Abstract
Purpose
Young age is associated with poor prognosis in ductal carcinoma in situ (DCIS) of female breast and controversy exists regarding the optimal treatment modality for young patients. We aimed to compare treatment outcomes among breast conserving surgery (BCS), BCS with adjuvant radiotherapy (BCS + RT), and total mastectomy (MT) for young DCIS women.
Methods
PubMed, Cochrane, and Embase were searched for studies reporting comparative results among BCS, BCS + RT, or MT in ≤50 years old (y/o) DCIS females. Study quality was assessed and meta-analysis with subgroup analysis was performed to pool the effect sizes of the outcomes-of-interest.
Results
We included 3 randomized control trials and 18 observational studies. For DCIS women ≤50 y/o, RT following BCS significantly reduced the risk for ipsilateral breast tumor recurrence (IBTR) (HR = 0.66, 95% CI 0.50–0.87). However, the benefit was less robust in extremely young patients and with long follow-ups. RT revealed no statistically significant preventive effect on ipsilateral invasive recurrence (HR = 1.38, 95% CI 0.98–1.94). On the other hand, MT yielded the lowest IBTR (BCS + RT vs MT: HR = 4.4, 95% CI 2.06–9.40), both in ipsilateral DCIS recurrence and ipsilateral invasive recurrence. There was great heterogeneity and could not reach an evident conclusion concerning survival outcomes.
Conclusion
This study highlighted the varying effect of RT for young DCIS females. The local control benefit of MT was definite without survival differences observed. Our study provided a moderate certainty of evidence to guide the treatment for young DCIS women. Further age-specific prospective trial is warranted.
Keywords: Ductal carcinoma in situ, Young women, Breast conserving surgery, Adjuvant radiotherapy, Mastectomy, Ipsilateral breast tumor recurrence
Abbreviations: DCIS, Ductal carcinoma in situ; BCS, Breast conserving surgery; RT, Adjuvant radiotherapy; MT, Total mastectomy
Highlights
-
•
It's the largest meta-analysis including 114,619 young DCIS women from 21 studies.
-
•
Radiotherapy reduces ipsilateral breast events following breast conserving surgery.
-
•
Mastectomy yields the best local control outcomes.
-
•
No significant survival difference was found among local treatment modalities.
-
•
Shared-decision making could be suggested on local treatment options.
1. Introduction
Ductal carcinoma in situ (DCIS) of the female breast is a common pre-cancer lesion accounting for 20% of annual breast cancer diagnosis [1]. If not treated, 10–28% of DCIS patients may develop invasive breast cancers and have a higher risk for breast cancer deaths [2,3]. To reduce the risk of further breast cancer events, total mastectomy (MT) has been performed for DCIS patients. After breast conserving surgery (BCS) was introduced, it has become the mainstay of DCIS treatment due to better cosmetic outcomes [4,5]. Adjuvant radiotherapy (RT) is also widely applied after BCS with its local control benefit observed in four large clinical trials [[6], [7], [8], [9]].
In previous studies, young age at diagnosis, mostly defined as <40 or <50 years old (y/o), was recognized as a negative prognostic factor for DCIS women [10,11]. The 10-year breast tumor recurrence risk was 11.2–31% for women diagnosed ≤40 y/o and was 3–9% for those diagnosed >40 y/o [10,[12], [13], [14]]. Young age was also associated with a higher breast cancer mortality rate, with the hazard ratio (HR) of 2.58 for those diagnosed before 35 y/o compared to the older counterpart [15]. As a result, young DCIS patients tended to receive more intensive treatment in respect of surgical extent and RT [16,17].
In addition to the difference in prognosis, diagnostic age was found to influence the treatment effect of RT. The proportional and absolute risk reduction in ipsilateral breast events was less in younger than in older women with DCIS, which was not confounded by known prognostic factors [4,18]. Nevertheless, the young subgroup accounted for only a small portion of previous study populations and has not been comprehensively evaluated [5,19]. Literature regarding DCIS treatments for young women is sparse and no age-specific guideline is available. Without robust evidence to counsel the distinct subgroup, approaches to treating young DCIS women have been extrapolated largely from research conducted in women of greater age.
From 1975 to 2015, the incidence of DCIS had an approximately 4-fold increase in females aged 20–39 y/o [20]. With the growth of the extremely young DCIS population, we aimed to comprehensively review published literature for treatment outcomes among BCS, BCS + RT, and MT for young DCIS women.
2. Materials and methods
A systematic review with study-level meta-analysis divided by treatment modalities was performed. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [21].
Inclusion criteria were randomized control trials (RCTs) or observational studies reporting comparative outcomes among BCS, BCS + RT, and MT for female DCIS patients diagnosed before 50 y/o, whether as an age-specific study or in subgroup analysis. Outcomes should include at least one of the following: ipsilateral breast tumor recurrence (IBTR), disease-specific survival (DSS), or overall survival (OS) of the subgroup-of-interest.
In June 2018, we searched PubMed, Cochrane Controlled Register of Trials (CENTRAL), and Embase from their inception using keywords, including ductal carcinoma in situ, DCIS, non-invasive breast cancer, lumpectomy, BCS, radiotherapy, mastectomy, their synonyms, and associated MeSH terms; limitations such as publish date, language, or publication type were not set. The search results were tracked and last updated on November 7th, 2021. Reference lists of relevant literature were scrutinized to identify possible eligible studies.
The search results were reviewed by two independent authors, who used titles and abstracts to evaluate a study's eligibility for inclusion. For articles containing young DCIS women where inadequate data were reported for the subgroup-of-interest, the corresponding authors were contacted to request further information. When more than one publication reported on the same RCTs or cohorts derived from the same population, only data from the most sophisticated study design or the latest literature with the updated population and longest follow-up would be enrolled in the meta-analysis.
