Summary
Chimeric antigen receptor (CAR) T cell therapy has emerged as a cancer treatment with enormous potential, demonstrating impressive antitumor activity in the treatment of hematological malignancies. However, CAR T cell exhaustion is a major limitation to their efficacy, particularly in the application of CAR T cells to solid tumors. CAR T cell exhaustion is thought to be due to persistent antigen stimulation, as well as an immunosuppressive tumor microenvironment, and mitigating exhaustion to maintain CAR T cell effector function and persistence and achieve clinical potency remains a central challenge. Here, we review the underlying mechanisms of exhaustion and discuss emerging strategies to prevent or reverse exhaustion through modifications of the CAR receptor or CAR independent pathways. Additionally, we discuss the potential of these strategies for improving clinical outcomes of CAR T cell therapy.
Keywords: Chimeric antigen receptor, CAR T, T cell exhaustion, Cancer, Immunotherapy
Introduction
The field of immunotherapy encompasses a broad spectrum of treatments designed to induce, augment, or suppress the immune response, seeking to fine tune an immune system that has evolved to strike a balance between clearing harmful pathogens and protecting tissues from the collateral damage of an inflammatory response. Monoclonal therapeutic antibodies, immune checkpoint inhibitors, cytokines, and immunomodulators are methods of mediating the immune response. In addition to therapeutic molecules and proteins, immunotherapy also consists of cell-based therapeutic approaches. The transient delivery of disease targeting immune cells, known as adoptive cellular therapy (ACT), is a promising method for treating infection, autoimmune disease, and cancer.1, 2, 3, 4, 5 The foremost ACT approach is chimeric antigen receptor (CAR) T cell therapy, which involves the transfer of allogeneic or autologous T cells modified to express a CAR.
First proposed by Eshhar et al. in 1993,6 the CAR enables the modified T cells to mount an antigen-specific immune response to cells bearing the CAR target antigen independently of the major histocompatibility complex (MHC).7 Indeed, several ongoing clinical trials focus on the utilization of CAR T cell therapy to treat autoimmune disease (NCT04146051, NCT03030976), HIV (NCT03240328, NCT03980691, NCT03617198, NCT04648046), and solid tumors (NCT04981691, NCT04691713, NCT03932565, NCT04151186).7,8 However, CAR T cell therapies have been most notably successful in the treatment of hematological malignancies.
To date, the FDA has approved five CAR T cell therapies for the treatment of BCMA or CD19 antigen-expressing hematological cancers. However, durable remission following CAR T cell therapy is not guaranteed, as demonstrated by relapse occurring in up to 75% of patients treated with CD19 or CD22 CAR T cells for hematological malignancies.9, 10, 11 Most commonly, CAR T cell therapy failure is attributed to antigen escape, wherein selection pressure under CAR T surveillance leads to the emergence of antigen-negative tumors.12 However, relapse also occurs with antigen-positive disease, suggesting that CAR T cell-intrinsic factors can contribute to poor anti-tumor response. Analysis of clinical data collected from chronic lymphocytic leukemia (CLL) patients treated with CD19 CAR T cells identified overall CAR T cell fitness as a predictor of therapeutic success.13
The treatment of solid tumors is further constrained by the ability of the CAR T cells to infiltrate into the tumor and effectively kill target cells in an immunosuppressive microenvironment.14, 15, 16 The tumor microenvironment incorporates a barrier of stromal cells and extracellular matrix that limits CAR T cell access to tumor cells.14,17 Additionally, immunosuppressive cells accumulate in the tumor microenvironment to limit CAR T cell effector function. Tumor-infiltrating immune cells, such as regulatory T cells, generate an environment hostile to CAR T cells through the secretion of inhibitory cytokines and depletion of IL-2.14,18
These factors ultimately contribute to a failure to clear antigen, which occurs both in cancer and during chronic viral infection. Persistent antigen stimulation leads to T cell exhaustion, defined as the inhibition of T cell proliferation and effector function, which results in resistance and relapse in CAR T cell therapy.19,20 The persistent exposure of T cells to disease-specific antigens causes the T cells to differentiate into a dysfunctional state characterized by reduced proliferative capacity and effector function.21, 22, 23 Because successful CAR T cell therapy requires CAR T cells to kill target cells repeatedly, T cell exhaustion is associated with poor responses in cancer patients receiving immunotherapy.24, 25, 26 Hence, the development of methods that aim to counteract T cell exhaustion is essential to improving CAR T cell therapy efficacy.
Although it is clear that T cell receptor (TCR) and CAR signaling are quite different,27 most of the framework for understanding the influence of CAR signaling on CAR T cell function and differentiation is derived from comprehensive analogous work that has been done in the TCR field. In particular, the transcriptional and epigenetic mechanisms underlying T cell exhaustion in chronic viral infection are exquisitely described, whereas the parallels to CAR T exhaustion are still being elucidated. Thus, our review first describes the mechanism and influence of T cell exhaustion in the context of chronic stimulation of the TCR. We use the principles established in chronic TCR stimulation to then discuss approaches to overcoming exhaustion in CAR T cell therapy through targeting T cell intrinsic signaling and CAR engineering.
T cell exhaustion
T cell exhaustion was initially described as the failure of the immune system to mount a response to chronic viral infection.28,29 During chronic infection, as opposed to acute infection, the invading pathogen is not rapidly eliminated, and this persistence leads to prolonged antigen stimulation, inflammation, and subsequent T cell dysfunction.23,30 T cell exhaustion has also been described in cancer, where prolonged antigen exposure and the immunosuppressive tumor microenvironment contribute to a loss of effector function and sustained inhibitory receptor expression.22
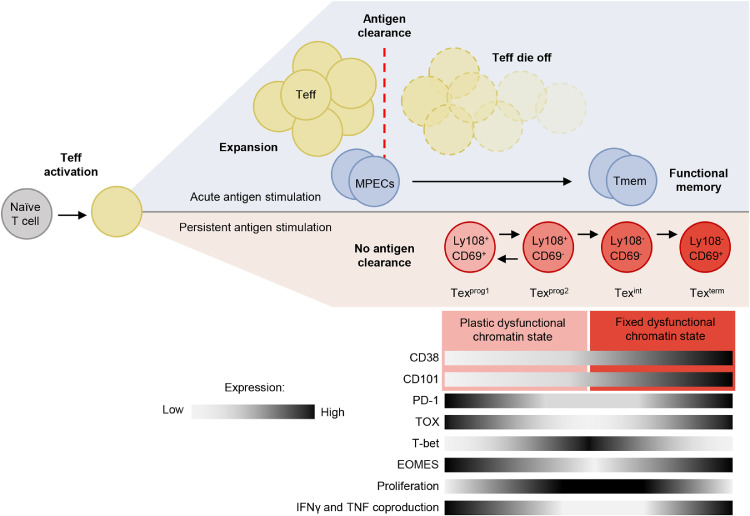
Exhaustion has been best characterized in CD8+ T cells, although dysfunctional states due to persistent antigen stimulation have also been identified in CD4+ T cells,31,32 Upon acute antigen stimulation, naïve CD8+ T (Tn) cells differentiate into effector T (Teff) cells.33 During differentiation, cells undergo functional and metabolic reprogramming, as well as vigorous clonal expansion, to establish an antigen-specific effector T cell population.34,35 The magnitude of this response is modulated by the density of antigen presented to the Tn cells, where an increase in antigen drives increased T cell expansion.36 However, T cell antigen sensitivity is inversely correlated with the density of antigen presented.36, 37, 38, 39 Following antigen clearance, the majority of effector T cells die off. The few surviving cells become memory T (Tmem) cells and persist in the host independent of the stimulatory antigen.35 However, persistent antigen stimulation during chronic infection or cancer can subvert CD8+ T cell differentiation into an exhausted state characterized by a loss of effector function and reduced proliferative capacity (Figure 1).23
Figure 1.
