase® inspired subsequent developments of new concepts and applications of FS laser technology, which has spurred the entry of newer FS laser models into the market [3]. One such model can perform the latest development in refractive laser surgery called SMILE (Small-incision lenticule extraction), a bladeless, all FS laser technique that utilizes the laser in the creation of an intrastromal lenticule and removal through a side cut in the cornea that corresponds to the desired refractive correction [1]. The FS laser mimics the cutting action of blade-based keratomes by scanning tightly focused patterns of laser pulses in the cornea, which results in a continuous cut in the cornea.
While refractive laser innovation continues to progress, the standardization of preclinical testing methods to demonstrate safety prior to clinical applications is lacking. Without existing regulations for preclinical requirements, manufacturers are challenged to create their own (unique) preclinical test plans, thus potentially providing more obstacles in the regulatory review process.
Inability to better assess and predict product safety leads to failures during clinical development. In the development of a new FS laser, preclinical animal studies are necessary to understand the effect of laser procedures on the cornea and internal eye structures and to assess the laser for safety and performance requirements prior to use in human patients [14]. International standards and FDA Regulatory Guidance Documents are key resources that guide the medical device industry in the methods of safety and biocompatibility testing, which typically involve demonstration through a combination of bench and performance testing using in vitro, ex vivo, and in vivo models [14]. Since the early 1990s, this biological evaluation has been guided by the International Standard (ISO) 10993-1 Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process that describes the assessment of medical devices based on risk mitigation, patient contact, and duration of patient contact [15]. The ISO 10993 series serves as a general framework for the development of a biological safety evaluation plan which defines how to demonstrate biocompatibility of a medical device. Furthermore, the US FDA has published a guidance document to provide clarification on the interpretation of ISO 10993-1 entitled Use of International Standard ISO 10993-1, Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process [16]. This document includes updated information regarding the use of a risk-based approach to determine if animal testing is needed and chemical assessment recommendations [16]. Vertical standards, or standards that are tailored to a specific medical device based on its use and unique characteristics, have also been published including several for ophthalmic devices, such as ISO 9394:2012 for contact lenses and contact lens care products, ISO 11979-5:2020 for intraocular lenses, and ISO 15798:2010 for viscosurgical devices [17–19].
Biocompatibility and physiochemical test evaluations are required on the Patient Interface (PI), a patient contacting accessory of the laser. The ISO 10993 standards guide this evaluation; however, a vertical standard or regulatory guidance specific for preclinical testing of ophthalmic laser effects to collateral tissues does not exist. Although an ophthalmic laser emits amplified light energy that impacts corneal tissue, this type of patient contact is not defined as either direct or indirect contact per ISO 10993-1; therefore, this general biocompatibility standard does not apply to ophthalmic lasers. Preclinical scientists are challenged to design testing plans using available medical device development guidance that can be applied to ophthalmic laser safety testing. As there is no specific regulatory industry guidance for ophthalmic lasers, preclinical test methods may vary between manufacturers. Here, we present a discussion of preclinical safety evaluation test methods and regulatory guidance that may be considered for the safety assessment of a new FS laser used for performing corneal lamellar resections in refractive surgery.
Patient Contacting Device
Precise delivery of the laser pulses into the eye requires an optomechanical interface that keeps the eye stable during the laser procedure [20]. A sterile single-use PI that directly contacts the anterior surface of the eye serves this function during refractive laser procedures. Typically, a new or modified patient-contacting device is subjected to several biocompatibility and physicochemical tests to ensure safety of the device prior to human clinical trials. The biological and physiochemical endpoints for consideration are largely determined based on the type and duration of patient contact. For the physiochemical evaluation, the PI is tested for aqueous leachables following the time/temperature recommendations of ISO 10993 and ISO 11979-5:2020 (Ophthalmic Implants-Intraocular Lenses-Part 5 Biocompatibility) [19]. The extractions are performed in water at 35 ± 2 °C for 72 ± 1 h and are assayed for leachables using ultraviolet–visible-near infrared spectroscopy and combined gas chromatography—mass spectrometry.