For quality assessment of enrolled studies, a six-domain risk-of-bias 2.0 (RoB 2.0) tool was used to appraise RCTs [22]. The nine-item Newcastle-Ottawa Scale was applied to assess the quality of observational studies [23]. Data extraction and quality assessment of each article were independently performed by two authors; a third author was consulted if a disagreement ensued. As BCS + RT is currently the most recognized local treatment, outcomes of BCS + RT were compared to those of BCS or MT, respectively. Age-specific HRs and their 95% confidence intervals (95%CI) were extracted for IBTR, DSS, and OS. If results of the multivariate analysis were reported, adjusted HRs were used. In articles reporting only cases and event numbers in the subgroup-of-interest, HRs were calculated from risk ratio based on the methodology published by Parmar et al. [24]; for studies with an inverse design of experimental versus the control arm, reciprocals of HRs and 95%CIs were recorded [25]. Meta-analysis of extracted data was performed with Review Manager (RevMan) ([Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and presented in a forest plot with heterogeneity tested by I2 analysis. Having cohorts from different countries and over a wide period of time, factors including median follow-up time, hormone therapy use, surgical margin status, application of boost irradiation, and patient-included year were taken into consideration as indexes of the quality of medical care for each cohort. Subgroup analyses stratified by the above-mentioned factors, along with study designs, the cutoff age for the young population, and the application of multivariate analysis were performed to evaluate the impact of these possible confounders.
3. Results
3.1. Search results and study inclusions
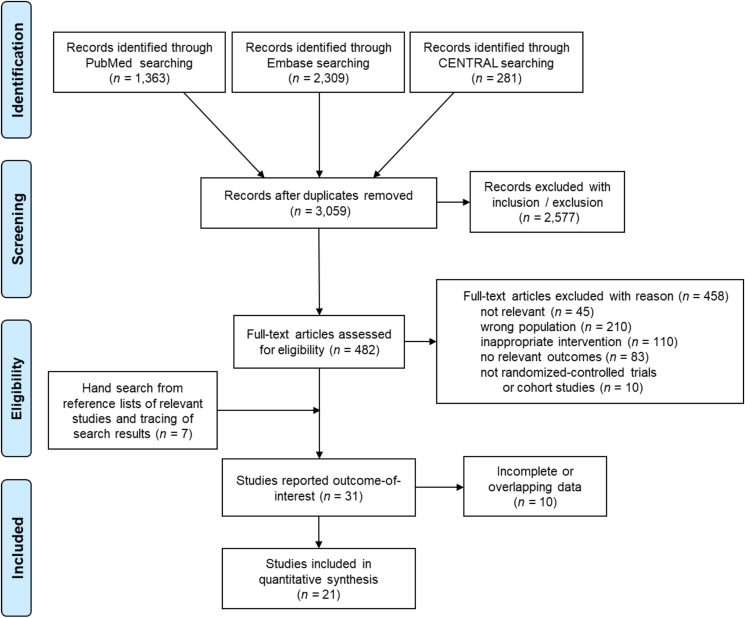
Excluding 894 duplications, the initial search included 3059 articles from electronic databases, with the earliest publication dated back to 1973. After screening by title, abstract, and full-text article, we found 31 articles, published between 1998 and 2021, that reported outcomes for young DCIS females. Ten articles were further excluded due to inadequate information about the subgroup-of-interest or overlapping data. Finally, 21 studies were included (Fig. 1). The overall population comprised 440,814 DCIS females, including 114,619 females diagnosed at 50 y/o or less. Only data regarding young DCIS women would be presented in the following text, including tables and figures. Among the included young patients, 23,395 cases received BCS (20.4%), 52,291 cases had BCS + RT (45.6%), and 38,933 cases underwent MT.
Fig. 1.
PRISMA flow diagram of summarizing study identification and selection
CENTRAL: Cochrane Controlled Register of Trials.
There were 3 RCTs [6,7,18] and 18 observational studies [16,17,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]] included: 13 compared outcomes between BCS and BCS + RT, 8 reported outcomes among BCS, BCS + RT, and MT; five were age-specific observational studies and 16 reported outcomes-of-interest in subgroup analysis. Other information regarding the enrolled studies were listed in Table 1.
Table 1.
Characteristics of enrolled studies.