The T cell response during acute and persistent antigen stimulation. For acute antigen stimulation, the expansion of Teff cells is followed by antigen clearance. After the antigen is cleared, the majority of antigen specific T cells die off, leaving a small number of Tmem cells. For persistent antigen stimulation, the antigen is not cleared leading to the differentiation of the Teff cells to an exhausted state. MPECs: memory precursor effector cells, Teff: effector T cells, Tmem: memory T cells. Original figure.
T cell exhaustion is believed to have evolved to prevent severe immunopathology from excessive CD8+ T cell response.34 Exhaustion describes a hyporesponsive T cell state, broadly characterized by decreased expression of effector cytokines and increased expression of inhibitory immune checkpoint receptors, which in combination mediate inhibitory signaling and diminish T cell cytotoxicity.40 Indeed, expression of PD-1, TIM-3, LAG-3, TIGIT, and CTLA-4 are hallmarks of T cell exhaustion, and exhausted T (Tex) cells are functionally distinct from Teff and Tmem cells.41, 42, 43 However, several of these inhibitory receptors are similarly upregulated in T cell activation as a mechanism of modulating co-stimulatory signaling.44 This suggests that inhibitory receptors alone are not sufficient to distinguish between exhausted and activated T cells.
In fact, T cell exhaustion comprises a differentiative process with several described stages.44,45 The transition of an effector T cell into an exhausted state is accompanied by significant epigenetic reorganization and distinct transcriptional signatures.45,46 In a study utilizing assay for Transposase Accessible Chromatin using Sequencing (ATAC-seq) and RNA sequencing (RNA-seq), Philip et al. identify two distinct chromatin states and key transcription factors associated with the exhaustion transition in tumor-specific T cells.47 The initial plastic dysfunctional chromatin state is reversible, and the later fixed chromatin state is irreversible, in PD-1high tumor-infiltrating CD8 T cells. Specifically, chromatin peaks containing TCF family transcription factor motifs close during the transition from the plastic to the fixed state of dysfunction, corresponding with a decrease in TCF1, a central transcription factor in early T cell exhaustion.
The chromatin transition is accompanied by a decrease in chromatin accessibility of genes associated with TCR signaling and cytokine response, complemented by an increase in expression of negative regulators of TCR signaling and a decrease in expression of costimulatory molecules. In murine tumor-specific T cells, two dysfunctional chromatin states of PD-1high T cells are associated with differential expression of the markers CD38 and CD101, which allows for the cell surface level identification of a reversible (CD38low/CD101low) and irreversible (CD38high/CD101high) exhausted state. The markers and dysfunctional chromatin accessibility state were similarly identified in human PD-1high tumor-infiltrating lymphocytes.47
Beltra et al. has since further characterized the stages of Tex differentiation, which can be categorized by the expression of cell surface receptors CD69 and Ly108.45 CD69 expression is inversely correlated with proliferation, and Ly108 is a surrogate for the expression of TCF1.45,48 Utilizing a mouse model infected with chronic lymphocytic choriomeningitis virus (LCMV), Beltra et al. define four hierarchical subsets of CD8+ Tex cells. The initial Tex cell state arising from Teff cells during exhaustion is the quiescent Ly108+/CD69+ progenitor population (Texprog1); this gives rise to a circulating population of proliferative Ly108+/CD69− cells (Texprog2). The Texprog2 population gives rise to an intermediate Ly108−/CD69− (Texint) stage; the loss of Ly108 is irreversible and indicates commitment to the exhausted phenotype coinciding with loss of the transcription factor TCF1. The terminal population, Texterm, differentiates from Texint Ly108− cells but re-expresses CD69. Unlike the progenitor exhausted states, Texint and Texterm populations do not possess the capacity to proliferate and have increased apoptosis (Figure 1). These Tex subsets were similarly identified in tumor-infiltrating lymphocytes from human melanoma patients.
This gradual progression into a terminally exhausted state has similarly been defined by the expression of CX3CR1 in subpopulations of Tex cells.49,50 Hudson et al. found that CX3CR1 was preferentially expressed in transitory (CD101−/Tim3+) compared to terminally differentiated (CD101+/Tim3+) Tex cells.50 Concurrently, Zander et al. describes three distinct exhaustion subpopulations including a self-renewing progenitor population (Ly108+/CX3CR1−) that can give rise to a cytolytic (Ly108+/CX3CR1+) or highly dysfunctional Tex cells (Ly108−/CX3CR1−).49 Together, these studies suggest that the loss of CX3CR1 expression in Tex cells is a marker for terminal differentiation.
High expression of high-mobility group (HMG)-box transcription factor TOX in response to persistent antigen stimulation induces the transcriptional and epigenetic signatures of T cell exhaustion.42,51 Persistent TCR activation leads to calcineurin-mediated dephosphorylation and subsequent nuclear localization of NFAT. NFAT inhibits TCF1 and promotes the expression of TOX and NR4A, inducing the terminal differentiation of TCF1-expressing (TCF1+) Tex progenitors.52, 53, 54, 55 The TCR-NFAT-TCF1-TOX/NR4A axis is central to the epigenetic commitment of T cells to an exhausted cell program.42 TOX overexpression induces Tex-specific epigenetic opening of an enhancer upstream of Pdcd1, the gene coding for PD-1.42 Conversely, TOX family transcription factor depletion increases cytokine production and decreases expression of PD-1, TIM3, and LAG-3, indicating a reversal of the exhausted phenotype.54 Specifically, TOX is a critical transcriptional checkpoint in the transition of Texint to Texterm cells.45 Whereas the establishment of the Texint population is driven by T-bet expression and the loss of TCF1 expression, TOX modulates the establishment of the Texterm population by repressing the expression of T-bet in TCF1− Texint cells.45 However, the role of TOX in T cell exhaustion is complex. Scott et al. found that Tox depleted tumor-specific T cells have lower expression of inhibitory markers, reduced persistence, and comparable effector function to wildtype tumor-specific T cells.56 Similarly, depletion of Tox impairs the persistence of exhausted virus-specific CD8+ T cells.57,58 These studies suggest that TOX has a role in sustaining a population of Tex cells during chronic infection and cancer.
In addition to TOX, T-bet interacts with Eomesodermin (EOMES) to regulate exhaustion. T-bet and EOMES have differential regulatory control of genes such as Pdcd1, where T-bet has stronger repression of Pdcd1 expression than EOMES.59 As such, high expression of EOMES and low expression of T-bet are associated with exhaustion and poor clinical outcomes.60,61 Indeed, Beltra et al. further described the Tex subtypes through the dynamics of EOMES expression. EOMES expression was greatest in the Texprog1 population and gradually decreased until its nadir in Texint, then increased again in the Texterm population. In contrast, T-bet expression increased, peaking in the Texint population before plummeting in the Texterm population. Although decreased PD-1 expression was observed in the TCF1−TOXintT-bethighEOMESlow Texint population, other inhibitory receptors such as TIM3 and LAG-3 gradually increased throughout the Tex subsets (Figure 1).45
Targeting T cell intrinsic pathways to overcome exhaustion in CAR T cell therapy
PD-1
Programmed death-1 (PD-1) is a cell surface receptor member of the CD28 coreceptor family. In contrast to CD28, which enhances TCR signaling, PD-1 attenuates immune cell function.62 Sustained expression of PD-1 on the surface of T cells is associated with dysfunction.63 Upon binding to PD-1 ligand-1 (PD-L1), PD-1 recruits the phosphatases SHP1 and SHP2, which inhibit activation of ZAP70 and PI3K to curb downstream activation of AKT and ERK.64 Additionally, PD-1 indirectly impacts TCR signaling through suppression of cyclin-dependent kinases (CDKs) to inhibit cell cycle progression and through downregulation of casein kinase II (CK2), which inhibits TCR expression.64, 65, 66 Blockade of PD-1/PD-L1 immunosuppression with specific antibodies markedly improves immune function in many cancer patients and has revolutionized clinical cancer care for some diseases.67 Interestingly, expression of CX3CR1 is predictive of clinical response to PD-1 blockade, suggesting that exhaustion state impacts therapeutic efficacy.68,69
Antibody PD-1/PD-L1 blockade has also been shown to augment CAR T cell therapy in a variety of tumors.70, 71, 72 Similarly, CAR T cells modified to secrete PD-1 single-chain variable fragment (scFv) had improved anti-tumor activity, comparable to PD-1 antibody + CAR T combination therapy.73, 74, 75 This localized delivery of PD-1 scFv is theorized to act in both a paracrine and autocrine manner, enhancing the function of delivered CAR T cells as well as the tumor-infiltrating endogenous T cells.
