Abstract
Many studies have demonstrated that curcumin has potential anticancer properties. This research aims to study the effect of iron (II, III) oxide (Fe3O4) nanoparticles coated with carboxymethyl chitosan containing curcumin combination with hyperthermia on breast cancer cells. Magnetic nanoparticles coated with carboxymethyl chitosan containing curcumin (MNP-CMC-CUR) were prepared and specified. MCF-7, MDA-MB-231, and human fibroblast cells were treated with free curcumin and MNP-CMC-CUR at concentrations of 0–60 µM and at different time points. A combined therapy of MNP-CMC-CUR and hyperthermia was performed on MCF-7 cells. The cytotoxicity of curcumin and MNP-CMC-CUR combined with hyperthermia was assessed by MTT. The changes in TP53 and CASPASE3 gene expression were evaluated using real-time PCR. Both cell apoptosis and cell cycle were studied by Annexin/PI staining. The results of MTT showed that the IC50 amount of MNP-CMC-CUR has significantly decreased compared to free curcumin (p < 0.05) and MNP-CMC-CUR in combination with the hyperthermia, and significantly reducing the metabolic activity of the cells (p < 0.05). Real-time PCR results revealed the up-regulation of TP53 and CASPASE3 (p < 0.05). The combinational therapy-induced cell apoptosis (64.51%) and sub-G1 cell cycle were arrested in MCF-7 cells. Based on these observations, a combination of MNP-CMC-CUR with hyperthermia could inhibit the proliferation of MCF-7 cells.
Keywords: Curcumin, Breast cancer, Carboxymethyl chitosan, Magnetic nanoparticle, Hyperthermia
Introduction
Cancer is a genetic disease associated with uncontrolled cell proliferation and cell death inhibition (Yahya and Alqadhi 2021). Breast cancer is the most common cancer in women that needs special attention due to its low response to conventional treatments (Miller et al. 2019). There are many ways to treat breast cancer, including surgery, chemotherapy, radiotherapy, and hormone therapy that have many side effects (Valencia et al. 2017; Waks and Winer 2019).
The ideal goal of treating cancers is to limit the toxicity of drugs to normal cells by targeting them (Klochkov et al. 2021). For this purpose, drug delivery systems have been developed that increase treatment efficacy (Mirzaie et al. 2016).
Some natural compounds have exhibited anticancer activity with minimal side effects (Manso et al. 2021). Curcumin is a polyphenolic compound extracted from the root of curcuma longa (turmeric). This bioactive drug has various biological properties, including anticancer, antiviral, antifungal, antioxidant, and anticoagulant (Wong et al. 2019). Curcumin could inhibit the growth of breast cancer cells and induce apoptosis (Ozkan et al. 2020). Curcumin limitation is due to the low solubility in water and its low cellular absorption (Moideen et al. 2021).
Nanocarriers could improve the solubility of drugs and deliver them to a specific target (Subramani et al. 2017). Magnetic nanoparticles (MNPs) have intrinsic magnetic properties and excellent biocompatibility (Materón et al. 2021). Fe3O4 has gained much attention from different iron-based magnetic nanoparticles due to its excellent magnetic properties and thermodynamic stability (Heydari et al. 2015). Fe3O4 is also a good option for clinical use due to its non-toxicity. Health officials only declare the use of Fe3O4-based magnetic nanoparticles safe for cancer treatment in patients with hyperthermia (Yalcin and Gündüz 2021). It should be noted that numerous studies have shown that at 100 mg/mL or higher concentrations, MNPs may cause toxicity or cytotoxicity by altering physicochemical properties (Vakili-Ghartavol et al. 2020). However, previous studies have shown that the use of nanoparticles for drug delivery and hyperthermia causes problems such as low drug loading capacity and accumulation of nanoparticles (Gu et al. 2020).
It has demonstrated that encapsulated curcumin in chitosan nanoparticles could improve bioavailability, cellular uptake, and anticancer activity on cervical cancer cells (Khan et al. 2016). Proper surface coatings can stabilize MNPs and prevent aggregation. It is also an effective way to prevent the dissolution and release of toxic ions, thus reducing overall toxicity (Laffon et al. 2018).
Several studies have been conducted on chitosan-coated Fe3O4 nanoparticles containing curcumin and A549 cancer cell treatment that suggest nanoparticle modification increases the uptake of nanoparticles into cancer cells resulting in increased cell death (Pham et al. 2016).
Using the appropriate surface coating for nanoparticles results in better dispersion and more stability of MNPs. Therefore, it is necessary to use polymers in the particle coating process to prevent the growth of iron oxide core and prevent the accumulation of nanoparticles (Barahuie et al. 2017). Chitosan is a derivative of glucan with chitin repeating units and an alkali polymer, non-toxic, biocompatible Saxena et al. (2021). Low solubility in water caused by chitosan hard crystalline structure limits its use in various processes. It is possible to increase the solubility by leveraging chitosan methylation modifications which result in CMC that has benefits for a wide range of applications in biomedical including drug delivery (Olanipekun et al. 2021). The active hydroxyl and amino groups of CMC polymer chains inhibit free radicals and enhance their antioxidant properties (Shariatinia 2018). In this study, CMC was used as a suitable coating to improve the solubility and performance of MNPs.
Hyperthermia is a therapeutic method that increases the temperature of the tumor regions by 41–45 °C, which may cause cell death and increase the toxicity of drugs (Espinosa et al. 2016). MNPs are targeted to absorb cancerous cells and produce hyperthermia under the influence of an alternating magnetic field (AMF). Usually, hyperthermia is used as an adjunct treatment to increase the effect of cytotoxic radiotherapy and chemotherapy (Chang et al. 2018; Jose et al. 2020). Researchers investigated the effect of MNPs loaded with Gemcitabine and NucAnt (N6L) and magnetic hyperthermia on pancreatic cancer cells. Their results showed that a combination therapy induces apoptosis, cell cycle arrest, and cell growth inhibition (Sanhaji et al. 2019).
Apoptosis suppression seems to play a key role in the onset and progression of cancer (Mortezaee and Rosengren 2019; Khan et al. 2017). It is recognized as one of the vital functions of the TP53 gene for tumor suppression (Zhou et al. 2019). Studies have also shown that CASPASE3 activation is necessary for inducing apoptosis (Boice and Bouchier 2020).