Per ISO 10993-1:2018, Table A.1, the FS laser PI is categorized as a surface medical device contacting mucosal membrane for a limited duration [15]. Based on this contact, the endpoints to be addressed in the biological evaluation include cytotoxicity, sensitization, and ocular irritation. Cytotoxicity testing can be performed using the MEM Elution method guided by 10993-5:2009 with test article extraction in minimum essential medium [21]. A sensitization study (Kligman Guinea Pig Maximization following ISO 10993-10:2010) and a Primary Ocular Irritation study (ISO 10993-10:2010) on the PI extract are also conducted [22].
Following the successful completion of a physicochemical and biology evaluation, including understanding the current literature and history of use in medical devices, the PI is considered biocompatible by the test methods used.
Bench and Ex Vivo Preclinical Models
Prior to human clinical trials, the effects of FS laser refractive treatment on corneal tissue as well as internal eye structures, such as the anterior chamber (AC), iris, lens, and retina, need thorough evaluation. A step-wise approach to this testing is presented in the following narrative that utilizes ex-vivo platforms in early development and progresses to in-vivo animal models in the later stages. All institutional and national guidelines for the care and use of laboratory animals were followed.
In early phases of development, FS laser refractive treatments are tested using glass, gel, and/or ex vivo tissue since these methods are the most efficient in terms of study execution time, resource availability, and cost. To verify laser pulse trajectories and segment placement, the laser patterns are initially cut into glass and then repeated in 10% agarose gel. The pliancy of the gel material allows for the manipulation and separation of the layers, mimicking the cornea, as a precursor to testing in ex vivo animal tissue. Ex vivo eye models such as porcine or rabbit eyes are commonly used as these models are similar in morphology to the human eye [23] and are readily available in comparison to human cadaver tissue. The porcine corneal surface area is larger than those of both the human and rabbit, allowing for greater experimental treatment area when evaluating corneal flap parameters [24]. Ex vivo sheep eyes are another commonly used model as the corneal thickness of sheep (approximately 620 µm) closely resembles human cornea (mean value of 550 µm) [25]. Finally, the laser patterns are evaluated in human cadaver eyes to demonstrate the effectiveness of the laser energy on the ocular tissues and repeatability of tissue manipulation and to establish pattern fidelity. Throughout ex vivo testing, the laser settings (pulse energy and location) are adjusted until optimal lamellar cuts are achieved (flap or lenticule) with minimal or no impact to surrounding eye tissue. These settings are later tested and confirmed during in vivo evaluation.
In Vivo Model
Animal models play important roles in ophthalmic research and the emerging technologies in cataract and refractive surgery [26]. Rabbit models are preferred largely because of the animal's docile nature and cost-effectiveness and the availability of a large normative database [27]. The popularity of the rabbit model also stems from its wide acceptance by regulatory bodies such as United States Food and Drug Administration (US FDA), American National Standards Institute (ANSI), and the International Standards Organization (ISO), who have published multiple standards and guidelines outlining test methods in rabbits as previously mentioned. Ophthalmic research is most frequently conducted in the New Zealand (NZ) species of rabbit. NZ White, NZ Red, or NZ Black rabbit models are used since their eye size and anatomy are similar to those of humans. NZ White rabbits lacking iris/retinal pigmentation are used for assessing laser energy on ocular tissues because of easy visibility of internal ocular structures [27]. Further verification of the laser treatments is tested in NZ black/red rabbits as these breeds contain pigmentation in the eye structures, similar to humans, which can absorb the laser pulse differently as it passes through the ocular tissues.
In vivo studies of the laser patterns are completed in NZ rabbits to demonstrate pattern fidelity and effectiveness as well as assess the postoperative healing response. Rabbits at approximately 4 months old are ideal as the AC at this age closely resembles the average AC size in adult humans (internal research data). As a side note, the rabbit corneal epithelium is thinner than that of human (approximately 30–40 µm thick [27]) and provides a “worst case” scenario for performance testing of the various laser treatments. Additionally, young rabbits up to 18 months of age can regenerate the corneal endothelium (via mitosis) as early as 36 h post injury [28]. For this reason, examination of the corneal endothelium post laser treatment for any signs of injury or cell loss would need to be completed at time points within this time frame.