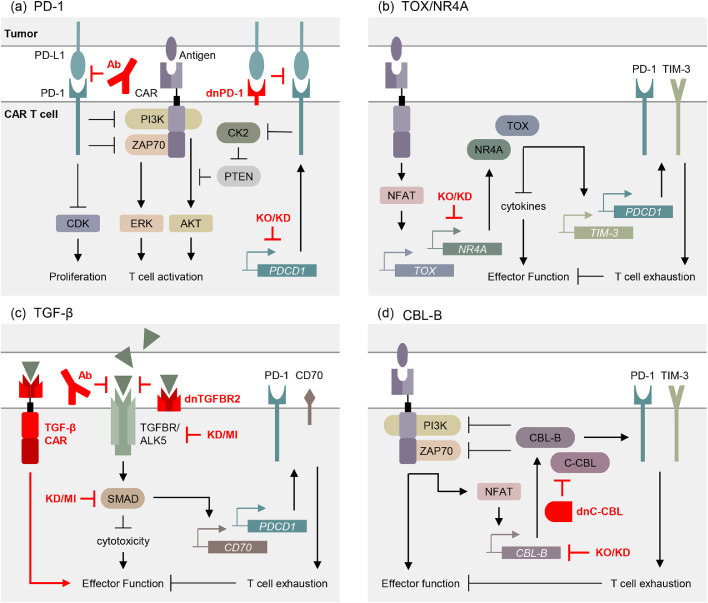
Cell intrinsic blockade of PD-1 signaling is another promising method of combating exhaustion in CAR T cells. The expression of a dominant-negative PD-1 receptor has been shown to improve CAR T functional persistence and protect against exhaustion.76 Recently, CD19 CAR T cells modified to express dominant-negative PD-1 receptors have been shown to be safe and effective in the treatment of refractory B cell lymphoma.77 Similarly, shRNA-mediated silencing of PDCD1 enhanced the therapeutic function of CLL-1 and mesothelin CAR T cells.78,79 Knockout of PDCD1 improved the anti-tumor activity and survival of EGFR, CD19, and GPC3 CAR T cells in mouse models.80, 81, 82 Phase I clinical trials demonstrated that CRISPR-Cas9 guided disruption of PDCD1 does not lead to uncontrolled CAR T cell proliferation or persistence.83 While the impact of PDCD1 knockout on CAR T immunotherapeutic clinical efficacy is still under investigation, these discoveries suggest that extrinsic and intrinsic blockade of the PD-1/PD-L1 axis protect CAR T cells from PD-1 induced exhaustion (Figure 2a).
Figure 2.
Intrinsic T cell approaches to overcoming exhaustion in CAR T cell therapy. Inhibition of T cell activation and promotion of exhaustion by the (a) PD-1, (b) TOX/NR4A, (c) TGF-β, and (d) CBL-B pathways. Means of targeting these pathways to resist exhaustion are depicted in red. KO: knockout, KD: knock down, MI: molecular inhibitor, dnPD-1: dominant negative PD-1, dnC-CBL: dominant negative C-CBL, dnTGFBR2: dominant negative TGFBR2. Original figure.
TOX and NR4A
Kim et al. utilized single-cell transcriptomics to differential expression of TOX between PD-1 high and low populations of tumor-infiltrating CD8+ T cells in patient tumor samples. An increase in TOX expression was associated with CD8+ T cell exhaustion and increased expression of the immune checkpoint genes PDCD1, CTLA-4, TIM-3, LAG3, and TIGIT, as well as increased protein level expression of PD-1 and TIM-3.51,52,56 Knockdown of TOX in tumor-infiltrating lymphocytes resulted in decreased expression of exhaustion-associated immune checkpoint molecules and increased IFNγ and TNFα production, suggesting that TOX modulates tumor infiltration-induced exhaustion in CD8+ T cells.42,51
The TOX protein subfamily consists of four members: TOX (also referred to as TOX1), TOX2, TOX3, and TOX4. In addition to their roles in CD4+ T cell and T follicular helper cell development, TOX and TOX2 are highly expressed in exhausted CAR T cells (PD-1highTIM3high).54, 55, 56,84,85 TOX and NR4A cooperate to induce exhaustion in CAR tumor-infiltrating T cells and depletion of Nr4a1 prevented exhaustion and improved anti-tumor immunity in tumor-infiltrating T cells.54,55,86 Tox and Tox2 depletion in CAR T cells improved anti-tumor activity and survival in tumor inoculated mice.54 In a similar study, the triple knockout of Nr4a1, Nr4a2, and Nr4a3 in tumor-infiltrating CAR T cells enhanced tumor regression and improved survival in tumor-bearing mice.55,87 Thus, targeting the T cell intrinsic TOX/NR4A axis is a promising method of improving the persistence and function of CAR T cells (Figure 2b).
TGF-β
Transforming growth factor beta (TGF-β) is a cytokine with a variety of biological functions in mammals, including the regulation of immune cell development and homeostasis.88 TGF-β is highly expressed in the tumor microenvironment, ultimately attenuating the immune response by suppressing T cell activation and proliferation.89,90 TGF-β signaling is activated when a TGF-β dimer binds to and assembles two TGF-β receptors II (TGFBR2) and two TGF-β receptors I (ALK5) into a heterotetramer. TGF-β signaling activates SMAD family proteins, which in turn suppress the expression of genes essential for CD8+ T cell migration and effector function.91, 92, 93, 94 In CD4+ and CD8+ T cells, TGF-β-induced activation of SMAD promotes the expression of CD70, a suppressor of T cell expansion and function that is associated with increased expression of the inhibitory receptors PD-1 and TIM-3.95, 96, 97 Moreover, TGF-β signaling in CD8+ T cells promotes an exhausted phenotype of enhanced PD-1 expression, impaired expansion, and decreased immunostimulatory cytokine production.98,99
Recently, multiple studies investigating TGF-β signaling blockade have demonstrated enhanced antitumor function of CAR T cells through the knockdown of TGFBR2,100 overexpression of dominant negative TGFBR2,101,102 small molecule inhibition of ALK5,103,104 or sequestration of TGF-β from the tumor microenvironment through the expression of membrane bound and soluble TGFBR2 extracellular domains.75,105 Fusion of a TGFBR2 binding domain to a PD-1 scFv effectively sequesters TGF-β from the tumor microenvironment, preventing TGF-β-mediated immunosuppression of T cells. Expression of this bispecific protein results in the blockade of both PD-1 and TGF-β signaling, ultimately improving T cell persistence and effector function.75 Currently, clinical trials are investigating the efficacy of TGFBR2 knock out (NCT04976218) and dominant negative TGFBR2 (NCT03089203 NCT00889954) in improving CAR T cell therapy of solid tumors.
TGF-β targeting CAR T cells have been demonstrated to improve the antitumor function of CAR T cell therapy.106 TGF-β CAR T cells transform the immunosuppressive TGF-β cytokine into a potent activator of T cells both by sequestering soluble TGF-β from the tumor microenvironment and by producing immunostimulatory cytokines when they bind TGF-β.106, 107, 108 A clinical trial utilizing TGF-β CAR T cells in combination with GPC3 CAR T cells has been initiated; however, results have yet to be published (NCT03198546).109
In addition to blocking activation, downstream effectors of the TGF-β/SMAD signaling cascade could be targeted to mediate TGF-β–induced immunosuppression in CAR T cells. Although not as well defined as upstream blockade of TGF-β ligand and receptor, inhibition of the ALK5 substrate SMAD3 has shown initial promise in improving immune response to tumors. In a mouse model, SMAD3 inhibition improved the anti-tumor activity of CD8+ T cells.98 Selective SMAD3 inhibitors have similar potential in improving CAR T cell therapy. Knockdown of SMAD3 in breast cancer cells resulted in a suppression of proliferation and metastasis.110 As such, the introduction of a SMAD3 inhibitor to the tumor microenvironment could simultaneously promote resistance to exhaustion in tumor-infiltrating CAR T cells and directly suppress tumor growth.98,110,111 Overall, the abrogation of TGF-β signaling in T cells has demonstrated significant promise in protecting against exhaustion and enhancing effector function (Figure 2c).