| Study design/Enrolled studies | Countries | Enroll years | Overall population |
Young population |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Case No. | Median f/u (y) | Hormone therapy use | Free margin | RT regimens | Cutoff agea | Case No. (%)b | |||
| Randomized control trialsc | |||||||||
| NSABP B-17 1998 [7] | US, Canada | 1985–1990 | 818 | 7.5 | 0% | 87% (positive or NA: 13%) | 50 Gy/25 frx; 9% with boost | 49 | 274 (33.5%) |
| EORTC 10853 2006 [6] | Europe | 1986–1996 | 1010 | 10.5 | 0% | 84% (Positive, < 1 mm, or NA: 16%) | 50 Gy/25 frx; 5% with boost | 40 | 51 (5.0%) |
| SweDCIS 2008 [18] | Sweden | 1987–1999 | 1067 | 8.4g | 0% | 80% (positive: 11%, NA: 9%) | 50 Gy/25 frx, or 48 Gy/20 frx, or 54 Gy/27 frxi; boost not recommended | 49 | 252 (23.6%) |
| Observational studies | |||||||||
| Van Zee 1999 [26] | US | 1978–1990 | 157 | 6.2 | 8% | 44% (positive: 3%, NA: 53%) | prescribed dose: 50 Gy; 100% with boost (10–20 Gy) | 39 | 15 (9.5%) |
| Cutuli 2002 [27] | France | 1985–1995 | 822 | 7.2 | NA | 78.6% (positive: 7.5%, NA: 13.9%) | minimal whole-breast dose: 45 Gy; 80% with boost (10–20 Gy) | 39 | 51 (6.2%) |
| Omlin 2006 [28] | Transnationald | 1978–2004 | 373 | 6 | 7% | 65.4% (positive: 5.4%, NA: 29.2%) | median whole breast dose: 50 Gy; 47.5% with boost (median boost dose: 10 Gy) | 45 | 373 (100%) |
| Gonzaga 2009 [29],e | Italy | 1996–2005 | 775 | 3.3 | 26.0% (ER+: 48%) | 86.3% (positive: 13.7%) | most common RT schedule: 50 Gy without boost | 40 | 72 (9.3%) |
| Tunon-de-Lara 2010 [30] | France | 1974–2003 | 207 | 13.3 | 1.0% | 85.7% in BCS (positive: 14.3%) NA in TM | 50 Gy whole breast RT; 45.5% with boost (10 Gy) | 39 | 207 (100%) |
| Alvarado 2012 [31] | US | 1996–2009 | 2037 | 5.2 | 35.7% (<40 y/o: 28.8%) | 99.8% (positive: 0.2%) | NA | 39 | 132 (6.5%) |
| Rakovitch 2013 [32] | Canada | 1994–2003 | 3762 | 10 | 17% in >65 y/o | 55.7% (positive: 13.9%, NA: 30.4%) | 50 Gy/25frx; or 40–44 Gy/16 frxj; without boost | 50 | 480 (12.8%) |
| NA in <65 y/o | |||||||||
| Worni 2015 [33] | US-SEER | 1991–2010 | 121,080 | 5.9 | NAh | NAh | NAh | 49 | 31,036 (25.6%) |
| Qian 2015 [34] | US-SEER | 1998–2007 | 56,968 | 7.6 | NAh | NAh | NAh | 50 | 15,554 (27.3%) |
| Elshof 2016 [35] | Netherland | 1989–2004 | 10,090 | 10.7 | 0% | NA | NA | 49 | 2159 (21.4%) |
| Cronin 2016 [36] | US | 1978–2010 | 2634 | 6.3 | 21% (<40 y/o: 11%) | 74% (positive: 19%, NA: 7%) < 40y/o: 71% (positive and close: 19, NA: 10%) | NA | 39 | 138 (5.2%) |
| Sagara 2016 [16] | US-SEER | 1988–2007 | 32,144 | 8 | NAh | NAh | NAh | 40 | 896 (2.8%) |
| Kim 2017 [37] | Korea | 1995–2010 | 286 | 6.4 | 61.5% | 93.7% (positive: 6.3%) | median dose: 50.4 Gy (45.0–50.4 Gy); median dose of boost: 12.6 Gy (9–20 Gy) | 50 | 286 (100%) |
| Park 2018 [17] f | US-SEER | 1998–2011 | 3648 | 7 | NAh | NAh | NAh | 40 | 3648 (100%) |
| Kuo 2018 [38] | Taiwan | 2003–2010 | 375 | 7.9 | 73.1% | 72.5% (positive and close: 27.5%) | 50 Gy/25 frx whole breast RT; 100% with boost: 10 Gy/5 frx | 39 | 45 (12%) |
| Giannakeas 2018 [39] | US-SEER | 1998–2014 | 140,366 | NA | NAh | NAh | NAh | 39 | 4657 (3.3%) |
| Van Seijen 2021 [40] | Netherland | 1989–2004 | 10,045 | 15.7 | 0% | NA | NA | 49 | 2143 (21.3%) |
| Byun 2021 [41] f | US-NCDB | 2004–2016 | 52,150 | 5.4 | 32.0% (ER+: 43.7%) | 94.5% | NA | 49 | 52,150 (100%) |
f/u: follow up, y: years, RT: radiotherapy, US: United States, SEER: Surveillance, Epidemiology, and End Results Program, NCDB: National Cancer Database, NA: not available, ER+: estrogen receptor positive, y/o: year-old, BCS: breast conserving surgery, TM: mastectomy.
The cutoff value of age as young population in each studies.
The proportion of defined young subgroup in each studies.
The UK/ANZ trial was not included due to inadequate information provided for outcome-of-interest in the young subgroup.
The cohorts included patients from Australia, Belgium, France, the UK, Israel, Italy, the Netherlands, Spain, Switzerland, Turkey, and the US.
For Gonzaga 2009, only those with grade II-III DCIS receiving breast conserving surgery were included for the meta-analysis.
For Park 2018 and Byun 2021, those receiving contralateral prophylactic mastectomy or post-mastectomy radiotherapy were not included in the meta-analysis.
The values presented were the mean values instead of median.
The prescription of tamoxifen, dose of radiotherapy, and surgical margin were not available in SEER database.
The distribution of whole breast radiation dose: 50 Gy/25 frx in 80%, 48 Gy/20 frx in 13%, 54 Gy/27 frx then 2 weeks gap in 7%.
The distribution of whole breast radiation dose: 50 Gy/25 frx in 56%, 40–44 Gy/16 frx in 36%, NA for others.