In Vivo Studies
The focus of in vivo studies is to demonstrate laser refractive treatment effectiveness, ocular tissue effects, and the postoperative healing response. There are currently no international standards that define preclinical regulatory expectations for FS laser applications and the effect on collateral tissues by the laser pulses. As such, scientists are challenged to create a biological evaluation plan to demonstrate safety and effectiveness of the laser treatments predictive of use in humans. The authors rely on the standard that governs nonclinical testing of intraocular lenses (ISO 11979-5) [19] as guidance. The safety testing endpoints for both intraocular lenses and refractive lasers focus on possible effects to internal ocular structures and functions [i.e., intraocular pressure (IOP) and corneal metabolism].
Early development studies are initially conducted in nonclinical feasibility studies to evaluate safety and performance in a small number of animals. Feasibility studies are typically shorter in duration, require less resources and may involve fewer evaluation parameters than those included in larger studies. Modifications to the laser parameter settings can be made as needed until a final version is produced for testing in a larger pivotal study following Good Laboratory Practices (GLP) for inclusion in a regulatory submission.
Refractive FS laser treatment platforms are currently focused on two modalities: the creation of a corneal flap (a step in the LASIK procedure) and a method for corneal reshaping. In corneal reshaping, a small, lens-shaped piece of tissue (lenticule) is created within the cornea that corresponds to the desired refractive correction, which is subsequently extracted through a small laser-created corneal side-cut incision.
Study objectives focus on evaluating the effectiveness of the laser treatments in producing the intended corneal reshaping (flap or lenticule) and the safety of the laser energy on internal ocular structures and ocular function. The treated tissue is evaluated for ease of manipulation (lenticule removal or flap lift) and healing response. All laser treatments applied to the eye have a “power range for use” specified by the manufacturer, for example, 40–150 nJ. Manufacturers are required to test the complete laser power range in consideration of user error. Maximum laser exposure is a combination of maximum laser power focused through the tissues and laser-on/firing treatment time. Rabbits undergo laser treatments to establish data for the range of use at both standards, to mimic real-world refractive surgical treatments and maximum laser exposure and to mimic worst case laser application.
Prestudy Evaluations
Baseline ocular status for each rabbit is determined for eye structures including the lids, conjunctiva, sclera, cornea, iris, lens, anterior and posterior chamber, and retina. Current standard-of-care modalities that are designed to assess ocular structures are used, including slit-lamp biomicroscopy, corneal and retinal optical coherence tomography (OCT), specular microscopy (corneal endothelial cell count and density), retinal fluorescein angiography (FA), and tonometry (IOP).
A slit-lamp examination is used to assess external eye structures such as the eye lids, conjunctiva, sclera, and cornea for abnormalities including conjunctival hyperemia (redness) and swelling and corneal opacities. Internal eye structures are additionally examined with the slit-lamp including the iris for redness and pupillary response, the AC for cells and flare, and the lens for lens opacities (cataract). The corneal endothelium is examined using a specular microscope, which produces a detailed image of the corneal endothelium and an endothelial cell count. Retinal OCT is used to examine the retina for abnormalities, and corneal OCT is used to assess corneal health and measure corneal thickness. Retinal FA evaluates the retinal vessels for vascular leakage and a tonometer is employed to measure IOP prior to the laser surgery.
Surgical and Postoperative Evaluations
Observations conducted during the surgical procedure and postoperatively focus on examining and evaluating external and internal ocular structures and functions for potential adverse effects of the laser pulse treatments to collateral tissue, the effectiveness of the laser pulses to create the intended corneal reshaping, and monitoring the healing process. The postoperative monitoring phase typically takes place over a 30-day period, which closely approximates normal human healing status.
