To the Editor
Actinic keratosis (AK) is a precursor to cutaneous squamous cell carcinoma. Lengthy treatment duration and severe side effects have limited the therapeutic efficacy of the Food and Drug Administration–approved topical AK treatments (Cornejo et al., 2020). In addition, the efficacy of these treatments against hypertrophic AK is unclear. We previously showed the high efficacy of topical calcipotriol (a low-calcemic vitamin D3 analog) in combination with 5-fluorouracil (5-FU, an established topical AK treatment) for the elimination of AK in patients with multiple AKs at baseline in a randomized, double-blind clinical trial (Cunningham et al., 2017). To enable the comparison between calcipotriol plus 5-FU treatment outcomes and other recent AK treatments following the standard inclusion criteria (Blauvelt et al., 2021; Lebwohl et al., 2012), we performed an exploratory secondary analysis on our clinical trial data. In addition, we examined the impact of combination therapy on the clearance of hypertrophic AKs on the face.
In our clinical trial, 64 subjects received 0.005% calcipotriol ointment plus 5% 5-FU cream (test) and 66 received Vaseline plus 5% 5-FU cream (control) twice-daily treatment for 4 consecutive days (Cunningham et al., 2017). Subjects underwent clinical evaluation before (day 0) and 8 weeks after treatment. In the original trial, the inclusion criteria were subjects with 4 to 15 clinically discrete and visible AKs within a 25 cm2 contiguous area on the face, scalp, right upper extremity, and/or left upper extremity. The inclusion criteria for this study were subjects with a minimum of four to a maximum of 10 clinically typical, discrete, and visible AKs in a defined anatomical boundary within any of the treated sites: forehead, cheek (left and/or right), temples (combined), vertex of the scalp, and one-third distal portion of the forearm (left and/or right). In addition, we used the presence of any hypertrophic AKs on the face as inclusion criteria to assess the clearance rate for hypertrophic AKs on the face in the test versus the control group. Among eligible subjects, we compared the complete (100%) clearance, partial (≥75%) clearance, the percent clearance of AKs in each anatomical boundary, and the percent total clearance of hypertrophic AKs on the face at week 8 after treatment between the test and the control group in a blinded manner. Hypertrophic AK was defined as ≥6 mm hyperkeratotic papule or plaque on the treated skin. Hypertrophic and typical AKs were identified during the trial and documented in the annotated clinical images. Statistical significance was determined by Pearson’s chi-squared test (categorical) or two-sample t-test (continuous).
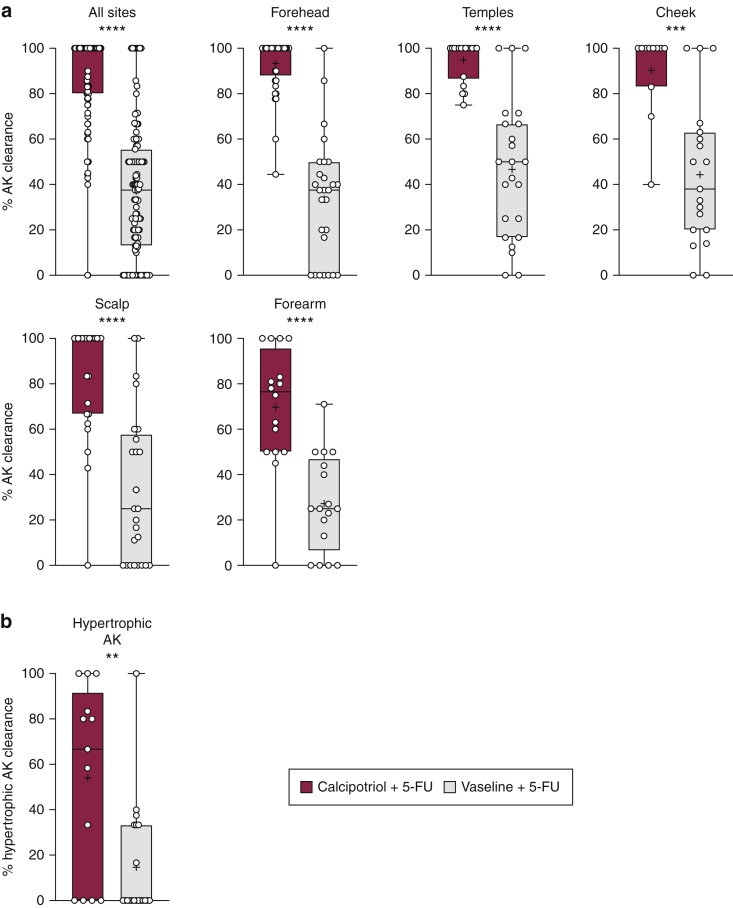
In this secondary analysis, 54 and 52 subjects were included in the test and control groups, respectively. In total, 24 subjects from the original trial were excluded in this secondary analysis because they had >10 AKs in the defined anatomical sites. No significant difference was noted between the two groups for age, sex, drug amount used, anatomical sites treated, and baseline AK counts on each site (Table 1). Calcipotriol plus 5-FU treatment showed a significantly higher complete (62% vs. 8%, P < 0.0001) and partial AK clearance (82% vs. 11%, P < 0.0001) for all anatomical sites combined in the test than in the control group (Table 2). All the anatomical sites assessed in this analysis showed a significantly higher percent AK clearance in the test than in the control group (Figure 1a). The percent AK clearance was 87.4% versus 37.1% in 107 test versus 111 control anatomical sites combined (P < 0.0001). Likewise, percent AK clearance was significantly higher for each anatomical site in test than in the control group: forehead: 93.4 versus 33.5, P < 0.0001; temples: 94.8 versus 46.6, P < 0.0001; cheek: 90.2 versus 44.3, P = 0.0002; scalp: 83.4 versus 33.3, P < 0.0001; and forearm: 69.7 versus 27.2, P < 0.0001 (Figure 1a). In total, 33 subjects (13 in the test and 20 in the control group) were eligible for the assessment of their hypertrophic AKs. The percent total clearance of hypertrophic AKs on the face was significantly higher in the test than in the control group (54.0% vs. 14.7%, P = 0.002, Figure 1b). The cumulative number of hypertrophic AKs on the face was 50 in the test and 66 in the control group before treatment, which was reduced to 18 in the test and 52 in the control group after treatment (64% vs. 21.2% reduction, respectively, P < 0.0001).