In this study, to improve curcumin solubility and increase cellular uptake, curcumin was encapsulated in Fe3O4 nanoparticles. In addition, we coated the nanoparticles with CMC to strengthen the drug delivery system. To increase the treatment efficiency, we used combination therapy with hyperthermia and investigated the effect of MNP-CMC-CUR with hyperthermia on cell viability, gene expression (expression of TP53 and CASPASE3 genes), cell cycle progression, and apoptosis in MCF-7 cell line. Finally, we compared the anticancer activity of MNP-CMC-CUR on two types of breast cancer cells: (1) MDA-MB-132, which are triple-negative, and (2) MCF-7 (ER/PR (+) and HER-2 (-)).
Materials and methods
Synthesis of curcumin-loaded Fe3O4 magnetic nanoparticle coated with carboxymethyl chitosan
MNPs were obtained from Daypetronic Co (Iran). The following protocol was then coated with CMC (250,000 kDa) and loaded with curcumin (Sigma, Germany). First, 0.2 g MNPs was added to 4 mL phosphate-buffered saline (PBS) at pH 6. Then, 1 mL of carbodiimide solution (0.025 g/L) was added to the previous solution and sonicated for 30 s at 100 watts. After that, 5 mL of CMC (Sigma, Germany) solution (50 g/L) and 1 mL curcumin solution was added to the same solution. The solution was then stirred vigorously for 1 h by mechanical stirrer. The ratio of curcumin to polymer was acquired at 10%.
Characterization of curcumin-loaded Fe3O4 magnetic nanoparticle coated with carboxymethyl chitosan
MNP-CMC-CUR were separated with centrifuged at 25,155 g for 15 min (MPW-260R, Poland) and washed 4 times with acetone. Finally, the nanoparticles were dried by vacuuming the oven in a droplet at 50 °C for 24 h. The morphology of nanoparticles was studied by field emission scanning electron microscopy (FE-SEM, Hitachi-4160-Japan) and in the range of 15 kV.
Particle size and its distribution and zeta potential of MNPs were determined by quasielastic laser light scattering with Malvern Zeta sizer (Nano ZS Malvern Instruments Ltd).
Infrared spectroscopy (FTIR) of carboxymethyl chitosan and Fe3O4 nanoparticles coated with carboxymethyl chitosan was obtained using KBR disk in the range of 4000–400 cm−1 reported by SRG1100G BOMEM Canada.
X-ray diffraction (XRD) has been reported by Equinox 3000 from the INEL Co to study the crystalline structure of bare and coated Fe3O4 nanoparticles. X-ray patterns were obtained using a Siemens D-5000 diffractometer with radiation CuKα (λ = 15.4 nm, 40 kV, and 30 mA) at 25 °C. Relative intensity was registered in a dispersion range (2θ) of 5–65 °C.
Cell culture
Human breast adenocarcinoma cell line (MCF-7), triple-negative human breast cancer cell line (MDA-MB-231), and human dermal fibroblasts (HDF) were obtained from the cell bank of Pasteur Institute, Iran. The cells were grown in high-glucose Dulbecco’s modified Eagle medium (DMEM, Gibco, USA) with 10% fetal bovine serum (FBS, Gibco, Germany) and 1% penicillin/streptomycin. The cell was incubated at 37 °C in 5% CO2. For cytotoxicity assay, 1 × 104 cells per well in 96-well plates were seeded. After 24 h, the cells were treated with free curcumin (10–60 µg), Fe3O4 magnetic nanoparticle coated with carboxymethyl chitosan (MNP-CMC) (0.25, 0.5, 1, 2 mg/mL) and MNP-CMC-CUR (10–40 µg/mL) for different time points. The experiments were carried out with three replicates, and untreated cells with culture medium served as control.
After 24, 48 and 72 h of incubation, the culture medium was removed, and the MTT (Sigma, Germany) solution (5 mg/mL in PBS) was added. The plates were incubated for 3–4 h. In the next step, the medium was removed, and 100 µL of dimethyl sulfoxide (DMSO, Sigma, Germany) was added to dissolve the formazan crystal. The absorbance was read at 570 nm using a microplate reader (BioTek ELx800, USA).
Hyperthermia
Design of hyperthermia device
The hyperthermia system includes a copper wire and a 30 V power supply. The number of rounds of the copper coil is 210 with a diameter of 1 (mm). The cell plate is placed in the middle of the coil, and two ends of the wire coil are connected to the power supply. The electric current is guided through the coil by turning on the power supply and creating an electromagnetic field (Fig. 1). The electromagnetic field can be calculated as the following:
where B, N, and are electromagnetic fields (µT), outer copper coil diameter (m), and electric current (A), respectively.
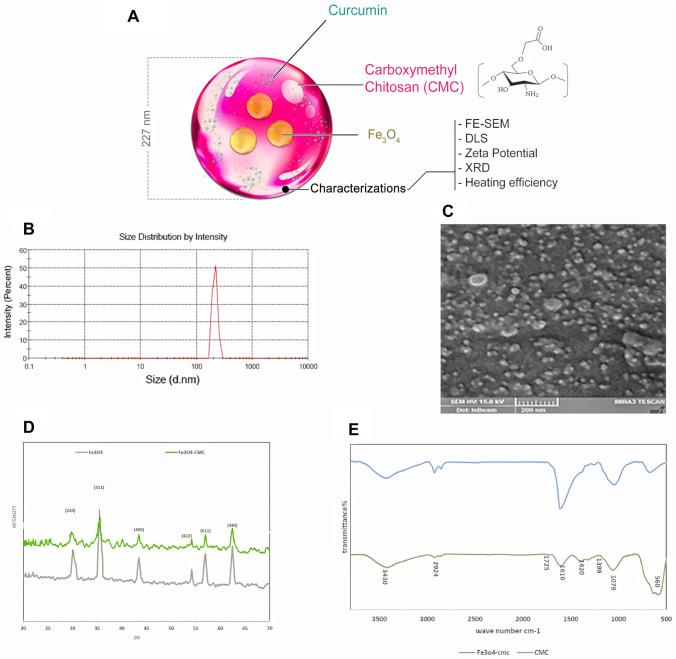
Fig. 1.
A Chemical structure of the MNP-CMC-Cur. B Size distribution of the nano-cur. C SEM images of nanoparticles (NPs). The spherical nanoparticles are observed with uniform dispersion. D XRD, X-ray patterns were obtained using a Siemens D-5000 diffractometer with radiation Cu Kα (λ = 15.4 nm, 40 kV, and 30 mA) at 25 °C. These patterns indicate that the coating process did not result in the crystalline phase change of Fe3O4. E FTIR of Fe3O4 nanoparticles and Fe3O4 nanoparticles coated with carboxymethyl chitosan. Comparing the two spectra indicates the presence of CMC in the coated nanoparticles and the successful synthesis of MNP-CMC
MNPs excited under the influence of this field are heated by their movements in the direction of the field of production. A digital thermometer accurately measured temperature changes.