CBL-B
TCR ligation activates NFAT, which induces the expression of the E2 ligase Casitas B-lineage lymphoma-b (CBL-B), a negative modulator of immune activation. CBL-B can influence multiple signaling pathways through the targeted ubiquitination and subsequent down-regulation of protein tyrosine kinases (PTKs).112 CBL-B is upregulated in PD-1 and TIM-3 expressing exhausted CD8+ T cells.113,114 Cbl-b deficient T cells have greater tumor clearing efficiency compared to wild type controls.115
Recently, CRISPR-Cas9 guided deletion of Cbl-b was shown to effectively reverse exhaustion and restore effector function in exhausted CAR T cells.113 These Cbl-b deficient CAR T cells had reduced expression of the exhaustion markers PD-1 and TIM-3, greater anti-tumor function, and restored expression of the immunostimulatory cytokines IFN-γ and TNF-α. Additionally, Cbl-b deficient T cells are resistant to PD-1/PD-L1 checkpoint-mediated inhibition of proliferation and IFN-γ production.116,117 Currently, the autologous transfer of CBL-B-silenced peripheral blood mononuclear cells is being investigated for its efficacy in the treatment of metastatic solid tumors (NCT03087591).
Interestingly, a case report from a CD22 CAR T cell clinical trial describes the clonal expansion of CAR T in a patient with relapse of pre-B cell ALL. Quantitative vector integration site analysis revealed that the CAR lentiviral vector integrated into the C-CBL gene, a closely related homolog of CBL-B (NCT02315612).118 The authors theorize that the observed clonal expansion is due to the formation of a dominant-negative C-CBL mutant. The expression of a dominant negative C-CBL would account for the clonal expansion, as heterozygous knock out of CBL-B is not known to enhance antitumor efficiency of T cells,119,120 warranting further investigation of dominant negative CBL proteins in CAR T cell activation and persistence (Figure 2d).
One caveat with these strategies is that Cbl-b depletion can induce autoimmunity.121 Indeed, whole-mouse Cbl-b knockout causes an increase in the incidence of autoimmune arthritis in a DBA/1 arthritis mouse model.122,123 However, the impact of conditional knockout or the adoptive transfer of Cbl-b deficient T cells on autoimmunity remains unclear.
CAR engineering approaches to overcoming exhaustion in CAR T cell therapy
Modulating the surface expression of the CAR
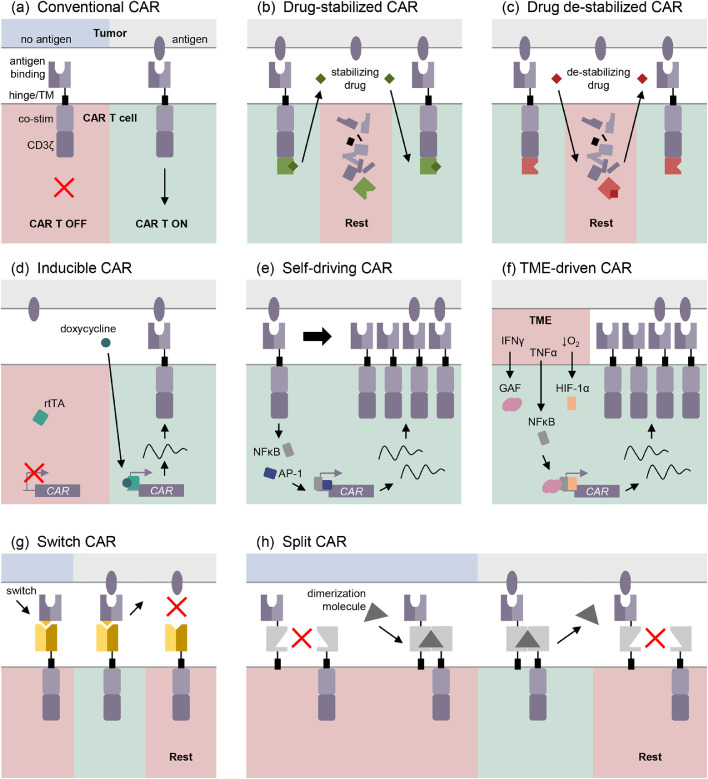
CAR T exhaustion is partly attributed to prolonged exposure to the immunosuppressive microenvironment, upregulation of inhibitory receptors, and persistent CAR stimulation by antigen. As such, limiting or interrupting CAR:antigen interaction is an attractive strategy for ameliorating exhaustion in CAR T cells. Currently, antigen presentation on tumor cells cannot be regulated, which has led to the development of methods for controlling CAR expression on the surface of T cells. Weber et al. employ inducible “transient rest” to restore effector function in exhausted T cells, using a CAR that degrades upon withdrawal of a particular small molecule.124 Induced transient loss of CAR expression restored effector function and enhanced the anti-tumor response of exhausted CAR T cells (Figure 3a and b). Similar systems in which the efficient modulation of CAR expression and activity through drug-induced destabilization have been reported, however, they do not detail the impact of CAR suppression on exhaustion (Figure 3c).125,126 Transcriptional control of CAR expression has also been explored as a strategy for mitigating prolonged antigen stimulation. Doxycycline-inducible CAR expression has been demonstrated to mitigate CAR T cell therapy toxicity and improve clinical safety.127, 128, 129
Figure 3.
CAR construct approaches to overcoming exhaustion in CAR T cell therapy. (a) Diagram of the conventional construction of a CAR. Post-transcriptional regulation of CAR at the surface of the T cell include drug-induced stabilization (b) and de-stabilization (c). Methods of regulating CAR expression at the transcriptional level include inducible systems (d), self-driving CAR promoters (e), and tumor microenvironment (TME) factor driven CAR promoters. Switchable CARs (g) and split CARs (h) uncouple the antigen recognition or co-stimulation from the CD3ζ domain. TM: transmembrane domain, co-stim: co-stimulatory domain, TME: tumor microenvironment. Original figure.
It is thus plausible that small molecule inducible expression systems will be useful for mitigating antigen-induced exhaustion (Figure 3d).
So-called “self-driving” CARs utilize transcriptional response elements such as AP1-NF-kB or STAT5 to control CAR expression, such that antigen stimulation leads to upregulation of the CAR. Compared to a traditional CD19 CAR, placing the CAR under the control of AP1-NF-kB resulted in greater expansion and exhaustion resistance with equivalent in vivo antitumoral function (Figure 3e).102 Recently, a synthetic promoter designed to respond to the tumor microenvironment has been shown to efficiently restrict CAR expression to the T cells at the tumor site.130 The synthetic promoter consists of IFNγ, NFκB, and hypoxia response elements and synergistically activates CAR expression in response to immunosuppressive conditions (Figure 3f). In addition to synthetic promoters, endogenous promoters can be appropriated to control the expression of CAR. Using CRISPR/Cas9, a CD19-CAR was delivered to the T cell receptor α constant (TRAC) locus which simultaneously knocked out the TCR and knocked in the CAR under the control of the TRAC promoter. Compared to conventional retrovirally produced CAR T cells, the TRAC-edited CAR T cells have more consistent CAR expression, greater tumor control, improved persistence, and reduced antigen-induced exhaustion.131
Uncoupling antigen recognition from CAR activation signaling
In addition to titrating CAR expression to modulate CAR signaling, the antigen recognition domain of the CAR can be uncoupled from the activation domain to combat prolonged antigen stimulation in CAR T cells.132 Switchable CAR constructs make use of a soluble antigen recognition domain, or switch, to recognize tumor-specific antigen (Figure 3g).133, 134, 135, 136, 137 Dosing of the switch, which binds to the cell-bound CAR portion to effect signaling, allows for reversible modulation of CAR activation in T cells, potentially preserving effector function through periods of rest. Additionally, switch designs permit substitution of CAR specificity on demand. Similarly, split CARs can be used to control overactivation in cells by dissociating CAR activation from antigen recognition or co-stimulation. Activation of a split CAR requires the presence of a dimerization molecule for CAR assembly, and the presence of tumor antigen for CAR activation (Figure 3h).134,138,139 Similar to modulating the expression of the CAR, uncoupling of antigen recognition and CAR signaling is a potential method of reducing the risk of exhaustion through cessation of persistent antigen stimulation.