Table 1.
Demographic and baseline clinical characteristics of the study subjects
| Characteristics | Calcipotriol + 5-FU (n = 54) | Vaseline + 5-FU (n = 52) | P-Value |
|---|---|---|---|
| Age, mean (SD), y | 68.0 (6.9) | 70.4 (9.5) | 0.143 |
| Range | 51–88 | 52–89 | |
| Sex, n (%) | 0.316 | ||
| Male | 44 (81) | 46 (88) | |
| Female | 10 (19) | 6 (12) | |
| Drug amount used, mean (SD), g | 15.42 (9.7) | 16.54 (9.2) | 0.547 |
| Baseline sites treated, n (%) | 0.447 | ||
| Forehead | 37 (69) | 27 (52) | |
| Temples | 18 (33) | 23 (44) | |
| Cheek | 11 (20) | 19 (37) | |
| Scalp | 25 (46) | 25 (48) | |
| Forearm | 16 (30) | 17 (33) | |
| Baseline actinic keratosis count on each site, median (IQR) | |||
| Forehead | 8 (3) | 7 (4) | 0.073 |
| Temples | 5 (1) | 6 (3) | 0.698 |
| Cheek | 9 (2.5) | 7 (3) | 0.095 |
| Scalp | 6 (2) | 6 (2) | 0.544 |
| Forearm | 8 (6) | 8 (6) | 0.929 |
Abbreviations: 5-FU, 5-fluorouracil; IQR, interquartile range.
Table 2.
Complete and partial clearance of AKs in the study subjects
| Variable | Calcipotriol + 5-FU n/Total n (%) | Vaseline + 5-FU n/Total n (%) | P-Value | Difference (95% CI) |
|---|---|---|---|---|
| Complete clearance | ||||
| All locations | 66/107 (62) | 9/111 (8) | <0.0001 | 54 (42–63) |
| Forehead | 26/37 (70) | 1/27 (4) | <0.0001 | 66 (44–78) |
| Temples | 13/18 (72) | 3/23 (13) | 0.0001 | 59 (29–77) |
| Cheek | 8/11 (73) | 3/19 (16) | 0.0022 | 57 (20–77) |
| Scalp | 15/25 (60) | 2/25 (8) | 0.0001 | 52 (26–70) |
| Forearm | 4/16 (25) | 0/17 (0) | 0.0301 | 25 (1–49) |
| Partial clearance | ||||
| All locations | 88/107 (82) | 12/111 (11) | <0.0001 | 71 (60–79) |
| Forehead | 35/37 (95) | 2/27 (7) | <0.0001 | 88 (68–94) |
| Temples | 18/18 (100) | 3/23 (13) | <0.0001 | 87 (61–95) |
| Cheek | 9/11 (82) | 3/19 (16) | 0.0005 | 66 (29–83) |
| Scalp | 17/25 (68) | 4/25 (16) | 0.0002 | 52 (25–70) |
| Forearm | 9/16 (56) | 0/17 (0) | 0.0004 | 56 (22–81) |
Abbreviations: 5-FU, 5-fluorouracil; AK, actinic keratosis; CI, confidence interval.
Complete clearance was defined as 100% reduction and partial clearance as at least 75% reduction in the number of AKs in the treated anatomical site at 8 weeks after treatment.
Figure 1.
Percent AK clearance at week 8 after treatment. (a) The reduction in AK counts on combined anatomical sites and each anatomical site after calcipotriol plus 5-FU versus Vaseline plus 5-FU treatment. Average clearance percentages were compared between treatment groups for subjects who had 4–10 AKs on an anatomical site at baseline. (b) The reduction in hypertrophic AK on the face after calcipotriol plus 5-FU (n = 13) versus Vaseline plus 5-FU treatment (n = 20). ∗∗∗∗P < 0.0001, ∗∗∗P = 0.0002, and ∗∗P = 0.002. 5-FU, 5-fluorouracil; AK, actinic keratosis.