Heating efficiency of MNPs under an alternating current (AC) external magnetic field
To evaluate the MNP-CMC effect to raise the temperature, nanoparticles dissolved in PBS (at different concentrations) were added to 1 well of 6-well plates, and in another well, only PBS was added. This plate was exposed to an AC magnetic field. Next, the temperature of each well at different time points and voltages were measured to set the system for the desired temperature. Finally, It was found that the instrument so constructed could generate magnetic fields in the frequency of about 39/564 µT to generate 43 °C (mild hyperthermia) through MNP-CMC-CUR.
In vitro hyperthermia experiments
MCF-7 cells at a density of 1 × 104 cells were seeded in 96-well plates. After 24 h, the cells were treated with MNP-CMC-CUR (10–40 µg/mL) and incubated for 48 h. Then, the plate was rapidly exposed to the electromagnetic field, under a specified time and temperature. In the next step, the MTT solution was added to each well and continued as in previous section.
Real-time quantitative PCR analysis
Total RNA extraction and cDNA synthesis
The MCF-7 breast cancer cell was seeded in 6-well plates at a density of 1 × 105 cells per well. After 48 h, the cells were treated with MNP-CMC-CUR and incubated for 48 h. After this time, one of the plates was heated at 43 °C for 60 min (effective time and temperature). Then, the total RNA of the treated and untreated cells was extracted by Trizole (Easy-BLUE total RNA extraction solution) according to the kit’s protocol (GeneAll® RiboEx, Korea). The extracted RNA in DEPC-treated water was stored at − 80 °C. According to the defined protocol, Pars Tous Biotechnology (Iran) Revert Aid TM First-Strand synthesized complementary DNA (cDNA).
Primer design was performed using the Primer premier v6 software (Thermo Fisher Scientific), and analyzed by Gene Runner software. The primer TP53 was performed as: F:5′-GCGAGCACTGCCCAACAACAC-3′ and R:5′-TCACGCCCACGGATCTGAAGG-3′ (amplicon size, 93 bp) and the CASPASE3 primer as F:5′-TGTCCATCCAGCAAACCTCAG-3′ and R:5′-TCCTCCTTAGAAACATCACGCATC-3′ (amplicon size, 117 bp). In addition, the gene sequence β2-microglobulin (β2M) used as the reference gene was F:5′-AGATGAGTATGCCTGCCGTG-3′ and R:5ʹ-GCGGCAATCTTCAAACCTCCA-3ʹ (amplicon size, 106 bp).
Real-time PCR
Real-time PCR reaction was performed by Rotor-Gene 6000 (Corbett Research, Sydney, Australia) and by the protocol SYBR green (Yektatajhiz, Iran). All reactions were conducted with 10 μL of SYBR Green PCR Master Mix, 3 μL cDNA (2 ng), 0.5 μL of each primer, and 6 μL of double-distilled water (final volume of 25 μL). The thermal cycling conditions are 95 °C for 15 s, 60 °C for 20 s, and 30 s for 72 °C for 40 consecutive cycles.
Apoptosis and cell cycle study
Cell apoptosis and cell cycle assay were performed based on 4 groups: MCF-7 cells with media (control group); cells treated with free-curcumin at IC50 dose (20 μg for 48 h); cells treated with MNP-CMC-CUR at IC50 dose (15 μg concentration for 48 h); cell treated with MNP-CMC-CUR (10 μg) and hyperthermia. Flow cytometry device evaluated the apoptosis and cell cycle assay (BD Biosciences, San Jose, CA, USA). For these tests, two 6-well plates were considered. In each well, 1 × 105 cells were seeded and incubated for 24 h. Then cells were treated with curcumin, MNP-CMC-CUR, and combination therapy of MNP-CMC-CUR and hyperthermia at 43 °C for 60 min. After a specific time, the cells were washed with PBS, trypsinized, and subjected to suspension with PBS. Annexin-V (Biolegend) was added to the suspension and then incubated for 10 min. Cell cycle assay was performed by staining (Propidium Iodide PI). The data were restored using Partec Flomax software.
Statistical analysis
p value was calculated using SPSS version 16 software, and the significance level was considered p < 0.05. The significance of the data obtained from Real-time PCR was investigated by the Relative Expression Software Tool (REST, Germany) 2009. Data were analyzed by one-way ANOVA.
Results
Physical and chemical characterization of bare Fe3O4 and MNP-CUR-CMC nanoparticles
The chemical composition of the MNP-CMC-CUR is presented in Fig. 2A. The results of the zeta sizer analysis of bare Fe3O4 and MNP-CUR-CMC showed − 4 mV and − 21 mV, respectively, of the zeta potential, and the results of particle size showed 227 nm for MNP-CMC-CUR. Based on the bare Fe3O4 datasheet, the particle size measured by BET (Brunauer–Emmett–Teller) was reported 10–20 nm (Fig. 2B, Table 1).
Fig. 2.

The hyperthermia system includes a copper wire and a high-voltage power supply. The number of rounds of the copper coil is 210. The cell plate is placed in the middle of the coil, and two ends of the wire coil are connected to the power supply. The electric current is guided through the coil by turning on the power supply and creates an electromagnetic field
Table 1.
Properties of bare Fe3O4 and MNM-CMC-CUR
| Sample | Size (nm) | Zeta | Loading efficiency | Loading capacity (Wt of curcumin/Wt of nanoparticles |
|---|---|---|---|---|
| MNP-CMC-CUR | 227 | − 21 | 90% | 0.047 |
| Bare Fe3O4 | Less than 20 | − 4 | – | – |
The micrograph of SEM showed that the nanoparticles are spherical and almost uniform dispersion (Fig. 2C).
FTIR spectra of CMC and MNP-CMC are presented in Fig. 2D-a. The absorption spectrum shows characteristic peaks at 3423 cm−1 OH stretching, 2924 cm−1 CH stretching, 1151 cm−1 O-bridge stretching, and 1079 cm−1 C–O stretching 1610 cm−1 also corresponds to C=O, indicating the binding of carboxymethyl groups to the chitosan chains. FTIR spectrum of MNP-CMC shows all the peaks related to CMC and a new peak at 560 cm−1 corresponding to the Fe–O group. Comparing the two spectra indicates the presence of CMC in the coated nanoparticles and the successful synthesis of MNP-CMC (Fig. 2E).