Delivery of the Laser Pattern and Tissue Separation
Eyes are examined using OCT imaging of the cornea to show that the laser pattern was delivered to the pre-programed trajectory location for each pattern segment confirming uniformity of depth and symmetry of placement (Fig. 1). Corneal topography imaging is another useful tool that can detail corneal curvature changes post surgery. In vivo corneal topography imaging was previously studied by the authors in the rabbit model using a Pentacam HR Scheimpflug camera to better understand corneal biometric measurements (Gray et al. Invest Ophthalmol Vis Sci. 2010;51:ARVO E-Abstract 4200). Effectiveness of the laser treatment is further evaluated through the manipulation of lenticle or flap (either the lenticule is extracted or the corneal flap is lifted) to evaluate ease of tissue separation (flap) and for ease of lenticule removal. A successful treatment occurs when the tissue manipulation does not disturb any collateral tissues. Tissue separation (cleavage) performed by the laser is a key factor for healing; if not cleaved well, tearing of tissues may occur leading to an extended healing process. It is critical to assess the completeness of tissue separation, as incomplete separation and tearing may impact stromal tissue causing higher order aberrations and visual degradation. This assessment may be accomplished by evaluation of adhesion levels and ease of separation during the surgical procedure and then adjusting laser settings as needed. Measurement of wavefront aberrations is another method that may be used to assess the cornea post surgery that has been previously performed in the rabbit model in vivo by the authors using a Wavescan Wavefront System [26].
Fig. 1.
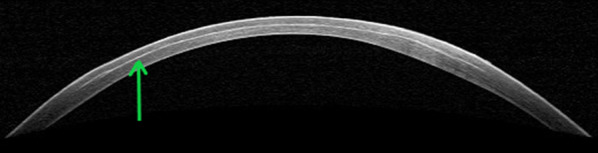
OCT image of cornea immediately post laser treatment with lenticule removed. Green arrow indicates post laser lamellar bubbles. The image allows assessment of collateral tissue effects of the laser pulse as well as laser pulse depth and location
Effects of the Laser Energy and Patient Interface on Anterior and Posterior Ocular Structures
Delivery of laser energy into the eye for surgical procedures requires a PI. The PI allows efficient delivery of the laser beam into the transparent ocular tissues and maintains mechanical stability of the eye during laser application [20]. Common PI designs include:
Flat corneal applanation (CA): a flat transparent window pressed against the cornea using a suction ring placed on the limbus/sclera to fix the eye in place.
Curved contact lens interface (CCI): approximates the natural radius of curvature of the anterior cornea reducing globe deformation when applanating the cornea using a suction ring to affix the eye.
Liquid optical immersion interface (LOI): uses a layer of transparent fluid placed between the cornea and an optical window [20] with a suction ring to affix the eye.
During laser refractive surgery, the PI device directly contacts the lids, cornea, and conjunctiva. Slit-lamp biomicroscopy is used to examine these ocular structures for any changes from contact with the PI or changes from the laser treatment (Fig. 2). The AC is examined for cells, a sign of inflammation, the iris for pigment change, pupillary response anomalies or changes to the blood vessels, and the lens for color, capsular disturbance, and cortical changes.
Fig. 2.
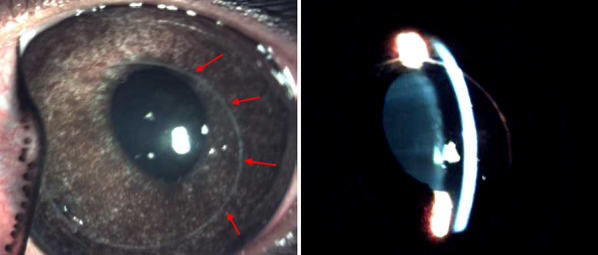
Rabbit eye slit-lamp images following LASIK procedure. Left, decentered nasal placement of LASIK flap, red arrows note the edge of the flap. Anterior eye structures and flap placement are examined with the slit-lamp biomicroscope. Right, slit-lamp beam illuminating the corneal epithelial layer
As previously stated, the PI works to stabilize the eye during the flap/lenticule cutting procedure through the application of suction, which is known to cause a temporary increase in intraocular pressure (IOP) experienced during the surgical procedure. Complications of refractive surgery, including damage to retinal tissue, relating to the fluctuating increases in IOP have been reported [29]. In vivo real-time IOP variations seen during LASIK flap creation have been studied in rabbits [30] by cannulation of the anterior chamber and direct IOP measurement during suction. Similar investigations have been performed by the authors using ex vivo porcine eyes. Histopathological evaluation of posterior eye structures, such as the retina and optic nerve, of animals subject to refractive surgery can further explore possible effects of this inevitable spike in IOP resulting from the application of suction.