Concluding remarks
An important consideration when attempting to block resistance in CAR T cell therapy is that T cell exhaustion is not without evolutionary function; attenuating immune tolerance to chronic antigen stimulation risks inducing immunotoxicity.140,141 Although PD-1 blockade enhances CAR T cell therapy, it is associated with T cell autoreactivity, which can enhance tumor growth.142, 143, 144 Similarly, Cbl-b is essential for the regulation of immune tolerance and preventing autoimmunity.121,122 To avoid swinging the pendulum too far, suicide or safety switches have been implemented to induce CAR T cell death in the event of toxic side effects. The inducible Caspase9 (iC9) safety switch system efficiently induces apoptosis in CAR T and effectively controls adverse events in patients after hematopoietic stem cell transplantation (NCT01494103).107,145,146
The antigen density of the CAR target can also impact the efficacy of CAR T cell therapy. Antigen escape is a common mechanism of tumor evasion of CAR T cell therapy and highlights the need to improve CAR T cell response to low antigen densities. Altering the antigen density threshold is one strategy of optimizing CAR T cell response to clinically relevant antigen density. Recently, Lynn et al. demonstrate that overexpression of c-Jun, which was previously shown to enhance persistence, improves GPC2 CAR T cell anti-tumor activity at low antigen densities without toxicity.147,148
In addition to T cell intrinsic factors, methods of counteracting T cell exhaustion by targeting the tumor microenvironment have been explored. “Armored” CAR T cells, which are so named for the protective effects in the tumor microenvironment, have become a prominent area of interest in cancer immunotherapy.14,149 These CAR T cells may express an immunostimulatory cytokine, an inhibitory receptor blocking antibody, or an inhibitory cytokine sink. In addition to the previously described PD-1 and TGF-β intercepting methods, a CAR T cell modified to constitutively secrete IL12 was able to overcome and modulate the tumor microenvironment in a model of ovarian peritoneal carcinomatosis, ultimately improving anti-tumor function, and is currently employed in a phase I clinical trial for high-grade serous ovarian cancer (NCT02498912).150 Alternatively, the physical barrier of the tumor microenvironment can be targeted. Remodeling of the extracellular matrix of the tumor microenvironment through degradation of hyaluronan has been shown to promote CAR T cell infiltration and anti-tumor activity.151
In conclusion, overcoming T cell exhaustion is a significant challenge for the success of CAR T cell therapy. Persistent antigen exposure and immunosuppression exacerbate intrinsic inhibitory signaling in the CAR T cell, driving terminal differentiation and dysfunction. In this review, we have summarized the mechanisms of exhaustion and highlighted current clinical and preclinical strategies of counteracting CAR T cell dysfunction. Methods of modulating intrinsic T cell pathways include the blockade of exhaustion-promoting signaling, inhibition of downstream effectors, the negation of TME immunosuppression, transformation of inhibitory signals to stimulatory signals, and modification of the CAR. Several of these strategies have demonstrated significant promise, and their safety and efficacy are currently being determined in clinical trials.
Outstanding questions
Despite the recent advancements in cancer immunotherapy, the prevention and reversal of exhaustion remains a focus for future research aimed at improving the CAR T cell therapeutic response. To develop more effective therapies, the deficiencies in our understanding of the mechanisms of exhaustion in the context of CAR T cells must be addressed. This would promote the development of more precise and effective methods of inhibiting T cell differentiation and hyporesponsiveness by targeting the intrinsic mechanisms of T cell exhaustion. Lastly, the safety of these methods must be exhaustively investigated as removing the brakes of the immune system could have devastating consequences.
Search strategy and selection criteria
Articles and clinical trials were selected from searches on Google Scholar, PUBMED, and ClinicalTrials.gov. Search terms used to identify relevant papers included “T cell exhaustion”, “CAR T cell”, “CAR T cell dysfunction” and “CAR T cell exhaustion”. Additionally, searches were supplemented with the search terms “mechanism”, “TME”, “PD-1”, “TOX”, “NR4A”, “TGF-B”, “Cbl-B” to identify papers relevant to the biology, molecular pathways, and mechanisms of CAR T cells and T cell exhaustion. The selected articles were published between 1993 and 2021 in English.
Declaration of interests
The authors declare no relevant conflicts of interest.
Acknowledgments
Acknowledgements
This work was supported by funding from the National Cancer Institute (K08CA201591, LDW), the California Institute for Regenerative Medicine (CIRM CLIN2-12153, LDW), and the Pediatric Cancer Research Foundation (LDW). The authors would like to thank Jennifer Shepphird for helpful critical review and editing of the manuscript.
Autor contributions
DG and LDW: conceived of and designed the manuscript. DG: performed the literature search, drafted the manuscript, and designed the figures. LDW and DG: edited and revised the manuscript. Both authors read and approved the final manuscript.