Figure 2D shows the XRD pattern for uncoated MNPs and MNP-CMC. Six characteristic peaks for the MNPs are identified with (220, 311,400) and (422, 440, 511) indices observed for all three samples. These patterns indicate that the coating process did not result in the crystalline phase change of Fe3O4.
The loading capacity (LC) of nanoparticles and curcumin loading efficacy (LE) are calculated from the following equations (Table 1):
where A is the total amount of curcumin in added solution, B is the total amount of curcumin in the supernatant after centrifuge, and C is the weight of the nanoparticles containing curcumin. The amount of drug in the solutions was quantified using UV–visible spectroscopy at of 440 nm.
MTT assay
The results illustrate that by increasing the dosage of free curcumin, the number of treated viable cells was significantly reduced compared to the control group (p < 0.05). IC50 values for MCF-7 cells treated with curcumin was 20 µg after 48 h (Fig. 3A). MCF-7 cells treated with MNP-CMC-CUR at different concentrations (0.25–2 mg/mL) showed no significant difference between the control and treated groups with MNPs (p < 0.05). Therefore, the lack of toxicity of MNP-CMC was confirmed for MCF-7 cells. MTT testing for HDF cell line at different concentrations and times was performed to confirm the non-toxicity of MNP-CMC-CUR for normal human cells. The results indicate that MNP-CMC-CUR did not affect the metabolic activity of normal cells at concentrations of 10–40 µg/mL for 72 h (Fig. 3B).
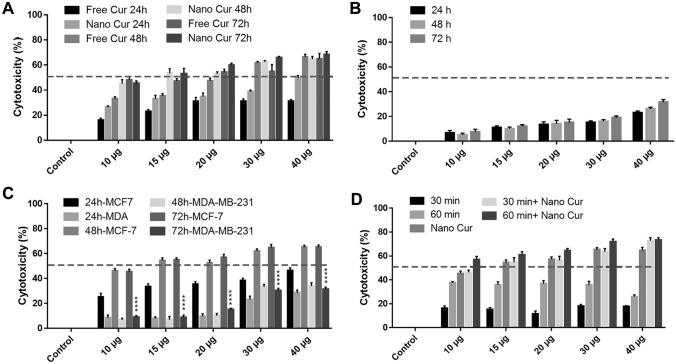
Fig. 3.
A The results of the MTT test of the MCF-7 treated cells with curcumin (10 to 60 μg/mL) after 24, 48, and 72 h. IC50 is the amount of free curcumin 20 μg (p ≤ 0.05) Statistical significance calculated by ANOVA. **p ≤ 0.05, ***p ≤ 0.001, B the results of the MTT test of the HDF cells treated with MNP- CMC-CUR (10–40 µg/mL) after 24, 48 and 72 h. The results indicate that MNP-CMC-CUR did not affect the metabolic activity of HDF cells. (p ≤ 0.05) Statistical significance calculated by ANOVA. **p ≤ 0.05, ***p ≤ 0.001, C comparison of MTT test results for MCF-7 and MDA-MB-231 treated with MNP-CMC-CUR at 24, 48, and 72 h. The results of the comparison of the viability of MCF-7 cells treated with free curcumin and MNP-CMC-CUR showed that IC50 for MNP-CMC-CUR significantly decreased (p < 0.05), D comparison of the effect of hyperthermia on the viability of MCF-7 cells at 30 and 60 min time. The cell viability rate was more than 60 min after 30 min, and the metabolic activity of the cells decreased significantly after 60 min (p ≤ 0.05). Statistical significance was calculated by ANOVA. **p ≤ 0.05, ***p ≤ 0.001
The results of MCF-7 cell treatment with MNP-CMC-CUR at different concentrations (10–40 µg/mL) and 24, 48, and 72 h showed that with increasing MNP-CMC-CUR dosage, the viable cells was reduced (p < 0.05). The IC50 value for MNP-CMC-CUR, 15 µg/mL, was obtained in 48 h (Fig. 3A). The amount of IC50 of MCF-7 treated with MNP-CMC-CUR cells was significantly reduced compared to free-curcumin (p < 0.05).
The MDA-MB-231 cells were exposed to different concentrations of MNP-CMC-CUR (10–40 µg/mL) for 24, 48, and 72 h (Fig. 3C). There was a significant (p < 0.05) difference between MCF-7 cells and MDA-MB-23 cells cytotoxicity (Fig. 4B).
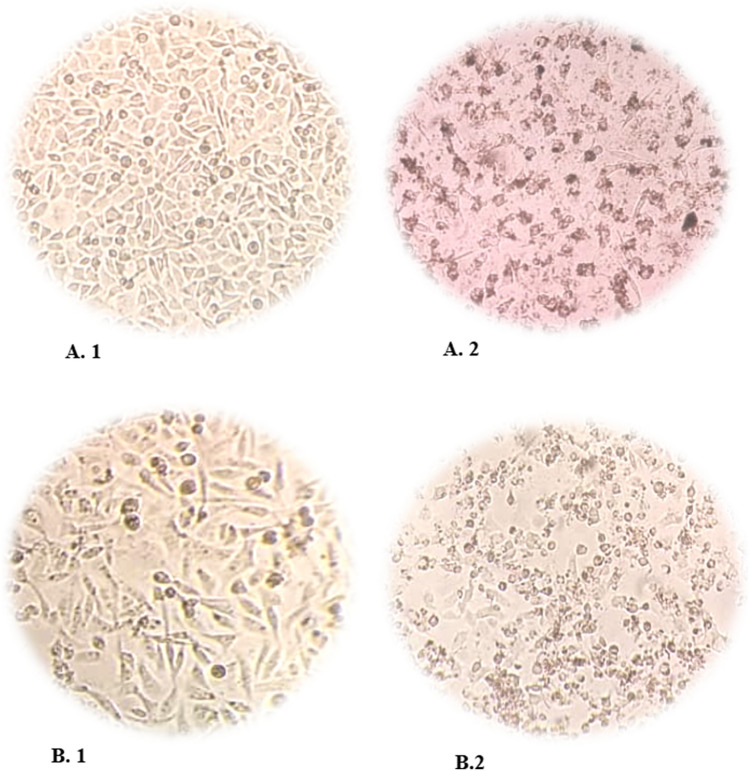
Fig. 4.