Among the 3 RCTs, concerns regarding the domain of allocation arose since data revealing balanced baseline characteristics for the young subgroup was not available; the NSABP B-17 trial was an exception as age-stratification was performed during randomization. Otherwise, risks of bias for other domains were low (Supplementary Table S1 A). The NOS score for the 18 observational studies ranged from 5 to 8 points. Most bias concerns were directed at the field of comparability; confounders were not controlled for the young subgroup in some studies (Supplementary Table S1 B).
3.2. Ipsilateral breast tumor recurrences risk after DCIS
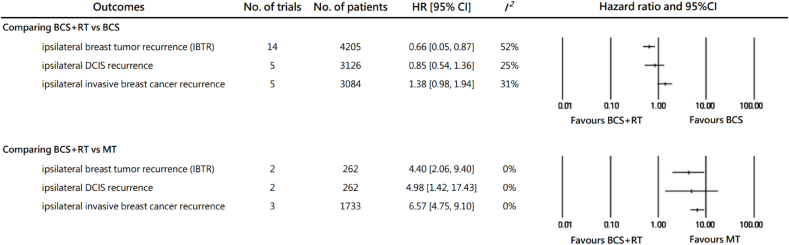
Comparative results in ipsilateral breast tumor recurrence among BCS + RT, BCS, and MT were pooled and presented in Fig. 2 and Supplementary Fig. S2. Comparing BCS + RT to BCS, RT presented an IBTR risk reduction for women ≤50 y/o, the pooled HR being 0.66 (95% CI 0.50–0.87; I2 = 52%). The protective effect of RT was less profound in pooled results of ipsilateral DCIS recurrence (HR = 0.85, 95% CI 0.54–1.36) and showed a non-significant trend for elevated risk for ipsilateral invasive events (HR = 1.38, 95% CI 0.98–1.94).
Fig. 2.
The recurrences of comparing breast conserving surgery with or without adjuvant radiotherapy, and mastectomy in DCIS patients ≤50 years of age.
HR: hazard ratio, CI: confidence interval, BCS + RT: breast conserving surgery with adjuvant radiotherapy, BCS: breast conserving surgery, IBTR: ipsilateral breast tumor recurrence, DCIS: ductal carcinoma in situ, MT: total mastectomy.
When compared to MT, BCS + RT had a higher IBTR risk. The pooled HR was 4.4 with great consistency (95% CI 2.06–9.40; I2 = 0%). The inferiority of BCS + RT was found significant in both ipsilateral DCIS (HR = 4.98, 95% CI 1.42–17.43) and invasive recurrence (HR = 6.57, 95% CI 4.75–9.10).
3.3. Subgroup analysis of IBTR risk comparing BCS + RT versus BCS
Subgroup analyses were performed for IBTR. The results were shown in Table 2 and Supplementary Fig. S3. When stratified by cutoff age for the young population, studies focusing on < 40 y/o females revealed a smaller IBTR risk reduction with RT, compared to studies including women aged 40–50 y/o. The pooled HR of IBTR for <40 y/o women was not able to support a statistically significant benefit of RT (HR = 0.82, 95% CI 0.48–1.39; I2 = 30%).
Table 2.
Subgroup analysis for ipsilateral breast tumor recurrence (IBTR) comparing BCS + RT to BCS in DCIS women diagnosis at 50 year-old or less.
| Stratification | No. of studies | HR | 95% CI | Intra-subgroup heterogeneity (I square) | p value for inter-subgroup interaction test | |
|---|---|---|---|---|---|---|
| Cutoff age for young subgroup | <40 y/o | 6 | 0.82 | 0.48, 1.39 | 30% | 0.31 |
| 40 - 50 y/o | 8 | 0.60 | 0.43, 0.83 | 64% | ||
| Median f/u timea | <7 y | 6 | 0.45 | 0.29, 0.68 | 8% | 0.04 |
| 7–10 y | 5 | 0.82 | 0.65, 1.04 | 13% | ||
| ≥10 y | 3 | 0.80 | 0.37, 1.74 | 75% | ||
| Hormone therapy usea,b | ≥50% | 2 | 0.28 | 0.09, 0.82 | 17% | 0.17f |
| <50% | 9 | 0.62 | 0.43, 0.89 | 47% | ||
| NA | 3 | 0.92 | 0.76, 1.12 | 0% | ||
| Free surgical margina,c | ≥80% | 7 | 0.60 | 0.36, 0.99 | 61% | 0.89f |
| <80% | 6 | 0.62 | 0.46, 0.85 | 0% | ||
| NA | 1 | 0.97 | 0.78, 1.21 | – | ||
| Study design | RCT | 3 | 0.59 | 0.42, 0.83 | 0% | 0.51 |
| Obs. | 11 | 0.69 | 0.49, 0.96 | 54% | ||
| Multivariate analysisd | With | 3 | 0.67 | 0.39, 1.17 | 76% | 0.88 |
| without | 11 | 0.64 | 0.46, 0.90 | 39% | ||
| Boost irradiationa | 100% | 2 | 0.48 | 0.17, 1.34 | 0% | 0.69f |
| 1–99% | 5 | 0.62 | 0.33, 1.18 | 71% | ||
| 0% | 3 | 0.74 | 0.54, 1.02 | 0% | ||
| NA | 4 | 0.56 | 0.27, 1.16 | 66% | ||
| Patient-included yeare | before 1990 | 2 | 0.55 | 0.34, 0.89 | 0% | 0.75 |
| cross years | 6 | 0.67 | 0.41, 1.12 | 63% | ||
| after 1990 | 6 | 0.69 | 0.46, 1.03 | 48% |
BCS + RT: breast conserving surgery with adjuvant radiotherapy, BCS: breast conserving surgery, HR: hazard ratio, 95% CI: 95% confidence interval, RCT: randomized control trials, Obs.: observational studies, y/o: years old, f/u: follow-up, y: years, NA: not available.