References
- 1.Tan A.T., Schreiber S. Adoptive T-cell therapy for HBV-associated HCC and HBV infection. Antivir Res. 2020;176 doi: 10.1016/j.antiviral.2020.104748. [DOI] [PubMed] [Google Scholar]
- 2.Laskowski T., Rezvani K. Adoptive cell therapy: living drugs against cancer. J Exp Med. 2020;217(12):e20200377. doi: 10.1084/jem.20200377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., Maldini C.R., Jadlowsky J., Riley J.L. Challenges and opportunities of using adoptive T-cell therapy as part of an HIV cure strategy. J Infect Dis. 2021;223(12 Suppl 2):38–45. doi: 10.1093/infdis/jiaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong KY. Immunotherapy in autoimmune diseases. Ann Acad Med Singap. 2002;31(6):702–706. [PubMed] [Google Scholar]
- 5.Duffy S.S., Keating B.A., Moalem-Taylor G. Adoptive transfer of regulatory T cells as a promising immunotherapy for the treatment of multiple sclerosis. Front Neurosci. 2019;13:1107. doi: 10.3389/fnins.2019.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshhar Z., Waks T., Gross G., Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marofi F., Motavalli R., Safonov V.A., et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12(1):81. doi: 10.1186/s13287-020-02128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zmievskaya E., Valiullina A., Ganeeva I., Petukhov A., Rizvanov A., Bulatov E. Application of CAR-T cell therapy beyond oncology: autoimmune diseases and viral infections. Biomedicines. 2021;9(1):59. doi: 10.3390/biomedicines9010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finney O.C., Brakke H.M., Rawlings-Rhea S., Hicks R., Doolittle D., Lopez M., et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest. 2019;129(5):2123–2132. doi: 10.1172/JCI125423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu L., Charwudzi A., Li Q., et al. Anti-CD19 CAR-T cell therapy bridge to HSCT decreases the relapse rate and improves the long-term survival of R/R B-ALL patients: a systematic review and meta-analysis. Ann Hematol. 2021;100(4):1003–1012. doi: 10.1007/s00277-021-04451-w. [DOI] [PubMed] [Google Scholar]
- 11.Xu X., Huang S., Xiao X., et al. Challenges and clinical strategies of CAR T-cell therapy for acute lymphoblastic leukemia: overview and developments. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.569117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majzner R.G., Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 13.Fraietta J.A., Lacey S.F., Orlando E.J., et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Garcia A., Palazon A., Noguera-Ortega E., Powell D.J., Guedan S. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front Immunol. 2020;11:1109. doi: 10.3389/fimmu.2020.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesch S., Benmebarek M.R., Cadilha B.L., et al. Determinants of response and resistance to CAR T cell therapy. Semin Cancer Biol. 2020;65:80–90. doi: 10.1016/j.semcancer.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Sterner R.C., Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G., Rui W., Zhao X., Lin X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol Immunol. 2021;18(5):1085–1095. doi: 10.1038/s41423-021-00655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmenate T., Ortiz Y., Enamorado M., et al. Blocking IL-2 signal in vivo with an IL-2 antagonist reduces tumor growth through the control of regulatory T cells. J Immunol. 2018;200(10):3475–3484. doi: 10.4049/jimmunol.1700433. [DOI] [PubMed] [Google Scholar]
- 19.Louis C.U., Savoldo B., Dotti G., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majzner R.G., Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25(9):1341–1355. doi: 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
- 21.Bucks C.M., Norton J.A., Boesteanu A.C., Mueller Y.M., Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol. 2009;182(11):6697–6708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauken K.E., Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 24.Fraietta J.A., Lacey S.F., Orlando E.J., et al. Author correction: determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2021;27(3):561. doi: 10.1038/s41591-021-01248-2. [DOI] [PubMed] [Google Scholar]
- 25.Wang D., Starr R., Alizadeh D., Yang X., Forman S.J., Brown CE. In vitro tumor cell rechallenge for predictive evaluation of chimeric antigen receptor T cell antitumor function. J Vis Exp. 2019;(144):e59275. doi: 10.3791/59275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z.Z., Kim H.J., Wu H., et al. TIGIT expression is associated with T-cell suppression and exhaustion and predicts clinical outcome and anti-PD-1 response in follicular lymphoma. Clin Cancer Res. 2020;26(19):5217–5231. doi: 10.1158/1078-0432.CCR-20-0558. [DOI] [PubMed] [Google Scholar]
- 27.Lindner S.E., Johnson S.M., Brown C.E., Wang LD. Chimeric antigen receptor signaling: functional consequences and design implications. Sci Adv. 2020;6(21):eaaz3223. doi: 10.1126/sciadv.aaz3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zajac A.J., Blattman J.N., Murali-Krishna K., et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeidi A., Zandi K., Cheok Y.Y., et al. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol. 2018;9:2569. doi: 10.3389/fimmu.2018.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong Y., Li X., Zhang L., et al. CD4+ T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol. 2019;20(1):27. doi: 10.1186/s12865-019-0309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han S., Asoyan A., Rabenstein H., Nakano N., Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci USA. 2010;107(47):20453–20458. doi: 10.1073/pnas.1008437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerlach C., van Heijst J.W., Swart E., et al. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med. 2010;207(6):1235–1246. doi: 10.1084/jem.20091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornberg M., Kenney L.L., Chen A.T., et al. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front Immunol. 2013;4:475. doi: 10.3389/fimmu.2013.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obar J.J., Lefrancois L. Memory CD8+ T cell differentiation. Ann N Y Acad Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosma G.L., Eisenlohr LC. Impact of epitope density on CD8+ T cell development and function. Mol Immunol. 2019;113:120–125. doi: 10.1016/j.molimm.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Kroger C.J., Alexander-Miller M.A. Cutting edge: CD8+ T cell clones possess the potential to differentiate into both high- and low-avidity effector cells. J Immunol. 2007;179(2):748–751. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- 38.Kroger C.J., Amoah S., Alexander-Miller M.A. Cutting edge: dendritic cells prime a high avidity CTL response independent of the level of presented antigen. J Immunol. 2008;180(9):5784–5788. doi: 10.4049/jimmunol.180.9.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S.K., Alexander-Miller M.A. Increased sensitivity to antigen in high avidity CD8+ T cells results from augmented membrane proximal T-cell receptor signal transduction. Immunology. 2011;133(3):307–317. doi: 10.1111/j.1365-2567.2011.03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curdy N., Lanvin O., Laurent C., Fournie J.J., Franchini D.M. Regulatory mechanisms of inhibitory immune checkpoint receptors expression. Trends Cell Biol. 2019;29(10):777–790. doi: 10.1016/j.tcb.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 41.McLane L.M., Abdel-Hakeem M.S., Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 42.Khan O., Giles J.R., McDonald S., et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571(7764):211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostroumov D., Duong S., Wingerath J., et al. Transcriptome profiling identifies TIGIT as a marker of T-cell exhaustion in liver cancer. Hepatology. 2021;73(4):1399–1418. doi: 10.1002/hep.31466. [DOI] [PubMed] [Google Scholar]
- 44.Jiang W., He Y., He W., et al. Exhausted CD8+T cells in the tumor immune microenvironment: new pathways to therapy. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.622509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beltra J.C., Manne S., Abdel-Hakeem M.S., et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020;52(5):825. doi: 10.1016/j.immuni.2020.04.014. -41 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu T., Zehn D. Charting the roadmap of T cell exhaustion. Immunity. 2020;52(5):724–726. doi: 10.1016/j.immuni.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Philip M., Fairchild L., Sun L., et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545(7655):452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z., Ji Z., Ngiow S.F., et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity. 2019;51(5):840. doi: 10.1016/j.immuni.2019.09.013. -55 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zander R., Schauder D., Xin G., et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity. 2019;51(6):1028. doi: 10.1016/j.immuni.2019.10.009. -42 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudson W.H., Gensheimer J., Hashimoto M., et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T Cells During Chronic Infection. Immunity. 2019;51(6):1043. doi: 10.1016/j.immuni.2019.11.002. -58 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim K., Park S., Park S.Y., et al. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med. 2020;12(1):22. doi: 10.1186/s13073-020-00722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang C., Huang S., Zhao Y., Chen S., Li Y. TOX as a potential target for immunotherapy in lymphocytic malignancies. Biomark Res. 2021;9(1):20. doi: 10.1186/s40364-021-00275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seo W., Jerin C., Nishikawa H. Transcriptional regulatory network for the establishment of CD8+ T cell exhaustion. Exp Mol Med. 2021;53(2):202–209. doi: 10.1038/s12276-021-00568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seo H., Chen J., Gonzalez-Avalos E., Samaniego-Castruita D., Das A., Wang Y.H., et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc Natl Acad Sci USA. 2019;116(25):12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J., Lopez-Moyado I.F., Seo H., et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567(7749):530–534. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott A.C., Dundar F., Zumbo P., et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571(7764):270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alfei F., Kanev K., Hofmann M., et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571(7764):265–269. doi: 10.1038/s41586-019-1326-9. [DOI] [PubMed] [Google Scholar]