Microscopic images of MCF-7 and MDA-MB-231 cell lines. A1 control group of MCF-7 cells, A2 MCF-7 cells image after treated with MNP-CMC-CUR in IC50 dose and time (15 µg/mL in 48 h) compared to the control group, the cell density decreased and the morphology of the cells changed, which indicates that the drug is toxic to the cell. B1 Control group of MDA-MB-231 cells, B2 MDA-MB-231 cells image after treated with MNP-CMC-CUR in IC50 dose and time
Combinational therapy
MCF-7-treated cells with MNP-CMC-CUR at various concentrations (10–40 µg/mL) were incubated at 60 °C for 30 min and 43 °C for 48 h. The MTT test was started immediately after hyperthermia so that the cells were not exposed to more stress by various external factors. The results showed that by increasing MNP-CMC-CUR dosage, the viability of the cells has decreased. In addition, the mortality rate of MCF-7 cells after 60 min of hyperthermia was significantly higher than 30 min (p < 0.05) (Fig. 3D).
Comparison of the effect of two treatments on MCF-7 cells showed that MNP-CMC-CUR combination therapy with hyperthermia (60 min 43 °C) significantly reduced the metabolic activity of cells compared to monotherapy (p < 0.05).
Real-time PCR
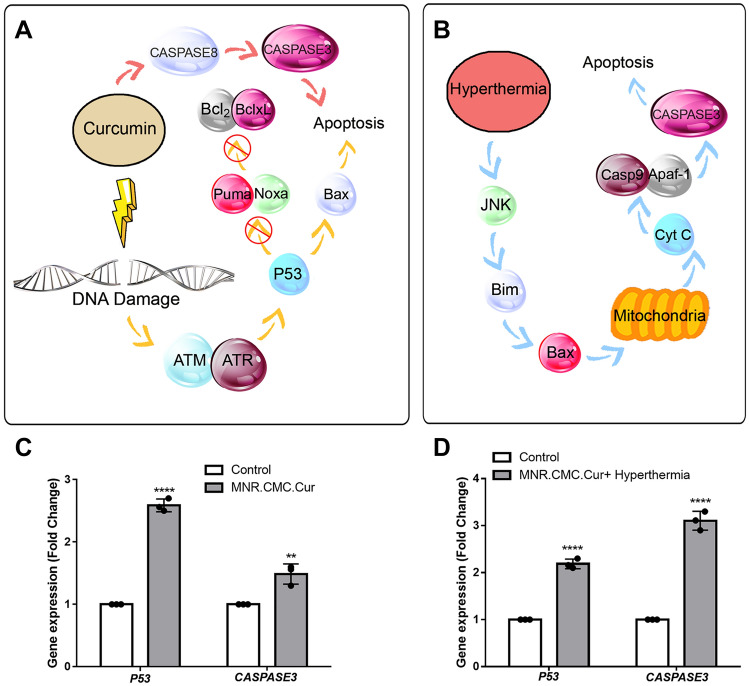
Real-time PCR results showed that the expression of TP53 and CASPASE3 genes in MCF-7 cells treated with MNP-CMC-CUR at an IC50 dosage significantly increased (p < 0.05). In addition, the results for the treatment of MCF-7 cells with MNP-CMC-CUR at 10 μg concentration after 48 h and hyperthermia with 43 °C for 60 min showed a significant increase in the expression of two TP53 and CASPASE3 genes (p < 0.05) (Fig. 5).
Fig. 5.
A Pathways of the effect of curcumin on apoptosis of the cells. B The pathways showed the effect of hyperthermia on apoptosis of the cells. C Real-time PCR results showing changes in the expression of the P53 and CASPASE3 genes and the control group compared to MCF-7 cells with MNP-CMC-CUR, the TP53 gene is 2.5-fold, and the CASPASE3 gene is 1.5 times compared to untreated control. D MCF-7 cells treated by MNP-CMC-CUR and hyperthermia, the TP53 gene was 2.16 times higher, and the CASPASE3 gene was 3.11-fold higher untreated cells. One-way ANOVA. **p ≤ 0.05, ****p ≤ 0.0001
Apoptosis and cell cycle analysis
The apoptosis percentage of MCF-7 cells treated with free curcumin, MNP-CMC-CUR, and combination therapy was 29.3%, 32.39%, and 95.9%, respectively (Fig. 6A). It indicates that the percentage of apoptotic cells in the combination treatment is approximately 63.5% greater than the apoptotic cells treated with MNP-CMC-CUR (Fig. 6B).
Fig. 6.
A, B Evaluation of MCF-7 cell apoptosis in response to treatment with curcumin, MNP-CMC-CUR, and combination therapy of MNP-CMC-CUR and hyperthermia. This indicates that the percentage of cells treated with apoptosis in the combination treatment is approximately 60% greater than the percentage of apoptosis in the cells treated with free curcumin and MNP-CUR. C, D cell cycle study of the MCF-7 treated by curcumin, MNP-CMC-CUR, and combination therapy of MNP-CMC-CUR and hyperthermia, which suggests that combination therapy, in contrast to other treatments, has further increased the sub-G1 percentage
Figure 6C indicates that MCF-7 cells treated with free curcumin at the dosage of IC50 increased the sub-G1 level by 7%, also increase in the number of cells in G1, and decrease in the number of cells in S and G2 phases were concluded accordingly compared to the control group. The MCF-7 cell cycle treated with MNP-CMC-CUR at the dosage of IC50 showed that the level of sub-G1 increased by about 10%, as well as the number of cells in the G1 phase and the number of cells in the S and G2 phases decreased compared to the control sample and the treated cells with free curcumin. The cell cycle chart for combination therapy shows that sub-G1 (cell death) has increased by 96% (Fig. 6D). These results indicate that combination therapy induces approximately 100% death of MCF-7 cells.
Discussion
Conventional treatments for breast cancer have many side effects and significantly lower the patient’s quality of life (Hossen et al. 2019). Today, the use of natural materials is more commonplace than chemicals. One of these is curcumin, which has attracted much attention in preventing and treating cancer (Mansouri et al. 2020). In this study, curcumin was used as an anticancer drug. It has shown several dose-dependent anticancer effects in many cancer models and pre-clinical trials. However, the clinical use of curcumin is limited due to its insolubility in water, low stability, and high metabolism in the body (Dhanavel et al. 2017). To overcome the problems encountered in the absorption of curcumin cells, magnetic nanoparticles coated with carboxymethyl chitosan (MNP-CMC) were used to load curcumin.