For non-age specific studies, data of these factors from the whole study population would be taken as the proxy for the young subgroup, assuming the distributions were similar among age groups as an indicator for quality of medical care.
The incidence of positive estrogen receptor in DCIS was ranged from 49 to 77.8%[59]. Therefore, ≥ 50% was chosen as the cutoff value for subgroup analysis, indicating a more regular use of hormone therapy in the study population.
In order to compare with the EBCTCG study including 4 large RCTs having the free surgical margin rate of 80% or more, ≥ 80% was chosen as the cutoff value for subgroup analysis.
Factors corrected in studies with multivariate analysis: Omlin 2006: age, detection method, tumor size, necrosis, tumor grade, margin status, oestrogen-receptor status; Park 2018: age, year of diagnosis, race, registry region, tumor grade, histology, size, hormone receptor status; SWeDCIS: tumor size, focality, margin status, mode of detection.
Patient-included year was taken as substitutional index for technique of radiation therapy. Case inclusion before 1990 was considered at risk for suboptimal RT techniques [60].
The p value if for inter-subgroup interaction analysis test excluding the NA subgroup.
Stratified by median follow-up time, there was a subgroup difference observed among studies with shorter versus longer follow-up. Results from studies with median follow-up < 7 years had low heterogeneity and indicated an IBTR risk reduction with RT. But for those with longer median follow-up, the benefit of RT was less consistent among studies and did not reach a statistically significant superiority. In other subgroup analyses, there was no specific trend observed among studies with different hormone therapy coverage, the proportion of patients achieving free surgical margin or receiving boost irradiation, or patient-included year. The pooled effect of RT on IBTR did not vary significantly between different study designs and those with and without multivariate analysis.
3.4. Survival outcomes
There were 7 retrospective cohort studies providing survival outcomes, 6 of which were derived from the database of the US including Surveillance, Epidemiology, and End Results (SEER) and National Cancer Database (NCDB). Considering possible repeated sampling among studies derived from SEER and NCDB while they were equal in quality, data from studies with coverage of the most recent population were selected for meta-analysis. The pooled results were shown in Supplementary Fig. S4 with moderate to high heterogeneity observed except for the DSS between BCS + RT and MT. It revealed no significant difference among BCS, BCS + RT, and MT concerning OS and DSS.
4. Discussion
To our knowledge, this is the largest series of young DCIS women including 114,619 ≤ 50 y/o DCIS females from 21 studies, where outcomes of BCS, BCS + RT, and MT were compared. With the systematic review and study-level meta-analysis, our study confirmed the IBTR risk reduction by RT in young DCIS females, while pointing out the difference of RT benefit among age groups. The benefit of RT was less robust in extremely young patients (<40 y/o), with long follow-up (>7 years), and in the prevention of invasive events. On the other hand, MT yielded a superior ipsilateral control in both DCIS and invasive recurrence. In terms of survival, currently available data was sparse and non-conclusive among these treatment modalities.
Unlike the proportional reduction of recurrence observed in invasive breast cancer [42], the local control benefit of RT was smaller in younger DCIS women, who were at higher risk for recurrence. Combining 4 RCTs, the EBCTCG review and the Cochrane meta-analysis both revealed a smaller IBTR risk reduction by RT in DCIS women younger than versus older than 50 y/o, having the 10-year absolute IBTR risk reduction of 10.6% vs 17% and the risk ratio of 0.67 vs 0.35, respectively [4,43]. In current clinical practice, young DCIS women were more likely to be symptomatic and the coverage of hormone therapy increased, which were different from the study populations of the 4 RCTs [4]. With the addition of more recent cohorts, our study revealed a 34% IBTR risk reduction with RT in ≤50 y/o DCIS females receiving BCS.
In the EBCTCG study, the trend in the decrease of RT benefit with younger age was also significant when patients were further divided into 5 age groups (<40, 40–49, 50–59, 60–69, and ≥70 y/o) [4]. Knowing the impact of age, results derived from women of different ages should not be applied to younger females without detailed consideration. However, the effect of RT for <40 y/o DCIS women has not been reported in the previous meta-analyses [4,43]. Focusing on women <40 y/o, neither the included studies nor the pooled result has revealed a difference between BCS + RT and BCS concerning IBTR risk in our study. Therefore, taking an extremely young age as an indication for RT might warrant further evaluation.
Having a pre-cancer lesion diagnosed early in life, young DCIS women have an optimistic 10-year OS rate of more than 94% [6,12,44]. Therefore, the long-term treatment effect, especially on preventing possible life-threatening invasive events, is essential. However, the protective effect of RT seemed to diminish over time and was less effective on invasive recurrence. A similar time-varying trend was also observed in young DCIS women from three population-based cohort studies with long-term follow-up. For DCIS women ≤40 y/o recruited from SEER database, BCS + RT yielded a 5-fold lower IBTR risk than BCS at 2 years, which reduced to 2-fold by 5 years and eventually diminished with longer follow-up [17]. Another study from the Netherlands found no difference in ipsilateral invasive breast cancer (iIBC) risk between young DCIS patients receiving BCS + RT and BCS beyond 5 years from diagnosis [35]. Based on the same cohort with longer follow-up, van Seijen et al. even reported a higher iIBC risk with BCS + RT beyond 10 years [40]. Nevertheless, this phenomenon was not seen for the older counterpart who sustained a lower iIBC risk with RT [35,40].