The synergy between calcipotriol and 5-FU results in a novel and effective AK immunotherapy; however, further studies are required to determine whether this therapy can effectively prevent squamous cell carcinoma (Cunningham et al., 2017; Rosenberg et al., 2019). The results of this secondary analysis are consistent with the outcomes of our primary trial, which showed a higher complete and partial AK clearance on the face (27% vs. 0% and 80% vs. 0%, P < 0.0001 for both) in the test versus the control group, respectively (Cunningham et al., 2017). This study confirms the high efficacy of calcipotriol plus 5-FU immunotherapy for AK treatment using standard inclusion criteria and outcome measures that are comparable to other AK treatment studies (Blauvelt et al., 2021; Lebwohl et al., 2012). Specifically, topical application of ingenol mebutate gel versus placebo to a 25 cm2 contiguous area on face and scalp once daily for 3 consecutive days led to complete and partial AK clearance of 42.2% versus 3.7% and 63.9% versus 7.4% (P < 0.001 for both), respectively (Lebwohl et al., 2012). Topical tirbanibulin ointment application to a 25 cm2 contiguous area on face and scalp once daily for 5 consecutive days led to complete and partial AK clearance of 44% versus 5% and 68% versus 16% (P < 0.001 for both) in the test versus the placebo group, respectively (Blauvelt et al., 2021). Unlike these AK clinical trials, which compare the test treatment with placebo (Blauvelt et al., 2021; Lebwohl et al., 2012), we show the efficacy of 0.005% calcipotriol plus 5% 5-FU combination compared with 4 days of Vaseline plus 5% 5-FU. Future studies are required to compare this combination immunotherapy with the full course (i.e., 2–4 weeks) of 5% 5-FU monotherapy. In addition, a randomized clinical trial designed and powered to specifically investigate the efficacy of calcipotriol plus 5-FU immunotherapy for squamous cell carcinoma prevention is warranted. Finally, we show the high efficacy of calcipotriol plus 5-FU for the treatment of facial hypertrophic AKs. Future clinical trials with the standard inclusion criteria are needed to further validate and expand these findings.
Human study
The exploratory secondary analysis of clinical trial data was conducted in accordance with Massachusetts General Hospital (Boston, MA) and Washington University in St. Louis (St. Louis, MO) Institutional Review Board guidelines. Trial subjects provided written, informed consent.
Data availability statement
All data needed to evaluate the conclusions in the paper are present in the paper. No datasets were generated or analyzed during this study.
ORCIDs
Marjan Azin: http://orcid.org/0000-0001-6950-4854
Andrew B. Mahon: http://orcid.org/0000-0001-5842-3944
Steven Isaacman: http://orcid.org/0000-0002-7529-4440
Julia E. Seaman: http://orcid.org/0000-0003-2528-9800
Isabel E. Allen: http://orcid.org/0000-0001-9029-9744
Michael Szarek: http://orcid.org/0000-0002-0046-0264
Lynn A. Cornelius: http://orcid.org/0000-0002-6329-2819
Shadmehr Demehri: http://orcid.org/0000-0002-7913-2641
Author Contributions
Conceptualization: MA, LAC, SD; Data Curation: MA; Formal Analysis: MA, JES, IEA, MS; Investigation: MA, ABM, SI, JES, IEA, MS; Project Administration: SD; Supervision: SD; Writing - Original Draft Preparation: MA, SD; Writing - Review and Editing: ABM, SI, JES, IEA, MS, LAC
Acknowledgments
This study was funded by PHD Biosciences. MA, JES, IEA, and MS received funding support from PHD Biosciences for this study.
Conflict of Interest
LAC and SD are coinventors on a filed patent for the use of calcipotriol plus 5-fluorouracil for the treatment of precancerous skin lesions (PCT/US2015/049434). ABM is a chief scientific officer at PHD Biosciences. Washington University has a potential financial interest in PHD Biosciences through Option Agreement.
Accepted manuscript published online XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2022;X:100104
References
- Blauvelt A., Kempers S., Lain E., Schlesinger T., Tyring S., Forman S., et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512–520. doi: 10.1056/NEJMoa2024040. [DOI] [PubMed] [Google Scholar]
- Cornejo C.M., Jambusaria-Pahlajani A., Willenbrink T.J., Schmults C.D., Arron S.T., Ruiz E.S. Field cancerization: treatment. J Am Acad Dermatol. 2020;83:719–730. doi: 10.1016/j.jaad.2020.03.127. [DOI] [PubMed] [Google Scholar]
- Cunningham T.J., Tabacchi M., Eliane J.P., Tuchayi S.M., Manivasagam S., Mirzaalian H., et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106–116. doi: 10.1172/JCI89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl M., Swanson N., Anderson L.L., Melgaard A., Xu Z., Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–1019. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
- Rosenberg A.R., Tabacchi M., Ngo K.H., Wallendorf M., Rosman I.S., Cornelius L.A., et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:e125476. doi: 10.1172/jci.insight.125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper. No datasets were generated or analyzed during this study.