MNP-CMC in this study has a size of 227 nm and zeta potential-21, and spherical morphology (Fig. 2). Rod-shaped nanoparticles have a higher binding efficiency than spherical nanoparticles, since the curved mode, like the surface of spherical nanoparticles, limits the number of nanoparticle binding sites to link target cell surface receptors (Salatin et al. 2015; Banerjee et al. 2016).
Carboxymethyl chitosan was used to cover the MNPs due to its structural and biological properties. Many studies have shown that MNPs coating with chitosan not only prevents the accumulation of these particles but also provides an internal cavity suitable for drug loading (Parsian et al. 2016; Zavisova et al. 2019).
Zou et al. (2015) conducted a study on the toxicity of Doxorubicin’s anticancer drug in free and loaded MNPs-coated chitosan on MCF-7 cells. Their results showed that these nanoparticles have excellent biocompatibility and exhibit 85% viability of the cells at the highest concentration of the drug, confirming current results.
The results of the MTT showed that this nano-drug did not have any toxic effects on normal cells even at the highest concentration. Therefore, it can be said that this therapeutic method is a targeted and selective method for treating breast cancer cells.
Comparing the viability of MCF-7 cells treated with free curcumin and MNP-CMC-CUR showed that IC50 for MNP-CMC-CUR significantly decreased (p < 0.05) (Fig. 3A). This can be due to the stability of curcumin in nanoparticles.
Tabatabaei et al. confirmed that curcumin-loaded PLGA-PEG has a more toxic effect than free curcumin on cells (Mirakabad et al. 2016).
Subsequently, due to the growing trend of combination therapy, hyperthermia with MNP-CMC-CUR was used to treat MCF-7 cells. The results showed a significant relationship between hyperthermia time and cell viability. After about 30 min of heat, 40% of the cells died on average, while after 60 min, the percentage of dead cells passed to the average of 60%. Hyperthermia affects the fluidity and stability of cell membranes. It prevents the function of transmembrane proteins and cell surface receptors, thus facilitating the entry of nanoparticles into cells and increasing their function. In combination, treatment of nanoparticles containing anticancer drugs along with hyperthermia was due to increased accumulation of the drug in tumor cells, increasing drug toxicity due to improved cellular absorption of drugs and increased sensitivity of cells to the drug (Salimi Bani et al. 2019; Sun et al. 2018). In this regard, researchers investigated the effect of magnetite nanoparticles as a carrier of Temozolomide (TMZ) functionalized with folic acid-ligand (TMZ-MNP-FA) combined with hyperthermia C6 glioblastoma cancer cells. Their results showed that combined magnetite chemo-hyperthermia (AMF + TMZ-MNP-FA) treatment was significantly more efficacious in cancer cells than hyperthermia or chemotherapy treatments (p < 0.0001) (Minaei et al. 2019).
Studies have shown that curcumin can induce apoptosis in cancer cells from different pathways as an anticancer agent (Ali and Smiley 2018). Multiple apoptosis signaling pathways, apoptotic proteins, and genes have been identified as curcumin targets (Rao et al. 2014; Zhou et al. 2012). In addition, hyperthermia has been observed to modify the structure of the cell’s skeleton, such as cell shape, mitotic formations, lysosomes, and endoplasmic reticulum (Liao et al. 2019).
Gene expression results showed that the expression of P53 and CASPASE3 genes in MCF-7 cells treated with both therapeutic methods, nanoparticle-containing curcumin and combination therapy, significantly increased (p < 0.0001). Since CASPASE3 plays a significant role in apoptosis, increasing its expression in combination therapy can confirm the results.
Reports suggest that curcumin enhances P53 gene expression in tumor cells and induces apoptosis from a P53-dependent pathway (Hallman et al. 2017). This study may confirm a significant increase (p < 0.0001) in the P53 gene expression due to the release of curcumin from the MNP-CMC. Hou et al. studied hyperthermia on osteosarcoma (U-2 OS) cells (Fig. 5). They reported that the treatment of cells at 43 °C for 60 min induced apoptosis. After hyperthermia treatment, a significant increase (p < 0.0001) in the stopping of cancer cells in the G1 phase, an increase in expression of the CASPASE3 gene, and an increase in ROS production in these cells were observed (Hou et al. 2014).
Cell apoptosis study indicates 96% of apoptotic cells in combined therapy, while apoptosis was 31% due to treatment with MNP-CMC-CUR. The reason for this can be the denaturation of proteins at temperatures above 40 °C and the accumulation of denatured proteins, which itself creates a signal for the planned death of the cell (Fig. 6).
Geo et al. conducted a study on skeletal muscle satellite cells and examined the effects of thermal stress of 41 °C. The results showed that in addition to inhibition of cell growth, some stopped in the G0/G1 phase and a large number in the S phase of the cell cycle, and the number of cells in the G2/M phase decreased significantly (Gao et al. 2015).
These findings indicate that thermal stress inhibits the growth of cancer cells and stops their cell cycle, ultimately leading to their apoptosis.
After obtaining impressive results for MCF-7 breast cancer cell apoptosis, we examined the effect of MNP-CMC-CUR on another category of this cancer, MDA-MB-231 triple-negative breast cancer cells. As a result, MCF-7 cells have a better response to MNP-CMC-CUR than MDA-MB-231 (Fig. 3B).
Conclusion
Our goal in this study was to investigate the effect of curcumin-loaded magnetic nanoparticles coated with carboxymethyl chitosan combined with hyperthermia on breast cancer cells. Our findings demonstrated: (a) an increase in treatment efficacy with MNP-CMC-CUR compared to free curcumin, (b) combination therapy caused the treatment efficacy increases compared to any other treatment alone and (c) combination therapy caused apoptosis of MCF-7 cells by increased expression of the CASPASE3 and TP53 genes. Our results suggest that using herbal remedies instead of synthetic drugs, increasing drug effectiveness by encapsulating it in MNPs, and combining it with hyperthermia can increase the effectiveness of the treatment. Therefore, this study offers a promising combination therapy for breast cancer therapy.
Author contributions
SBK and SI designed the research. NP, NO and SI conducted the experiments. SMA contributed to hyperthermia design and device preparation. All the authors read and approved the manuscript, and all data were generated in-house, and no paper mill was used.
Funding
This manuscript is not supported by any foundation.
Availability of data and materials
All the data are available in an Excel file.