The mechanism underlying the varying effect of RT on DCIS women of different ages is not clearly understood. It cannot be ascribed to by difference in histological grade, comedonecrosis, or by difference in nuclear grade or architecture in the EBCTCG study [4]. The age effect was not confounded by focality, lesion size, completeness of excision, or detection mode, as stated in the SweDCIS trial [9]. Some concerns were raised about the suboptimal radiation dose in the relatively high-risk population. Boost irradiation was reported to further improve local control in young DCIS women [28,45], at the cost of increased toxicity [46]. Nevertheless, this meta-analysis might be ineffectual at evaluating the impact of boost irradiation since it was not clearly provided in many studies. The local control result of the ongoing BIG 3–07/TROG 07.01 trial (NCT00470236) might provide a more precise answer on this issue. Other possible causes were the difference in endogenous hormone levels, the impact of pregnancy on disease control, and the possible existence of some difference in immunity or biogenetics for women to develop DCIS at a young age and, meanwhile, limiting the RT benefit. Evaluating the location of new breast events and the disease-free interval would help differentiate second primary cancer from true recurrence in these radiosensitive young females [47]. With further understanding of the mechanism, we may be able to optimize and personalize the treatment of DCIS.
Total mastectomy had never been compared to BCS in a clinical trial for DCIS. The results pooled from observational studies were highly consistent. It indicated that MT leads to the lowest IBTR risk, both in DCIS and invasive recurrence. Along with the rising incidence of DCIS in young women, MT is increasingly performed for this distinct population in the US [17,41]. Although the extent of MT may cause more sequelae in body imaging impact and psychosocial distress [48,49], the young population was found to recover from depressed mood faster and benefit the most from breast reconstruction which significantly corrects the negative impacts of MT [[50], [51], [52]].
In respect of survival between BCS + RT and BCS, results were highly consistent among SEER-derived studies, which indicated a superior survival with RT. With the adjustment of socioeconomic status, surgical margin, and hormone therapy, the NCDB-derived study agreed on the overall survival benefit, but the benefit was of less magnitude [41]. Despite sophisticated study designs, the results might warrant further validation considering the short follow-up and highly confounded distribution of patients among treatment groups [17,41]. The absence of a statistically significant superiority of BCS + RT in the pooled survival results could be accounted for by the retrospective cohort study with a median follow-up of 13.3 years [30]. Nevertheless, the relatively small sample size and absence of multivariate analysis in the result-of-interest might decrease the accuracy of the result [30]. On the other hand, no survival difference was observed between young DCIS women receiving BCS + RT and MT. Although evidence was emerging to support a superior survival with BCS + RT over MT in patients with invasive breast cancer [[53], [54], [55]], the benefit was stated to arise from the prevention of distal metastasis [56], which is extremely rare in the case of pure DCIS. Therefore, the authors could not reach a conclusive suggestion concerning survival outcomes with currently available data. Since the survival difference was less evident and might be trivial, long-term toxicities, such as second primary cancer, major cardiovascular events, and cosmetic outcome [57,58], should be carefully weighed against benefit. Deep-inspirational breath-hold (DIBH) technique or prone position could be considered to reduce exposure dose of heart. Young DCIS women and their physicians might need a personalized and thoughtful discussion before making their treatment decision.
Nevertheless, the findings of this study have to be seen in the light of some limitations. The influence of competing events on local control outcomes was not fully estimated. Some important outcomes such as contralateral breast events were not reported due to the limited available data. With the lack of age-specific RCTs, the data was mainly extracted from subgroup analysis of clinical trials and retrospective cohort studies, and some were thus not adjusted for possible confounders. Although subgroup analyses revealed no inter-group difference among study designs and application of multivariate analysis, possible imbalance of known risk factors, such as tumor size, histological grade, and comedonecrosis, among treatment groups might still cause selection bias that could lead to underestimation of RT benefit.
5. Conclusion
With the evidence available for young DCIS females at present, this study validated the reduction of IBTR with RT and highlighted its varying effect with diagnostic age, follow-up duration, and recurrent type. On the other hand, the local control benefit of MT was more evident and consistent. Without conclusive evidence for survival differences at present and having different adverse effects, it would be recommended that local treatment options, including BCS, BCS + RT, and MT, should be well discussed with young DCIS patients through a shared-decision making process. Further studies on the mechanism of the varying effect of RT and age-specific prospective trials are required.
6. Funding
This work was supported by Kaohsiung Veterans General Hospital [grant number: VGHKS107-D03-3].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.03.006.
Contributor Information
Yow-Ling Shiue, Email: shirley@imst.nsysu.edu.tw.