- 58.Yao C., Sun H.W., Lacey N.E., et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat Immunol. 2019;20(7):890–901. doi: 10.1038/s41590-019-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLane L.M., Ngiow S.F., Chen Z., et al. Role of nuclear localization in the regulation and function of T-bet and Eomes in exhausted CD8 T cells. Cell Rep. 2021;35(6) doi: 10.1016/j.celrep.2021.109120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J., He Y., Hao J., Ni L., Dong C. High levels of Eomes promote exhaustion of anti-tumor CD8+ T cells. Front Immunol. 2018;9:2981. doi: 10.3389/fimmu.2018.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia B., Zhao C., Rakszawski K.L., et al. Eomes+T-bet(low) CD8+ T cells are functionally impaired and are associated with poor clinical outcome in patients with acute myeloid leukemia. Cancer Res. 2019;79(7):1635–1645. doi: 10.1158/0008-5472.CAN-18-3107. [DOI] [PubMed] [Google Scholar]
- 62.Riley J.L. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jubel J.M., Barbati Z.R., Burger C., Wirtz D.C., Schildberg F.A. The role of PD-1 in acute and chronic infection. Front Immunol. 2020;11:487. doi: 10.3389/fimmu.2020.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuazo M., Gato-Canas M., Llorente N., et al. Molecular mechanisms of programmed cell death-1 dependent T cell suppression: relevance for immunotherapy. Ann Transl Med. 2017;5(19):385. doi: 10.21037/atm.2017.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bardhan K., Anagnostou T., Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patsoukis N., Brown J., Petkova V., Liu F., Li L., Boussiotis V.A. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding G., Shen T., Yan C., Zhang M., Wu Z., Cao L. IFN-gamma down-regulates the PD-1 expression and assist nivolumab in PD-1-blockade effect on CD8+ T-lymphocytes in pancreatic cancer. BMC Cancer. 2019;19(1):1053. doi: 10.1186/s12885-019-6145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan Y., Cao S., Liu X., et al. CX3CR1 identifies PD-1 therapy-responsive CD8+ T cells that withstand chemotherapy during cancer chemoimmunotherapy. JCI Insight. 2018;3(8):e97828. doi: 10.1172/jci.insight.97828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamauchi T., Hoki T., Oba T., et al. T-cell CX3CR1 expression as a dynamic blood-based biomarker of response to immune checkpoint inhibitors. Nat Commun. 2021;12(1):1402. doi: 10.1038/s41467-021-21619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serganova I., Moroz E., Cohen I., et al. Enhancement of PSMA-directed CAR adoptive immunotherapy by PD-1/PD-L1 blockade. Mol Ther Oncolytics. 2017;4:41–54. doi: 10.1016/j.omto.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.John L.B., Devaud C., Duong C.P., et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 72.Adusumilli P.S., Zauderer M.G., Riviere I., et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 2021;11(11):2748–2763. doi: 10.1158/2159-8290.CD-21-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rafiq S., Yeku O.O., Jackson H.J., et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S., Siriwon N., Zhang X., Yang S., et al. Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res. 2017;23(22):6982–6992. doi: 10.1158/1078-0432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]
- 75.Chen X., Yang S., Li S., et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-beta trap enhances antitumor efficacy of CAR-T cell therapy. Mol Ther Oncolytics. 2021;21:144–157. doi: 10.1016/j.omto.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherkassky L., Morello A., Villena-Vargas J., et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu X., Zhang Y., Li K., et al. A novel dominant-negative PD-1 armored anti-CD19 CAR T cell is safe and effective against refractory/relapsed B cell lymphoma. Transl Oncol. 2021;14(7) doi: 10.1016/j.tranon.2021.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin G., Zhang Y., Yu L., Wu D. Cytotoxic effect of CLL1 CART cell immunotherapy with PD1 silencing on relapsed/refractory acute myeloid leukemia. Mol Med Rep. 2021;23(3):208. doi: 10.3892/mmr.2021.11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu G., Zhang Q., Li D., et al. PD-1 silencing improves anti-tumor activities of human mesothelin-targeted CAR T cells. Hum Immunol. 2021;82(2):130–138. doi: 10.1016/j.humimm.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Zhu H., You Y., Shen Z., Shi L. EGFRvIII-CAR-T Cells with PD-1 knockout have improved anti-glioma activity. Pathol Oncol Res. 2020;26(4):2135–2141. doi: 10.1007/s12253-019-00759-1. [DOI] [PubMed] [Google Scholar]
- 81.Rupp L.J., Schumann K., Roybal K.T., Gate R.E., Ye C.J., Lim W.A., et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo X., Jiang H., Shi B., et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118. doi: 10.3389/fphar.2018.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z., Li N., Feng K., et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell Mol Immunol. 2021;18(9):2188–2198. doi: 10.1038/s41423-021-00749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aliahmad P., Kadavallore A., de la Torre B., Kappes D., Kaye J. TOX is required for development of the CD4 T cell lineage gene program. J Immunol. 2011;187(11):5931–5940. doi: 10.4049/jimmunol.1101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu W., Zhao X., Wang X., et al. The transcription factor Tox2 drives T follicular helper cell development via regulating chromatin accessibility. Immunity. 2019;51(5):826. doi: 10.1016/j.immuni.2019.10.006. -39 e5. [DOI] [PubMed] [Google Scholar]
- 86.Liu X., Wang Y., Lu H., et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature. 2019;567(7749):525–529. doi: 10.1038/s41586-019-0979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li F., Zhang Y. Targeting NR4As, a new strategy to fine-tune CAR-T cells against solid tumors. Signal Transduct Target Ther. 2019;4:7. doi: 10.1038/s41392-019-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Batlle E., Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dahmani A., Delisle JS. TGF-beta in T cell biology: implications for cancer immunotherapy. Cancers. 2018;10(6) doi: 10.3390/cancers10060194. Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmadzadeh M., Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174(9):5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gunderson A.J., Yamazaki T., McCarty K., et al. TGFbeta suppresses CD8+ T cell expression of CXCR3 and tumor trafficking. Nat Commun. 2020;11(1):1749. doi: 10.1038/s41467-020-15404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maurice N.J., McElrath M.J., Andersen-Nissen E., Frahm N., Prlic M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8+ T cells. Nat Commun. 2019;10(1):4987. doi: 10.1038/s41467-019-12980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li K., Zhu Z., Luo J., et al. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol. 2015;8(11):14725–14732. [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas D.A., Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 95.Yang Z.Z., Grote D.M., Xiu B., et al. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin's lymphoma. Leukemia. 2014;28(9):1872–1884. doi: 10.1038/leu.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Neill R.E., Du W., Mohammadpour H., et al. T cell-derived CD70 delivers an immune checkpoint function in inflammatory T cell responses. J Immunol. 2017;199(10):3700–3710. doi: 10.4049/jimmunol.1700380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leigh N.D., O'Neill R.E., Du W., et al. Host-derived CD70 suppresses murine graft-versus-host disease by limiting donor T cell expansion and effector function. J Immunol. 2017;199(1):336–347. doi: 10.4049/jimmunol.1502181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park B.V., Freeman Z.T., Ghasemzadeh A., et al. TGFbeta1-mediated SMAD3 enhances PD-1 expression on antigen-specific T cells in cancer. Cancer Discov. 2016;6(12):1366–1381. doi: 10.1158/2159-8290.CD-15-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gabriel S.S., Tsui C., Chisanga D., et al. Transforming growth factor-beta-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity. 2021;54(8):1698. doi: 10.1016/j.immuni.2021.06.007. -714 e5. [DOI] [PubMed] [Google Scholar]