Declarations
Conflict of interest
No author has declared a conflict of interests.
Ethical approval
No humans or animals were used in this study.
Consent to participate
All the authors consent for their participation in doing the experiment and writing the paper.
Consent to publish
All the authors consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali M, Smiley R. Curcumin induces apoptosis via the capase-8 activated extrinsic pathway in MDA-MB-231 breast cancer cells. FASEB J. 2018;32:664–666. [Google Scholar]
- Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Cont Rel. 2016 doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani MS, Hatamie S, Haghpanahi M, Bahreinizad H, Alavijeh MHS, Eivazzadeh-Keihan R, Wei ZH. Casein-coated iron oxide nanoparticles for in vitro hyperthermia for cancer therapy. SPIN. 2019;9(2):1–10. doi: 10.1142/S2010324719400034. [DOI] [Google Scholar]
- Barahuie F, Dorniani D, Saifullah B, Gothai S, Hussein MZ, Pandurangan AK, et al. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int J Nanomed. 2017 doi: 10.2147/IJN.S126245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice A, Bouchier-Hayes L. Targeting apoptotic caspases in cancer. Biochimica Et Biophysica Acta - Mol Cell Res. 2020;1867:118688. doi: 10.1016/j.bbamcr.2020.118688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Lim M, Goos JACM, Qiao R, Ng YY, Mansfeld FM, et al. Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol. 2018 doi: 10.3389/fphar.2018.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanavel S, Nivethaa EAK, Narayanan V, Stephen A. In vitro cytotoxicity study of dual drug loaded chitosan/palladium nanocomposite towards HT-29 cancer cells. Mater Sci Eng C. 2017 doi: 10.1016/j.msec.2017.03.058. [DOI] [PubMed] [Google Scholar]
- Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C. Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano. 2016 doi: 10.1021/acsnano.5b07249. [DOI] [PubMed] [Google Scholar]
- Gao C, Zhao Y, Li H, Sui W, Yan H, Wang X. Heat stress inhibits proliferation, promotes growth, and induces apoptosis in cultured Lantang swine skeletal muscle satellite cells. J Zhejiang Univ Sci B. 2015 doi: 10.1631/jzus.B1400339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Yoshikiyo M, Namai A, Bonvin D, Martinez A, Piñol R, et al. Magnetic hyperthermia with ϵ-Fe2O3 nanoparticles. RSC Adv. 2020;10(48):28786–28797. doi: 10.1039/d0ra04361c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman K, Aleck K, Dwyer B, Lloyd V, Quigley M, Sitto N, et al. The effects of turmeric (Curcumin) on tumor suppressor protein (p53) and estrogen receptor (ERα) in breast cancer cells. Breast Cancer Targets Therapy. 2017;9:153–161. doi: 10.2147/BCTT.S125783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari M, Javidi M, Attar MM, Karimi A, Navidbakhsh M, Haghpanahi M, Amanpour S. Magnetic fluid hyperthermia in a cylindrical gel contains water flow. J Mech Med Biol. 2015;15(5):1–16. doi: 10.1142/S0219519415500888. [DOI] [Google Scholar]
- Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18. doi: 10.1016/j.jare.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CH, Lin FL, Hou SM, Liu JF. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human Osteosarcoma cells. Int J Mol Sci. 2014 doi: 10.3390/ijms151017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose J, Kumar R, Harilal S, Mathew GE, Parambi DGT, Prabhu A, et al. Magnetic nanoparticles for hyperthermia in cancer treatment: an emerging tool. Environ Sci Pollut Res. 2020;27(16):19214–19225. doi: 10.1007/s11356-019-07231-2. [DOI] [PubMed] [Google Scholar]
- Khan MA, Zafaryab M, Mehdi SH, Ahmad I, Rizvi MMA. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int J Biol Macromol. 2016 doi: 10.1016/j.ijbiomac.2016.08.050. [DOI] [PubMed] [Google Scholar]
- Khan MN, Haggag YA, Lane ME, McCarron PA, Tambuwala MM. Polymeric nano-encapsulation of curcumin enhances its anticancer activity in breast (MDA-MB231) and lung (A549) cancer cells through reduction in expression of hif-1α and nuclear p65 (Rel A) Curr Drug Deliv. 2017 doi: 10.2174/1567201814666171019104002. [DOI] [PubMed] [Google Scholar]
- Klochkov SG, Neganova ME, Nikolenko VN, Chen K, Somasundaram SG, Kirkland CE, Aliev G. Implications of nanotechnology for the treatment of cancer: recent advances. Semin Cancer Biol. 2021;69(August):190–199. doi: 10.1016/j.semcancer.2019.08.028. [DOI] [PubMed] [Google Scholar]
- Laffon B, Fernández-bertólez N, Costa C, Brandão F, Teixeira JP, Pásaro E, Valdiglesias V (2018) Cellular and molecular toxicity of iron oxide nanoparticles. In: Saquib Q, Faisal M, Al-Khedhairy A, Alatar A (eds) Cellular and Molecular Toxicology of Nanoparticles. Advances in Experimental Medicine and Biology, vol 1048. Springer, Cham [DOI] [PubMed]
- Liao S, Hu X, Liu Z, Lin Y, Liang R, Zhang Y, et al. Synergistic action of microwave-induced mild hyperthermia and paclitaxel in inducing apoptosis in the human breast cancer cell line MCF-7. Oncol Lett. 2019 doi: 10.3892/ol.2018.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso J, Sharifi-Rad J, Zam W, Tsouh Fokou PV, Martorell M, Pezzani R. Plant natural compounds in the treatment of adrenocortical tumors. Int J Endocrinol. 2021;2021:1–18. doi: 10.1155/2021/5516285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, Mohammadi M, et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20:1–11. doi: 10.1186/s12885-020-07256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materón EM, Miyazaki CM, Carr O, Joshi N, Picciani PHS, Dalmaschio CJ, et al. Magnetic nanoparticles in biomedical applications: a review. Appl Surf Sci Adv. 2021;6:100163. doi: 10.1016/j.apsadv.2021.100163. [DOI] [Google Scholar]