Effects of the Laser Energy on the Corneal Endothelium
The corneal endothelium is the closest layer effected after the laser pulse focus point. The endothelium aids in maintaining corneal transparency by functioning as both a barrier to fluid movement into the cornea and an active pump that moves ions, and draws water osmotically, from the stroma into the aqueous humor [31]. A specular microscope is used to visualize the corneal endothelium and determine endothelial cell density (Fig. 3). Assessment of the endothelium at intervals post surgery can aid in determining whether there is damage to the endothelium. It is important to assess the endothelium not more than 36 h post surgery as younger rabbits (≤ 18 months) have been shown to regenerate the corneal endothelium post injury [28], and therefore any cell loss due to the refractive treatment may not be identified.
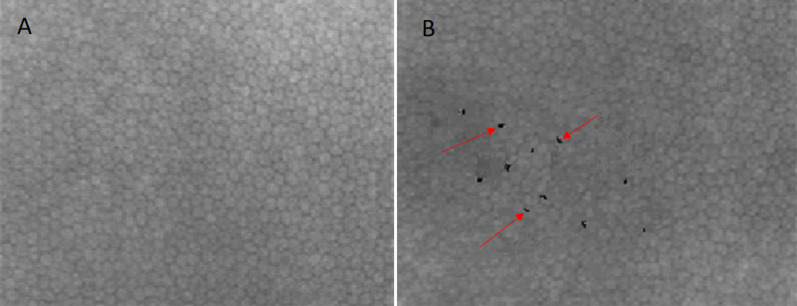
Fig. 3.
Heidelberg retinal tomography (HRT) of NZW rabbit corneal endothelium. Assessment of the corneal endothelium is important as this vital structure cannot regenerate if damaged by laser procedures. Normal cell count and density (A). Normal corneal endothelium image rendered to illustrate the appearance of induced corneal endothelium cell loss and density changes (indicated by red arrows) (B)
Careful imaging using confocal microscopy shows endothelial layer cell counts and density. The images below illustrate a normal corneal endothelium and an image rendering of a normal corneal endothelium to show how an abnormal endothelium with induced alterations may appear.
Effects of the Laser Energy on the Iris
The iris plays a key role in the regulation of light reaching the retina by way of the pupillary light reflex. This involuntary adjustment of the pupil size facilitates clear vision in varying light intensities and contributes to the ability to switch focus from near to far objects. Effects of the laser treatments on the iris are evaluated with the aid of the slit-lamp biomicroscope for signs of iris vessel congestion or redness, swelling, and any abnormalities of the iris tissue. The pupillary reflex is evaluated using the slit-lamp diffuse illumination setting for the natural dilation and constriction response.
Effects of the Laser Energy on the Retina
Retinal complications after refractive surgery include retinal tears, detachments, and hemorrhages [32]. Effects of laser treatments on the retina can be evaluated for structure and functional changes using fundoscopy, Heidelberg retina tomography (HRT), and optical coherence tomography (OCT). For minor vascular injury or leaking, retinal fluorescence angiography is commonly used. Figure 4 shows normal retinal structure on OCT.
Fig. 4.