Pei-Chin Lin, Email: pclin@vghks.gov.tw.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Vatovec C., Erten M.Z., Kolodinsky J., et al. Ductal carcinoma in situ: a brief review of treatment variation and impacts on patients and society. Crit Rev Eukaryot Gene Expr. 2014;24:281–286. doi: 10.1615/critreveukaryotgeneexpr.2014011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eusebi V., Foschini M.P., Cook M.G., Berrino F., Azzopardi J.G. Long-term follow-up of in situ carcinoma of the breast with special emphasis on clinging carcinoma. Semin Diagn Pathol. 1989;6:165–173. [PubMed] [Google Scholar]
- 3.Eusebi V., Feudale E., Foschini M.P., et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994;11:223–235. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative G., Correa C., McGale P., et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuart K.E., Houssami N., Taylor R., Hayen A., Boyages J. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer. 2015;15:890. doi: 10.1186/s12885-015-1904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group E.B.C.C., Group E.R., Bijker N., et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for research and treatment of cancer randomized phase III trial 10853--a study by the EORTC breast cancer cooperative group and EORTC radiotherapy group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B., Dignam J., Wolmark N., et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J., Sestak I., Pinder S.E., et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warnberg F., Garmo H., Emdin S., et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 2014;32:3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 10.Vicini F.A., Shaitelman S., Wilkinson J.B., et al. Long-term impact of young age at diagnosis on treatment outcome and patterns of failure in patients with ductal carcinoma in situ treated with breast-conserving therapy. Breast J. 2013;19:365–373. doi: 10.1111/tbj.12127. [DOI] [PubMed] [Google Scholar]
- 11.Mamtani A., Nakhlis F., Downs-Canner S., et al. Impact of age on locoregional and distant recurrence after mastectomy for ductal carcinoma in situ with or without microinvasion. Ann Surg Oncol. 2019;26:4264–4271. doi: 10.1245/s10434-019-07693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhingran A., Kim J.S., Buchholz T.A., et al. Age as a predictor of outcome for women with DCIS treated with breast-conserving surgery and radiation: the University of Texas M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 2002;54:804–809. doi: 10.1016/s0360-3016(02)02988-7. [DOI] [PubMed] [Google Scholar]
- 13.Solin L.J., Kurtz J., Fourquet A., et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. J Clin Oncol. 1996;14:754–763. doi: 10.1200/JCO.1996.14.3.754. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B., Costantino J., Redmond C., et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 15.Narod S.A., Iqbal J., Giannakeas V., Sopik V., Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1:888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 16.Sagara Y., Freedman R.A., Vaz-Luis I., et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: a population-based longitudinal cohort study. J Clin Oncol. 2016;34:1190–1196. doi: 10.1200/JCO.2015.65.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park H.L., Chang J., Lal G., Lal K., Ziogas A., Anton-Culver H. Trends in treatment patterns and clinical outcomes in young women diagnosed with ductal carcinoma in situ. Clin Breast Cancer. 2018;18:e179–e185. doi: 10.1016/j.clbc.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmberg L., Garmo H., Granstrand B., et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 19.Meattini I., Lambertini M., Desideri I., De Caluwe A., Kaidar-Person O., Livi L. Radiation therapy for young women with early breast cancer: current state of the art. Crit Rev Oncol Hematol. 2019;137:143–153. doi: 10.1016/j.critrevonc.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Guo F., Kuo Y.F., Shih Y.C.T., Giordano S.H., Berenson A.B. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer. 2018;124:3500–3509. doi: 10.1002/cncr.31638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. w264. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Sterne J.A.C., Savovic J., Page M.J., Hróbjartsson A., Boutron I. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10:3. [Google Scholar]
- 23.Wells G.A.S.B., O'Connell D., Peterson J., Welch V., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2011. www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 24.Parmar M.K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Zee K.J., Liberman L., Samli B., et al. Long term follow-up of women with ductal carcinoma in situ treated with breast-conserving surgery: the effect of age. Cancer. 1999;86:1757–1767. doi: 10.1002/(sici)1097-0142(19991101)86:9<1757::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Cutuli B., Cohen-Solal-le Nir C., de Lafontan B., et al. Breast-conserving therapy for ductal carcinoma in situ of the breast: the French Cancer Centers' experience. Int J Radiat Oncol Biol Phys. 2002;53:868–879. doi: 10.1016/s0360-3016(02)02834-1. [DOI] [PubMed] [Google Scholar]
- 28.Omlin A., Amichetti M., Azria D., et al. Boost radiotherapy in young women with ductal carcinoma in situ: a multicentre, retrospective study of the Rare Cancer Network. Lancet Oncol. 2006;7:652–656. doi: 10.1016/S1470-2045(06)70765-3. [DOI] [PubMed] [Google Scholar]
- 29.Guerrieri-Gonzaga A., Botteri E., Rotmensz N., et al. Ductal intraepithelial neoplasia: postsurgical outcome for 1,267 women cared for in one single institution over 10 years. Oncol. 2009;14:201–212. doi: 10.1634/theoncologist.2008-0203. [DOI] [PubMed] [Google Scholar]
- 30.Tunon-de-Lara C., Lemanski C., Cohen-Solal-Le-Nir C., et al. Ductal carcinoma in situ of the breast in younger women: a subgroup of patients at high risk. Eur J Surg Oncol. 2010;36:1165–1171. doi: 10.1016/j.ejso.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Alvarado R., Lari S.A., Roses R.E., et al. Biology, treatment, and outcome in very young and older women with DCIS. Ann Surg Oncol. 2012;19:3777–3784. doi: 10.1245/s10434-012-2413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakovitch E., Nofech-Mozes S., Narod S.A., et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res Treat. 2013;138:581–590. doi: 10.1007/s10549-013-2455-8. [DOI] [PubMed] [Google Scholar]