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019 doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- Minaei SE, Khoei S, Khoee S, Vafashoar F, Mahabadi VP. In vitro anticancer efficacy of multi-functionalized magnetite nanoparticles combining alternating magnetic hyperthermia in glioblastoma cancer cells. Mater Sci Eng C. 2019;101(April):575–587. doi: 10.1016/j.msec.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Mirakabad FST, Akbarzadeh A, Milani M, Zarghami N, Taheri-Anganeh M, Zeighamian V, et al. A Comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif Cells Nanomed Biotechnol. 2016 doi: 10.3109/21691401.2014.955108. [DOI] [PubMed] [Google Scholar]
- Mirzaie ZH, Irani S, Mirfakhraie R, Atyabi SM, Dinarvand M, Dinarvand R, et al. Docetaxel-Chitosan nanoparticles for breast cancer treatment: cell viability and gene expression study. Chem Biol Drug Des. 2016 doi: 10.1111/cbdd.12814. [DOI] [PubMed] [Google Scholar]
- Moideen J, Mohamed M, Alqahtani A, Khan BA, Fatease AA, Alqahtani T, et al. Preparation of soluble complex of curcumin for the potential antagonistic effects on human colorectal adenocarcinoma cells. Pharmaceuticals. 2021 doi: 10.3390/ph14090939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortezaee K, Rosengren RJ. Mechanisms of apoptosis modulation by curcumin: implications for cancer therapy. J Cell Physiol. 2019 doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- Olanipekun EO, Ayodele O, Olatunde OC, Olusegun SJ. Comparative studies of chitosan and carboxymethyl chitosan doped with nickel and copper: characterization and antibacterial potential. Int J Biol Macromol. 2021;183(March):1971–1977. doi: 10.1016/j.ijbiomac.2021.05.162. [DOI] [PubMed] [Google Scholar]
- Ozkan D, Eskiler G, Kucukkara S. The anti-proliferative and apoptotic effects of curcumin on feline mammary gland tumor cells in vitro. Iran J Vet Res. 2020;22:222. doi: 10.22099/ijvr.2021.40452.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsian M, Unsoy G, Mutlu P, Yalcin S, Tezcaner A, Gunduz U. Loading of Gemcitabine on chitosan magnetic nanoparticles increases the anticancer efficacy of the drug. Eur J Pharmacol. 2016 doi: 10.1016/j.ejphar.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Pham XN, Nguyen TP, Pham TN, Tran TTN, Tran TVT. Synthesis and characterization of chitosan-coated magnetite nanoparticles and their application in curcumin drug delivery. Adv Nat Sci Nanosci Nanotechnol. 2016 doi: 10.1088/2043-6262/7/4/045010. [DOI] [Google Scholar]
- Rao W, Zhang W, Poventud-Fuentes I, Wang Y, Lei Y, Agarwal P, et al. Thermally responsive nanoparticle-encapsulated curcumin and its combination with mild hyperthermia for enhanced cancer cell destruction. Acta Biomater. 2014 doi: 10.1016/j.actbio.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salatin S, Maleki Dizaj S, Yari Khosroushahi A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int. 2015 doi: 10.1002/cbin.10459. [DOI] [PubMed] [Google Scholar]
- Sanhaji M, Göring J, Couleaud P, Aires A, Cortajarena AL, Courty J, et al. The phenotype of target pancreatic cancer cells influences cell death by magnetic hyperthermia with nanoparticles carrying gemicitabine and the pseudo-peptide NucAnt. Nanomed Nanotechnol Biol Med. 2019 doi: 10.1016/j.nano.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Saxena V, Hasan A, Pandey LM. Antibacterial nano-biocomposite scaffolds of Chitosan, Carboxymethyl Cellulose and Zn & Fe integrated Hydroxyapatite (Chitosan-CMC-FZO@HAp) for bone tissue engineering. Cellulose. 2021;28(14):9207–9226. doi: 10.1007/s10570-021-04072-6. [DOI] [Google Scholar]
- Shariatinia Z. Carboxymethyl chitosan: Properties and biomedical applications. Int J Biol Macromol. 2018;120:1406–1419. doi: 10.1016/j.ijbiomac.2018.09.131. [DOI] [PubMed] [Google Scholar]
- Subramani PA, Panati K, Narala VR. Curcumin nanotechnologies and its anticancer activity. Nutr Cancer. 2017 doi: 10.1080/01635581.2017.1285405. [DOI] [PubMed] [Google Scholar]
- Sun L, Cui ZG, Zakki SA, Feng QW, Li ML, Inadera H. Mechanistic study of nonivamide enhancement of hyperthermia-induced apoptosis in U937 cells. Free Radical Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Vakili-Ghartavol R, Momtazi-Borojeni AA, Vakili-Ghartavol Z, Aiyelabegan HT, Jaafari MR, Rezayat SM, Arbabi Bidgoli S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif cells Nanomed Biotechnol. 2020;48(1):443–451. doi: 10.1080/21691401.2019.1709855. [DOI] [PubMed] [Google Scholar]
- Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast cancer a review. JAMA Surg. 2017 doi: 10.1001/jamasurg.2017.0552. [DOI] [PubMed] [Google Scholar]
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA J Am Med Assoc. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- Wong KE, Ngai SC, Chan K-G, Lee L-H, Goh B-H, Chuah L-H. Curcumin nanoformulations for colorectal cancer: a review. Front Pharmacol. 2019 doi: 10.3389/fphar.2019.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahya EB, Alqadhi AM. Recent trends in cancer therapy: a review on the current state of gene delivery. Life Sci. 2021;269(December 2020):119087. doi: 10.1016/j.lfs.2021.119087. [DOI] [PubMed] [Google Scholar]
- Yalcin S, Gündüz U. Iron oxide-based polymeric magnetic nanoparticles for drug and gene delivery: in vitro and in vivo applications in cancer. Handb Polym Ceram Nanotechnol. 2021 doi: 10.1007/978-3-030-40513-7_38. [DOI] [Google Scholar]
- Zavisova V, Koneracka M, Gabelova A, Svitkova B, Ursinyova M, Kubovcikova M, et al. Effect of magnetic nanoparticles coating on cell proliferation and uptake. J Magn Magn Mater. 2019 doi: 10.1016/j.jmmm.2018.09.116. [DOI] [Google Scholar]
- Zhou H, Beevers SC, Huang S. The targets of curcumin. Curr Drug Targets. 2012 doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hao Q, Lu H. Mutant p53 in cancer therapy-the barrier or the path. J Mol Cell Biol. 2019;11:293–305. doi: 10.1093/jmcb/mjy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Liu P, Liu CH, Zhi XT. Doxorubicin-loaded mesoporous magnetic nanoparticles to induce apoptosis in breast cancer cells. Biomed Pharmacother. 2015 doi: 10.1016/j.biopha.2014.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available in an Excel file.