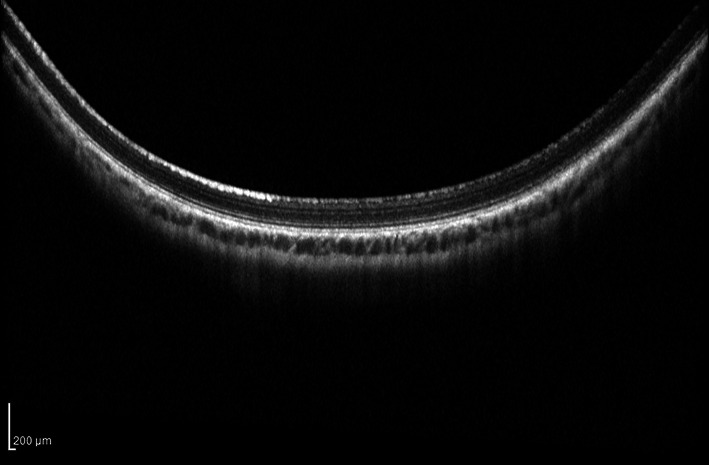
HRT OCT scan of retina, 30 days post surgery. Standard laser exposure with lenticule removed from the cornea; cross-section of retinal layers shows normal tissue
Effects of the Laser Energy on Ocular Function
Laser surgery has an effect on central corneal thickness, the corneal curvature, and corneal biomechanics change, which may affect intraocular pressure (IOP) measurements [33]. To assess these possible effects, IOP and corneal thickness (central, superior, and inferior locations) are measured immediately post surgery and at periodic intervals throughout the study period for expected changes and recovery after laser treatment. Experience with the rabbit model has shown an immediate postoperative increase in IOP measurements thought to be a result of the PI contact with the smaller rabbit eye. During refractive surgery, the PI is designed to fit the larger human eye, rather than the smaller rabbit eye; a higher portion of the rabbit globe is pulled up into the PI suction ring thereby applanating increased corneal surface area. This deforms the AC and blocks the trabecular meshwork (TM) causing an increase in IOP. However, experience with this model has shown that most rabbit IOPs return to normal within a 24-h period. Corneal thickness typically increases immediately post treatment because of tissue edema from manipulation of the flap or lenticule extraction.
Histopatholgical Evaluations
At the end of the study, rabbits are humanely killed for subsequent necropsy. The globes and extraocular tissues and glands are harvested and prepared by standard histopathological processing techniques including hematoxylin and eosin (H&E) staining (Figs. 5 and 6). Tissue slides are microscopically evaluated by a board-certified ophthalmic veterinary pathologist for any abnormalities from laser treatments focusing on the laser patterns within the targeted layers of the cornea as well as for possible collateral tissue damage to vital ocular structures including the lens, iris, retina, sclera, choroid, and optic nerve. At this microscopic level, the details of the laser patterns produced at the tested energy levels (standard vs. maximum) can be evaluated and compared to the pre-programmed laser specifications. These pattern details may include shape, depth, thickness, and line trajectories.
Fig. 5.
Histopathological section sampling of globes for high resolution imaging describing sections of interest
Fig. 6.
Examples of histology slides. A Stitched image showing appearance of laser incision lines (blue arrow, anterior segment; red arrow, posterior segment of the lenticule). B, C Lenticule laser incisions appear as thin purple/black lines
Conclusion
The path to market is long, costly, and inefficient due in large part to current reliance on cumbersome assessment methods. A new product development toolkit is urgently needed to improve predictability and efficiency along with a critical path from laboratory concept to commercial product. Laser standards regarding the safety of laser systems do exist, providing requirements and recommendations for safe use with which the personnel who operate, maintain, and service lasers must be familiar. However, current guidelines fail to provide specialized standards in the pre-clinical testing of FS lasers for developers.
This article discusses the preclinical safety evaluation test methods and regulatory guidance considered for the safety assessment of a new FS laser used for performing corneal lamellar resections in refractive surgery. Without proper controls in place and no existing regulations for preclinical testing of ophthalmic lasers to show safety prior to human use, most manufacturers have formulated their own testing techniques. Here, we have presented various studies and endpoints to consider for pre-clinical evaluation of ophthalmic lasers. We have shown that the lasers may have adverse effects on ocular tissue that require assessment during development. While each endpoint may not be appropriate for every laser, the overall aim is to demonstrate a potential evaluation plan in the preclinical testing of FS lasers in the absence of a standardized approach.
Bench testing and ex vivo studies first guide laser pattern assessment for trajectory and placement. Ex vivo eye models with animal and cadaver eyes are invaluable in demonstrating the effectiveness of laser energy and repeatability of tissue manipulation. In vivo studies utilizing rabbit models reveal the laser treatment effectiveness as well as the effects on the ocular tissue, including postoperative response. The in vivo studies we prescribe include examining the cornea to demonstrate the laser pattern was delivered and the expected tissue manipulation occurred. Effects of the laser energy on the anterior ocular structures, corneal endothelium, iris, and retina are critical to identify unintended damage or adverse events. Finally, by measuring IOP and corneal thickness, ocular function following laser energy treatment can be determined. Through this evaluation of patient contacting components, bench and ex vivo testing, followed by exhaustive in vivo surgical studies, a complete pre-clinical assessment of the ophthalmic laser can be achieved.