- 33.Worni M., Akushevich I., Greenup R., et al. Trends in treatment patterns and outcomes for ductal carcinoma in situ. J Natl Cancer Inst. 2015;107:djv263. doi: 10.1093/jnci/djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian G.W., Ni X.J., Wang Z., Jiang Y.Z., Yu K.D., Shao Z.M. Effect of radiotherapy on survival of women with locally excised ductal carcinoma in situ of the breast: a Surveillance, Epidemiology, and End Results population-based analysis. OncoTargets Ther. 2015;8:1407–1418. doi: 10.2147/OTT.S82087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elshof L.E., Schaapveld M., Schmidt M.K., Rutgers E.J., van Leeuwen F.E., Wesseling J. Subsequent risk of ipsilateral and contralateral invasive breast cancer after treatment for ductal carcinoma in situ: incidence and the effect of radiotherapy in a population-based cohort of 10,090 women. Breast Cancer Res Treat. 2016;159:553–563. doi: 10.1007/s10549-016-3973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cronin P.A., Olcese C., Patil S., Morrow M., Van Zee K.J. vol. 23. 2016. pp. 2816–2824. (Impact of age on risk of recurrence of ductal carcinoma in situ: outcomes of 2996 women treated with breast-conserving surgery over 30 years). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K., Kim J.H., Kim Y.B., et al. Selective radiation therapy for ductal carcinoma in situ following breast-conserving surgery according to age and margin width: Korean radiation oncology group 11-04 and 16-02 studies. J Breast Cancer. 2017;20:327–332. doi: 10.4048/jbc.2017.20.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo S.H., Lo C., Chen Y.H., et al. Prognostic significance of clinicopathologic features in patients with breast ductal carcinoma-in-situ who received breast-conserving surgery. Clin Breast Cancer. 2018;18 doi: 10.1016/j.clbc.2018.04.002. 441-450 e442. [DOI] [PubMed] [Google Scholar]
- 39.Giannakeas V., Sopik V., Narod S.A. Association of radiotherapy with survival in women treated for ductal carcinoma in situ with lumpectomy or mastectomy. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Seijen M., Lips E.H., Fu L., et al. Long-term risk of subsequent ipsilateral lesions after surgery with or without radiotherapy for ductal carcinoma in situ of the breast. Br J Cancer. 2021;125(10):1443–1449. doi: 10.1038/s41416-021-01496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byun D.J., Wu S.P., Nagar H., Gerber N.K. Ductal carcinoma in situ in young women: increasing rates of mastectomy and variability in endocrine therapy use. Ann Surg Oncol. 2021;28:6083–6096. doi: 10.1245/s10434-021-09972-2. [DOI] [PubMed] [Google Scholar]
- 42.Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 43.Goodwin A., Parker S., Ghersi D., Wilcken N. Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev. 2013:CD000563. doi: 10.1002/14651858.CD000563.pub7. [DOI] [PubMed] [Google Scholar]
- 44.Vicini F.A., Kestin L.L., Goldstein N.S., et al. Impact of young age on outcome in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Clin Oncol. 2000;18:296–306. doi: 10.1200/JCO.2000.18.2.296. [DOI] [PubMed] [Google Scholar]
- 45.Moran M.S., Zhao Y., Ma S., et al. Association of radiotherapy boost for ductal carcinoma in situ with local control after whole-breast radiotherapy. JAMA Oncol. 2017;3:1060–1068. doi: 10.1001/jamaoncol.2016.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartelink H., Maingon P., Poortmans P., et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 47.Narendran N., Luzhna L., Kovalchuk O. Sex difference of radiation response in occupational and accidental exposure. Front Genet. 2019;10:260. doi: 10.3389/fgene.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berhili S., Ouabdelmoumen A., Sbai A., Kebdani T., Benjaafar N., Mezouar L. Radical mastectomy increases psychological distress in young breast cancer patients: results of A cross-sectional study. Clin Breast Cancer. 2019;19:e160–e165. doi: 10.1016/j.clbc.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg S.M., Tamimi R.M., Gelber S., et al. Body image in recently diagnosed young women with early breast cancer. Psycho Oncol. 2013;22:1849–1855. doi: 10.1002/pon.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fanakidou I., Zyga S., Alikari V., Tsironi M., Stathoulis J., Theofilou P. Mental health, loneliness, and illness perception outcomes in quality of life among young breast cancer patients after mastectomy: the role of breast reconstruction. Qual Life Res. 2018;27:539–543. doi: 10.1007/s11136-017-1735-x. [DOI] [PubMed] [Google Scholar]
- 51.Kim M.S., Kim S.Y., Kim J.H., Park B., Choi H.G. Depression in breast cancer patients who have undergone mastectomy: a national cohort study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dauplat J., Kwiatkowski F., Rouanet P., et al. Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg. 2017;104:1197–1206. doi: 10.1002/bjs.10537. [DOI] [PubMed] [Google Scholar]
- 53.Almahariq M.F., Quinn T.J., Siddiqui Z., et al. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother Oncol. 2020;142:186–194. doi: 10.1016/j.radonc.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Li H., Chen Y., Wang X., Tang L., Guan X. T1-2N0M0 triple-negative breast cancer treated with breast-conserving therapy has better survival compared to mastectomy: a SEER population-based retrospective analysis. Clin Breast Cancer. 2019;19:e669–e682. doi: 10.1016/j.clbc.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Christiansen P., Carstensen S.L., Ejlertsen B., et al. Breast conserving surgery versus mastectomy: overall and relative survival-a population based study by the Danish Breast Cancer Cooperative Group (DBCG) Acta Oncol. 2018;57:19–25. doi: 10.1080/0284186X.2017.1403042. [DOI] [PubMed] [Google Scholar]
- 56.Wang J., Wang S., Tang Y., et al. Comparison of treatment outcomes with breast-conserving surgery plus radiotherapy versus mastectomy for patients with stage I breast cancer: a propensity score-matched analysis. Clin Breast Cancer. 2018;18:e975–e984. doi: 10.1016/j.clbc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Taylor C., Correa C., Duane F.K., et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobse J.N., Duane F.K., Boekel N.B., et al. Radiation dose-response for risk of myocardial infarction in breast cancer survivors. Int J Radiat Oncol Biol Phys. 2019;103:595–604. doi: 10.1016/j.ijrobp.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lari S.A., Kuerer H.M. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–261. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawrence BM, Louis SC, Jacob A., et al. Cardiotoxicity of radiation therapy for breast cancer and other malignancies. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on January 010, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.