- 100.Tang N., Cheng C., Zhang X., et al. TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 2020;5(4):e133977. doi: 10.1172/jci.insight.133977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kloss C.C., Lee J., Zhang A., et al. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther. 2018;26(7):1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Webster B., Xiong Y., Hu P., et al. Self-driving armored CAR-T cells overcome a suppressive milieu and eradicate CD19+ Raji lymphoma in preclinical models. Mol Ther. 2021;29(9):2691–2706. doi: 10.1016/j.ymthe.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stuber T., Monjezi R., Wallstabe L., et al. Inhibition of TGF-beta-receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J Immunother Cancer. 2020;8(1):e000676. doi: 10.1136/jitc-2020-000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uhl M., Aulwurm S., Wischhusen J., et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64(21):7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 105.Li Y., Wu H., Chen G., et al. Arming anti-EGFRvIII CAR-T With TGFbeta trap improves antitumor efficacy in Glioma mouse models. Front Oncol. 2020;10:1117. doi: 10.3389/fonc.2020.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hou A.J., Chang Z.L., Lorenzini M.H., Zah E., Chen YY. TGF-beta-responsive CAR-T cells promote anti-tumor immune function. Bioeng Transl Med. 2018;3(2):75–86. doi: 10.1002/btm2.10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hartley J., Abken H. Chimeric antigen receptors designed to overcome transforming growth factor-beta-mediated repression in the adoptive T-cell therapy of solid tumors. Clin Transl Immunol. 2019;8(6):e1064. doi: 10.1002/cti2.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang Z.L., Lorenzini M.H., Chen X., Tran U., Bangayan N.J., Chen YY. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol. 2018;14(3):317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pang N., Shi J., Qin L., et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14(1):118. doi: 10.1186/s13045-021-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singha P.K., Pandeswara S., Geng H., et al. Increased Smad3 and reduced Smad2 levels mediate the functional switch of TGF-beta from growth suppressor to growth and metastasis promoter through TMEPAI/PMEPA1 in triple negative breast cancer. Genes Cancer. 2019;10(5-6):134–149. doi: 10.18632/genesandcancer.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu N., Lian G., Sheng J., et al. Discovery of a novel selective water-soluble SMAD3 inhibitor as an antitumor agent. Bioorg Med Chem Lett. 2020;30(17) doi: 10.1016/j.bmcl.2020.127396. [DOI] [PubMed] [Google Scholar]
- 112.Lutz-Nicoladoni C., Wolf D., Sopper S. Modulation of Immune cell functions by the E3 ligase Cbl-b. Front Oncol. 2015;5:58. doi: 10.3389/fonc.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar J., Kumar R., Kumar Singh A., et al. Deletion of Cbl-b inhibits CD8+ T-cell exhaustion and promotes CAR T-cell function. J Immunother Cancer. 2021;9(1):e001688. doi: 10.1136/jitc-2020-001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sitaram P., Uyemura B., Malarkannan S., Riese M.J. Beyond the cell surface: targeting intracellular negative regulators to enhance T cell anti-tumor activity. Int J Mol Sci. 2019;20(23):5821. doi: 10.3390/ijms20235821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiang J.Y., Jang I.K., Hodes R., Gu H. Ablation of Cbl-b provides protection against transplanted and spontaneous tumors. J Clin Invest. 2007;117(4):1029–1036. doi: 10.1172/JCI29472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fujiwara M., Anstadt E.J., Clark R.B. Cbl-b deficiency mediates resistance to programmed death-ligand 1/programmed death-1 regulation. Front Immunol. 2017;8:42. doi: 10.3389/fimmu.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peer S., Baier G., Gruber T. Cblb-deficient T cells are less susceptible to PD-L1-mediated inhibition. Oncotarget. 2017;8(26):41841–41853. doi: 10.18632/oncotarget.18360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shah N.N., Qin H., Yates B., et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019;3(15):2317–2322. doi: 10.1182/bloodadvances.2019000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loeser S., Loser K., Bijker M.S., et al. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med. 2007;204(4):879–891. doi: 10.1084/jem.20061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zha Y., Gajewski T.F. An adenoviral vector encoding dominant negative Cbl lowers the threshold for T cell activation in post-thymic T cells. Cell Immunol. 2007;247(2):95–102. doi: 10.1016/j.cellimm.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou X., Sun S.C. Targeting ubiquitin signaling for cancer immunotherapy. Signal Transduct Target Ther. 2021;6(1):16. doi: 10.1038/s41392-020-00421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeon M.S., Atfield A., Venuprasad K., et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21(2):167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 123.Chiang Y.J., Kole H.K., Brown K., et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403(6766):216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 124.Weber E.W., Parker K.R., Sotillo E., et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372(6537) doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Richman S.A., Wang L.C., Moon E.K., Khire U.R., Albelda S.M., Milone MC. Ligand-induced degradation of a CAR permits reversible remote control of CAR T cell activity in vitro and in vivo. Mol Ther. 2020;28(7):1600–1613. doi: 10.1016/j.ymthe.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Juillerat A., Tkach D., Busser B.W., et al. Modulation of chimeric antigen receptor surface expression by a small molecule switch. BMC Biotechnol. 2019;19(1):44. doi: 10.1186/s12896-019-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang R.Y., Wei D., Liu Z.K., et al. Doxycycline inducible chimeric antigen receptor T cells targeting CD147 for hepatocellular carcinoma therapy. Front Cell Dev Biol. 2019;7:233. doi: 10.3389/fcell.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gu X., He D., Li C., Wang H., Yang G. Development of inducible CD19-CAR T cells with a Tet-on system for controlled activity and enhanced clinical safety. Int J Mol Sci. 2018;19(11):3455. doi: 10.3390/ijms19113455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Drent E., Poels R., Mulders M.J., et al. Feasibility of controlling CD38-CAR T cell activity with a Tet-on inducible CAR design. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0197349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Greenshpan Y., Sharabi O., Ottolenghi A., et al. Synthetic promoters to induce immune-effectors into the tumor microenvironment. Commun Biol. 2021;4(1):143. doi: 10.1038/s42003-021-01664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eyquem J., Mansilla-Soto J., Giavridis T., et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brandt L.J.B., Barnkob M.B., Michaels Y.S., Heiselberg J., Barington T. Emerging approaches for regulation and control of CAR T cells: a mini review. Front Immunol. 2020;11:326. doi: 10.3389/fimmu.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu X., Wen J., Yi H., et al. Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920910347. 1758835920910347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leung W.H., Gay J., Martin U., et al. Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization. JCI Insight. 2019;5(11):e124430. doi: 10.1172/jci.insight.124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Viaud S., Ma J.S.Y., Hardy I.R., et al. Switchable control over in vivo CAR T expansion, B cell depletion, and induction of memory. Proc Natl Acad Sci USA. 2018;115(46):E10898–E1E906. doi: 10.1073/pnas.1810060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zajc C.U., Dobersberger M., Schaffner I., et al. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc Natl Acad Sci USA. 2020;117(26):14926–14935. doi: 10.1073/pnas.1911154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee Y.G., Chu H., Lu Y., et al. Regulation of CAR T cell-mediated cytokine release syndrome-like toxicity using low molecular weight adapters. Nat Commun. 2019;10(1):2681. doi: 10.1038/s41467-019-10565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu C.Y., Roybal K.T., Puchner E.M., Onuffer J., Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350(6258):aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mata M., Gerken C., Nguyen P., Krenciute G., Spencer D.M., Gottschalk S. Inducible activation of MyD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov. 2017;7(11):1306–1319. doi: 10.1158/2159-8290.CD-17-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poorebrahim M., Melief J., Pico de Coana Y., S L.W., Cid-Arregui A., Kiessling R. Counteracting CAR T cell dysfunction. Oncogene. 2021;40(2):421–435. doi: 10.1038/s41388-020-01501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Catakovic K., Klieser E., Neureiter D., Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal. 2017;15(1):1. doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du S., McCall N., Park K., et al. Blockade of tumor-expressed PD-1 promotes lung cancer growth. Oncoimmunology. 2018;7(4) doi: 10.1080/2162402X.2017.1408747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McGowan E., Lin Q., Ma G. PD-1 disrupted CAR-T cells in the treatment of solid tumors: promises and challenges. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109625. [DOI] [PubMed] [Google Scholar]
- 144.Wang X., Yang X., Zhang C., et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc Natl Acad Sci USA. 2020;117(12):6640–6650. doi: 10.1073/pnas.1921445117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Diaconu I., Ballard B., Zhang M., et al. Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol Ther. 2017;25(3):580–592. doi: 10.1016/j.ymthe.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou X., Dotti G., Krance R.A., et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125(26):4103–4113. doi: 10.1182/blood-2015-02-628354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lynn R.C., Weber E.W., Sotillo E., et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576(7786):293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heitzeneder S., Bosse K.R., Zhu Z., et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell. 2022;40(1):53–69. doi: 10.1016/j.ccell.2021.12.005. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yeku O.O., Brentjens R.J. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans. 2016;44(2):412–418. doi: 10.1042/BST20150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yeku O.O., Purdon T.J., Koneru M., Spriggs D., Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep. 2017;7(1):10541. doi: 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao R., Cui Y., Zheng Y., et al. Human hyaluronidase PH20 potentiates the antitumor activities of mesothelin-specific CAR-T cells against gastric cancer. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.660488. [DOI] [PMC free article] [PubMed] [Google Scholar]