Acknowledgements
Funding
These studies and the journal’s Rapid Service Fees were funded by Johnson & Johnson Surgical Vision, Inc. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Lisa J. Kloft, James E. Hill, Ronika S. Leang, and Ling C. Huang drafted the manuscript; Lisa J. Kloft, James E. Hill, Ronika S. Leang, Arlene E. Gwon, and Ling C. Huang were involved in the concept, design, and/or review of the studies conducted.
Compliance with Ethics Guidelines
All institutional and national standards and guidelines for the care and use of laboratory animals were followed including National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and ISO 10993-2:2006 Biological evaluation of medical devices—Animal welfare requirements.
Disclosures
Lisa J. Kloft, James E. Hill, Ronika S. Leang, and Ling C. Huang are employees of Johnson & Johnson Surgical Vision, Inc. Arlene E. Gwon is a paid consultant of Johnson & Johnson Surgical Vision, Inc.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Marino GK, Santhiago MR, Wilson SE. Femtosecond lasers and corneal surgical procedures. Asia Pac J Ophthalmol (Phila) 2017;6(5):456–464. doi: 10.22608/APO.2017163. [DOI] [PubMed] [Google Scholar]
- 2.Roszkowska AMU, Mario S, Alberto AP. Use of the femtosecond lasers in ophthalmology. EPJ Web Conf. 2018;167:05004. doi: 10.1051/epjconf/201816705004. [DOI] [Google Scholar]
- 3.Callou TP, Garcia R, Mukai A, Giacomin NT, de Souza RG, Bechara SJ. Advances in femtosecond laser technology. Clin Ophthalmol. 2016;10:697–703. doi: 10.2147/OPTH.S99741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palanker DV, Blumenkranz MS, Marmor MF. Fifty years of ophthalmic laser therapy. Arch Ophthalmol. 2011;129(12):1613–1619. doi: 10.1001/archophthalmol.2011.293. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson KE, Braga-Mele R, Cabot F, et al. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753–1763. doi: 10.1016/j.jcrs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Han SB, Liu YC, Mohamed-Noriega K, Mehta JS. Application of femtosecond laser in anterior segment surgery. J Ophthalmol. 2020;2020:8263408. doi: 10.1155/2020/8263408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu L, Huang Y, Lin M. Excimer laser and femtosecond laser in ophthalmology. In: Viskup R, editor. High energy and short pulse lasers. InTechOpen; 2016. [Google Scholar]
- 8.Raoof-Daneshvar DS, Roni M. Femtosecond lasers in ophthalmology. US Ophthalmic Rev. 2013;6(1):38–41. doi: 10.17925/USOR.2013.06.01.38. [DOI] [Google Scholar]
- 9.McAlinden C. Corneal refractive surgery: past to present. Clin Exp Optom. 2012;95(4):386–398. doi: 10.1111/j.1444-0938.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 10.Bashir ZS, Ali MH, Anwar A, Ayub MH, Butt NH. Femto-lasik: the recent innovation in laser assisted refractive surgery. J Pak Med Assoc. 2017;67(4):609–615. [PubMed] [Google Scholar]
- 11.Durrie DS, Kezirian GM. Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis: prospective contralateral eye study. J Cataract Refract Surg. 2005;31(1):120–126. doi: 10.1016/j.jcrs.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Feng Y, Stojanovic A, Jankov MR, 2nd, Wang Q. IntraLase femtosecond laser vs mechanical microkeratomes in LASIK for myopia: a systematic review and meta-analysis. J Refract Surg. 2012;28(1):15–24. doi: 10.3928/1081597X-20111228-02. [DOI] [PubMed] [Google Scholar]
- 13.Chan A, Ou J, Manche EE. Comparison of the femtosecond laser and mechanical keratome for laser in situ keratomileusis. Arch Ophthalmol. 2008;126(11):1484–1490. doi: 10.1001/archopht.126.11.1484. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan AV, Baim DS, Smith JJ, et al. Medical device development: from prototype to regulatory approval. Circulation. 2004;109(25):3068–3072. doi: 10.1161/01.CIR.0000134695.65733.64. [DOI] [PubMed] [Google Scholar]
- 15.ISO 10993–1 biological evaluation of medical devices. Part 1: evaluation and testing within a risk management process. International Organization for Standardization; 2018.
- 16.U.S. Food & Drug Administration. Use of International Standard ISO 10993–1, “Biological evaluation of medical devices—part 1: evaluation and testing within a risk management process.” U.S. FDA; 2020.
- 17.ISO 15798 ophthalmic implants—ophthalmic viscosurgical devices. International Organization for Standardization; 2010.
- 18.ISO 9394 ophthalmic optics—contact lenses and contact lens care products—determination of biocompatibility by ocular study with rabbit eyes. International Organization for Standardization; 2012.
- 19.ISO 11979–5 ophthalmic implants—Intraocular lenses. Part 5: biocompatibility. International Organization for Standardization; 2020.
- 20.Talamo JH, Gooding P, Angeley D, et al. Optical patient interface in femtosecond laser-assisted cataract surgery: contact corneal applanation versus liquid immersion. J Cataract Refract Surg. 2013;39(4):501–510. doi: 10.1016/j.jcrs.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 21.ISO 10993–5 biological evaluation of medical devices. Part 5: tests of in vitro cytotoxicity. International Organization for Standardization; 2009.
- 22.ISO 10993–10 Biological evaluation of medical devices. Part 10: Tests for irritation and skin sensitization. Switzerland: International Organization for Standardization; 2010.
- 23.Menduni F, Davies LN, Madrid-Costa D, Fratini A, Wolffsohn JS. Characterisation of the porcine eyeball as an in-vitro model for dry eye. Contact Lens Anterior Eye. 2018;41(1):13–17. doi: 10.1016/j.clae.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Fyffe JG, Neal TA, Butler WP, Johnson TE. The ex vivo pig eye as a replacement model for laser safety testing. Comp Med. 2005;55(6):503–509. [PubMed] [Google Scholar]
- 25.Greene CA, Misra SL, Lee H, et al. The sheep cornea: structural and clinical characteristics. Curr Eye Res. 2018;43(12):1432–1438. doi: 10.1080/02713683.2018.1510970. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Huang LC, Gray B, Chernyak DA. Comparison of wavefront aberrations in rabbit and human eyes. Clin Exp Optom. 2014;97(6):534–539. doi: 10.1111/cxo.12184. [DOI] [PubMed] [Google Scholar]
- 27.Gwon A. The rabbit in cataract/IOL surgery. In: Tsonis P, editor. Animal models in eye research. Dayton: Elsevier Ltd; 2008. pp. 184–204. [Google Scholar]
- 28.Valdez-Garcia JE, Lozano-Ramirez JF, Zavala J. Adult white New Zealand rabbit as suitable model for corneal endothelial engineering. BMC Res Notes. 2015;8:28. doi: 10.1186/s13104-015-0995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahay P, Bafna RK, Reddy JC, Vajpayee RB, Sharma N. Complications of laser-assisted in situ keratomileusis. Indian J Ophthalmol. 2021;69(7):1658–1669. doi: 10.4103/ijo.IJO_598_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaurasia SS, Luengo Gimeno F, Tan K, et al. In vivo real-time intraocular pressure variations during LASIK flap creation. Invest Ophthalmol Vis Sci. 2010;51(9):4641–4645. doi: 10.1167/iovs.10-5228. [DOI] [PubMed] [Google Scholar]
- 31.Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond) 2003;17(8):912–918. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- 32.Arevalo JF, Freeman WR, Gomez L. Retina and vitreous pathology after laser-assisted in situ keratomileusis: is there a cause–effect relationship? Ophthalmology. 2001;108(5):839–840. doi: 10.1016/S0161-6420(00)00471-1. [DOI] [PubMed] [Google Scholar]
- 33.Shih CY, Graff Zivin JS, Trokel SL, Tsai JC. Clinical significance of central corneal thickness in the management of glaucoma. Arch Ophthalmol. 2004;122(9):1270–1275. doi: 10.1001/archopht.122.9.1270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.